Abstract
Mutations in the SFTPC gene, encoding surfactant protein–C (SP-C), are associated with interstitial lung disease (ILD). Knowledge of the intracellular fate of mutant SP-C is essential in the design of therapies to correct trafficking/processing of the proprotein, and to prevent the formation of cytotoxic aggregates. We assessed the potential of a chemical chaperone to correct the trafficking and processing of three disease-associated mutant SP-C proteins. HEK293 cells were stably transfected with wild-type (SP-CWT) or mutant (SP-CL188Q, SP-CΔexon4, or SP-CI73T) SP-C, and cell lines with a similar expression of SP-C mRNA were identified. The effects of the chemical chaperone 4-phenylbutyric acid (PBA) and lysosomotropic drugs on intracellular trafficking to the endolysosomal pathway and the subsequent conversion of SP-C proprotein to mature peptide were assessed. Despite comparable SP-C mRNA expression, proprotein concentrations varied greatly: SP-CI73T was more abundant than SP-CWT and was localized to the cell surface, whereas SP-CΔexon4 was barely detectable. In contrast, SP-CL188Q and SP-CWT proprotein concentrations were comparable, and a small amount of SP-CL188Q was localized to the endolysosomal pathway. PBA treatment restored the trafficking and processing of SP-CL188Q to SP-CWT concentrations, but did not correct the mistrafficking of SP-CI73T or rescue SP-CΔexon4. PBA treatment also promoted the aggregation of SP-C proproteins, including SP-CL188Q. This study provides proof of the principle that a chemical chaperone can correct the mistrafficking and processing of a disease-associated mutant SP-C proprotein.
Keywords: pulmonary surfactant protein–C, interstitial lung disease, 4-phenylbutyric acid, chemical chaperone
Clinical Relevance
Mutations in the gene encoding surfactant protein–C (SFTPC) are associated with interstitial lung disease in both children and adults. The pathogenesis is thought to be related to an accumulation of cytotoxic aggregates of misfolded surfactant protein–C proprotein (proSP-C). No treatment options are currently available. Treatment with 4-phenylbutyric acid (PBA) rescues at least one form of mutant proSP-C from proteasome degradation, and restores trafficking to the endolysosomal pathway as well as the conversion of the proprotein to the mature peptide. This study shows that PBA may be a viable treatment option for some patients with SFTPC mutations.
Interstitial lung disease (ILD) is the result of a multiplicity of pathological processes, from infections to autoimmune diseases (1). In addition to idiopathic forms of the disease, familial ILD has also been reported. Mutations in the gene encoding surfactant protein–C (SP-C) are associated with heritable ILD in both children (2) and adults (1), and are the focus of this study.
SP-C, encoded by the SFTPC gene on chromosome 8 in humans, is a hydrophobic protein produced by alveolar Type II epithelial cells. Human SP-C is synthesized as a 197–amino acid precursor protein (proSP-C) that undergoes multiple proteolytic cleavages as it traffics through the endolysosomal pathway to the lamellar body. The fully processed 35–amino acid mature SP-C peptide is packaged with SP-B and phospholipids into bilayer membranes that are stored in lamellar bodies before secretion into the alveolar airspaces.
Nogee and colleagues described the first disease-associated SFTPC mutation in an infant and her mother in 2001 (3). An SFTPC mutation that resulted in the skipping of exon 4 (SP-CΔexon4) was identified in one allele in both subjects, consistent with autosomal dominant inheritance. A number of other autosomal dominant SFTPC mutations were subsequently identified with a range of histological phenotypes, even within kindreds. For example, a mutation leading to the substitution of glutamine for leucine at position 188 of proSP-C (SP-CL188Q) resulted in a variable disease onset, with nonspecific interstitial pneumonia (NSIP) in children and usual interstitial pneumonia (UIP) in adults of the same family (4, 5).
Very little is known about the pathogenesis of lung disease associated with SFTPC mutations. The overexpression of mutant proSP-C can cause cytotoxicity in transfected cells (4) and transgenic mice (6). However, not all individuals who carry a disease-associated mutant SFTPC allele are symptomatic (4, 5). This observation led to the hypothesis that misfolded proSP-C is normally rapidly degraded but, under specific conditions (e.g., viral infection), might accumulate to form cytotoxic aggregates that, in turn, could trigger disease onset (7). In this model of disease pathogenesis, the goal of therapy would involve accelerating the turnover of the misfolded proprotein to prevent aggregation. This therapeutic approach comes at the expense of reduced SP-C in the airspaces. An alternative approach would involve the rescue of misfolded proprotein by promoting folding to a stable conformation that will pass quality control in the endoplasmic reticulum (ER) and traffic to the endolysosomal pathway for processing to the mature peptide.
