Abstract
Mechanical ventilation is necessary for patients with acute respiratory failure, but can cause or propagate lung injury. We previously identified cyclooxygenase-2 as a candidate gene in mechanical ventilation–induced lung injury. Our objective was to determine the role of cyclooxygenase-2 in mechanical ventilation–induced lung injury and the effects of cyclooxygenase-2 inhibition on lung inflammation and barrier disruption. Mice were mechanically ventilated at low and high tidal volumes, in the presence or absence of pharmacologic cyclooxygenase-2–specific inhibition with 3-(4-methylsulphonylphenyl)-4-phenyl-5-trifluoromethylisoxazole (CAY10404). Lung injury was assessed using markers of alveolar–capillary leakage and lung inflammation. Cyclooxygenase-2 expression and activity were measured by Western blotting, real-time PCR, and lung/plasma prostanoid analysis, and tissue sections were analyzed for cyclooxygenase-2 staining by immunohistochemistry. High tidal volume ventilation induced lung injury, significantly increasing both lung leakage and lung inflammation relative to control and low tidal volume ventilation. High tidal volume mechanical ventilation significantly induced cyclooxygenase-2 expression and activity, both in the lungs and systemically, compared with control mice and low tidal volume mice. The immunohistochemical analysis of lung sections localized cyclooxygenase-2 expression to monocytes and macrophages in the alveoli. The pharmacologic inhibition of cyclooxygenase-2 with CAY10404 significantly decreased cyclooxygenase activity and attenuated lung injury in mice ventilated at high tidal volume, attenuating barrier disruption, tissue inflammation, and inflammatory cell signaling. This study demonstrates the induction of cyclooxygenase-2 by mechanical ventilation, and suggests that the therapeutic inhibition of cyclooxygenase-2 may attenuate ventilator-induced acute lung injury.
Keywords: cyclooxygenase-2, mechanical ventilation, lung injury
Clinical Relevance
Mechanical ventilation is a key supportive therapy for patients with acute respiratory failure, but can also injure the lungs. A better understanding of the mechanisms by which mechanical ventilation contributes to lung injury is necessary in identifying new therapeutic targets. We demonstrate increased cyclooxygenase-2 (COX-2) expression and activity in response to injurious mechanical ventilation, and further demonstrate attenuation of lung injury by COX-2 inhibition. These findings have important implications in the potential therapeutic use of pharmacologic COX-2 inhibition for patients requiring mechanical ventilation.
Mechanical ventilation is a life-saving therapy for patients with acute respiratory failure. However, it can cause or worsen lung injury, particularly at larger tidal volumes (1, 2). Positive-pressure mechanical ventilation may expose the lungs, and particularly the alveoli, to overdistention, resulting in volutrauma with the subsequent release of inflammatory and vasoactive cytokines and prostanoids that propagate lung injury (1, 3). Therapeutic trials aimed at decreasing lung injury have focused on minimizing the damage from mechanical ventilation with the use of “protective” low tidal volume ventilator strategies. However, because of the heterogeneity of lung injury, even at lower tidal volumes, different regions of the lung may be subjected to higher stretch (4). Although using lower tidal volumes decreases markers of injury and inflammation and significantly improves survival in patients with acute lung injury, mortality remains high (1). A better understanding of the mechanisms by which mechanical ventilation injures the lungs will be key in identifying new therapeutic targets for pharmacologic therapy.
We have identified cyclooxygenase-2 (COX-2) as an important candidate gene in ventilator-induced lung injury (5, 6). In contrast to COX-1, which is constitutively expressed and involved in housekeeping functions, COX-2 is an inducible enzyme that catalyzes the conversion of arachidonic acid to downstream prostanoids involved in inflammation and vascular homeostasis. COX-2 is expressed in resident inflammatory cells in the lung as well as in the pulmonary endothelium and epithelium, where it may be induced by a variety of insults, including LPS, acid aspiration, pancreatitis, and bleomycin (7–10). The induction of COX-2 leads to the increased expression of prostanoids, including prostaglandin E2 (PGE2) and prostacyclin, which play key roles in modulating inflammation and governing vascular tone and barrier function in the lung. COX-2 appears to play an important role in the resolution of pulmonary inflammation, and decreased COX-2 activity has been implicated in the pathogenesis of pulmonary fibrosis in humans and in animal models (11, 12). Likewise, aberrant COX-2 expression and a shift in the balance of vasodilatory and antiproliferative prostanoids have been demonstrated in animal models of pulmonary hypertension (13, 14). In contrast, accumulating evidence points to an important role for COX-2 in carcinogenesis and tumor metastasis (15, 16), as well as the injurious pulmonary response to acute infection (17, 18). Evidence points to cyclooxygenase in the pathophysiology of ventilator-induced lung injury. However, attempts at nonspecific cyclooxygenase inhibition have yielded mixed results (19–21). Little is known about the roles of COX-2 and of specific COX-2 inhibition in governing acute lung injury in response to mechanical stress.
Our present study aimed to determine the role of COX-2 inhibition in attenuating mechanical ventilation–induced lung injury. We show here that high tidal volume mechanical ventilation induces lung inflammation and barrier disruption. This lung injury is associated with increased COX-2 expression and activity, whereas the pharmacologic inhibition of COX-2 ameliorates the injury caused by harmful ventilation.
Materials and Methods
Antibodies and Reagents
Antibodies to COX-1, COX-2, and CAY10404 were obtained from Cayman Chemical (Ann Arbor, MI). Antibodies to intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) were obtained from Abcam (Cambridge, MA). All other reagents were obtained from Sigma Chemical Co. (St. Louis, MO).
COX-2 Inhibition
CAY10404 (3-(4-methylsulphonylphenyl)-4-phenyl-5-trifluoromethylisoxazole) at 50 mg/kg/day was administered by intraperitoneal injection daily for 3 days plus 1 hour before the initiation of ventilation. This dose was chosen to give an optimal balance of efficacy and toxicity, based on our preliminary dose range studies and previous published studies (22, 23).
