Abstract
Oxidative stress is emerging as a key factor underpinning life history and the expression of sexually selected traits. Resolving the role of oxidative stress in life history and sexual selection requires a pluralistic approach, which investigates how age affects the relationship between oxidative status (i.e., antioxidants and oxidative damage) and the multiple traits contributing to variation in reproductive success. Here, we investigate the relationship between oxidative status and the expression of multiple sexually selected traits in two-age classes of male red junglefowl, Gallus gallus, a species which displays marked male reproductive senescence. We found that, irrespective of male age, both male social status and comb size were strongly associated with plasma oxidative status, and there was a nonsignificant tendency for sperm motility to be associated with seminal oxidative status. Importantly, however, patterns of plasma and seminal antioxidant levels differed markedly in young and old males. While seminal antioxidants increased with plasma antioxidants in young males, the level of seminal antioxidants remained low and was independent of plasma levels in old males. In addition, old males also accumulated more oxidative damage in their sperm DNA. These results suggest that antioxidant allocation across different reproductive traits and somatic maintenance might change drastically as males age, leading to age-specific patterns of antioxidant investment.
Keywords: Oxidative stress, reproductive restraints, reproductive senescence, sexual selection, sperm competition
Introduction
Oxidative stress occurs when antioxidants defenses cannot fully compensate for the oxidant activity of reactive molecules (i.e., reactive oxygen species, ROS; Halliwell and Gutteridge 2007), and is thought to be a universal cause of aging and a fundamental factor in life history evolution (Finkel and Holbrook 2000). Recent work suggests that oxidative stress might be particularly relevant to male traits affecting reproductive success and targeted by sexual selection (von Schantz et al. 1999; Dowling and Simmons 2009; Monaghan et al. 2009). Sexual selection operates on traits that affect a male's ability to compete for mates, such as ornaments and competitive ability, and those that affect the ability of his ejaculates to compete over fertilization, when females mate multiply (Birkhead and Møller 1992; Andersson 1994; Pizzari and Parker 2009). Oxidative stress might mediate the condition-dependent expression of sexual ornaments, via antioxidant allocation trade-offs (von Schantz et al. 1999; Blount et al. 2003; Pike et al. 2007; Pérez et al. 2008; Dowling and Simmons 2009; Monaghan et al. 2009), and has also been identified as an important factor shaping the fertilization efficiency of an ejaculate under sperm competition (Blount et al. 2001; Poiani 2006; Velando et al. 2008; Dowling and Simmons 2009; Almbro et al. 2011). For example, sperm motility and viability can be affected by the antioxidant capacity of seminal fluid, which protects sperm from oxidative damage (Poiani 2006; see also den Boer et al. 2010; Simmons and Beveridge 2011). Oxidative stress deteriorates sperm plasma membrane and DNA, leading to a decline in fertilizing efficiency and sperm competitiveness (Aitken and Baker 2006; Poiani 2006).
As their physiological performance declines (Rose 1991), aging organisms may suffer from less efficient antioxidant systems, and as a consequence, become more vulnerable to oxidative stress (Sohal and Weindruch 1996; Finkel and Holbrook 2000; Torres and Velando 2007). Therefore, antioxidant demands exacted by reproductive investment may be become relatively more costly as individuals age, and this might contribute to age-related declines in reproductive success (i.e., reproductive senescence; Torres and Velando 2007; Monaghan et al. 2009). Age-related declines in male mating and fertilizing success have been documented in a number of taxa (Radwan et al. 2005; Pizzari et al. 2008a; Møller et al. 2009; Dean et al. 2010; Carazo et al. 2011), and some studies have indicated a role for oxidative stress in the senescence of certain reproductive traits (Sikka 2001; Torres and Velando 2007; Pizzari et al. 2008a; Dowling and Simmons 2009; Velando et al. 2011).
Determining the extent to which oxidative stress contributes to the senescence of sexually selected traits is key to better understand the evolution of male life-history strategies and the fitness consequences of mate preferences (e.g., preference for old partners, e.g., Kokko and Lindström 1996). However, it is difficult to establish the role of oxidative stress without a pluralistic approach, which investigates how age affects the relationship between oxidative status (i.e., antioxidants and oxidative damage) and the multiple traits contributing to reproductive success. For example, in species with both pre- and postcopulatory sexual selection, males may adopt different mating strategies as they age, modifying their relative antioxidant investment to different components of reproductive effort, including sexual ornaments versus sperm quality (Preston et al. 2011). Previous studies showed that oxidative stress may negatively affect pre- and postcopulatory sexually selected traits (Metcalfe and Alonso-Alvarez 2010 and reference therein) as well as the age-related decline in such traits (Pizzari et al. 2008a; Møller et al. 2009; Velando et al. 2011; Dean et al. 2010). However, the way in which age-related changes in male oxidative status influence strategies in antioxidant allocation among different components of reproductive success remains to be elucidated. As a consequence, the contribution of such age-related patterns of antioxidant allocation to male reproductive senescence remains unresolved.
Here, we examine the relationships between oxidative status and the expression of multiple pre- and postmating reproductive traits in two-age classes of male red junglefowl, Gallus gallus (Fig. 1). The red junglefowl is an appropriate system to explore age-specific changes in antioxidants allocation strategies. First, natural populations are structured in social groups (Sullivan 1991) in which multiple males compete for access to females, and male mating success is strongly influenced by male social status (Lill 1966; Johnsen et al. 2001; see also Pizzari et al. 2002). Male social status is also sexually selected after copulation, as females preferentially retain semen of dominant males (Pizzari and Birkhead 2000; Dean et al. 2011). Second, the male comb is a fleshy sexual ornament whose size is consistently associated with female preference in mate choice experiments (Zuk et al. 1990; Parker and Ligon 2003). Comb size is strongly condition-dependent in this species (Zuk et al. 1995) and has been suggested to reflect a male's oxidative stress (von Schantz et al. 1999). Finally, despite a skew in male mating success, females often mate with multiple males within a breeding season (Ligon and Zwartjes 1995), and sperm remain viable within the female sperm storage tubules for approximately 2 weeks (Etches 1996), creating a risk of sperm competition. Experiments in the domestic fowl, G. g. domesticus, indicate that sperm competition favors large inseminations (Martin et al. 1974), and particularly ejaculates with prolonged sperm motility and viability (Pizzari et al. 2008b). A significant proportion of inter- and intramale variation in sperm motility and viability in this species appears to be determined by seminal fluid, the nonsperm physiological component of an ejaculate (Pizzari et al. 2007; Cornwallis and O'Connor 2009). Importantly, male social status, comb size, and sperm motility tend to decline with male age (Dean et al. 2010).
Figure 1.

Adult red junglefowl male Gallus gallus. During breeding season, males show large combs, a trait important in both mate choice and male competition. Photo credit: Jose C. Noguera.
