Abstract
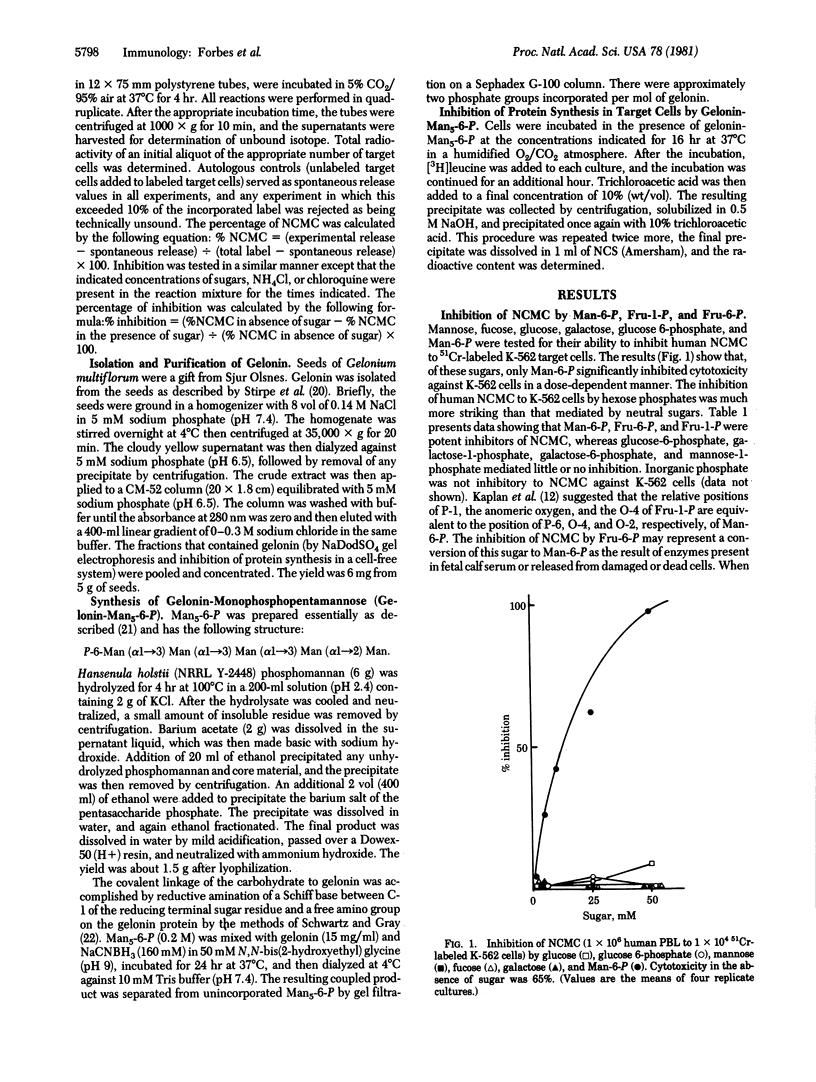
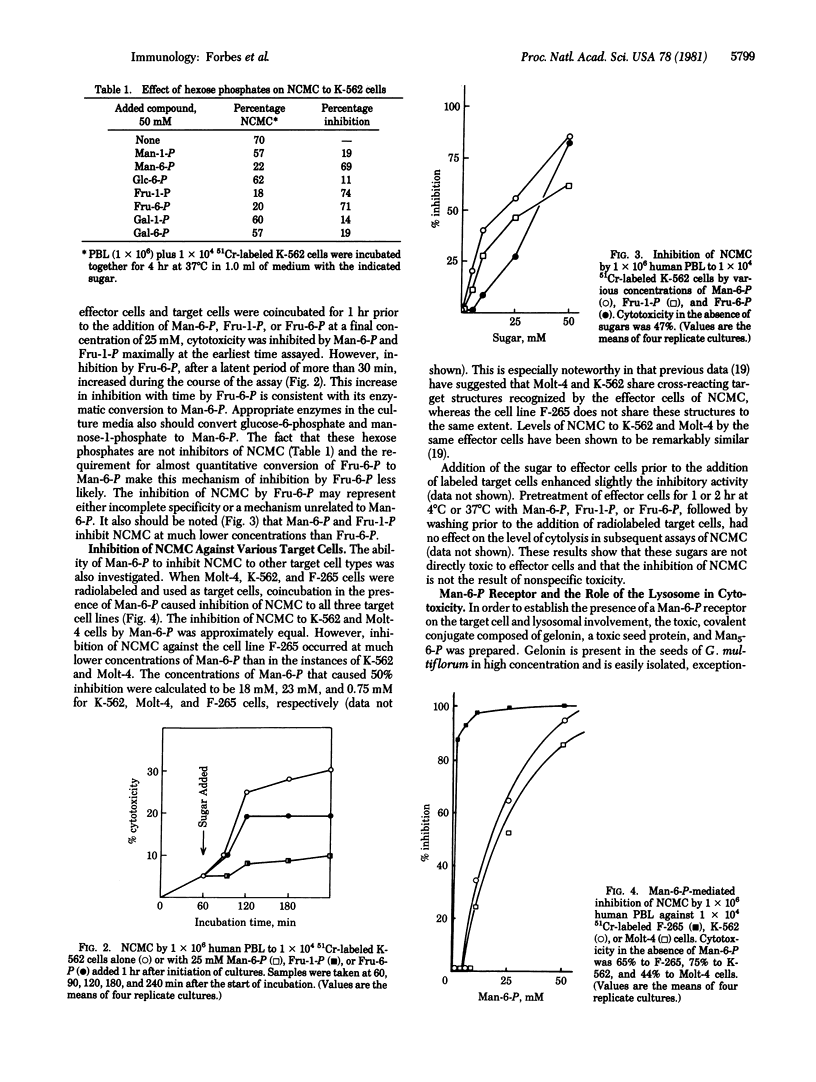
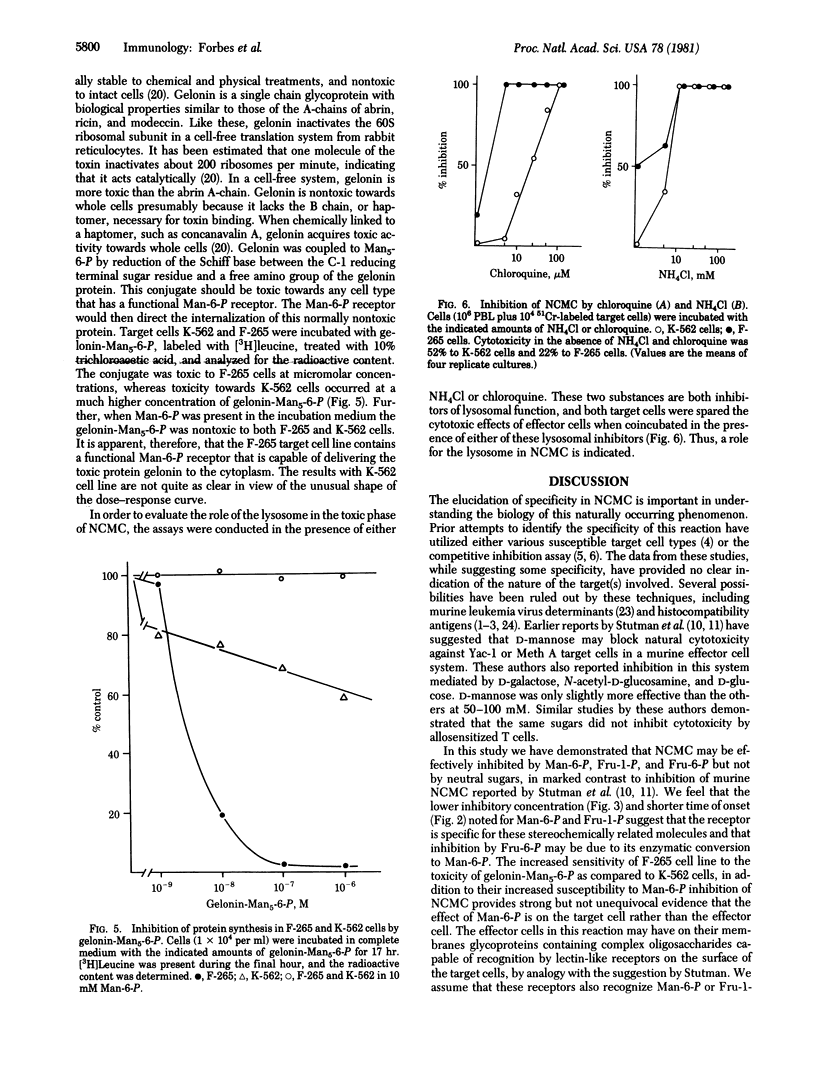
In vitro human natural cell-mediated cytotoxicity (NCMC) to K-562, Molt-4, and F-265 cells is inhibited in a dose-dependent manner by mannose 6-phosphate, fructose 1-phosphate and fructose 6-phosphate. This inhibition is not observed with mannose, glucose, fucose, glucose 6-phosphate, mannose 1-phosphate, galactose 1-phosphate, or galactose 6-phosphate. Preincubation of the effector cells, obtained from fresh whole blood, with mannose-6-phosphate, fructose-1-phosphate, or fructose-6-phosphate did not inhibit cytotoxicity, which indicated that these hexose phosphates are not nonspecifically toxic towards the effector lymphocytes. Mannose-6-phosphate and the stereochemically similar fructose-1-phosphate are more potent inhibitors than fructose-6-phosphate in terms of concentration required and time of onset of effect. Inhibition of cytotoxicity by mannose-6-phosphate varied with target cell type: F-265 is protected at much lower concentrations of mannose-6-phosphate (less than 1 mM) than is either Molt-4 or K-562. The inhibition of NCMC is also observed with the inhibitors of lysosomal function, NH4Cl, and chloroquine. The presence of a functional mannose-6-phosphate receptor on target cells was demonstrated: (i) Gelonin, a seed protein that inactivates the eukaryotic ribosome but is nontoxic to intact cells, was covalently linked to monophosphopentamannose, and this conjugate ws toxic to both K-562 and F-265 target cells, the latter being by far the more sensitive; and (ii) chloroquine, NH4Cl, and mannose-6-phosphate all inhibited the toxicity of gelonin-monophosphopentamannose. These results suggest either that a cytolytic lymphokine contains a hexose phosphate residue and may be taken up by target cells through the lysosomal/mannose 6-phosphate pathway or that such a residue is involved in target cell-effector cell recognition.