Abstract
Recent studies suggest the importance of the transition of airway epithelial cells (EMT) in pulmonary fibrosis, and also indicate a role for Wingless protein (Wnt)/β-catenin signaling in idiopathic pulmonary fibrosis. We investigated the possible role of the Wnt signaling pathway in inducing EMT in lung epithelial cells, and sought to unravel the role of c-Jun–N-terminal-kinase–1 (JNK1). The exposure of C10 lung epithelial cells or primary mouse tracheal epithelial cells (MTECs) to Wnt3a resulted in increases in JNK phosphorylation and nuclear β-catenin content. Because the role of β-catenin as a transcriptional coactivator is well established, we investigated T-cell factor/lymphocyte-enhancement factor (TCF/LEF) transcriptional activity in C10 lung epithelial cells after the activation of Wnt. TCF/LEF transcriptional activity was enhanced after the activation of Wnt, and this increase in TCF/LEF transcriptional activity was diminished after the small interfering (si)RNA-mediated ablation of JNK. The activation of the Wnt pathway by Wnt3a, or the expression of either wild-type or constitutively active β-catenin (S37A), led to the activation of an EMT transcriptome, manifested by the increased mRNA expression of CArG box-binding factor–A, fibroblast-specific protein (FSP)–1, α–smooth muscle actin (α-SMA), and vimentin, increases in the content of α-SMA and FSP1, and the concomitant loss of zona occludens–1. The siRNA-mediated ablation of β-catenin substantially decreased Wnt3a-induced EMT. The siRNA ablation of JNK1 largely abolished Wnt3a, β-catenin, and β-catenin S37a-induced EMT. In MTECs lacking Jnk1, Wnt3a-induced increases in nuclear β-catenin, EMT transcriptome, and the content of α-SMA or FSP1 were substantially diminished. These data show that the activation of the Wnt signaling pathway is capable of inducing an EMT program in lung epithelial cells through β-catenin, and that this process is controlled by JNK1.
Keywords: lung, epithelium, Wnt3a, fibrosis, epithelial to mesenchymal transition
Clinical Relevance
These findings provide new insights into the role of c-Jun–N-terminal-kinase–1 (JNK1) in the Wingless protein (Wnt3a)–induced mesenchymal expression program in lung epithelial cells, and suggest that JNK1 is a relevant therapeutic target in patients with tissue fibrosis.
The development of fibrosis represents an important feature of pulmonary remodeling, and the critical role of epithelial cells in fibrogenesis is emerging. Studies from our laboratory identified a critical role for c-Jun–N-terminal–kinase–1 (JNK1) in augmenting the profibrotic effects of TGF-β1, in association with the causation of a mesenchymal transition of airway epithelial cells (EMT) (1). EMT is an important process during embryonic development, tumor progression, and fibrotic tissue repair after injury (2). We recently demonstrated that JNK1-induced phosphorylation in the linker domain of SMAD3 enhanced its ability to induce an EMT transcriptome. Consequently, JNK1-dependent, TGF-β1–induced EMT was greatly diminished in epithelial cells expressing a variant of SMAD3 refractory to phosphorylation in the linker domain (3).
Recent studies indicated a role of β-catenin signaling in the induction of EMT. Moreover, the Wingless protein (Wnt)/β-catenin axis was recently implicated to play a role in the development of idiopathic pulmonary fibrosis (IPF). Increased nuclear staining of β-catenin in bronchiolar lung epithelium and Type II alveolar epithelial cells from human IPF lung biopsies has been reported (4, 5), and consistent with these results, a microarray analysis showed increases in genes associated with the Wnt/β-catenin pathway in IPF (6). Furthermore, various studies in a murine model of pulmonary fibrosis showed that β-catenin is activated in lung epithelium, and the inhibition of the Wnt/β-catenin pathway can attenuate (7) or reverse pulmonary fibrosis (8).