Chemical chaperones are small molecules that promote the stabilization and folding of nascent proteins (8). The chemical chaperone 4-phenylbutyric acid (PBA) has been shown to rescue the trafficking of a wide range of misfolded proteins, including the ATP-binding cassette (ABC) transporters ABCC6 (9) and ABCC7/cystic fibrosis transmembrane conductance regulator (10). PBA is one of only two chemical chaperones currently approved by the United States Food and Drug Administration for use in humans. The present study evaluated the potential of PBA to restore the trafficking and processing of three different mutant SP-C proproteins. The most common SFTPC allele associated with ILD in both pediatric and adult patients is a substitution of threonine for isoleucine at position 73 of proSP-C (SP-CI73T) (11–13). This mutation occurs in a highly conserved region of the linker domain, a 34 amino acid juxtamembrane portion of the C-terminal (luminal) region of the proprotein. In contrast, the SP-CΔexon4 and SP-CL188Q mutations are located in the 105–amino acid, C-terminal Brichos domain, a region to which the majority of SFTPC mutations map. The Brichos domain functions as an intramolecular chaperone for the metastable transmembrane domain of the proprotein (14–16). Disease-associated Brichos mutations are thought to compromise chaperone activity, leading to terminal misfolding and the proteasomal degradation of the proprotein via the ER-associated degradation (ERAD) pathway.
In this study, we used cell lines that stably (constitutively) express nontagged SP-CWT, SP-CI73T, SP-CL188Q, or SP-CΔexon4 (7, 17) to avoid artifacts associated with the transient overexpression or tagging of SP-C proproteins. The stability of each mutant proprotein and its ability to traffic to the endolysosomal pathway for processing to mature peptide were evaluated in the presence or absence of chemical chaperones and lysosomotropic drugs. The results demonstrate for the first time, to the best of our knowledge, that PBA at clinically achievable and tolerable concentrations can rescue SP-CL188Q from ERAD, and restore both trafficking and processing of the proprotein to its mature peptide. A portion of these studies was previously reported in abstract form (18).
Materials and Methods
Reagents
PBA, chloroquine, formic acid, brefeldin A, and glycerol were purchased from Sigma (St. Louis, MO). Bafilomycin A1 was purchased from Calbiochem (San Diego, CA). Antibodies directed against the N-terminal propeptide of SP-C or the mature SP-C peptide were obtained from Seven Hills Bioreagents (Cincinnati, OH). The generation of the antibody directed against the C-terminal (luminal) domain of SP-C was previously described (16).
Generation of Stably Transfected Lines
Full-length human wild-type (SP-CWT) or mutant (SP-CL188Q, SP-CΔexon4, or SP-CI73T) SP-C was subcloned into pTRE2-Hyg (Clontech, Mountain View, CA), and stably transfected cell lines were generated in HEK293 Tet-Off cells, as previously described (7).
Analyses of SP-C mRNA and Protein Expression
SDS-soluble and insoluble proteins were isolated, normalized to the total cell lysate protein concentration, and analyzed by SDS-PAGE/Western blotting, as previously described (6, 7, 15). Pulse-chase experiments were performed as previously described (17). The isolation of RNA for quantitative PCR and microarray analyses is described in the online supplement. Unfolded protein response element luciferase reporter assays and confocal microscopy were performed exactly as previously described (7, 19). Immunogold labeling and ultrastructural analyses of HEK293 cells are described in the online supplement.
Statistical Analysis
All experiments were performed at least three times, except where noted. Data were analyzed using GraphPad Prism, version 5.0 (GraphPad Software, Inc., La Jolla, CA). The data are representative of at least three independent transfection experiments, with each group performed in triplicate. Data are plotted as means ± SD, and were analyzed by the Tukey multiple comparisons test. Statistical significance was defined as P < 0.05.
Results
SFTPC Mutations Result in Widely Variable Proprotein Stability
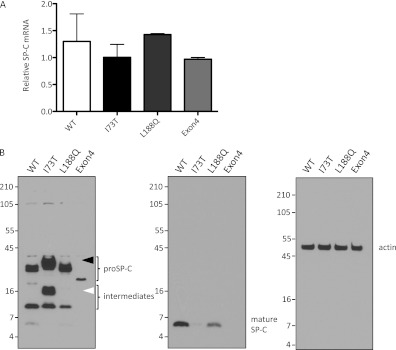
Except where noted, all experiments in this study were performed in stably transfected HEK293 cells to model the constitutive expression of SP-C. Multiple cell lines stably expressing untagged SP-CWT or mutant SP-C (SP-CI73T, SP-CL188Q, or SP-CΔexon4) were grown in the absence of doxycycline to induce the expression of the proprotein, and screened to identify four cell lines that expressed similar concentrations of SP-C mRNA (Figure 1A). Cell lysates (30 μg) of each line were subsequently immunoblotted with antibodies directed against the N-terminus of the SP-C proprotein or the mature SP-C peptide, to assess relative protein expression (Figure 1B). Despite comparable SP-C mRNA concentrations, proSP-C expression varied greatly among the four cell lines (Figure 1B). SP-CWT proprotein was considerably more abundant than SP-CΔexon4 proprotein, which was previously shown to be rapidly degraded by the proteasome (7, 17). SP-CL188Q mutant proprotein was also more stable than SP-CΔexon4 proprotein and similar to SP-CWT proprotein. Notably, SP-CI73T proprotein accumulated to a much greater extent than either SP-CWT or the other two mutant proproteins. The abundance of the SP-CI73T mutant was particularly striking when all forms of the proprotein were considered, including two unique bands recently identified by Beers and colleagues (20), composed of a prominent processing intermediate (Figure 1B, white arrowhead) and a slower-migrating form of the proprotein (Figure 1B, black arrowhead, and Figure 2C). Thus the stability of proSP-C varies widely with the mutation: SP-CI73T accumulates, SP-CΔexon4 is rapidly degraded, and SP-CL188Q exhibits an intermediate stability similar to that of SP-CWT.