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bretthauer R. K., Kaczorowski G. J., Weise M. J. Characterization of a phosphorylated pentasaccharide isolated from Hansenula holstii NRRL Y-2448 phosphomannan. Biochemistry. 1973 Mar 27;12(7):1251–1256. doi: 10.1021/bi00731a002. [DOI] [PubMed] [Google Scholar]
- Chism S. E., Burton R. C., Grail D. L., Bell P. M., Warner N. L. In vitro induction of tumor-specific immunity. VI: analysis of specificity of immune response by cellular competitive inhibition: limitations and advantages of the technique. J Immunol Methods. 1977;16(3):245–262. doi: 10.1016/0022-1759(77)90202-2. [DOI] [PubMed] [Google Scholar]
- Fischer H. D., Gonzalez-Noriega A., Sly W. S., Morré D. J. Phosphomannosyl-enzyme receptors in rat liver. Subcellular distribution and role in intracellular transport of lysosomal enzymes. J Biol Chem. 1980 Oct 25;255(20):9608–9615. [PubMed] [Google Scholar]
- Herberman R. B., Djeu J., Kay H. D., Ortaldo J. R., Riccardi C., Bonnard G. D., Holden H. T., Fagnani R., Santoni A., Puccetti P. Natural killer cells: characteristics and regulation of activity. Immunol Rev. 1979;44:43–70. doi: 10.1111/j.1600-065x.1979.tb00267.x. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Kaplan A., Achord D. T., Sly W. S. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc Natl Acad Sci U S A. 1977 May;74(5):2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling R., Wigzell H. An analysis of the murine NK cell as to structure, function and biological relevance. Immunol Rev. 1979;44:165–208. doi: 10.1111/j.1600-065x.1979.