The canonical Wnt pathway involves the transcriptional coactivator β-catenin (9). Under basal circumstances, β-catenin is regulated by a protein complex containing Axin, adenomatous Polyposis coli (APC), and glycogen synthase kinase 3β(GSK3β), which phosphorylates β-catenin at its N-terminus (10), which in turn targets β-catenin for destruction by the ubiquitin–proteosome pathway (11). Canonical Wnt ligands bind to a receptor complex containing the seven-transmembrane protein Frizzled (Fz) and the low-density lipoprotein receptor–related protein 5 or 6 (12). Wnt binding leads to the activation of the downstream element, Dishevelled (Dvl), which recruits FRAT (frequently rearranged in T-cell lymphoma) (13). Dvl dissociates the GSK3β/APC/Axin complex, inactivating GSK3β, which results in reduced β-catenin phosphorylation, thereby stabilizing β-catenin. Subsequently, β-catenin accumulates in complexes with cadherins at the cell membrane, which are involved in cell–cell interactions (14, 15). Alternatively, β-catenin translocates to the nucleus, where it interacts with transcriptional coactivators such as members of the T-cell factor/lymphocyte-enhancement factor–1 (TCF/LEF-1) family and induces the expression of growth-associated genes such as c-myc and cyclin D1 (9, 14).
Originally, JNK was implicated to play a role in noncanonical Wnt signaling (16). However, JNK activation was also reported recently after the stimulation of canonical Wnt signaling by Wnt3a (17). This observation, together with our finding that the absence of JNK1 protects against different murine models of lung fibrosis (18), led us to hypothesize that the JNK activation induced by canonical Wnt/β-catenin is important in the induction of a mesenchymal expression profile in lung epithelial cells. Therefore, this study was designed to investigate the possible role of the Wnt signaling pathway in inducing EMT in lung epithelial cells, and to unravel the role of JNK1 therein.
Materials and Methods
Cell Culture
Primary murine tracheal epithelial (MTE) cell cultures were isolated as previously published (3, 19). A line of spontaneously transformed murine alveolar Type II epithelial cells (C10) (20) was cultured as described elsewhere (3). When applicable in both culture systems, recombinant Wnt3a (R&D Systems, Minneapolis, MN) was added for the indicated time points. Chemicals were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise noted. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Vermont.
Western Blotting
MTE or C10 cell lysates were obtained as previously described (3). Total protein was assessed by the Bio-Rad DC Protein Assay kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions, and 5–30 μg of protein were loaded. α-Smooth muscle actin (α-SMA) antibody was purchased from Sigma (St. Louis MO). Full-length JNK 1/2, phosphorylated (P)–JNK 1/2, β-catenin, histone H3, (P-)Smad2 and (P-)Smad3 (Cell Signaling Technology, Danvers, MA), E-cadherin, fibroblast-specific protein (FSP)–1, and β-actin (Santa Cruz biotechnology, Santa Cruz, CA) protein abundance was evaluated as previously described (3).
Transfections and Plasmids
Transient transfections were performed using Nanofectin (PAA, Pasching, Austria) according to the manufacturer’s instructions. TCF/LEF luciferase reporter (TOP Flash) plasmid was used to measure β-catenin–induced transcriptional activity (0.25 μg per transfection). A scrambled TCF/LEF luciferase reporter (FOP Flash) was used for measuring unspecific activation (0.25 μg per transfection). Plasmids encoding TOP and FOP Flash, β-catenin, and β-catenin S37A were provided by Dr. W. M. Blankesteijn (21). pSV–β-sal (0.25 μg per transfection; Promega, Madison, WI) was used to correct for differences in transfection efficiency. To determine luciferase and β-galactosidase activity, cells were lysed in luciferase lysis buffer (Promega), and β-galactosidase (Tropix, Bedford, MA) was measured.
JNK1 Small Interfering RNA
C10 cells were incubated with Dharmacon SMARTpool control nontargeting small interfering (si)RNA (100 nM) or Dharmacon SMARTpool siRNA specific against JNK1 (100 nM) (Dharmacon, Lafayette, CO), subsequently transfected as already described, and exposed to Wnt3a for the indicated time points for evaluation by luciferase activity or Taqman analysis.
Immunofluorescence
C10 cells were grown on glass coverslips and transfected with pcDNA3 (control), β-catenin, and β-catenin S37A (as already described), or exposed to Wnt3a for 48 hours. After transfection, cells were fixed and stained for zonula occludens–1 (ZO-1) and α-SMA. AlexaFluor (Molecular Probes, Eugene, OR) secondary antibodies were used for imaging. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole, and images were taken with confocal microscopy (Zeiss, Thornwood, NY).