In Vivo Model of Acute Lung Injury/Ventilator-Induced Lung Injury
All animal protocols were approved by the Institutional Animal Care and Use Committee of Oregon Health and Science University, and conform to National Institutes of Health guidelines. Details of the ventilation protocol are available in the online supplement.
Adult male C57Bl/6J mice weighing 24–30 g (Jackson Laboratories, Sacramento, CA) were anesthetized with ketamine/xylazine, intratracheally intubated, and ventilated with room air at either low tidal volume (LTV, 7 ml/kg) or high tidal volume (HTV, 20 ml/kg) for 4 hours at a respiratory rate of 160 breaths/minute, with 3 cm H2O positive end-expiratory pressure. Control animals were anesthetized and allowed to breathe spontaneously. External dead space was applied to the HTV mice. All ventilated animals received an intravenous bolus of 0.5 ml sterile Ringer’s lactate at the onset of mechanical ventilation to prevent hypotension.
Bronchoalveolar lavage.
Bronchoalveolar lavage (BAL) was performed via endotracheal tube with 1 ml PBS for inflammatory cell count and protein, cytokine, and prostanoid measurements.
Histology and Immunohistochemical Staining
The left lung was removed, fixed in buffered formalin, and processed for hematoxylin and eosin staining and histologic analysis. The tissue scoring of lung injury was performed as previously described (24) by a histopathologist (D.S.) blinded to experimental conditions. Immunohistochemistry for COX-2, and for CD45/CD68 in a subset of mice, was performed in formalin-fixed sections, and sections were counterstained with toluidine blue. Negative control sections were incubated with an isotype-matched control antibody at a concentration of 1 mg/ml.
Assessment of alveolar–capillary leak.
Evan’s blue dye (EBD) deposition and BAL protein in lung tissue were used as markers of vascular permeability, as previously described (5, 25). The tissue deposition of EBD was measured and normalized to serum EBD for each animal. BAL protein concentrations were determined by a modified Lowry colorimetric assay, using a DC protein assay kit (Bio-Rad, Hercules, CA) as previously described (5).
Prostanoid and cytokine measurement.
PGE2, 6-keto prostaglandin F1α (6-keto PGF1α), and 2,3-dinor-6-keto prostaglandin F1α (2,3-dinor-6-keto PGF1α) were measured using commercially available immunoassays (Cayman Chemical). BAL and plasma IL-6 were measured according to immunoassays (R&D Systems, Minneapolis, MN).
Statistical Methods
All data are presented as means ± SEM. One-way ANOVA was used to compare baseline values between groups (5, 25, 26). Two-way ANOVA for repeated measures was used to determine the interactions between COX-2 inhibition and mechanical ventilation. Group comparisons were evaluated by post hoc Newman-Keuls testing. P < 0.05 was considered statistically significant.
Detailed methods are available in the online supplement.
Results
Physiologic Parameters
To test the role of COX-2 in ventilator-induced lung injury, we developed a murine model of mechanical ventilation–induced lung injury induced by HTV mechanical ventilation. Because both hypercapnia and hypoxia have been shown to influence COX-2 expression in the lung, we developed a model in which all three ventilation groups were ventilated on room air and were well matched for partial pressure of carbon dioxide (CO2). In preliminary studies, blood gas sampling was performed by cardiac puncture at the end of the experiment to determine pH, Pco2, and concentrations of bicarbonate (Hco3). To isolate the specific effects of tidal volume on lung injury, mice in both the LTV and HTV groups were ventilated at a constant respiratory rate of 160/minute, which was matched to that of anesthetized but spontaneously breathing mice. As shown in Table 1, no statistically significant differences in blood pH, Pco2, and Hco3 were evident between the three groups (control, LTV, and HTV). All mice demonstrated acute hypercapnic acidosis, as previously described in anesthetized mice. In separate mice, oximetry was intermittently measured throughout the experiment by the use of a Mouse Ox system (STARR Life Sciences Corp., Oakmont, PA). Mice from all three experimental groups were normoxic (O2 saturation, > 92%) throughout the duration of the 4-hour experiment, consistent with early lung injury.
TABLE 1.
BLOOD GASES AND OXYGENATION
| pH | Pco2 (mm Hg) | Hco3 (mmol/L) | O2 Saturation | |
| Control | 7.195 ± 0.0295 | 62.03 ± 9.00 | 24.03 ± 1.86 | > 92% |
| LTV | 7.194 ± 0.0251 | 57.15 ± 5.05 | 21.66 ± 0.81 | > 92% |
| HTV | 7.187 ± 0.0381 | 55.89 ± 5.60 | 20.05 ± 1.03 | > 92% |
| NS | NS | NS | NS |
Definition of abbreviations: HTV, high tidal volume; LTV, low tidal volume; NS, no significance; ± SEM.
In a subset of mice, a terminal blood sample was obtained by cardiac puncture for blood gas analysis. Oximetry was measured at 15-minute intervals during ventilation, using a Mouse Ox system. No differences in pH, Pco2, or Hco3 were evident between the three experimental groups. All mice demonstrated acute hypercapnic acidosis, consistent with previously published studies, and were well matched to spontaneously breathing anesthetized control mice. No measurable hypoxia occurred in any of the mice studied during these 4-hour experiments.
HTV Mechanical Ventilation Causes Lung Injury
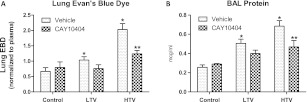
HTV mechanical ventilation increased lung injury, compared with mice ventilated with “protective” LTV, as measured by lung leakage and inflammation. As seen in Figure 1, HTV ventilation induced a 3-fold increase of EBD deposition in tissue, compared with both LTV mice and spontaneously breathing control mice (P < 0.01; Figure 1A). LTV mechanical ventilation did not significantly increase EBD deposition in lung tissue, compared with control mice. LTV mechanical ventilation induced a modest 65% increase in BAL protein, and HTV ventilation induced a nearly 3-fold increase in BAL protein, compared with spontaneously breathing control mice (Figure 1B; P < 0.05).
Figure 1.