The aim of this study was to determine whether these patterns of senescence are associated with changes in oxidative status. Specifically, we addressed the following questions: (i) Is social status associated with plasma oxidative status in young and old males? (ii) Does comb size covary with plasma oxidative status in young and old males? (iii) Does the oxidative status of seminal fluid change with male age? (iv) Does sperm motility covary with seminal oxidative status? (v) Does sperm DNA damage covary with seminal oxidative status in young and old males?
If male traits sexually selected before and after copulation are influenced by male oxidative status in this species, one would expect that; (1) dominant males have better oxidative status than subordinates, (2) males with better plasma oxidative status have larger combs, and (3) that males with better seminal oxidative status also have higher sperm motility and reduced levels of sperm oxidative damage. Moreover, if the availability of antioxidant resources to sexually selected traits becomes limited as a male ages, three alternative scenarios can be predicted depending on the antioxidant allocation strategy adopted by old males. First, old males could experience a decline in both comb size and sperm quality. Second, old males could allocate proportionally more antioxidant resources to either comb size or sperm quality, in which case we would expect a strong decline in only one trait (sperm quality or comb size), but less so in the other trait.
Methods
Study population
The study was carried out in a population of red junglefowl housed at the John Krebs Field Station of the University of Oxford, during breeding (May–July 2010). We selected sexually mature males of two-age classes: six “young” (1-year-old), and 15 “old” (4-year-old). In this species, males live at least up to 5.5 years under seminatural conditions (Collias and Collias 1996), and recent data on feral populations of domestic fowl, G. g. domesticus, suggest that the onset of male reproductive senescence (i.e., strong decline in the number of copulations or sperm motility) occurs on average at the age of four (Dean et al. 2010). Body mass (±1 g) and tarsus length (±0.01 mm) were measured for each male. To prevent any age-related effect of copulation rate on seminal and somatic oxidative status, males did not have access to females until the end of the study.
A subset of males (n = 14) were kept in groups of three for at least a week prior to sampling to allow stable social hierarchies to form. Social hierarchy was determined through behavioral observations of pairwise interactions. Males that ranked top in the trio were classified as dominant and males that were either second or third were classified as subordinate.
Semen samples were collected through abdominal massage for each male in between 11:00–16:00 h, and spermatozoa and seminal fluid were separated within 15 min by centrifugation (1 min × 14,000g at 4°C). To standardize sperm age across all males (Reinhardt 2007; Pizzari et al. 2008b), 48 h before sampling, males were depleted of sperm reserves through abdominal massage (Burrows and Quinn 1937). A blood sample (∼250 μL) was also obtained from the brachial vein through heparinized capillary tubes. Plasma was separated from blood cells within 15 min after collection by centrifugation (10 min × 6500g at 4°C). All samples (seminal fluid, spermatozoa, blood plasma, and blood cells) were immediately transferred to cryovials after centrifugation and stored in liquid nitrogen during transfer back to the laboratory for storage at –80°C until their analysis (within a month). To estimate the effect of sperm oxidative status on sperm motility in old males, we collected a semen sample in an additional group of 11 old males (4-year-old). Sperm motility was measured as sperm average path velocity (sperm swimming speed: μms-1) using computer assisted sperm analysis (Sperm Class Analyzer: SCA v. 3.0.3). One microliter of semen was diluted in 50 μL of Dolbecco's modified Eagle's medium, and 5μL of this was placed on a mounted microscope slide on a heated microscope stage at 41°C and video recorded at 200× magnification. Median sperm average path velocity was calculated per ejaculate.
Comb size was measured using digital photographs of the right- and left-side of the bird's head under standardized lighting conditions and against a standard white background together with a milimetric scale (Cornwallis and Birkhead 2007). The comb area (mm2) in the right- and left-side photographs of each male was measured by the same person (J. C. N.) using image analysis software (analySIS FIVE) blindly with respect to male age and status, and the mean of the left- and right-side was used all further analyses.
Measures of antioxidant capacity and oxidative damage
Analysis of antioxidant capacity
The antioxidant capacity of plasma (hereafter, “plasma antioxidants”) and seminal fluid (hereafter, “seminal antioxidants”) was measured using the method described by Erel (2004). Main antioxidants contributing to the assay are hydrophilic and hydrophobic antioxidants, such as –SH group of proteins, uric acid, vitamin-C, and vitamin-E. Briefly, the method consists of mixing plasma or seminal fluid (5 μL) with acetate solution and 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate) (ABTS), which is decolorized by antioxidant compounds according to their concentration and antioxidant capacity. The change in color was measured as the change in absorbance at 660 nm (Synergy™ 2 Multi-Mode Microplate Reader, Bio-Tek Instruments, Inc., Winooski, VT). Samples were assayed in duplicate and showed high level of repeatability (blood plasma: r = 0.97, F17,18 = 66.377, P < 0.001, CV = 0.06; seminal fluid: r = 0.84, F29,30 = 11.379, P < 0.001, CV = 0.07; as described by Lessells and Boag 1987). Levels of plasma and seminal antioxidants were expressed as millimoles of Trolox equivalent per liter.
Analysis of lipid peroxidation
Lipid peroxidation (level of oxidative damage to lipids) in plasma and seminal fluid (20 μL) was assessed by quantifying malondialdehydes (MDA), using high-performance liquid chromatography, according to Karatas et al. (2002), but modifying the volume of sample as described in Noguera et al. (2011). The absorbance of the eluent was monitored at 254 nm and 1,1,3,3-tetraethoxypropane (Sigma-Aldrich, St. Louis, MO) was used as external standard (calibration curves, R2 = 0.999). Plasma and seminal fluid samples were assayed in triplicate and duplicated, respectively, and showed high level of repeatability (blood plasma: r = 0.99, F17,36 = 3.694, P < 0.001; seminal fluid: r = 0.99, F9,10 = 21.142, P < 0.001). Lipid peroxidation was expressed as μg of MDA per milliliter.
Analysis of oxidative DNA damage
The analysis of oxidative DNA damage present in sperm samples (spermatozoa) were assessed as described in Velando et al. (2011). Basically, sperm DNA was extracted by a Chaotropic NaI-based method, as recommended by European Standars Committee on Oxidative DNA Damage (ESCODD) to avoid artifactual oxidation. The amount of isolated DNA was determined using high sensitivity fluorescent assay (Quant-iT™ High-Sensitivity DNA Assay Kit, Invitrogen, USA), and protein contamination checked. DNA damage was estimated as apurinic–apyrimidinic (AP) sites using a biotin-labeled reagent specific for the aldehyde group in the ring-open form of AP site, designated as the aldehyde reactive probe (ARP), and according to manufacturer's instructions (ARP; Oxidative DNA Damage Quantitation kit-AP sites; Cell Biolabs, San Diego, California). ARP specifically binds to AP sites in isolated genomic DNA, and the biotin molecular in ARP can then be detected spectrophotometrically at 450 nm (Synergy™ 2 Multi-Mode Microplate Reader, Bio-Tek Instruments, Inc., Winooski). The quantities of AP sites in sperm DNA samples were assayed in duplicate in a single bout (repeatability: r = 0.97, F17,18 = 73.046, P < 0.001) and expressed as the number of AP sites per 100,000 base pair.