Gene Expression
Total RNA was isolated from C10 cells using the RNeasy Mini-Kit (Qiagen, Valencia, CA), and subjected to reverse transcription and DNase treatment to produce cDNA for Taqman gene analysis, using SYBR green or Assays on Demand for the individual target genes (Applied Biosystems, Foster City, CA). Primer sequences are provided in Table 1. Sequences were taken from Genbank. All accession numbers are denoted.
TABLE 1.
PRIMER SEQUENCES
| Gene | Accession Number | Sequences (5′ → 3′) | Amplicon (Base Pairs) | |
| HMGA2 | NM_010441 | Forward | AAGGCAGCAAAAACAAGAGC | 121 |
| Reverse | GCAGGCTTCTTCTGAACGAC | |||
| CBF-A | L36663 | Forward | GGGAAAAATGTTCGTTGGTG | 130 |
| Reverse | CCCTCTTGATCGTCCAGTGT | |||
| FSP-1 | NM_0113111 | Forward | CTGGGGAAAAGGACAGATGA | 109 |
| Reverse | TGCAGGACAGGAAGACACAG | |||
| E-cadherin | NM_009864.2 | Forward | AGCCATTGCCAAGTACATCC | 133 |
| Reverse | AAAGACCGGCTGGGTAAACT | |||
| Axin2 | NM_015732.4 | Forward | CCAACACTTTGGCACAGCTA | 103 |
| Reverse | TTCCTGTCCCTCTGCTGACT | |||
| Vimentin | NM_011701.4 | Forward | TGAAGGAAGATGGCTCGT | 100 |
| Reverse | TCCAGCAGCTTCCTGTAGGT | |||
| Tjp1 (ZO-1) | NM_009386.2 | Forward | CCACCTCTGTCCAGCTCTTC | 249 |
| Reverse | CACCGGAGTGATGGTTTTCT | |||
| α-SMA | NM_007392.2 | Forward | CTGACAGAGGCACCACTGAA | 160 |
| Reverse | CATCTCCAGAGTCCAGCACA | |||
| β-catenin | NM_007614 | Forward | GCTTCTGGGTTCCGATGATA | 101 |
| Reverse | CCTGGCACACCATCATCTTG | |||
| Cyclophilin | NM_008907 | Forward | TTCCTCCTTTCACAGAATTATTCCA | 57 |
| Reverse | CCGCCAGTGCCAGTGCCATTATGG |
Definition of abbreviations: α-SMA, α–smooth muscle actin; CBF-A, CArG box-binding factor–A; FSP-1, fibroblast-specific protein–1; HMGA2, high mobility group AT-hook 2; Tjp1, tight junction protein-1; ZO-1, zona occludens–1.
Primer sequences for quantitative PCR cycling conditions with similar efficiencies to obtain simultaneous amplification in the same run. Sequences were taken from GeneBank, and all accession numbers are denoted.
Statistical Analysis
All experiments were performed three times, with two independent samples/group/time point. For real-time PCR analyses, each experiment consisted of three independent samples/group/time point. Data from combined experiments were evaluated according to one-way ANOVA, using the Tukey Honestly Significant Difference test to adjust for multiple comparisons. Results at P < 0.05 or less were considered statistically significant.