Figure 1.
Variable expression of mutant surfactant protein–C proprotein (proSP-C). (A) HEK293 cell lines stably expressing SP-CWT, SP-CI73T, SP-CL188Q, or SP-CΔexon4 were screened by quantitative RT-PCR to identify lines that expressed similar concentrations of SP-C mRNA. (B) Cell lines were grown in the absence of doxycycline to induce the expression of SP-C. Cell lysates (30 μg) were analyzed by SDS-PAGE, followed by Western blotting. Blots were sequentially probed with antibody directed against mature SP-C peptide (middle), proSP-C (left), and actin (right). The detection of SP-CΔexon4 required extended exposure of the blot for proSP-C (left). Unique forms of SP-CI73T proprotein (black arrowhead) and processing intermediate (white arrowhead) are indicated. WT, wild-type.
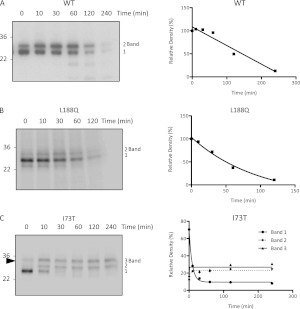
Figure 2.
Differential stability of mutant SP-C proproteins. SP-CWT (A), SP-CL188Q (B), and SP-CI73T (C) cell lines were grown in the absence of doxycycline. Cells (2.5 × 106 cells/well of a six-well plate) were labeled with 35(S) cysteine/methionine for 10 minutes, followed by transfer to chase media for the indicated periods of time. Cell lysates were immunoprecipitated with antibody directed against proSP-C, and analyzed by SDS-PAGE/autoradiography. Densitometric analyses appear to the right of each image. The sum of proSP-C bands 1 and 2 is plotted for SP-CWT (A) and SP-CL188Q (B).
The expression of SP-CWT in cells lacking lamellar bodies, such as HEK293 cells, results in the anterograde trafficking of the proprotein to the lysosome, where it is processed to the mature peptide (19) (Figure 1B, center). In contrast, the SP-CL188Q and SP-CΔexon4 mutants are ERAD substrates that are retained in the ER and degraded by the proteasome (17). The Western blotting of cell lysates detected a small amount of mature peptide in cells stably expressing SP-CL188Q, suggesting that some of the proprotein escaped ER quality control when constitutively expressed in HEK293 cells (Figure 1B, center). No mature peptide was identified in cells expressing SP-CΔexon4 or SP-CI73T, although longer exposure of the blot detected some mature peptide in cells expressing SP-CI73T (not shown).
The block in processing of the SP-CI73T proprotein to the mature peptide, coupled with the accumulation of proprotein, suggested that the I73T mutation may impart increased stability to the proprotein. To test this hypothesis, pulse-chase experiments were performed in HEK293 cells stably expressing WT or mutant SP-C. SP-CWT was synthesized as a proprotein (Figure 2A, band 1) that was posttranslationally modified to a larger proprotein (Figure 2A, band 2), with an estimated t1/2 of 145 minutes. The conversion of band 1 to band 2 was incomplete and blocked by brefeldin A (Figure E1A in the online supplement), consistent with proprotein modification in a compartment distal to the ER. In contrast, very little SP-CL188Q was converted to band 2 and the proprotein was rapidly degraded, with a t1/2 of 43 minutes (Figure 2B). Band 1 of SP-CI73T was rapidly converted to band 2 and a higher molecular weight protein (Figure 2C, band 3). The formation of band 3 was inhibited by brefeldin A (Figure E1B) or by the mutation of isoleucine at position 73 to serine (I73S) or lysine (I73K) (Figure E1C). Both bands 2 and 3 of SP-CI73T were very stable and did not decrease over the 2-hour chase period (Figure 2C). Thus the I73T mutation is associated with decreased proprotein processing/degradation that leads to accumulation.