Mechanical ventilation induces lung leakage. High tidal volume mechanical ventilation significantly increased Evans blue dye deposition in lung tissue, compared with control and low tidal volume mice (A, light bars). Similarly, mechanical ventilation caused a stepwise increase in bronchoalveolar lavage protein compared with spontaneously breathing control mice (B, light bars). These effects were significantly attenuated in high tidal volume mice by the pharmacologic inhibition of cyclooxygenase-2 with 3-(4-methylsulphonylphenyl)-4-phenyl-5-trifluoromethylisoxazole (CAY10404; hatched bars) (n = 4–7 mice per condition). LTV, low tidal volume ventilation; HTV, high tidal volume ventilation; COX-2, cyclooxygenase-2; BAL, bronchoalveolar lavage; EBD, Evans blue dye. *P < 0.01 versus control values. **P < 0.05 versus HTV alone.
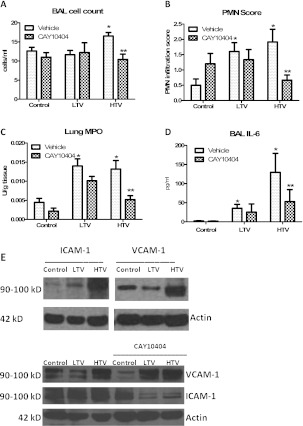
HTV mechanical ventilation induced mild alveolar leukocytosis, with a 30% increase in BAL leukocytes compared with control mice (Figure 2A; P < 0.05); macrophages comprised the vast majority (> 99%) of the BAL cell macrophages. No significant differences in alveolar hemorrhage, vascular congestion, or hyaline membrane formation were evident between the ventilation groups. However, the histologic analysis of hematoxylin and eosin–stained lung sections demonstrated a greater than 3-fold increase in tissue polymorphonuclear leukocyte (PMN) score in both the LTV and HTV groups, compared with spontaneously breathing control mice (Figure 2B; P < 0.05). Lung homogenate myeloperoxidase (MPO) activity was used as a marker of tissue inflammatory cell infiltration/activity. Similar to tissue PMNs, mechanical ventilation at both low and high tidal volumes led to a significant, greater than 3-fold increase in total lung MPO, compared with spontaneously breathing control mice (Figure 2C; P < 0.05). BAL IL-6 was used as a representative proinflammatory cytokine. BAL IL-6 concentrations in control mice were at the lower limit of detection in the assay, but were substantially increased in both the LTV and HTV groups (Figure 2D; P < 0.05). Plasma IL-6 concentrations were below the limit of detection in all three groups. Taken together, our model of early ventilator-induced lung injury recapitulates many of the defining characteristics of human acute lung injury, with alveolar barrier disruption and lung inflammation. Given the increase in inflammatory infiltrates, Western blotting for ICAM-1 and VCAM-1 were used to assess inflammatory cell adhesion signaling in our model (Figure 2E, top). Mechanical ventilation induced a stepwise increase in ICAM-1 expression compared with spontaneously breathing control mice. LTV caused a mild increase in ICAM-1 expression, and HTV caused a significant increase in ICAM-1 expression. In contrast, LTV caused a decrease in VCAM-1, and HTV caused an increase in VCAM-1, compared with control mice.
Figure 2.
Mechanical ventilation induces lung inflammation. HTV ventilation induced a mild, but statistically significant, increase in alveolar leukocytes (A, light bars) that was significantly attenuated by the pharmacologic inhibition of COX-2 with CAY10404 (A, dark bars). Both LTV and HTV ventilation increased tissue polymorphonuclear leukocytes (PMNs) and tissue myeloperoxidase (MPO) activity compared with spontaneously breathing control mice (B and C, light bars), indicative of acute tissue inflammation. COX-2 inhibition with CAY10404 significantly decreased both tissue PMNs and tissue MPO activity in HTV mice, with a trend toward decreased MPO in control and LTV mice (B and C, dark bars). (D) Finally, mechanical ventilation induced a tidal volume–dependent increase in BAL IL-6, which was significantly attenuated in HTV mice treated with CAY10404. Lung homogenates were stained for ICAM-1 and VCAM-1 as representative inflammatory cell adhesion molecules. (Results were repeated in triplicate, and representative images are shown). (E, top left) Mechanical ventilation induced a stepwise increase in ICAM-1 expression compared with control mice. (E, top right) In contrast, whereas HTV ventilation increased VCAM-1 expression, LTV ventilation was associated with a decrease in VCAM-1 expression compared with control mice. (E, bottom) The pharmacologic inhibition of COX-2 significantly decreased ICAM-1 expression in both the LTV and HTV groups, but increased VCAM-1 in the LTV and HTV groups. LTV, low tidal volume ventilation; HTV, high tidal volume ventilation; BAL, bronchoalveolar lavage; ICAM-1, intercellular adhesion molecule–1; VCAM-1, vascular cell adhesion protein–1. *P < 0.05 versus control values. **P < 0.05 versus HTV alone.
HTV Mechanical Ventilation Increases COX-2 Expression and Activity
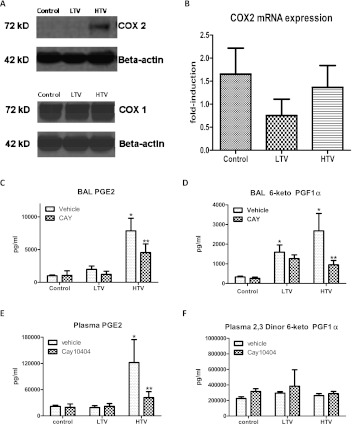
Lung homogenate protein from control, LTV, and HTV mice were probed by Western blotting for COX-1 and COX-2. As seen in Figure 3A, HTV mechanical ventilation significantly increased the expression of COX-2, compared with both control and LTV mice. This induction was specific for COX-2, because no significant differences were evident in the expression of COX-1, the constitutively expressed cyclooxygenase isoform. We then measured lung homogenate total mRNA for COX-2 expression. No significant differences in COX-2 mRNA expression were evident between the three groups in our model (Figure 3B).
Figure 3.