Results
Canonical Wnt Stimulation in Lung Type II Alveolar Epithelial Cells Results in β-Catenin Nuclear Translocation and TCF/LEF Transcriptional Activity
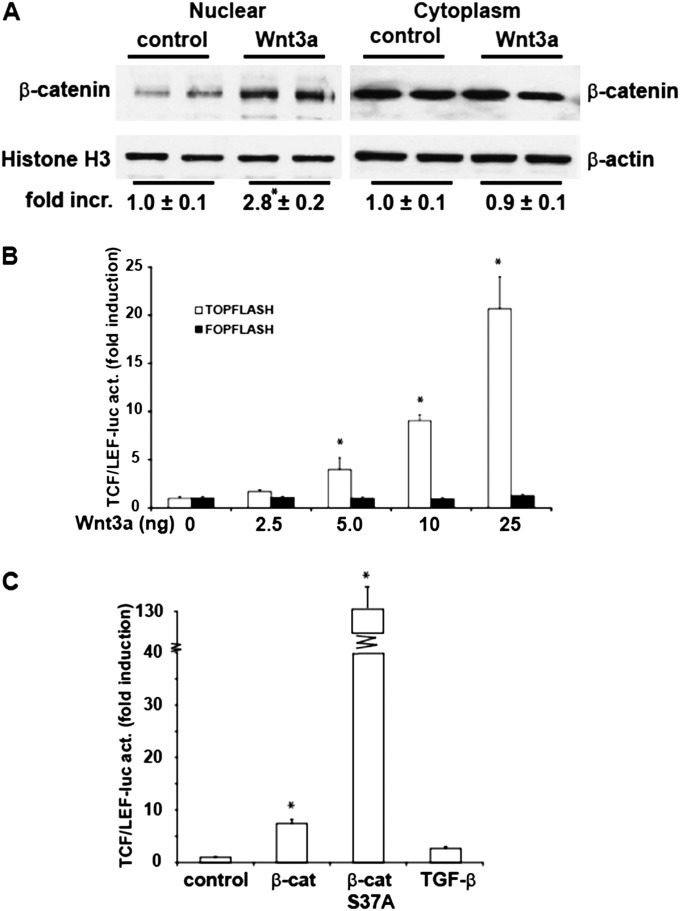
To investigate whether Wnt3a induced canonical Wnt signaling in C10 murine lung Type II epithelial cells, we assessed the nuclear translocation of β-catenin. The results in Figure 1Ademonstrate that Wnt3a stimulation resulted in an increase in nuclear β-catenin compared with unstimulated cells. Cytoplasmic β-catenin concentrations were not affected. In addition, the activity of the β-catenin–dependent TCF/LEF transcriptional activator was evaluated via the overexpression of a TCF/LEF-sensitive promoter reporter construct (TOP Flash). In a dose-dependent fashion, Wnt3a stimulation led to a significant increase in β-catenin transcriptional activity, compared with control samples (Figure 1B). The transfection of cells with a vector containing mutated copies of TCF/LEF (FOP Flash) to measure unspecific transactivation did not result in increases of luciferase activity after Wnt3a stimulation (Figure 1B). Furthermore, the overexpression of wild-type (WT) β-catenin increased TCF/LEF transcriptional activity. A mutant form of β-catenin (S37A), which cannot be degraded by GSK3β, led to an even greater increase in TCF/LEF promoter activity compared with WT β-catenin in lung epithelial cells (Figure 1C). In contrast, TGF-β1, a known inducer of the epithelial-to-mesenchymal transition, exerted minimal, nonsignificant effects on TCF/LEF transcriptional activity (Figure 1C). Overall, these results show that canonical Wnt activation, either by Wnt3a or the overexpression of β-catenin, increases TCF/LEF transcriptional activity in murine lung epithelial cells.
Figure 1.
Activation of Wingless protein (Wnt)/β-catenin signaling in C10 lung epithelial cells after stimulation with Wnt3a or the expression of β-catenin. (A) C10 cells were stimulated with 10 ng/ml Wnt3a, and after 1 hour, nuclear and cytosolic extracts were prepared for the assessment of β-catenin content. Histone H3 and β-actin were used as loading control. Replicate lanes reflect independent samples. Band intensity was determined and expressed as fold increase (fold incr.) of β-catenin over histone H3 or β-actin, respectively. *P < 0.05 (ANOVA), compared with control samples. (B) Activation of T-cell factor/lymphocyte-enhancement factor (TCF/LEF) reporter activity (act.) after stimulation with Wnt3a. C10 cells were transfected with TOP Flash or FOP Flash (0.25 μg) and β-galactosidase (0.25 μg). Twenty-four hours after transfection, cells were stimulated with the indicated concentrations of Wnt3a, and 24 hours afterward were lysed for the assessment of luciferase and β-galactosidase activities. Results are normalized to β-galactosidase, and are expressed as fold increases from sham control samples. *P < 0.05 (ANOVA), compared with control samples. (C) Activation of TCF/LEF reporter activity after the expression of 1 μg wild-type (WT) β-catenin (β-cat), 1 μg S37A β-catenin (β-cat S37A), or 5 ng/ml TGF-β1. Twenty-four hours after transfection with plasmids or stimulation with agents, cells were lysed for the assessment of luciferase and β-galactosidase activities, as described in B. *P < 0.05 (ANOVA), compared with control samples.