Statistical analysis
Age and plasma oxidative status
We studied age-related differences on oxidative status of males by fitting two separate general linear models (GLMs) with plasma antioxidants and lipid peroxidation level as response variables. In these models, age (two-level factor), male body condition (covariate), and their interaction were fitted as fixed effects. Male body condition was calculated as residuals of linear regression of tarsus length on body mass (Schulte-Hostedde et al. 2005).
Comb size and plasma oxidative status
To investigate whether comb size covaries with oxidative status (“antioxidant trade-off hypothesis”), and whether age reduces antioxidant availability to comb, we fitted a GLM with comb size as the response variable, and age (two-level fixed factor) and plasma antioxidants (covariate), lipid peroxidation (covariate), and their interaction with male age as covariates. To control for any possible effect of male condition on comb size, we also included male body condition and its interaction with age as covariates in the model.
Seminal oxidative status and male age
To further test the antioxidant trade-off hypothesis we fitted the same model as described for the above analysis (Comb size and plasma oxidative status), but this time with seminal antioxidants as the response variable.
Seminal oxidative status and sperm motility
We also explored whether sperm oxidative status influences sperm motility of old males by fitting a model (GLM), which included sperm motility as dependent variable, and seminal antioxidants and lipid peroxidation levels as response variables. In this case, lipid peroxidation was previously log-transformed to fit normal distribution.
Seminal oxidative status and sperm DNA damage
Finally, we investigated the effect of age and seminal antioxidants on sperm DNA damage by fitting a model (GLM) with age as a fixed factor, and male body condition, seminal antioxidants, and their interactions with age as covariates. Sperm DNA damage (response variable) was log-transformed to meet model requirements. We also ran the same models including male social status (two-level factor) and its interaction with male age for the subset of males (n = 14) in which social status was recorded.
Multicollinearity diagnostics were examined in all models by calculating the collinearity statistic tolerance and the corresponding variance inflation factor (Quinn and Keough 2002). Tolerance values ranged from 0.69 to 0.96 indicating that the degrees of multicollinearity among the independent variables were acceptable. Following Whittingham et al. (2006), full models were also reported including nonsignificant fixed effects terms, but excluding nonsignificant interactions. Models limited to significant effects (minimal adequate models) provided similar results. All models were simplified by removing nonsignificant terms (in a backward deletion procedure), starting from two-way interactions. In four cases, digital photographs of combs were not available. Discrepancies in sample sizes between some analyses reflect missing values due to insufficient volume of blood or ejaculates samples to perform the biochemical assays (i.e., seminal lipid peroxidation or DNA damage). All statistical analyses were performed using SPSS (SPSS v.18), and the significance level was set at 0.05.
Results
Social status and plasma oxidative status
Oxidative status in plasma did not differ between male age classes. Old males had similar levels of plasma antioxidants and lipid peroxidation to young males (Table 1a). Similarly, plasma antioxidants and lipid peroxidation levels were not affected by male body condition or its interaction with age (Table 1a). When the model included social status, there was a significant effect of social status; dominant males had on average 33% higher levels of plasma antioxidants than subordinates (F1,12 = 7.172, P = 0.02; Fig. 2), whereas the levels of lipid peroxidation did not differ between dominant and subordinate males (estimate = –0.048, F1,11 = 1.952, P = 0.19). The interaction between age and social status did not explain variation in plasma antioxidants (estimate = 0.782, F1,9 = 1.653, P = 0.23) or lipid peroxidation levels (estimate = 0.018, F1,9 = 0.070, P = 0.79).
Table 1.
Summary of general lineal models (GLMs) showing the influence of (a) male age on plasma oxidative status, (b) plasma oxidative status on comb size, (c) age on seminal oxidative status, (d) seminal oxidative status on sperm swimming velocity, and (e) seminal antioxidant status on oxidative DNA damage in red junglefowl (Gallus gallus). Full models and the models that retained only variables that caused a significant increase in deviance (minimal adequate models) are shown. Significant values in full and minimal adequate model are shown in bold
| Full model | Variables | Minimal model | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dependent variable | Variables | Parameter estimate | F | df | P | Observed power | Effect size | Retained term | Removed term | Parameter estimate | F | df | P |
| (a) Plasma antioxidants | Intercept | 2.606 | 2.289 | ||||||||||
| Age | –0.123 | 0.067 | 1, 15 | 0.800 | 0.057 | 0.004 | Age | –0.123 | 0.067 | 1, 15 | 0.800 | ||