Mechanical ventilation induces COX-2 expression and activity. Lung expression of COX-2 was determined by Western blotting and quantitative real-time PCR analysis. (A) HTV mechanical ventilation significantly increased COX-2 protein expression, compared with control and LTV mice. No significant differences in COX-1 expression were evident between the three groups. (Results were repeated in triplicate, and representative images are shown). (B) No differences were evident in COX-2 mRNA expression between the three groups. BAL and plasma prostanoids were measured as markers for cyclooxygenase activity. HTV mechanical ventilation significantly increased lavage concentrations of both prostaglandin E2 (PGE2) (C) and 6-keto prostaglandin F1α (D), a stable prostacyclin metabolite, compared with control samples. Likewise, HTV mechanical ventilation increased plasma PGE2 (E) compared with control and LTV mice, but exerted no effect on plasma 2,3-dinor-6-keto prostaglandin F1α (2,3-dinor-6-keto PGF1α) (F), a stable prostacyclin metabolite. The pharmacologic inhibition of COX-2 with CAY10404 significantly decreased BAL PGE2 and 6-keto PGF1α as well as plasma PGE2 in HTV mice. LTV, low tidal volume ventilation; HTV, high tidal volume ventilation; BAL, bronchoalveolar lavage. *P < 0.05 versus control values. **P < 0.05 versus HTV alone.
Because cyclooxygenase governs the rate-limiting step in the conversion of arachidonic acid to downstream prostanoids, we measured PGE2, a prominent proinflammatory prostanoid, and 6-keto PGF1α, a stable metabolite of prostacyclin, as markers for cyclooxygenase activity. As seen in Figures 3B and 3C, HTV mechanical ventilation increased BAL concentrations of both PGE2 and 6-keto PGF1α nearly 8-fold compared with control mice (P < 0.05), confirming the function of the increased protein expression seen in Western blotting. In contrast, LTV ventilation did not significantly increase BAL PGE2, but a trend toward increased BAL 6-keto PGF1α was evident, involving a nearly 5-fold increase compared with control mice (P > 0.05). HTV mechanical ventilation also increased plasma PGE2 by more than 5-fold, compared with both LTV and control mice (P < 0.05). No significant changes in plasma 2,3-dinor-6-keto PGF1α, a plasma stable prostacyclin metabolite, were evident.
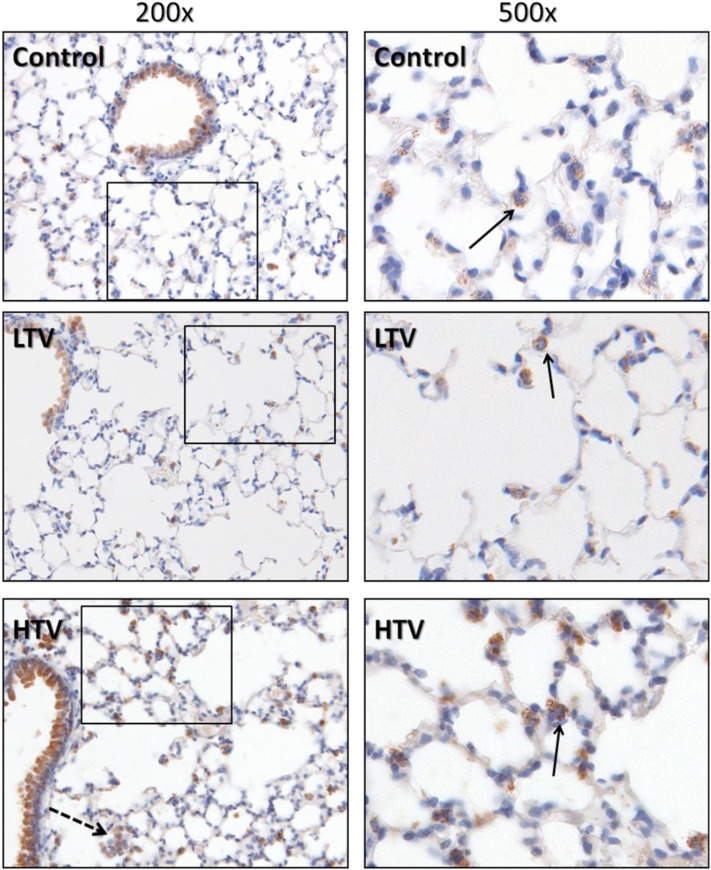
An immunohistochemical analysis of lungs stained with COX-2–specific antibody was performed to localize COX-2 expression within the lung (Figure 4). In addition to the expected staining of the bronchiolar epithelium, prominent cytoplasmic staining for COX-2 was evident in alveolar and interstitial mononuclear cells. These cells were confirmed to be of monocyte/macrophage lineage by subsequent staining for CD45 and CD68. The COX-2–positive cells were found both in the alveolar space and in the alveolar interstitium (Figure 4, arrows). In contrast, PMNs did not show significant COX-2 staining.
Figure 4.
COX-2 expression in murine lungs. Murine lungs were formalin-fixed in situ and immunohistochemically stained with antibodies directed against COX-2. In addition to the expected prominent staining of bronchiolar epithelium in all groups, significant staining of mononuclear cells was evident, showing granular cytoplasmic positivity to COX-2. These cells were confirmed to be of the monocyte/macrophage lineage by costaining for CD45 and CD68 (not shown). Note the presence of COX-2–positive alveolar macrophages in the HTV group (dashed arrow), in addition to interstitial monocytes and macrophages located in close proximity to the alveolar space (solid arrows). Images represent 5-μm histologic sections. Boxed areas in left panels are presented at ×500 magnification in right panels.