Inhibition of JNK1 Attenuates Wnt-Induced TCF/LEF Transcriptional Activity in C10 Type II Alveolar Epithelial Cells
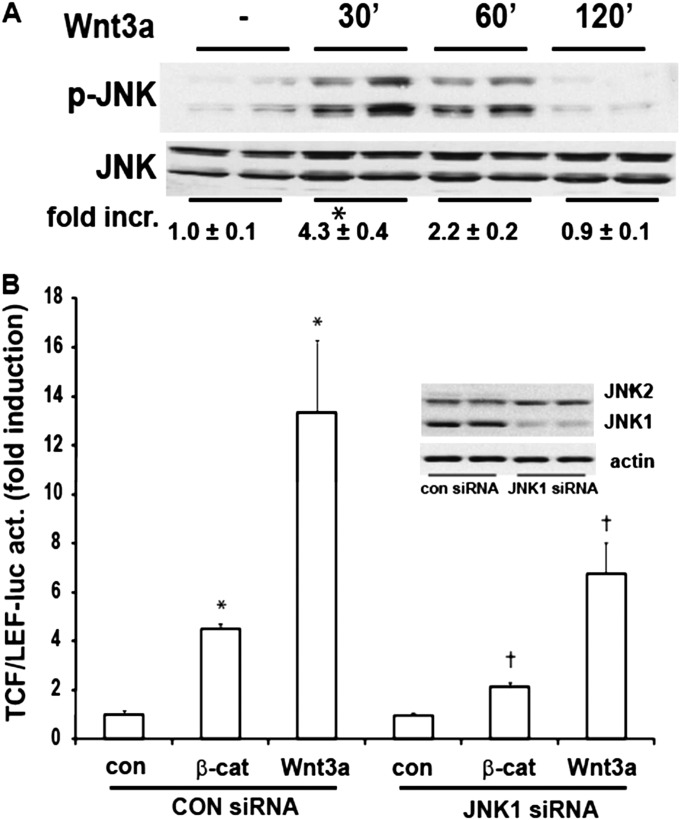
Wnt3a has been shown to activate JNK (17). We therefore assessed whether Wnt3a stimulation activates JNK in lung epithelial cells. Evaluation of the phosphorylation of JNK after Wnt3a stimulation revealed a rapid phosphorylation of JNK (Figure 2A), confirming that Wnt3a is capable of inducing JNK phosphorylation in lung epithelial cells. We next determined the role of JNK1 in TCF/LEF transcriptional activity. The siRNA-mediated knockdown of JNK1 in C10 lung epithelial cells diminished both the β-catenin–induced or Wnt3a-induced stimulation of TCF/LEF transcriptional activity (Figure 2B). These results demonstrate a role for JNK1 in canonical Wnt signaling in C10 Type II lung alveolar epithelial cells.
Figure 2.
Activation of JNK in C10 lung epithelial cells after stimulation with Wnt3a, and requirement for JNK1 in Wnt/β-catenin–induced TCF/LEF transcriptional activity. (A) Assessment of JNK phosphorylation in C10 cells exposed to 10 ng/ml Wnt3a for the indicated times. Cells were harvested, and phospho-JNK and total JNK content were assessed by Western blot analysis. Replicate lanes reflect independent samples. Band intensity was determined and expressed as fold increase of phosphorylated (p)–JNK over total JNK. *P < 0.05 (ANOVA), compared with control samples. (B) Assessment of β-catenin–induced or Wnt3a-induced TCF/LEF transcriptional activity in C10 cells after the ablation of JNK1 via siRNA. C10 cells were transfected with control or JNK1-specific siRNA, and 24 hours later were transfected with TCF/LEF–luciferase, β-galactosidase, and when indicated, with β-catenin (β-cat), and harvested 24 hours later. Selected dishes were stimulated with 10 ng/ml Wnt3a and harvested 24 hours later. Cells were lysed for the assessment of luciferase and β-galactosidase activities. Results are normalized to β-galactosidase, and are expressed as fold increases from sham control samples. *P < 0.05 (ANOVA), compared with control (con) samples. Inset: Confirmation of the ablation of JNK1 via small interfering (si)RNA, according to Western blot analysis of total JNK1 and JNK2, with β-actin as a loading control.