PBA Treatment Stabilizes SP-C Proprotein and Promotes Conversion to Mature Peptide
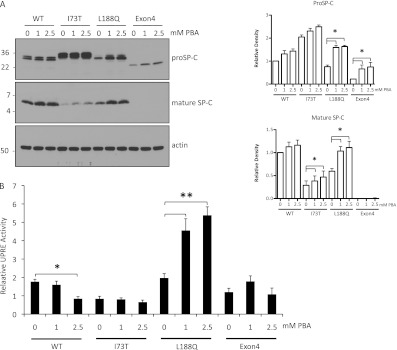
To determine if chemical chaperones would promote processing of the mutant proproteins to the mature peptide, cell lines were treated with PBA or glycerol. PBA was added at up to 2.5 mM and glycerol at up to 2.5% (higher doses of either reagent result in toxicity in all cell lines), and the cell lysates were probed for both proSP-C and mature SP-C. Because glycerol treatment did not alter SP-C concentrations, only the PBA data are reported here. PBA treatment did not significantly alter concentrations of SP-CWT proprotein or mature peptide (Figure 3A). In contrast, PBA treatment promoted a significant accumulation of SP-CL188Q and SP-CΔexon4 proproteins (Figure 3A, top). Proprotein stabilization was accompanied by a corresponding increase in mature peptide in cells expressing SP-CL188Q but not SP-CΔexon4 (Figure 3A, center). Although PBA did not alter SP-CI73T proprotein concentrations, treatment was associated with a small but significant increase in mature peptide. However, the proprotein/mature peptide ratio remained much higher for SP-CI73T than for SP-CL188Q or SP-CWT, consistent with the hypothesis that generation of the mature peptide is partly blocked in cells expressing SP-CI73T. Overall, PBA treatment resulted in the stabilization of the SP-CL188Q proprotein and conversion to mature peptide at concentrations approximating those of SP-CWT. Importantly, the effect of PBA was reproduced in three different HEK293 cell lines stably expressing SP-CL188Q (Figure E2A), as well as in mouse lung epithelial (MLE-12) cells transiently transfected with SP-CL188Q (Figure E2B).
Figure 3.
4-phenylbutyric acid (PBA) promotes SP-C proprotein stabilization and processing. (A) Cell lines were grown in the absence of doxycycline and treated with PBA for 48 hours. Cell lysates (30 μg) were analyzed by SDS-PAGE/Western blotting. Blots were sequentially probed with antibody directed against proSP-C (top), mature SP-C peptide (middle), and actin (bottom). The quantitation of the effects of PBA on proSP-C and mature peptide are shown to the right (n = 3). (B) Cell lines were grown in the absence of doxycycline and transiently transfected with plasmids encoding unfolded protein response element (UPRE) reporter and pRL-TK (plasmid expressing Renilla Luciferase under the herpes simplex virus thymidine kinase promoter). Cells were harvested 48 hours later, and luciferase activity was measured using the dual-luciferase reporter assay system (Promega). Data are plotted as ratios of Firefly/Renilla activity to correct for transfection efficiency. *P < 0.05. **P < 0.001.
The accumulation of mutant protein is frequently associated with the induction of ER stress. In this study, no evidence of ER stress was detected in any of the untreated SP-C cell lines (Figure 3B), consistent with our previous finding that the constitutive/stable expression of SP-CΔexon4 in HEK293 cells is not accompanied by ER stress (7). The absence of ER stress in cells expressing SP-CI73T suggested that the proprotein might accumulate in a compartment outside the ER (to be described later). PBA treatment resulted in ER stress in cells stably expressing SP-CL188Q, but not in cells expressing SP-CWT or either of the other two mutant proproteins (Figure 3B). PBA-induced ER stress (Figure 3B) may be related to the stabilization and retention of the proprotein in the ER that, in turn, allows more time for folding.
PBA Treatment Induces Detergent-Insoluble Aggregates of SP-C Proprotein
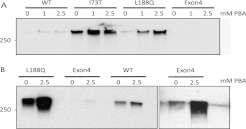
Mutant proSP-C has a high propensity to form intracellular SDS-insoluble aggregates. To assess aggregate formation, lysates of cells stably expressing SP-CWT or mutant SP-C were extracted with 1% SDS, and the detergent-insoluble fractions were analyzed by Western blotting. High molecular weight (> 250 kD) SP-C aggregates were consistently detected in SDS-insoluble fractions of all four cell lines, indicating that both mutant and WT SP-C can aggregate (Figures 4A and 4B). In the absence of PBA treatment, SDS-insoluble SP-C proprotein was much more abundant in cells expressing SP-CI73T (Figure 4A) compared with cells expressing SP-CWT, SP-CL188Q (Figures 4A and 4B), or SP-CΔexon4 proprotein (Figure 4B, right). We note that the aggregates were located at the top of the gels, leaving open the possibility that not all the sample entered the gel, leading to an underestimation of the amount of SDS-insoluble SP-C. These data indicate that SP-CI73T is the most aggregate-prone of the three mutant proteins tested in this study, and that the total amount of SP-CI73T proprotein (detergent-soluble + detergent-insoluble) is greater than initially estimated (Figure 1B). Treatment with PBA promoted the accumulation of SDS-insoluble SP-C aggregates in a dose-dependent manner. This effect was most pronounced for SP-CL188Q, but was also apparent for SP-CWT and SP-CΔexon4 after the extended exposure of Western blots (Figure 4B). At the highest dose of PBA, the amount of detergent-insoluble SP-CL188Q was similar to that observed for SP-CI73T proprotein (Figure 4A). Thus PBA treatment increases both soluble and insoluble forms of SP-CL188Q.