Inhibition of COX-2 Attenuates Ventilator-Induced Lung Injury
Mice were treated with the COX-2–specific inhibitor CAY10404 (50 mg/kg/day, intraperitoneal) × 4 days before the onset of mechanical ventilation. Treatment with the COX-2–specific inhibitor CAY10404 (50 mg/kg/day for 4 days) attenuated cyclooxygenase activity, significantly decreasing BAL PGE2 and 6-keto PGF1α (Figure 3). Likewise, systemic COX-2 inhibition decreased plasma PGE2 by 66% compared with untreated HTV mice (P < 0.05). The pharmacologic inhibition of COX-2 with CAY10404 significantly decreased alveolar–capillary leakage caused by HTV mechanical ventilation (Figure 1, dark bars; P < 0.05). Treatment with CAY10404 exerted no significant effect on tissue EBD or BAL protein in control or LTV mice. Likewise, inhibiting COX-2 decreased lung inflammation in HTV mice (Figures 2A–2D, dark bars; P < 0.05), decreasing BAL leukocytes, tissue PMNs, tissue MPO, and BAL IL-6 compared with untreated HTV mice. Treatment with CAY10404 exerted no significant effect on BAL cell count, PMN score, or IL-6 in control or LTV mice, although a nonsignificant trend toward decreased lung MPO was evident in LTV mice receiving COX-2 inhibition. COX-2 inhibition exerted divergent effects on leukocyte adhesion molecules, markedly decreasing ICAM-1 expression in both ventilation groups, but increasing VCAM-1 in both ventilation groups (Figure 2E, bottom). Of note, the treatment of control mice with CAY10404 increased basal VCAM-1 expression.
Discussion
In this study, we used a model of mechanical ventilation–induced lung injury to discern the role of COX-2 in the development of acute lung injury. HTV mechanical ventilation induced the expression of COX-2, which was accompanied by the production of PGE2 and prostacyclin. Pretreatment with the COX-2–selective inhibitor CAY10404 attenuated the pulmonary inflammation and barrier disruption caused by injurious mechanical ventilation, suggesting that COX-2 plays a central role in the development of lung injury in our model.
The major finding of this study involves the deleterious role of COX-2 in the initiation of lung injury. Although COX-1 is constitutively expressed and plays key physiologic roles, COX-2 is rapidly inducible in response to a variety of acute insults. For this reason, we chose to use CAY10404, which has a selectivity index of > 500,000 for COX-2 versus COX-1 (1,000-fold greater than celecoxib). In contrast to previous studies showing that nonspecific cyclooxygenase inhibition exerted a minimal effect on the inflammatory response to mechanical ventilation (19–21), we show a significant attenuation of inflammatory markers with COX-2–specific inhibition. Multiple studies investigated the role of COX-2 in regulating inflammation and tissue injury, with varying results. The inhibition of COX-2 attenuates the pulmonary inflammatory response to acute bacterial (18) and viral (17, 27) infection, and decreases the severity of illness during acute infection. In the lung, COX-2 expression is vital to the resolution of experimental inflammatory lung injury (28, 29), and a loss or reduction in COX-2 expression has been linked to the development of pulmonary fibrosis in patients and in experimental animal models (12). One explanation for the contrasting effects of COX-2 inhibition may involve an issue of timing.
COX-2 appears to exert different effects in the acute and subacute settings. Gilroy and colleagues demonstrated a biphasic response of COX-2 in a rat model of carrageenin-induced pleurisy (30). An initial increase in COX-2 expression and activity correlated with both PGE2 production and neutrophilic infiltration. A second peak in COX-2 expression and activity 48 hours later was not associated with PGE2 production, and coincided with the resolution of acute inflammation. The inhibition of COX-2 during the first peak significantly attenuated inflammation, whereas conversely, inhibition during the second peak worsened pleural inflammation. Fukunaga and colleagues (29) demonstrated a similar effect with an impaired resolution of oleic acid–induced lung injury with delayed COX-2 inhibition. In our experiments, pretreatment with CAY10404 prevented the development of ventilator-induced lung injury (VILI). In regard to these short-duration experiments, we cannot comment on the delayed effects of COX-2 inhibition in the progression or ultimate resolution of lung injury, or on the effects of postventilation COX-2 inhibition. Nonetheless, our results suggest a role for COX-2 inhibition in the prevention of VILI.
COX-2 exerts its effects via the production of downstream prostanoids, including prostaglandin D2, PGE1, PGE2, and prostacyclin, acting via their respective prostanoid receptors. In our model, VILI was associated with the increased production of both 6-keto PGF1α, a stable metabolite of prostacyclin, and PGE2. Endothelial cells are the main source for prostacyclin, which exerts its effects largely in an autocrine and paracrine fashion to govern vascular tone, thrombosis, and endothelial barrier function (31). Prostacyclin and its analogues have long been used as pulmonary vasodilators in pulmonary hypertension, and were more recently examined as barrier-enhancing agents in ALI (31, 32). Previous in vitro studies demonstrated the potent barrier-enhancing effects of prostacyclin in cultured pulmonary endothelial cells (32), and more recently demonstrated the protective effects of exogenous prostacyclin analogues in an animal model of VILI (31). Given these known barrier-enhancing effects, our finding of improved barrier function with COX-2 inhibition was somewhat unexpected, and likely reflects a greater role of the proinflammatory PGE2-mediated effects in our model. Lung injury in response to mechanical ventilation (MV) is likely attributable to a combination of direct barrier-disruptive effects and inflammatory cell recruitment, with secondary damage to the alveolar–capillary membrane because of cytokine release, oxidant damage, and cellular diapedesis. We believe that the ability of COX-2 inhibition to attenuate VILI in our model reflects its acute effects in the inflammatory cascade.
We show that mechanical ventilation caused a magnitude-dependent increase in IL-6 production and ICAM-1 expression with concomitant tissue neutrophilia, suggesting that, as in the clinical setting, a true “safe” threshold for positive pressure ventilation may not exist. The mild increase in IL-6 and ICAM-1 expression by LTV MV may be partly explained by regional differences in alveolar overdistention, even when lower volumes were applied globally. In our model, COX-2–specific inhibition decreased IL-6 and ICAM-1, and importantly, decreased acute tissue inflammation, as measured by tissue PMNs and MPO activity. Interestingly, COX-2 inhibition exerted a minimal effect on VCAM-1. Both ICAM-1 and VCAM-1 are members of the immunoglobulin superfamily, and serve as key mediators of leukocyte adhesion to endothelial cells, constituting the first step of inflammatory migration into tissue. VCAM-1 is more specific for monocytes/macrophages and lymphocytes, whereas ICAM-1 exerts a greater effect on neutrophil adhesion, providing a potential explanation for the decrease in tissue neutrophils with CAY10404. If the monocyte is the key cell type modulating COX-2 production in VILI, as suggested by our immunohistochemical staining, the differential ICAM-1/VCAM-1 expression may be important in controlling the acute inflammatory response to mechanical stretch. Ongoing in vivo and in vitro experiments in our laboratory seek a better understanding for the role of monocytes and macrophages in our VILI model and the specific role for leukocyte adhesion molecule expression in response to COX-2 inhibition.