| Body condition | 0.001 | 0.405 | 0.534 | 0.092 | 0.026 | Body condition | 0.001 | 0.411 | 1, 16 | 0.530 | |||
| Age × Body condition | 0.003 | 2.616 | 1, 14 | 0.128 | |||||||||
| Plasma lipid peroxidation | Intercept | 0.211 | 0.242 | ||||||||||
| Age | 0.039 | 0.808 | 1, 15 | 0.383 | 0.134 | 0.051 | Age | 0.039 | 0.808 | 1, 15 | 0.383 | ||
| Body condition | –8.660e−5 | 0.882 | 0.363 | 0.142 | 0.056 | Body condition | –3.141e−5 | 0.211 | 1, 16 | 0.652 | |||
| Age × Body condition | 0.000 | 0.585 | 1, 14 | 0.457 | |||||||||
| (b) Comb size | Intercept | 1048.984 | 1053.348 | ||||||||||
| Plasma antioxidants | 113.139 | 4.265 | 1, 10 | 0.066 | 0.463 | 0.299 | Plasma antioxidants | 112.732 | 5.695 | 1, 12 | 0.034 | ||
| Plasma lipid peroxidation | –2119.030 | 12.036 | 0.006 | 0.877 | 0.546 | Plasma lipid peroxidation | –2097.537 | 18.132 | 1, 12 | 0.001 | |||
| Age | 14.334 | 0.021 | 0.889 | 0.052 | 0.002 | Age | 12.034 | 0.040 | 1, 11 | 0.845 | |||
| Body condition | –0.006 | 0.001 | 0.977 | 0.050 | <0.001 | Body condition | –0.006 | 0.001 | 1, 10 | 0.997 | |||
| Age × Plasma lipid peroxidation | 2282.606 | 1.528 | 1, 9 | 0.248 | |||||||||
| Age × Plasma antioxidants | –224.511 | 1.749 | 1, 8 | 0.223 | |||||||||
| Age × Body condition | 0.435 | 0.214 | 1, 7 | 0.657 | |||||||||
| (c) Seminal antioxidants | Intercept | –3.351 | –2.762 | ||||||||||
| Age | 6.686 | 18.144 | 1, 11 | 0.001 | 0.972 | 0.623 | Age | 6.573 | 12.283 | 1, 13 | 0.004 | ||
| Plasma antioxidants | 3.373 | 11.069 | 0.007 | 0.422 | 0.253 | Plasma antioxidants | 3.512 | 20.522 | 1, 13 | 0.001 | |||
| Age × Plasma antioxidants | –4.227 | 43.693 | <0.001 | 1.000 | 0.799 | Age × Plasma antioxidants | –3.731 | 26.337 | 1, 13 | <0.001 | |||
| Body condition | 0.003 | 6.641 | 0.026 | 0.651 | 0.376 | Body condition | 0.003 | 4.409 | 1, 12 | 0.058 | |||
| Plasma lipid peroxidation | 7.002 | 2.786 | 0.123 | 0.332 | 0.202 | Plasma lipid peroxidation | 7.002 | 2.786 | 1, 11 | 0.123 | |||
| Age × Body condition | –0.003 | 1.497 | 1, 10 | 0.249 | |||||||||
| Age × Plasma lipid peroxidation | –32.233 | 0.417 | 1, 9 | 0.535 | |||||||||
| (d) Sperm motility | Intercept | 57.759 | 64.401 | ||||||||||
| Seminal lipid peroxidation | –24.156 | 3.916 | 1,7 | 0.088 | 0.401 | 0.359 | Seminal lipid peroxidation | –23.955 | 4.671 | 1, 8 | 0.063 | ||
| Seminal antioxidants | –0.089 | 0.005 | 0.948 | 0.050 | 0.001 | Seminal antioxidants | –0.089 | 0.005 | 1, 7 | 0.948 | |||
| (e) Sperm DNA damage | Intercept | 1.116 | 1.041 | ||||||||||
| Age | 0.115 | 6.273 | 1,14 | 0.025 | 0.645 | 0.309 | Age | 0.085 | 5.982 | 1, 16 | 0.026 | ||
| Body condition | –9.488e−5 | 0.832 | 0.377 | 0.136 | 0.056 | Body condition | 0.000 | 1.034 | 1, 15 | 0.325 | |||
| Seminal antioxidants | 0.004 | 0.172 | 0.684 | 0.067 | 0.012 | Seminal antioxidants | 0.004 | 0.172 | 1, 14 | 0.684 | |||
| Age × Body condition | 0.000 | 0.799 | 1, 13 | 0.388 | |||||||||
| Age × Seminal antioxidants | –0.003 | 0.027 | 1, 12 | 0.871 | |||||||||
Figure 2.
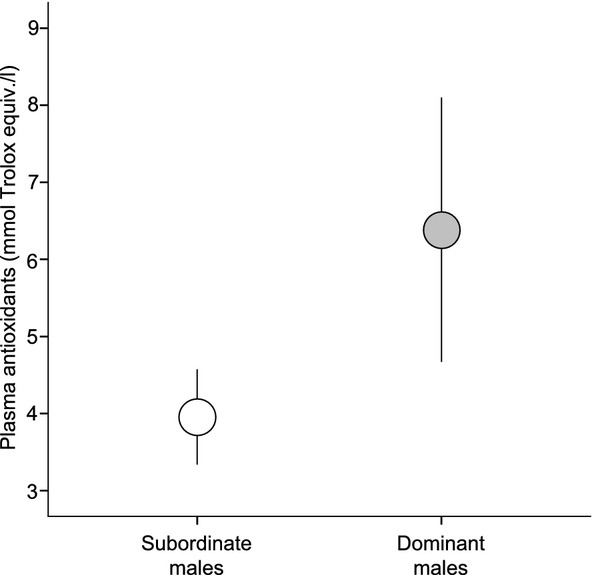
Level of plasma antioxidants of red junglefowl males, measured as mmol Trolox equivalents per liter (mean ± SE), in relation to male social status.
Comb size and plasma oxidative status
Male comb size was significantly correlated with the levels of plasma antioxidants and lipid peroxidation (Table 1b). Males with higher levels of plasma antioxidants displayed a larger comb (Fig. 3a), and this relationship was similar in both age classes (Table 1b). Moreover, the level of lipid peroxidation in plasma was negatively correlated with comb size (Table 1b; Fig. 3b). Again, this relationship was similar in both age classes (Table 1b). Male body condition, age, as well as the interaction of age with plasma antioxidants, lipid peroxidation, and body condition did not explain a significant source of variation in comb size (Table 1b). When we reran the model including social status, neither social status nor its interaction with male age explained a significant amount of variation in comb size (social status: estimate = 38.250, F1,7 = 0.152, P = 0.71; age × social status: estimate = –2970.80, F1,3 = 1.516, P = 0.31).
Figure 3.
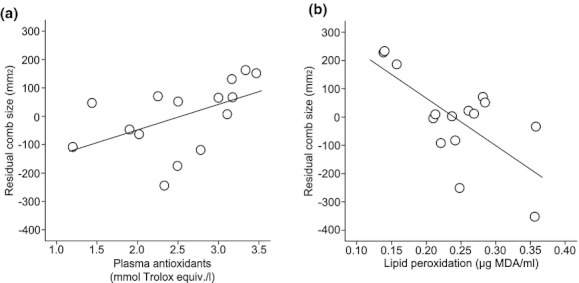
Comb size of red junglefowl males (residuals from final model after correcting by lipid peroxidation or plasma antioxidants, respectively) in relation to (a) plasma antioxidant and (b) plasma lipid peroxidation level. Lines show an adjusted linear regression (plasma antioxidants: y = 88.55x – 225.32, r = 0.50; lipid peroxidation: y = –1647.62x + 397.17, r = 0.68).
Seminal oxidative status and male age
The level of seminal antioxidants covaried positively with the levels of plasma antioxidants in young males but not in old males (Table 1c; Fig. 4a). In mean, old males had 37% less seminal antioxidants than young males (F1,17 = 4.976; P = 0.039; Table 1c; Fig. 4b). Levels of seminal antioxidants did not vary with male's body condition, lipid peroxidation in plasma, or their interactions with age (Table 1c). Social status and its interaction with age had no effect on seminal antioxidants when they were included in the model (social status: estimate = 1.373, F1,8 = 4.019, P = 0.08; age × social status: estimate = 1.871, F1,7 = 1.413, P = 0.27).
Figure 4.