Activation of Wnt Signaling Increases the Expression of EMT-Associated Proteins
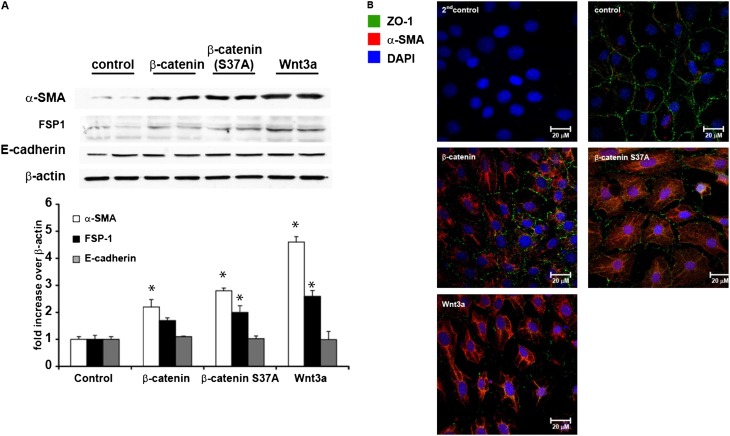
We next evaluated the content of mesenchymal and epithelial proteins after the activation of the Wnt/-β-catenin signaling pathway in C10 lung epithelial cells. The results in Figure 3A demonstrate that the overexpression of β-catenin and β-catenin S37A or the stimulation of cells with Wnt3a resulted in increases of α-SMA and FSP1 content. Perhaps surprisingly, E-cadherin concentrations were not affected in cells expressing β-catenin, or after exposure to Wnt3a. However, evaluation of the epithelial tight junction protein ZO-1 (22) via immunofluorescence demonstrated strong decreases in ZO-1 content and concomitant increases in α-SMA in cells expressing WT or S37A β-catenin, or after exposure to Wnt3a (Figure 3B).
Figure 3.
Activation of mesenchymal proteins in C10 lung epithelial cells after stimulation with Wnt3a or the expression of β-catenin. (A, top) Assessment of α–smooth muscle actin (α-SMA), fibroblast-specific protein–1 (FSP1), and E-cadherin content by Western blot analysis in C10 cells transfected with WT or S37A β-catenin, or after the stimulation of 10 ng/ml Wnt3a for 48 hours. β-actin was used as a loading control. Replicate lanes reflect independent samples. (A, bottom) Band intensity was determined and expressed as fold increase of α-SMA, FSP1, and E-cadherin, respectively, over β-actin. *P < 0.05 (ANOVA), compared with control samples. (B) Evaluation of zona occludens–1 (ZO-1) (green) and α-SMA (red) in C10 cells transfected with WT or S37A β-catenin, or after the stimulation of 10 ng/ml Wnt3a for 48 hours by immunofluorescence confocal microscopy. Nuclei were stained using 4′,6-diamidino-2-phenylindole (DAPI) (blue).
Genetic Ablation of Jnk1 in Lung Epithelial Cells Attenuates Wnt-Induced Expression of Mesenchymal Markers and Proteins
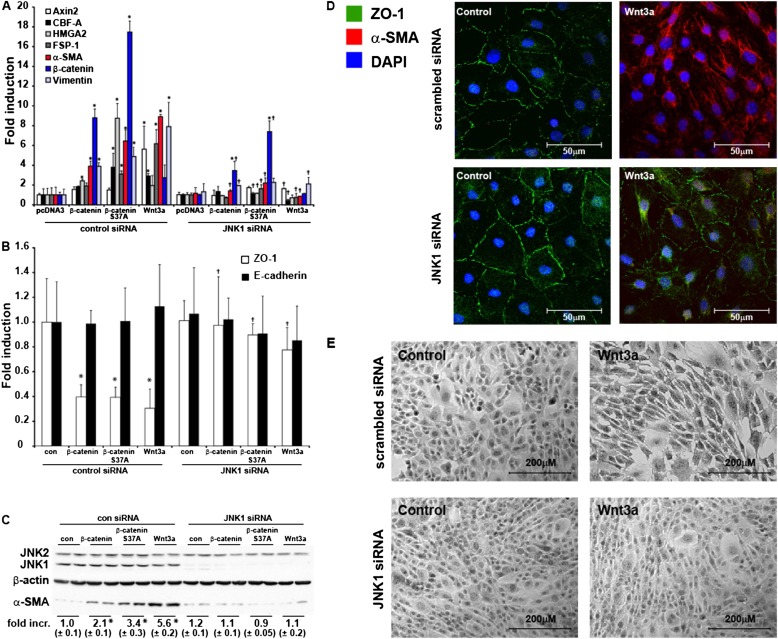
We next sought to characterize the EMT transcriptional response further in C10 epithelial cells stimulated with Wnt3a, or in cells expressing WT or S37A β-catenin, and to address the functional importance of JNK1 therein. We therefore ablated JNK1 in C10 cells with siRNA, before stimulation with Wnt3a or the transfection of WT or S37A β-catenin. The results in Figure 4A demonstrate robust increases in the mRNA expression of mesenchymal markers such as CArG box-binding factor–A (CBF-A) (23), fibroblast specific protein–1 (FSP1) (23), α-SMA (24), and vimentin (25). Significantly, the ability of β-catenin, β-catenin S37A, or Wnt3a to increase these EMT expression profiles was almost completely abrogated after the siRNA-mediated ablation of JNK1 (Figure 4A). Interestingly, the increased expression of β-catenin mRNA after the overexpression of WT or S37A β-catenin was also attenuated in cells lacking JNK1. In addition, Wnt3a-mediated increases in Axin2 mRNA were almost completely abolished in cells transfected with siRNA, consistent with the dampened TCF/LEF transcriptional activity in those cells (Figure 2B).
Figure 4.