The contrast of our results with those using isolated perfused lungs (19, 20), which failed to show a barrier-protective effect of cyclooxygenase inhibition, further points to the importance of leukocyte adhesion and subsequent migration across endothelial and epithelial monolayers in governing barrier disruption in our model. PGE2 may be key in mediating acute inflammation in our model. In contrast with prostacyclin, there are many cellular sources of PGE2, including endothelial cells, alveolar epithelial cells, fibroblasts, macrophages, and neutrophils. An immunohistochemical analysis of our murine lungs exposed to ventilation showed prominent COX-2 staining of alveolar monocytes and macrophages, but not alveolar epithelial or endothelial cells. Because alveolar macrophages are a major source of PGE2, which itself mediates the production of inflammatory cytokines, procoagulant factors, and neutrophil chemotactic factors during acute inflammation (33, 34), the COX-2–dependent production of PGE2 monocytes and macrophages appears key in orchestrating the acute response to mechanical stretch in our model.
PGE2 is a key prostanoid produced in the lung in response to a variety of insults, from LPS to tobacco smoke (35, 36). Although it exhibits direct barrier-enhancing effects on pulmonary endothelium (32), PGE2 also displays barrier-disruptive effects, particularly in epithelial cells (37), degrading occludin protein in pulmonary epithelium (38) and potentiating the development of pulmonary edema (39). Perhaps more importantly, PGE2 itself acts as a proinflammatory mediator, governing the production of inflammatory cytokines, procoagulant factors, and neutrophil chemotactic factors during acute inflammation (33, 34). However, PGE2 production can also exert anti-inflammatory and antifibrotic effects (40), particularly in the chronic setting. PGE2 production by pulmonary macrophages appears key in the resolution of inflammation in the lung, stomach, and joints (41), and PGE2 inhibits the proliferation of pulmonary fibrosis, inducing fibroblast apoptosis, in animal models (42).
The varied and contrasting effects of PGE2 are explained by its multiple downstream targets. PGE2 acts via four distinct G protein–coupled receptors, EP1–4, the distribution of which governs much of the effect of PGE2 in various cell and tissue types (43). Of the EP receptors, EP3 is the most widely expressed in the lung, followed by EP4, whereas EP1 and EP2 exhibit very low lung expression. The actions of PGE2 via the EP3 receptor include the promotion of platelet aggregation (44), pulmonary vasoconstriction (45), matrix metallopeptidase 9 (MMP-9) and vascular endothelial growth factor (VEGF) production (46), and the formation of pulmonary edema (39). EP4 mediates the induction of monocyte chemotactic protein-1 (MCP-1) (47), VEGF (48), and IL-6 production by macrophages (49) in response to PGE2. Because EP3 and EP4 have the highest binding affinities for PGE2 and the widest expression in the lung, their effects tend to predominate and may explain our reported findings. Although beyond the scope of this study, ongoing in vivo and in vitro experiments in our laboratory seek a better understanding for the role of individual prostanoid receptors in governing lung injury in our VILI model.
In conclusion, the pretreatment of mice with a COX-2–selective inhibitor attenuated lung injury caused by mechanical ventilation, decreasing both inflammation and alveolar barrier disruption. We believe that this effect most likely reflects a change in the balance of inflammatory versus barrier-enhancing prostanoids, with the acute anti-inflammatory effects outweighing the potential barrier-disruptive effects of decreased prostacyclin production. Further studies are needed to clarify the role of COX-2 in response to mechanical ventilation in both the uninjured lung and the lung primed by previous insult, as well as the precise role of prostanoid production by pulmonary monocytes and macrophages in lung injury. In the meantime, our results suggest a role for COX-2 inhibition in the prevention of mechanical ventilation–induced lung injury.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. David Jacoby for helpful discussions and critical review of the manuscript.
Footnotes
Author Contributions: J.A.R. performed data acquisition and analysis, helped with the study design, and was involved in drafting the manuscript. D.S. is a board-certified pathologist and performed blinded histologic analyses of hematoxylin and eosin–stained murine lungs for lung injury scoring as well as analyses of cyclooxygenase-2 immunohistochemistry. J.A.G. contributed to the study design, aided in data analysis, and was involved in the revision of the manuscript. S.A.N. was the principle investigator and was responsible for the overall conception, delineation of hypotheses and study design, data analysis and interpretation, and writing and revision of the final manuscript. All authors approved the final draft for publication.