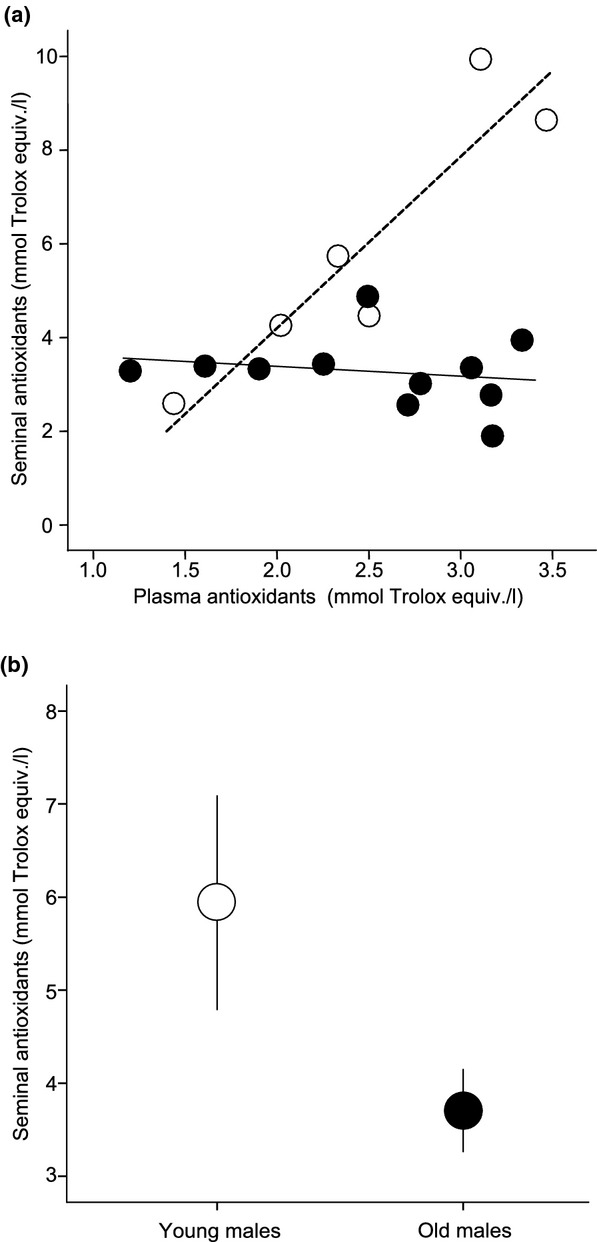
Seminal antioxidants levels in sperm of red junglefowl males. (a) seminal antioxidants in relation to plasma antioxidants (b) mean seminal antioxidants levels (±SE) in young (open circles) and old males (filled circles). Lines show an adjusted lineal regression (young males: y = 3.5x – 2.7, r = 0.91; old males: y = –0.2x + 3.8, r = 0.20).
Seminal oxidative status and sperm motility
Sperm motility was not correlated with seminal antioxidant levels, but there was a nonsignificant (P = 0.063) trend for sperm motility to decline with increasing lipid peroxidation (Table 1d; Fig. 5).
Figure 5.
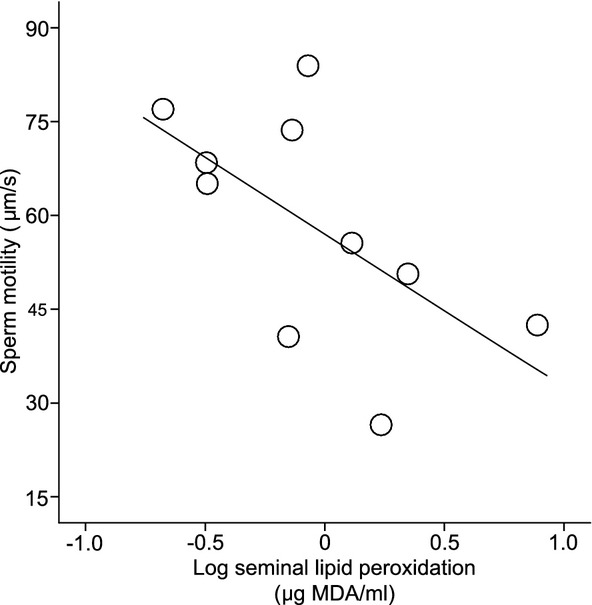
Sperm swimming velocity (average path velocity, VAP) of old junglefowls in relation to lipid peroxidation level present in the seminal fluid. Fitted line shows an adjusted linear regression (y = –23.95x + 57.3, r = 0.61).
Seminal oxidative stress and sperm DNA damage
Sperm DNA damage, measured as the number of AP sites, was positively correlated with male age: old males had on average 24% more AP sites in sperm DNA than young males (Table 1e; Fig. 6). Seminal antioxidants, male body condition, or their interactions with age did not explain a significant amount of variation in sperm DNA damage (Table 1e). Neither social status nor its interaction with age had a significant effect when they were included in the models (social status: estimate = –0.030, F1,8 = 0.782, P = 0.40; age × social status: estimate = 0.170, F1,6 = 1.939, P = 0.21).
Figure 6.
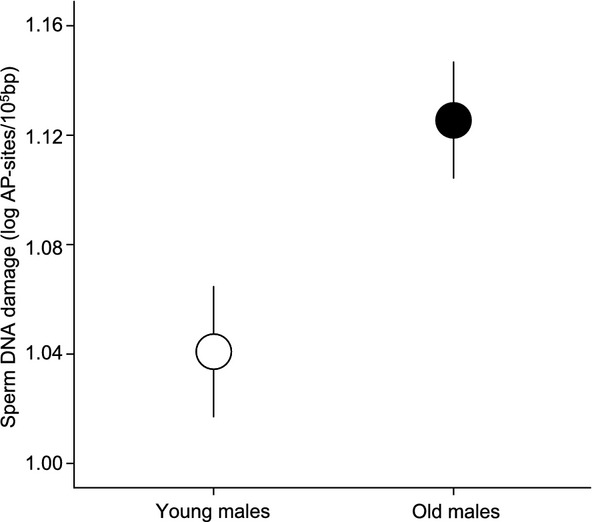
Level DNA damage in sperm of red junglefowls, measured as the number of apuric/apyrimidinic sites (mean ± SE), in relation to age classes.
Discussion
In this study, we examined the expression of multiple pre- and postmating reproductive traits in relation to age-related changes in oxidative status. Both male social status and comb size were strongly associated with plasma oxidative status, and there was a nonsignificant tendency for sperm motility to be associated with seminal oxidative status. In addition, we found that young and old males differed in patterns of plasma and seminal antioxidant levels. Levels of seminal antioxidants increased with levels of plasma antioxidants in young males, but remained low and independent of the level of plasma antioxidants in old males, which also accumulated more oxidative damage in their sperm DNA.