Figure 4.
PBA promotes the formation of detergent-insoluble SP-C aggregates. (A) Cell lines were grown in the absence of doxycycline and treated with PBA for 48 hours. SDS-insoluble protein was isolated from equivalent amounts of cell lysates and analyzed by SDS-PAGE, followed by Western blotting with proSP-C antibody. (B) To detect detergent-insoluble SP-CWT, the experiment in A was repeated, and exposure times for blots were increased (left). The detection of SP-CΔexon4 required the longest exposure times (right).
SP-CI73T and SP-CL188Q Traffic through Distinct Intracellular Pathways
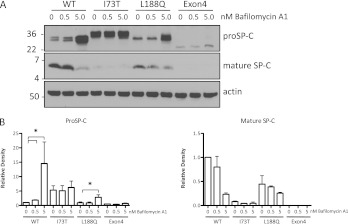
The detection of mature peptide in cells expressing SP-CWT or SP-CL188Q (Figure 1) is consistent with the trafficking of these proproteins to the endolysosomal pathway. Treatment with the lysosomotropic drugs bafilomycin A1 (Figure 5) or chloroquine (not shown) resulted in the accumulation of proprotein and inhibited the conversion to mature peptide, further supporting this conclusion. Treatment with azithromycin, a drug recently shown to inhibit lysosomal acidification (21), produced similar effects on proprotein stabilization and processing (not shown). In contrast, SP-CI73T proprotein was not affected, and the mature peptide was barely detectable. This result suggests that, unlike SP-CWT and SP-CL188Q, relatively little SP-CI73T proprotein traffics to the lysosome. Treatment with lysosomotropic drugs also exerted no effect on SP-CΔexon4, likely because this proprotein is rapidly degraded by the proteasome and is not exported from the ER.
Figure 5.
Trafficking of SP-C to the Golgi apparatus and endolysosomal compartments. (A) Cell lines were gown in the absence of doxycycline and treated with bafilomycin A1 for 24 hours. Cell lysates (30 μg) were analyzed by SDS-PAGE/Western blotting. Blots were sequentially probed for proSP-C (top), mature SP-C peptide (middle), and actin (bottom). (B) Quantitation of the effects of bafilomycin A1 on proSP-C (n = 3) and mature peptide concentrations (n = 2).
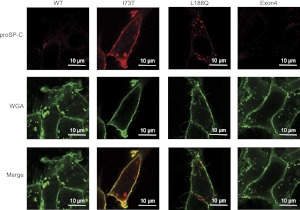
The differential response of SP-CL188Q and SP-CI73T to PBA and lysosomotropic drugs likely reflects distinct intracellular trafficking patterns for the two mutant proproteins. Unlike SP-CL188Q, SP-CI73T did not accumulate in response to proteasome inhibition (not shown), and did not engage the ERAD chaperones endoplasmic reticulum–localized DnaJ homologue 4 (ERdj4) or ERdj5 (Figure E3A). Further, unlike SP-CΔexon4, the transient expression of SP-CI73T did not induce ER stress (Figure E3B). These data, coupled with the finding that brefeldin A blocks the formation of band 3 in SP-CI73T (Figure E1B), indicate that SP-CI73T passes ER quality control, resulting in rapid export to the Golgi apparatus. Confocal microscopy with an antibody directed against the C-terminal (luminal) domain of proSP-C confirmed that SP-CI73T colocalized with wheat germ agglutinin (WGA) at the surface of nonpermeabilized cells (Figure 6), as recently reported (20). Immunogold labeling further confirmed the cell-surface localization of SP-CI73T and the absence of proprotein on the surface of cells expressing SP-CWT (Figure E4). Little or no colocalization of SP-C with WGA was detected in cells expressing SP-CWT, SP-CL188Q, or SP-CΔexon4 (Figure 6). To assess the turnover of cell-surface SP-C, cells stably expressing SP-CI73T were grown in the presence of doxycycline to suppress the expression of SP-C. Confocal microscopy confirmed the extended residency of SP-CI73T at the cell surface (Figure E5). These results suggest that an accumulation of SP-CI73T in the plasma membrane likely accounts for the unusual stability of the proprotein and the inhibition of proprotein conversion to mature peptide.
Figure 6.
SP-CI73T is localized to the cell surface. Cells were grown on coverslips in the absence of doxycycline, fixed in situ with 4% paraformaldehyde, and processed for confocal microscopy without permeabilization. Cells were incubated with antibody directed against the C-terminus of proSP-C (top row) or wheat germ agglutinin (WGA) to label the cell surface (middle row). No signal was detected when cells were incubated with antibody directed against the N-terminus of proSP-C, confirming that the Brichos domain is extracellular (not shown).