This work was supported by National Institutes of Health grant K08HL089178 (S.A.N.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0005OC on May 3, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301–1308 [DOI] [PubMed] [Google Scholar]
- 2.Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 1999;282:54–61 [DOI] [PubMed] [Google Scholar]
- 3.Maniatis NA, Kotanidou A, Catravas JD, Orfanos SE. Endothelial pathomechanisms in acute lung injury. Vascul Pharmacol 2008;49:119–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puybasset L, Gusman P, Muller JC, Cluzel P, Coriat P, Rouby JJ. Regional distribution of gas and tissue in acute respiratory distress syndrome: III. Consequences for the effects of positive end-expiratory pressure. CT Scan ARDS Study Group. Adult Respiratory Distress Syndrome. Intensive Care Med 2000;26:1215–1227 [DOI] [PubMed] [Google Scholar]
- 5.Nonas SA, Moreno-Vinasco L, Ma SF, Jacobson JR, Desai AA, Dudek SM, Flores C, Hassoun PM, Sam L, Ye SQ, et al. Use of consomic rats for genomic insights into ventilator-associated lung injury. Am J Physiol Lung Cell Mol Physiol 2007;293:L292–L302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nonas SA, Peng X FJ, Skirball J, Hassoun PM. The role of cyclooxygenase 2 in ventilator-induced lung injury [abstract]. Am J Respir Crit Care Med 2007;175:A1517633759 [Google Scholar]
- 7.Ermert L, Ermert M, Merkle M, Goppelt-Struebe M, Duncker HR, Grimminger F, Seeger W. Rat pulmonary cyclooxygenase-2 expression in response to endotoxin challenge: differential regulation in the various types of cells in the lung. Am J Pathol 2000;156:1275–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gust R, Kozlowski JK, Stephenson AH, Schuster DP. Role of cyclooxygenase-2 in oleic acid–induced acute lung injury. Am J Respir Crit Care Med 1999;160:1165–1170 [DOI] [PubMed] [Google Scholar]
- 9.Hodges RJ, Jenkins RG, Wheeler-Jones CP, Copeman DM, Bottoms SE, Bellingan GJ, Nanthakumar CB, Laurent GJ, Hart SL, Foster ML, et al. Severity of lung injury in cyclooxygenase-2–deficient mice is dependent on reduced prostaglandin E(2) production. Am J Pathol 2004;165:1663–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien G, Shields CJ, Winter DC, Dillon JP, Kirwan WO, Redmond HP. Cyclooxygenase-2 plays a central role in the genesis of pancreatitis and associated lung injury. Hepatobiliary Pancreat Dis Int 2005;4:126–129 [PubMed] [Google Scholar]
- 11.Bonner JC, Rice AB, Ingram JL, Moomaw CR, Nyska A, Bradbury A, Sessoms AR, Chulada PC, Morgan DL, Zeldin DC, et al. Susceptibility of cyclooxygenase-2–deficient mice to pulmonary fibrogenesis. Am J Pathol 2002;161:459–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coward WR, Watts K, Feghali-Bostwick CA, Knox A, Pang L. Defective histone acetylation is responsible for the diminished expression of cyclooxygenase 2 in idiopathic pulmonary fibrosis. Mol Cell Biol 2009;29:4325–4339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rakotoniaina Z, Guerard P, Lirussi F, Rochette L, Dumas M, Goirand F, Bardou M. Celecoxib but not the combination of celecoxib + atorvastatin prevents the development of monocrotaline-induced pulmonary hypertension in the rat. Naunyn Schmiedebergs Arch Pharmacol 2008;378:241–251 [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Sheares KK, Davie N, Upton PD, Taylor GW, Horsley J, Wharton J, Morrell NW. Hypoxic induction of COX-2 regulates proliferation of human pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol 2002;27:688–696 [DOI] [PubMed] [Google Scholar]
- 15.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009;30:377–386 [DOI] [PubMed] [Google Scholar]
- 16.Ikezoe T, Yang Y, Saitoh T, Heber D, McKenna R, Taguchi H, Koeffler HP. PC-SPES down-regulates COX-2 via inhibition of NF-kappaB and C/EBPbeta in non–small cell lung cancer cells. Int J Oncol 2006;29:453–461 [PubMed] [Google Scholar]
- 17.Carey MA, Bradbury JA, Seubert JM, Langenbach R, Zeldin DC, Germolec DR. Contrasting effects of cyclooxygenase-1 (COX-1) and COX-2 deficiency on the host response to influenza A viral infection. J Immunol 2005;175:6878–6884 [DOI] [PubMed] [Google Scholar]
- 18.Goldmann O, Hertzen E, Hecht A, Schmidt H, Lehne S, Norrby-Teglund A, Medina E. Inducible cyclooxygenase released prostaglandin E2 modulates the severity of infection caused by Streptococcus pyogenes. J Immunol 2010;185:2372–2381 [DOI] [PubMed] [Google Scholar]
- 19.Jaecklin T, Engelberts D, Otulakowski G, O'Brodovich H, Post M, Kavanagh BP. Lung-derived soluble mediators are pathogenic in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 2011;300:L648–L658 [DOI] [PubMed] [Google Scholar]
- 20.Miyahara T, Hamanaka K, Weber DS, Anghelescu M, Frost JR, King JA, Parker JC. Cytosolic phospholipase A2 and arachidonic acid metabolites modulate ventilator-induced permeability increases in isolated mouse lungs. J Appl Physiol 2008;104:354–362 [DOI] [PubMed] [Google Scholar]
- 21.Niitsu T, Tsuchida S, Peltekova V, Engelberts D, Copland I, Otulakowski G, Post M, Kavanagh BP. Cyclooxygenase inhibition in ventilator-induced lung injury. Anesth Analg 2011;112:143–149 [DOI] [PubMed] [Google Scholar]
- 22.Katada J, Saito H, Ohashi A. Significance of cyclooxygenase-2 induced via p38 mitogen–activated protein kinase in mechanical stimulus–induced peritoneal adhesion in mice. J Pharmacol Exp Ther 2005;313:286–292 [DOI] [PubMed] [Google Scholar]
- 23.Rajagopalan G, Asmann YW, Lytle AK, Tilahun AY, Theuer JE, Smart MK, Patel R, David CS. Cyclooxygenase 2 pathway and its therapeutic inhibition in superantigen-induced toxic shock. Shock 2008;30:721–728 [DOI] [PubMed] [Google Scholar]
- 24.Imanaka H, Shimaoka M, Matsuura N, Nishimura M, Ohta N, Kiyono H. Ventilator-induced lung injury is associated with neutrophil infiltration, macrophage activation, and TGF-beta 1 mRNA upregulation in rat lungs. Anesth Analg 2001;92:428–436 [DOI] [PubMed] [Google Scholar]