Our results show that dominant males had substantially higher plasma antioxidant levels than subordinates, irrespective of age without suffering a reduction in body condition, even though dominant males spend less time feeding and resting than subordinates (Pizzari 2003; Cornwallis and Birkhead 2008). In addition, as predicted and consistent with the “antioxidant trade-off” hypothesis (von Schantz et al. 1999), we found that males with higher level of circulating antioxidants and lower lipid peroxidation had larger combs. Comb size depends on the integrity of hyaluronic acid molecules, the main component responsible for comb viscousity (Laurent and Fraser 1992; von Schantz et al. 1999). Hyaluronic acid is depolymerized by free radicals, losing viscosity, and water content (Hawkins and Davies 1996). Thus, our results suggest that males with better oxidative status are able to invest more antioxidants into sexual signaling (comb size). In addition, both male social status and comb size are strongly testosterone dependent in the fowl (Ligon et al. 1990; Johnsen and Zuk 1995; Zuk et al. 1995; Parker et al. 2002), indicating that oxidative status might be related to testosterone levels. If testosterone has pro-oxidant effects (Isaksson et al. 2011 and references therein), the present results might suggest that some males are able to sustain higher levels of oxidative stress and achieve high status and/or develop large combs compared with other males. An alternative explanation is that testosterone might in fact facilitate the bioavailability of some kind of antioxidant compounds in this species, as has been observed in adult birds by other studies (Blas et al. 2006; but see also Noguera et al. 2011). Consistent with the latter scenario, in our study population, neither plasma antioxidants decreased nor lipid peroxidation increased in dominant males, as expected, if testosterone had increased the production of pro-oxidant molecules. Thus, the testosterone-mediated effect on bioavailability of antioxidant resources might explain why dominant and/or large-combed males, with high levels of testosterone, had elevated level of plasma antioxidants. Together, these results indicate that social dominance and large combs are likely to be reliable indicators of male condition, consistent with previous work indicating the condition dependence of both traits in this species (Zuk et al. 1990, 1995; Pizzari 2003; Cornwallis and Birkhead 2008). Therefore, our results are broadly consistent with the view that oxidative stress may be a proximal mechanism underlying the honesty of sexually selected signals (von Schantz et al. 1999; Torres and Velando 2007; Monaghan et al. 2009; Metcalfe and Alonso-Alvarez 2010).
The results of our study also suggest a marked age-specific pattern in oxidative status. Aging organisms are more vulnerable to oxidative stress probably because their antioxidant systems and repair mechanisms become less efficient with age (Sohal and Weindruch 1996; Finkel and Holbrook 2000). Thus, this physiological senescence may impose a constraint to divert antioxidant resources in other functions than somatic maintenance at old ages (Finkel and Holbrook 2000; McNamara et al. 2009). Accordingly, old males had similar somatic oxidative status (i.e., plasma antioxidants and lipid peroxidation) than young males, suggesting similar investment in somatic maintenance. In contrast, old males showed lower levels of seminal antioxidants than young males. Moreover, seminal antioxidants correlated with plasma antioxidants in young but not in old males. Importantly, comb size did not differ between young and old males, a sexually selected trait that correlated with antioxidant availability. Three alternative mechanisms can explain these patterns. First, this might reflect a phenotypically plastic response whereby aging males strategically mobilize antioxidant resources toward somatic maintenance away from seminal fluid while maintaining their investment in comb size. Interestingly, even at old ages, males can be socially dominant and therefore monopolize access to females (Dean et al. 2010). This response might represent a reproductive restraint strategy (McNamara et al. 2009), with individuals restraining their reproductive effort to slow down somatic damage accumulation as they age (McNamara et al. 2009). In this case, the reduction in seminal antioxidants in old males may not (or not wholly) be a direct effect of aging, but may instead reflect a strategy aimed at slowing down somatic damage accumulation at the time that males continue to have mating opportunities. Second, the present results might reflect inter-male variation in fixed life-history strategies. In this scenario, males with more limited antioxidant investment in ejaculates are more likely to survive to old age, and as a result, our group of “old” males might be overrepresented by such males. Finally, the differences in patterns of oxidative status detected between “young” and “old” males might reflect random variation between cohorts. Because only one cohort of birds was used in each age category, we cannot rule out this possibility. Nonetheless, this result should be taken with caution because of the small sample size in the group of “young” males. Future work should seek to distinguish between these scenarios through the longitudinal analysis of multiple cohorts.
Traditional good genes models of mate choice predict the evolution of female preferences for old males (reviewed in Brooks and Kemp 2001). Nevertheless, it has been recently shown that male senescence may act as potential source of sexual conflict (e.g., Dean et al. 2010; Carazo et al. 2011; Velando et al. 2011). For example, in feral populations of domestic fowl, potential sexual conflict arises when old males are able to achieve dominant status and therefore monopolize sexual access to females, but are unable to fertilize all their eggs (Dean et al. 2010). In this study, we showed that old males do not differ from young males in terms of comb size, but suffer from worse oxidative status of seminal fluid. Furthermore, we also found a non-significant negative relationship between seminal lipid peroxidation level and sperm motility, a trait closely related to male fertility in fowl (Froman et al. 1999; Pizzari et al. 2008b). Similar results have been recently reported in other avian models (Helfenstein et al. 2010; Losdat et al. 2011). Old males had not only lower level of seminal antioxidants but also higher sperm DNA damage than young males. Similarly, it has been recently found that sperm DNA damage increases with age in the blue-footed booby, Sula nebouxii (Velando et al. 2011). Seminal antioxidants prevent oxidative damage in sperm (Poiani 2006; Velando et al. 2008), thereby increasing its fertilizing efficiency (Velando et al. 2008). The accumulation of oxidative damage in the sperm of older males could contribute to the age-related decline in reproductive success observed in a range of bird and mammal species (Kidd et al. 2001; Møller et al. 2009; Dean et al. 2010). Importantly, these results challenge the traditional view that germ cells are adequately protected from DNA-damaging agents and constantly rejuvenated (Kirkwood 1977; Vijg 2007). Unexpectedly, sperm DNA damages did not correlate with seminal antioxidants, but note that measures of oxidative damage informs about past exposure to high levels of oxidative stress, whereas our antioxidant analysis provided information on standing antioxidants defenses at the moment of sampling. Consequently, females mating preferentially with old males may pay a cost in terms of reduced fertility (Carazo et al. 2011; Dean et al. 2010) or viability of their young (Pizzari et al. 2008a). Our results open up the possibility that sexual conflict arising over male reproductive senescence could be at least in part, modulated by age-specific patterns of antioxidant levels.
To summarize, we found evidence of a possible role of oxidative stress as a proximal mechanism involved in the evolution of male investment in sexually selected traits. The extent to which age-specific antioxidant allocation patterns are modulated by environmental and genetic factors underpinning male's oxidative status remains to be explored.
Acknowledgments
We are grateful to A. Tato for helping with lab analyses. We also thank two anonymous reviewers for their helpful and constructive comments. The study was supported by the Spanish Ministerio de Ciencia e Innovación MICINN (CGL2009-10883-C02-01), and by the Philip Leverhulme Trust Award to T. P.; J. C. N. was supported by a grant from MICINN (BES-2007-16432).