Discussion
In this study, we used the stable expression of constructs encoding WT or mutant SP-C to model the constitutive expression of SP-C in vivo. The selection of cell lines that express similar concentrations of SP-C mRNAs revealed that proprotein stability varied widely with the SFTPC mutation. Unexpectedly, both detergent-soluble and insoluble forms of SP-CI73T accumulated to a much greater extent than did the Brichos mutants, SP-CL188Q and SP-CΔexon4. SP-CI73T proprotein did not engage the ERAD chaperones previously reported to be involved in the turnover of Brichos mutants (17) or induce ER stress, as previously noted by Mulugeta and colleagues (22). These data, coupled with the results of pulse-chase experiments, suggest that SP-CI73T does not accumulate in the ER, but rather is rapidly folded and exported from the ER to the Golgi apparatus. For this reason, PBA treatment, which promotes the folding of some protein substrates in the ER, exerted no effect on SP-CI73T. Confocal microscopy detected SP-CI73T at the plasma membrane, confirming a recent report by Beers and colleagues (20). The loss of SP-CI73T from the plasma membrane occurred slowly, suggesting that cell-surface localization accounts for much of the accumulated proprotein. Consistent with this idea, relatively little proprotein was processed to mature peptide, an event that occurs in the endolysosomal pathway. Further, lysosomotropic drugs did not cause an accumulation of SP-CI73T proprotein. Collectively, these data suggest that the trafficking of SP-CI73T to the plasma membrane occurs rapidly, but that internalization and routing to the lysosome for processing/degradation constitute much slower events. Overall, the development of therapies for this “common” SFTPC mutation will require a better understanding of the molecular basis for SP-CI73T mistrafficking.
In contrast to SP-CI73T, SP-CΔexon4 was the least stable of the mutant proproteins, and frequently required an overexposure of immunoblots to detect its signal (Figure 1B). This result is in marked contrast to the reported tendency of SP-CΔexon4 to form aggresomes (23), a property that may well be related to the transient overexpression and saturation of the disposal pathway. The robust overexpression of SP-CΔexon4 in Type II epithelial cells of transgenic mice resulted in aggregation, cytotoxicity, and lung dysmorphogenesis (6). In the present study, the constitutive expression of SP-CΔexon4 at concentrations that did not induce ER stress resulted in the formation of very little detergent-insoluble proprotein, particularly when compared with SP-CI73T. PBA treatment increased the amount of SP-CΔexon4 proprotein, but the concentration was still much lower than for SP-CWT, and was not accompanied by detectable processing to the mature peptide. Moreover, treatment with bafilomycin A1, chloroquine, or azithromycin did not cause proprotein accumulation, suggesting that little or no SP-CΔexon4 was trafficked to the endolysosomal pathway. Proteasome inhibition resulted in a robust accumulation of proprotein (7) and a stable association with ER chaperones (Figure E3A), confirming that SP-CΔexon4 is a canonical ERAD substrate. Taken together, these results suggest that this SFTPC mutation leads to severe misfolding, and that the rescue of the mutant proprotein by treatment with chemical chaperones will be very challenging.
Like SP-CΔexon4, SP-CL188Q is also an ERAD substrate (Figure E3A) (17). The constitutive expression of SP-CL188Q at concentrations that did not cause ER stress resulted in the trafficking of some proprotein to the endolysosomal pathway, as indicated by our detection of mature peptide and the accumulation of proprotein in response to bafilomycin A1, chloroquine, or azithromycin. Treatment with concentrations of PBA widely used in other in vitro studies (10, 24–26) promoted the export of SP-CL188Q from the ER, leading to increased proprotein concentrations and the enhanced conversion of proprotein to mature peptide. At a dose of 1 mM PBA, the mature peptide concentration in SP-CL188Q–expressing cells approximated that in SP-CWT cells, with a minimal aggregation of proprotein. This dose is similar to that used in two clinical trials in which the concentration of PBA in serum ranged from 0.04–2.0 mM (27, 28). Collectively, these results suggest that this SFTPC mutation is amenable to rescue by PBA, and may offer a viable treatment option for patients.
The results of the present study provide support for the screening of other Brichos domain mutants to determine which mutants may be amenable to PBA therapy. However, this study also underscores the need for further investigation of the long-term effects of PBA treatment. PBA caused an increase in SDS-insoluble proprotein in cells expressing SP-CL188Q. Mutant proSP-C, including SP-CL188Q, can form amyloid-like fibrils (15) related to the loss of chaperone activity of the Brichos domain (14, 29) and subsequent transition of the α-helical transmembrane domain to a β-sheet structure (30, 31). Artificial β-sheet proteins that form amyloid-like fibrils have been shown to sequester proteins required for critical cellular functions (32). In the present study, the accumulation of SDS-insoluble proprotein was not associated with cytotoxicity (data not shown). However, the effect of extended PBA treatment on SP-CL188Q aggregation/cytotoxicity remains to be evaluated. Overall, analyses of PBA treatment in mice expressing SP-CL188Q (currently in development) will provide important insight into the risks and benefits of this potential therapy.