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Cytotoxicity of a factor isolated from human spleen. J Natl Cancer Inst. 1973 Feb;50(2):535–538. doi: 10.1093/jnci/50.2.535. [DOI] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Oldham R. K., Ortaldo J. R., Holden H. T., Herberman R. B. Cytotoxicity inhibition assay: cryopreservation and standardization: brief communication. J Natl Cancer Inst. 1977 Oct;59(4):1321–1323. doi: 10.1093/jnci/59.4.1321. [DOI] [PubMed] [Google Scholar]
- Pavie-Fischer J., Kourilsky F. M., Picard F., Banzet P., Puissant A. Cytotoxicity of lymphocytes from healthy subjects and from melanoma patients against cultured melanoma cells. Clin Exp Immunol. 1975 Sep;21(3):430–441. [PMC free article] [PubMed] [Google Scholar]
- Roder J. C., Ahrlund-Richter L., Jondal M. Target-effector interaction in the human and murine natural killer system: specificity and xenogeneic reactivity of the solubilized natural killer-target structure complex and its loss in a somatic cell hybrid. J Exp Med. 1979 Sep 19;150(3):471–481. doi: 10.1084/jem.150.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder J. C., Argov S., Klein M., Petersson C., Kiessling R., Andersson K., Hansson M. Target-effector cell interaction in the natural killer cell system. V. Energy requirements, membrane integrity, and the possible involvement of lysosomal enzymes. Immunology. 1980 May;40(1):107–116. [PMC free article] [PubMed] [Google Scholar]
- Sando G. N., Neufeld E. F. Recognition and receptor-mediated uptake of a lysosomal enzyme, alpha-l-iduronidase, by cultured human fibroblasts. Cell. 1977 Nov;12(3):619–627. doi: 10.1016/0092-8674(77)90262-8. [DOI] [PubMed] [Google Scholar]