Conflict of Interest
None declared
References
- Aitken RJ, Baker MA. Oxidative stress, sperm survival and fertility control. Mol. Cell Endocrinol. 2006;250:66–69. doi: 10.1016/j.mce.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Almbro M, Dowling DK, Simmons LW. Effects of vitamin E and beta-carotene on sperm competitiveness. Ecol. Lett. 2011;14:891–895. doi: 10.1111/j.1461-0248.2011.01653.x. [DOI] [PubMed] [Google Scholar]
- Andersson M. Sexual selection. Princeton, NJ: Princeton University Press; 1994. [Google Scholar]
- Birkhead TR, Møller AP. Sperm competition in birds. London: Academic Press; 1992. [Google Scholar]
- Blas J, Pérez-Rodríguez L, Bortolotti GR, Viñuela J, Marchant TA. Testosterone increases bioavailability of carotenoids: insights into the honesty of sexual signalling. Proc. Natl Acad. Sci. USA. 2006;103:18633–18637. doi: 10.1073/pnas.0609189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount JD, Møller AP, Houston DC. Antioxidants, showy males and sperm quality. Ecol. Lett. 2001;4:393–396. [Google Scholar]
- Blount JD, Metcalfe NB, Birkhead TR, Surai PF. Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science. 2003;300:125–127. doi: 10.1126/science.1082142. [DOI] [PubMed] [Google Scholar]
- den Boer SPA, Baer B, Boomsma JJ. Seminal fluid mediates ejaculate competition in social insects. Science. 2010;327:1506–1509. doi: 10.1126/science.1184709. [DOI] [PubMed] [Google Scholar]
- Brooks R, Kemp DJ. Can older males deliver the good genes? Trends Ecol. Evol. 2001;16:308–313. doi: 10.1016/s0169-5347(01)02147-4. [DOI] [PubMed] [Google Scholar]
- Burrows WH, Quinn JP. The collection of spermatozoa from domestic fowl and turkey. Poult. Sci. 1937;16:19–24. [Google Scholar]
- Carazo P, Molina-Vila P, Font E. Male reproductive senescence as a potential source of sexual conflict in beetle. Behav. Ecol. 2011;22:192–198. [Google Scholar]
- Collias NE, Collias E. Social organization of a red junglefowl, Gallus gallus, population related to evolutionary theory. Anim. Behav. 1996;51:1337–1354. [Google Scholar]
- Cornwallis CK, Birkhead TR. Experimental evidence that female ornamentation increases the acquisition of sperm and signals fecundity. Proc. R. Soc. Lond. B. 2007;274:583–590. doi: 10.1098/rspb.2006.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwallis CK, Birkhead TR. Plasticity in reproductive phenotypes reveals status-specific correlations between behavioral, morphological, and physiological sexual traits. Evolution. 2008;62:1149–1161. doi: 10.1111/j.1558-5646.2008.00346.x. [DOI] [PubMed] [Google Scholar]
- Cornwallis CK, O'Connor EA. Sperm seminal fluid interactions and the adjustment of sperm quality in relation to female attractiveness. Proc. R. Soc. Lond. B. 2009;276:3467–3475. doi: 10.1098/rspb.2009.0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R, Cornwallis CK, Løvlie H, Worley K, Richardson DS, Pizzari T. Male reproductive senescence causes potential for sexual conflict over mating. Curr. Biol. 2010;20:1192–1196. doi: 10.1016/j.cub.2010.04.059. [DOI] [PubMed] [Google Scholar]
- Dean R, Nakagawa S, Pizzari T. The risk and intensity of sperm ejection in female birds. Am. Nat. 2011;178:343–354. doi: 10.1086/661244. [DOI] [PubMed] [Google Scholar]
- Dowling DK, Simmons LW. Reactive oxygen species as universal constraints in life-history evolution. Proc. R. Soc. Lond. B. 2009;276:1737–1745. doi: 10.1098/rspb.2008.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Etches RJ. Reproduction in poultry. Ofon: CAB International; 1996. [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Froman DP, Feltmann AJ, Rhoads ML, Kirby JD. Sperm mobility: a primary determinant of fertility in the domestic fowl (Gallus domesticus. Biol. Reprod. 1999;61:400–405. doi: 10.1095/biolreprod61.2.400. [DOI] [PubMed] [Google Scholar]
- Halliwell BH, Gutteridge JMC. Free radicals in biology and medicine. 4th ed. Oxford: Oxford University Press; 2007. [Google Scholar]
- Hawkins CL, Davies MJ. Direct detection and identification of radicals generated during the hydroxyl radical-induced degradation of hyaluronic acid and related materials. Free Radical Biol. Med. 1996;21:275–290. doi: 10.1016/0891-5849(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Helfenstein F, Losdat S, Møller AP, Blount JD, Richner H. Sperm of colourful males are better protected against oxidative stress. Ecol. Lett. 2010;13:213–222. doi: 10.1111/j.1461-0248.2009.01419.x. [DOI] [PubMed] [Google Scholar]
- Isaksson C, While GM, McEvoy J, Olsson J, van de Crommenacker M, Groothuis TGG, et al. Aggression, but not testosterone, is associated to oxidative status in a free-living vertebrate. Behaviour. 2011;148:713–731. [Google Scholar]
- Johnsen TS, Zuk M. Testosterone and aggression in male red jungle fowl. Horm. Behav. 1995;29:593–598. doi: 10.1006/hbeh.1995.1288. [DOI] [PubMed] [Google Scholar]
- Johnsen TS, Zuk M, Fessler EA. Social dominance, male behaviour and mating in mixed-sex flocks of red jungle fowl. Behaviour. 2001;138:1–18. [Google Scholar]
- Karatas F, Karatepe M, Baysar A. Determination of free malondialdehyde in human serum by high performance liquid chromatography. Anal. Biochem. 2002;311:76–79. doi: 10.1016/s0003-2697(02)00387-1. [DOI] [PubMed] [Google Scholar]
- Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil. Steril. 2001;75:237–248. doi: 10.1016/s0015-0282(00)01679-4. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB. Evolution of ageing. Nature. 1977;270:301–304. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- Kokko H, Lindström J. Evolution of female preference for old mates. Proc. R. Soc. Lond. B. 1996;263:1533–1538. [Google Scholar]
- Laurent TC, Fraser JRE. Hyaluronan. FASEB J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- Lessells CM, Boag PT. Unrepeatable repeatabilities: a common mistake. Auk. 1987;104:116–121. [Google Scholar]
- Ligon JD, Zwartjes PW. Female junglefowl choose to mate with multiple males. Anim. Behav. 1995;49:127–135. [Google Scholar]
- Ligon JD, Thornhill R, Zuk M. Male-male competition, ornamentation and the role of testosterone in sexual selection in red jungle fowl. Anim. Behav. 1990;40:367–373. [Google Scholar]
- Lill A. Some observations on social organization and non-random mating in captive Burmese Red Jungle Fowl (Gallus gallus spadiceus. Behaviour. 1966;26:228–242. [Google Scholar]