In conclusion, PBA treatment substantially restored intracellular trafficking and processing of the SP-CL188Q proprotein to its mature peptide. Whether this correction will be sufficient to prevent or slow pathogenesis remains to be established, as do any potential adverse effects of prolonged PBA treatment. Given the propensity of all SP-C proproteins, including SP-CWT, to form detergent-insoluble aggregates, the use of drugs promoting the simultaneous proprotein stabilization and inhibition of processing, such as chloroquine and azithromycin (33, 34), should be carefully reevaluated.
Supplementary Material
Footnotes
Author Contributions: G.A.S., R.R., Y.X., and T.E.W. made substantial contributions to the conception and design of the study. E.P.M., C.-L.N., and K.M. made substantial contributions to the acquisition and interpretation of data. All authors participated in writing the manuscript.
This study was supported by National Institutes of Health grants HL086492 and HL103923 (T.E.W.). G.A.S. received a Medical Research Council United Kingdom Clinician Scientist Fellowship.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0003OC on March 29, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.King TEJ, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet 2011;378:1949–1961 [DOI] [PubMed] [Google Scholar]
- 2.Das S, Langston C, Fan LL. Interstitial lung disease in children. Curr Opin Pediatr 2011;23:325–331 [DOI] [PubMed] [Google Scholar]
- 3.Nogee LM, Dunbar AE, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med 2001;344:573–579 [DOI] [PubMed] [Google Scholar]
- 4.Thomas AQ, Lane K, Phillips J, Prince M, Markin C, Speer M, Schwartz DA, Gaddipati R, Marney A, Johnson J, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med 2002;165:1322–1328 [DOI] [PubMed] [Google Scholar]
- 5.Chibbar R, Shih F, Baga M, Torlakovic E, Ramlall K, Skomro R, Cockcroft DW, Lemire EG. Nonspecific interstitial pneumonia and usual interstitial pneumonia with mutation in surfactant protein C in familial pulmonary fibrosis. Mod Pathol 2004;17:973–980 [DOI] [PubMed] [Google Scholar]
- 6.Bridges JP, Wert SE, Nogee LM, Weaver TE. Expression of a human surfactant protein C mutation associated with interstitial lung disease disrupts lung development in transgenic mice. J Biol Chem 2003;278:52739–52746 [DOI] [PubMed] [Google Scholar]
- 7.Bridges JP, Xu Y, Na CL, Wong HR, Weaver TE. Adaptation and increased susceptibility to infection associated with constitutive expression of misfolded SP-C. J Cell Biol 2006;172:395–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engin F, Hotamisligil GS. Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes Obes Metab 2010;12:108–115 [DOI] [PubMed] [Google Scholar]
- 9.Le Saux O, Fulop K, Yamaguchi Y, Ilias A, Szabo Z, Brampton CN, Pomozi V, Huszar K, Aranyi T, Varadi A. Expression and in vivo rescue of human ABCC6 disease-causing mutants in mouse liver. PLoS ONE 2011;6:e24738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubenstein RC, Zeitlin PL. Sodium 4-phenylbutyrate downregulates HSC70: implications for intracellular trafficking of Delta F508-CFTR. Am J Physiol Lung Cell Mol Physiol 2000;278:C259–C267 [DOI] [PubMed] [Google Scholar]
- 11.Cameron HS, Somaschini M, Carrera P, Hamvas A, Whitsett JA, Wert SE, Deutsch G, Nogee LM. A common mutation in the surfactant protein C gene associated with lung disease. J Pediatr 2005;146:370–375 [DOI] [PubMed] [Google Scholar]
- 12.Brasch F, Griese M, Tredano M, Johnen G, Ochs M, Rieger C, Mulugeta S, Muller KM, Bahuau M, Beers MF. Interstitial lung disease in a baby with a de novo mutation in the SFTPC gene. Eur Respir J 2004;24:30–39 [DOI] [PubMed] [Google Scholar]
- 13.van Moorsel CH, van Oosterhout MF, Barlo NP, de Jong PA, van der Vis JJ, Ruven HJ, van Es HW, van den Bosch JM, Grutters JC. Surfactant protein C mutations are the basis of a significant portion of adult familial pulmonary fibrosis in a Dutch cohort. Am J Respir Crit Care Med 2010;182:1419–1425 [DOI] [PubMed] [Google Scholar]
- 14.Johansson H, Eriksson M, Nordling K, Presto J, Johansson J. The Brichos domain of prosurfactant protein C can hold and fold a transmembrane segment. Protein Sci 2009;18:1175–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nerelius C, Martin E, Peng S, Gustafsson M, Nordling K, Weaver T, Johansson J. Mutations linked to interstitial lung disease can abrogate anti-amyloid function of prosurfactant protein C. Biochem J 2008;416:201–209 [DOI] [PubMed] [Google Scholar]