Activation of transition of airway epithelial cells (EMT) regulatory genes and requirement for JNK1 in the activation of EMT transcriptome genes in C10 lung epithelial cells after the overexpression of β-catenin or stimulation with Wnt3a. C10 cells were transfected with scrambled or JNK1-specific siRNA, before the overexpression of WT or S37A β-catenin or stimulation with Wnt3a. Cells were harvested for analyses of EMT transcriptome genes (A) or epithelial genes (B) via real-time PCR analysis. Data were normalized to the housekeeping gene cyclophilin, and all results are expressed as fold change compared with scrambled siRNA transfected control cells. *P < 0.05 (ANOVA), compared with scrambled siRNA transfected control cells (con). †P < 0.05 (ANOVA), compared with respective control siRNA groups. (C) Cells were harvested, and total JNK1/2 (conformation of siRNA-mediated ablation), β-actin, and α-SMA content was assessed by Western blot analysis. Replicate lanes reflect independent samples. Band intensity was determined and expressed as fold increase of α-SMA over β-actin. *P < 0.05 (ANOVA), compared with control samples. (D) Evaluation of ZO-1 (green) and α-SMA (red) immunofluorescence in C10 cells transfected with scrambled or JNK1 siRNA after the stimulation of 10 ng/ml Wnt3a for 48 hours. Nuclei were stained using DAPI (blue). Cells were analyzed by confocal microscopy. (E) Assessment of morphological changes induced by Wnt3a in control siRNA–transfected cells or after the siRNA-mediated ablation of JNK1. Seventy-two hours after the stimulation of cells with Wnt3a, cells were fixed and stained with hematoxylin. Photographs were taken using an inverted microscope connected to a digital camera.
We next evaluated the impact on the expression of epithelial markers after Wnt activation. Consistent with the observed lack of changes in E-cadherin protein concentrations (Figure 3A), no differences in E-cadherin mRNA concentrations were observed in cells exposed to Wnt3a or after the expression of WT or S37A β-catenin. However, more than 50% decreases in ZO-1 mRNA were evident in cells exposed to Wnt3a, or after the expression of WT or S37A β-catenin, and the ablation of JNK1 almost completely prevented decreases in ZO-1 mRNA (Figure 4B). Furthermore, the observed increases in α-SMA protein abundance after Wnt activation were completely abolished in JNK1 siRNA-treated cells (Figures 4C and 4D). The Wnt3a-induced loss of ZO-1 content was also largely prevented in cells after JNK1 ablation with siRNA (Figure 4D). Lastly, the stimulation of C10 epithelial cells with Wnt3a resulted in striking morphological changes, apparent in terms of elongated morphology, characteristic of the transition of an epithelial cell into a cell with a mesenchymal signature. As shown in Figure 4E, Wnt3a-induced morphological changes were not observed after the ablation of JNK1. Altogether, these findings demonstrate that the activation of Wnt/β-catenin signaling in lung epithelial cells leads to the increased expression of mesenchymal proteins and EMT regulatory genes, and a decrease in ZO-1, along with striking morphological changes that are dependent on JNK1.