- Schwartz B. A., Gray G. R. Proteins containing reductively aminated disaccharides. Synthesis and chemical characterization. Arch Biochem Biophys. 1977 Jun;181(2):542–549. doi: 10.1016/0003-9861(77)90261-2. [DOI] [PubMed] [Google Scholar]
- Stirpe F., Olsnes S., Pihl A. Gelonin, a new inhibitor of protein synthesis, nontoxic to intact cells. Isolation, characterization, and preparation of cytotoxic complexes with concanavalin A. J Biol Chem. 1980 Jul 25;255(14):6947–6953. [PubMed] [Google Scholar]
- Stutman O., Dien P., Wisun R. E., Lattime E. C. Natural cytotoxic cells against solid tumors in mice: blocking of cytotoxicity by D-mannose. Proc Natl Acad Sci U S A. 1980 May;77(5):2895–2898. doi: 10.1073/pnas.77.5.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi M., Koide D., Ramseyer A. Specificities in natural cell-mediated cytotoxicity by the cross-competition assay. Int J Cancer. 1977 Mar 15;19(3):291–297. doi: 10.1002/ijc.2910190303. [DOI] [PubMed] [Google Scholar]
- Takasugi M., Mickey M. R. Interaction analysis of selective and nonselective cell-mediated cytotoxicity. J Natl Cancer Inst. 1976 Aug;57(2):255–261. doi: 10.1093/jnci/57.2.255. [DOI] [PubMed] [Google Scholar]
- Whisnant C. C., Singer K. H., Amos D. B. Interaction of cytotoxic T lymphocytes with target cells. I. Specific inhibition by detergent-solubilized, partially purified mouse histocompatibility antigens. J Immunol. 1978 Dec;121(6):2253–2256. [PubMed] [Google Scholar]
- Wright S. C., Bonavida B. Selective lysis of NK-sensitive target cells by a soluble mediator released from murine spleen cells and human peripheral blood lymphocytes. J Immunol. 1981 Apr;126(4):1516–1521. [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]