- 16.Johansson H, Nordling K, Weaver TE, Johansson J. The Brichos domain–containing C-terminal part of pro–surfactant protein C binds to an unfolded poly-val transmembrane segment. J Biol Chem 2006;281:21032–21039 [DOI] [PubMed] [Google Scholar]
- 17.Dong M, Bridges JP, Apsley K, Xu Y, Weaver TE. ERdj4 and ERdj5 are required for endoplasmic reticulum–associated protein degradation of misfolded surfactant protein C. Mol Biol Cell 2008;19:2620–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart GA, Weaver TE. Surfactant protein–C mutations and 4-phenylbutyrate (PBA) therapy: beneficial or deleterious? Am J Respir Crit Care Med 2009;179:A6262 [Google Scholar]
- 19.Conkright JJ, Apsley KS, Martin EP, Ridsdale R, Rice WR, Na CL, Yang B, Weaver TE. NEDD4-2–mediated ubiquitination facilitates processing of surfactant protein-C. Am J Respir Cell Mol Biol 2010;42:181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beers MF, Hawkins A, Maguire JA, Kotorashvili A, Zhao M, Newitt JL, Ding W, Russo S, Guttentag S, Gonzales L, et al. A nonaggregating surfactant protein C mutant is misdirected to early endosomes and disrupts phospholipid recycling. Traffic 2011;12:1196–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renna M, Schaffner C, Brown K, Shang S, Tamayo MH, Hegyi K, Grimsey NJ, Cusens D, Coulter S, Cooper J, et al. Azithromycin blocks autophagy and may predispose cystic fibrosis patients to mycobacterial infection. J Clin Invest 2011;121:3554–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulugeta S, Nguyen V, Russo SJ, Muniswamy M, Beers MF. A surfactant protein C precursor protein Brichos domain mutation causes endoplasmic reticulum stress, proteasome dysfunction, and caspase 3 activation. Am J Respir Cell Mol Biol 2005;32:521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang WJ, Mulugeta S, Russo SJ, Beers MF. Deletion of exon 4 from human surfactant protein C results in aggresome formation and generation of a dominant negative. J Cell Sci 2003;116:683–692 [DOI] [PubMed] [Google Scholar]
- 24.Rubenstein RC, Egan ME, Zeitlin PL. In vitro pharmacologic restoration of CFTR-mediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells containing Delta F508-CFTR. J Clin Invest 1997;100:2457–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi H, Sugiyama Y. 4-phenylbutyrate enhances the cell surface expression and the transport capacity of wild-type and mutated bile salt export pumps. Hepatology 2007;45:1506–1516 [DOI] [PubMed] [Google Scholar]
- 26.Iordache C, Duszyk M. Sodium 4-phenylbutyrate upregulates ENaC and sodium absorption in T84 cells. Exp Cell Res 2007;313:305–311 [DOI] [PubMed] [Google Scholar]
- 27.Collins AF, Pearson HA, Giardina P, McDonagh KT, Brusilow SW, Dover GJ. Oral sodium phenylbutyrate therapy in homozygous beta thalassemia: a clinical trial. Blood 1995;85:43–49 [PubMed] [Google Scholar]
- 28.Rubenstein RC, Zeitlin PL. A pilot clinical trial of oral sodium 4-phenylbutyrate (Buphenyl) in DeltaF508-homozygous cystic fibrosis patients: partial restoration of nasal epithelial CFTR function. Am J Respir Crit Care Med 1998;157:484–490 [DOI] [PubMed] [Google Scholar]
- 29.Willander H, Hermansson E, Johansson J, Presto J. Brichos domain associated with lung fibrosis, dementia and cancer: a chaperone that prevents amyloid fibril formation? FEBS J 2011;278:3893–3904 [DOI] [PubMed] [Google Scholar]
- 30.Nerelius C, Fitzen M, Johansson J. Amino acid sequence determinants and molecular chaperones in amyloid fibril formation. Biochem Biophys Res Commun 2010;396:2–6 [DOI] [PubMed] [Google Scholar]
- 31.Kallberg Y, Gustafsson M, Persson B, Thyberg J, Johansson J. Prediction of amyloid fibril-forming proteins. J Biol Chem 2001;276:12945–12950 [DOI] [PubMed] [Google Scholar]
- 32.Olzscha H, Schermann SM, Woerner AC, Pinkert S, Hecht MH, Tartaglia GG, Vendruscolo M, Hayer-Hartl M, Hartl FU, Vabulas RM. Amyloid-like aggregates sequester numerous metastable proteins with essential cellular functions. Cell 2011;144:67–78 [DOI] [PubMed] [Google Scholar]
- 33.Mechri M, Epaud R, Emond S, Coulomb A, Jaubert F, Tarrant A, Feldmann D, Flamein F, Clement A, de Blic J, et al. Surfactant protein C gene (SFTPC) mutation–associated lung disease: high-resolution computed tomography (HRCT) findings and its relation to histological analysis. Pediatr Pulmonol 2010;45:1021–1029 [DOI] [PubMed] [Google Scholar]
- 34.Rosen DM, Waltz DA. Hydroxychloroquine and surfactant protein C deficiency. N Engl J Med 2005;352:207–208 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.