- 25.Nonas S, Miller I, Kawkitinarong K, Chatchavalvanich S, Gorshkova I, Bochkov VN, Leitinger N, Natarajan V, Garcia JG, Birukov KG. Oxidized phospholipids reduce vascular leak and inflammation in rat model of acute lung injury. Am J Respir Crit Care Med 2006;173:1130–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nonas SA, M.L, Ma SF, Jacobson JR, Desai AA, Dudek S, Flores C, Hassoun PM, Sam L, Ye SQ, et al. Use of consomic rats for genomic insights into ventilator-associated lung injury. Am J Physiol Lung Cell Mol Physiol 2008;12:R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu T, Zaman W, Kaphalia BS, Ansari GA, Garofalo RP, Casola A. RSV-induced prostaglandin E2 production occurs via cPLA2 activation: role in viral replication. Virology 2005;343:12–24 [DOI] [PubMed] [Google Scholar]
- 28.Bonnans C, Fukunaga K, Levy MA, Levy BD. Lipoxin A(4) regulates bronchial epithelial cell responses to acid injury. Am J Pathol 2006;168:1064–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukunaga K, Kohli P, Bonnans C, Fredenburgh LE, Levy BD. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J Immunol 2005;174:5033–5039 [DOI] [PubMed] [Google Scholar]
- 30.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med 1999;5:698–701 [DOI] [PubMed] [Google Scholar]
- 31.Birukova AA, Fu P, Xing J, Cokic I, Birukov KG. Lung endothelial barrier protection by iloprost in the 2-hit models of ventilator-induced lung injury (VILI) involves inhibition of Rho signaling. Transl Res 2010;155:44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birukova AA, Zagranichnaya T, Fu P, Alekseeva E, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res 2007;313:2504–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rola-Pleszczynski M, Stankova J. Leukotriene B4 enhances interleukin-6 (IL-6) production and IL-6 messenger RNA accumulation in human monocytes in vitro: transcriptional and posttranscriptional mechanisms. Blood 1992;80:1004–1011 [PubMed] [Google Scholar]
- 34.Williams JG, Garcia I, Maier RV. Prostaglandin E2 mediates lipopolysaccharide-induced macrophage procoagulant activity by a cyclic adenosine monophosphate–dependent pathway. Surgery 1993;114:314–323 [PubMed] [Google Scholar]
- 35.Alba-Loureiro TC, Martins EF, Miyasaka CK, Lopes LR, Landgraf RG, Jancar S, Curi R, Sannomiya P. Evidence that arachidonic acid derived from neutrophils and prostaglandin E2 are associated with the induction of acute lung inflammation by lipopolysaccharide of Escherichia coli. Inflamm Res 2004;53:658–663 [DOI] [PubMed] [Google Scholar]
- 36.Vassallo R, Kroening PR, Parambil J, Kita H. Nicotine and oxidative cigarette smoke constituents induce immune-modulatory and pro-inflammatory dendritic cell responses. Mol Immunol 2008;45:3321–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka MN, Diaz BL, de Souza W, Morgado-Diaz JA. Prostaglandin E2–EP1 and EP2 receptor signaling promotes apical junctional complex disassembly of Caco-2 human colorectal cancer cells. BMC Cell Biol 2008;9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tai HY, Tam MF, Chou H, Peng HJ, Su SN, Perng DW, Shen HD. Pen ch 13 allergen induces secretion of mediators and degradation of occludin protein of human lung epithelial cells. Allergy 2006;61:382–388 [DOI] [PubMed] [Google Scholar]
- 39.Goggel R, Hoffman S, Nusing R, Narumiya S, Uhlig S. Platelet-activating factor–induced pulmonary edema is partly mediated by prostaglandin E(2), E-prostanoid 3–receptors, and potassium channels. Am J Respir Crit Care Med 2002;166:657–662 [DOI] [PubMed] [Google Scholar]
- 40.Scher JU, Pillinger MH. The anti-inflammatory effects of prostaglandins. J Investig Med 2009;57:703–708 [DOI] [PubMed] [Google Scholar]
- 41.Chan MM, Moore AR. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2–mediated lipoxin A4 production. J Immunol 2010;184:6418–6426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lama V, Moore BB, Christensen P, Toews GB, Peters-Golden M. Prostaglandin E2 synthesis and suppression of fibroblast proliferation by alveolar epithelial cells is cyclooxygenase-2–dependent. Am J Respir Cell Mol Biol 2002;27:752–758 [DOI] [PubMed] [Google Scholar]
- 43.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem 2007;282:11613–11617 [DOI] [PubMed] [Google Scholar]
- 44.Philipose S, Konya V, Sreckovic I, Marsche G, Lippe IT, Peskar BA, Heinemann A, Schuligoi R. The prostaglandin E2 receptor EP4 is expressed by human platelets and potently inhibits platelet aggregation and thrombus formation. Arterioscler Thromb Vasc Biol 2010;30:2416–2423 [DOI] [PubMed] [Google Scholar]
- 45.Norel X, de Montpreville V, Brink C. Vasoconstriction induced by activation of EP1 and EP3 receptors in human lung: effects of ONO-AE-248, ONO-DI-004, ONO-8711 or ONO-8713. Prostaglandins Other Lipid Mediat 2004;74:101–112 [DOI] [PubMed] [Google Scholar]
- 46.Amano H, Ito Y, Suzuki T, Kato S, Matsui Y, Ogawa F, Murata T, Sugimoto Y, Senior R, Kitasato H, et al. Roles of a prostaglandin E-type receptor, EP3, in upregulation of matrix metalloproteinase–9 and vascular endothelial growth factor during enhancement of tumor metastasis. Cancer Sci 2009;100:2318–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tajima T, Murata T, Aritake K, Urade Y, Hirai H, Nakamura M, Ozaki H, Hori M. Lipopolysaccharide induces macrophage migration via prostaglandin D(2) and prostaglandin E(2). J Pharmacol Exp Ther 2008;326:493–501 [DOI] [PubMed] [Google Scholar]
- 48.Liu H, Yang Y, Xiao J, Lv Y, Liu Y, Yang H, Zhao L. COX-2–mediated regulation of VEGF-C in association with lymphangiogenesis and lymph node metastasis in lung cancer. Anat Rec (Hoboken) 2010;293:1838–1846 [DOI] [PubMed] [Google Scholar]
- 49.Ma W. Up-regulation of interleukin-6 induced by prostaglandin E from invading macrophages following nerve injury: an in vivo and in vitro study. J Neurochem 2005;93:664–673 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.