- Losdat S, Richner H, Blount JD, Helfenstein F. Immune activation reduces sperm quality in the great tit. PLoS ONE. 2011;6:e22221. doi: 10.1371/journal.pone.0022221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PA, Reimers TJ, Lodge JR, Dziuk PJ. The effect of ratios and numbers of spermatozoa mixed from two males on proportions of offspring. J. Reprod. Fertil. 1974;39:251–258. doi: 10.1530/jrf.0.0390251. [DOI] [PubMed] [Google Scholar]
- McNamara JM, Houston AL, Barta Z, Scheuerlein A, Fromhage L. Deterioration, death and the evolution of reproductive restraint in late life. Proc. R. Soc. Lond. B. 2009;276:4061–4066. doi: 10.1098/rspb.2009.0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe NB, Alonso-Alvarez C. Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Funct. Ecol. 2010;24:984–996. [Google Scholar]
- Møller AP, Mousseau TA, Rudolfsen G, Balbontin J, Marzal A, Hermosell I, et al. Senescent sperm performance in old male birds. J. Evol. Biol. 2009;22:334–344. doi: 10.1111/j.1420-9101.2008.01650.x. [DOI] [PubMed] [Google Scholar]
- Monaghan P, Metcalfe NB, Torres R. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurement and interpretation. Ecol. Lett. 2009;12:75–92. doi: 10.1111/j.1461-0248.2008.01258.x. [DOI] [PubMed] [Google Scholar]
- Noguera JC, Alonso-Alvarez C, Kim SY, Morales J, Velando A. Yolk testosterone reduces levels of oxidative damages during postnatal development. Biol. Lett. 2011;7:93–95. doi: 10.1098/rsbl.2010.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker TH, Ligon JD. Female mating preferences in red junglefowl: a meta-analysis. Ethol. Ecol. Evol. 2003;15:63–72. [Google Scholar]
- Parker TH, Knapp R, Rosenfield JA. Social mediation of sexually selected ornamentation and steroid hormone levels in male junglefowl. Anim. Behav. 2002;64:291–298. [Google Scholar]
- Pérez C, Lores M, Velando A. Availability of nonpigmentary antioxidant affects red coloration in gulls. Behav. Ecol. 2008;19:967–973. [Google Scholar]
- Pike TW, Blount JD, Lindstrom J, Metcalfe NB. Availability of non-carotenoid antioxidants affects the expression of a carotenoid-based sexual ornament. Biol. Lett. 2007;3:353–356. doi: 10.1098/rsbl.2007.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzari T. Food, vigilance, and sperm: the role of male direct benefits in the evolution of female preference in a polygamous bird. Behav. Ecol. 2003;14:593–601. [Google Scholar]
- Pizzari T, Birkhead TR. Female feral fowl eject sperm of subdominant males. Nature. 2000;405:787–789. doi: 10.1038/35015558. [DOI] [PubMed] [Google Scholar]
- Pizzari T, Parker GA. Sperm competition and sperm phenotype. In: Birkhead TR, Hosken DJ, Pitnick S, editors. Sperm biology: an evolutionary perspective. San Diego: Academic Press; 2009. pp. 207–245. [Google Scholar]
- Pizzari T, Froman DP, Birkhead TR. Pre- and postinsemination episodes of sexual selection in the fowl, Gallus g. domesticus. Heredity. 2002;88:112–116. doi: 10.1038/sj.hdy.6800014. [DOI] [PubMed] [Google Scholar]
- Pizzari T, Cornwallis CK, Froman DP. Social competitiveness associated with rapid fluctuations in sperm quality in male fowl. Proc. R. Soc. Lond. B. 2007;274:853–860. doi: 10.1098/rspb.2006.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzari T, Dean R, Pacey A, Moore H, Bonsall MB. The evolutionary ecology of pre- and post-meiotic sperm senescence. Trends Ecol. Evol. 2008a;23:131–140. doi: 10.1016/j.tree.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Pizzari T, Worley K, Burke T, Froman DP. Sperm competition dynamics: ejaculate fertilizing efficiency changes differentially with time. BMC Evol. Biol. 2008b;8:332. doi: 10.1186/1471-2148-8-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiani A. Complexity of seminal fluid: a review. Behav. Ecol. Sociobiol. 2006;60:289–310. [Google Scholar]
- Preston BT, Jalme MS, Hingrat Y, Lacroix F, Sorci G. Sexually extravagant males age more rapidly. Ecol. Lett. 2011;14:1017–1024. doi: 10.1111/j.1461-0248.2011.01668.x. [DOI] [PubMed] [Google Scholar]
- Quinn GP, Keough MJ. Experimental design and data analysis for biologists. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Radwan J, Michalczyk Ł, Prokop Z. Age-dependence of male mating ability and sperm competition success in the bulb mite. Anim. Behav. 2005;69:1101–1105. [Google Scholar]
- Reinhardt K. Evolutionary consequences of sperm cell aging. Q. Rev. Biol. 2007;82:375–393. doi: 10.1086/522811. [DOI] [PubMed] [Google Scholar]
- Rose MR. Evolutionary biology of ageing. New York: Oxford University Press; 1991. [Google Scholar]
- von Schantz T, Bensch S, Grahn M, Hasselquist D, Wittzell H. Good genes, oxidative stress and condition-dependent sexual signals. Proc. R. Soc. Lond. B. 1999;266:1–12. doi: 10.1098/rspb.1999.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Hostedde AI, Zinner B, Millar JS, Hickling GJ. Restitution of mass-size residuals: validating body condition indices. Ecology. 2005;86:155–163. [Google Scholar]
- Sikka SC. Relative impact of oxidative stress on male reproductive function. Curr. Med. Chem. 2001;8:851–862. doi: 10.2174/0929867013373039. [DOI] [PubMed] [Google Scholar]
- Simmons LW, Beveridge M. Seminal fluid affects sperm viability in a Cricket. PLoS ONE. 2011;6:e17975. doi: 10.1371/journal.pone.0017975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MS. Flock structure in red jungle fowl. Appl. Anim. Behav. Sci. 1991;30:381–686. [Google Scholar]
- Torres R, Velando A. Male reproductive senescence: the price of immune induced oxidative damage on sexual attractiveness in the blue-footed booby. J. Anim. Ecol. 2007;76:1161–1168. doi: 10.1111/j.1365-2656.2007.01282.x. [DOI] [PubMed] [Google Scholar]
- Velando A, Torres R, Alonso-Alvarez C. Avoiding bad genes: oxidatively damaged DNA in germ line and mate choice. BioEssays. 2008;30:1–8. doi: 10.1002/bies.20838. [DOI] [PubMed] [Google Scholar]
- Velando A, Noguera JC, Drummond H, Torres R. Senescent males carry premutagenic lesion in sperm. J. Evol. Biol. 2011;24:693–697. doi: 10.1111/j.1420-9101.2010.02201.x. [DOI] [PubMed] [Google Scholar]
- Vijg J. Aging of the genome: the dual role of DNA in life and death. Oxford: Oxford University Press; 2007. [Google Scholar]
- Whittingham MJ, Stephens PA, Bradbury RB, Freckleton RP. Why do we still use stepwise modelling in ecology and behaviour? J. Anim. Ecol. 2006;75:1182–1189. doi: 10.1111/j.1365-2656.2006.01141.x. [DOI] [PubMed] [Google Scholar]
- Zuk M, Thornhill R, Ligon JD, Johnson K. Parasites and choise in red jungle fowl. Am. Zool. 1990;30:235–244. doi: 10.1111/j.1558-5646.1990.tb05933.x. [DOI] [PubMed] [Google Scholar]
- Zuk M, Johnsen TS, MacLarty T. Endocrine-immune interactions, ornaments and mate choice in red jungle fowl. Proc. R. Soc. Lond. B. 1995;260:205–210. [Google Scholar]