Genetic Ablation of β-Catenin in Lung Epithelial Cells Attenuates Wnt3a-Induced Expression of Mesenchymal Markers and Protein without Affecting JNK Phosphorylation
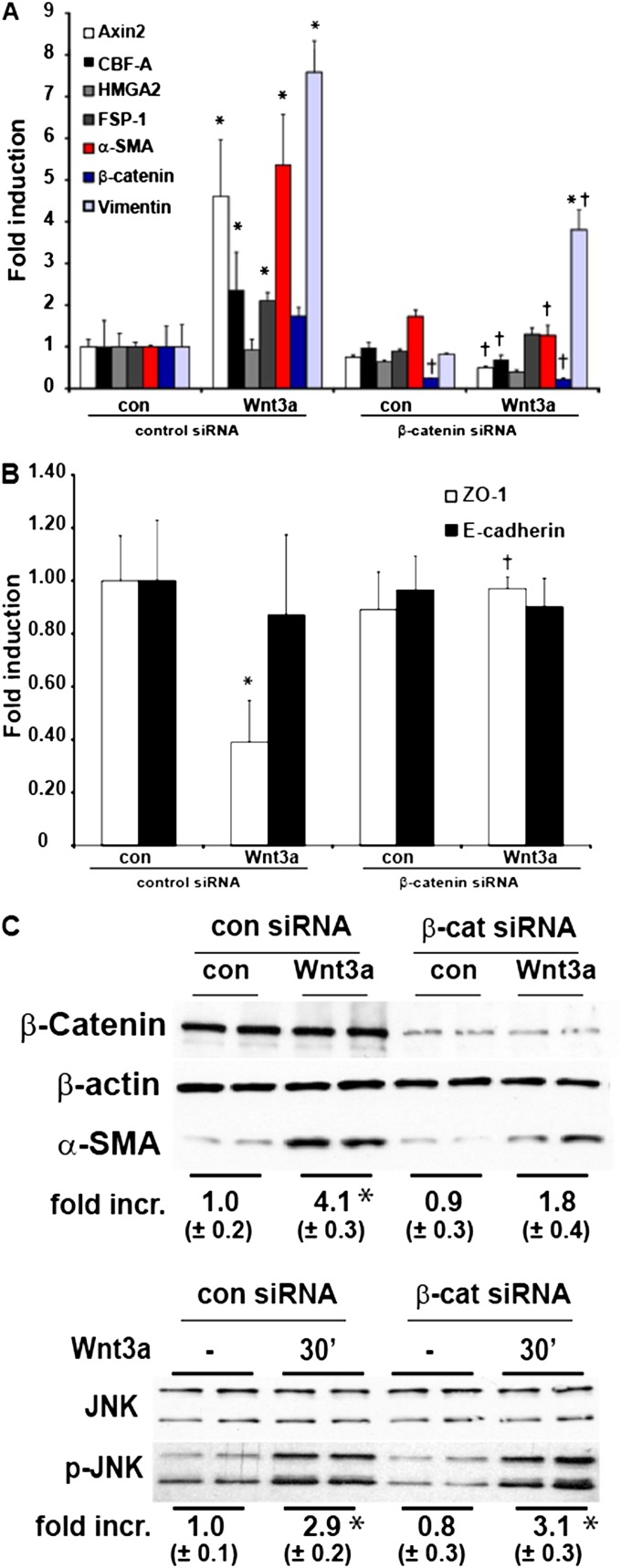
We next sought to explore further the functional role of β-catenin in the Wnt3a-induced mesenchymal transition. We therefore ablated β-catenin in C10 cells, using a siRNA approach. After β-catenin knockdown, the Wnt3a-induced expression of the mesenchymal markers CBF-A, FSP-1, α-SMA, and vimentin was significantly down-regulated compared Wnt3a-treated siRNA control samples (Figure 5A). The observed decreases in ZO-1 mRNA concentrations after stimulation with Wnt3a were no longer apparent after the knockdown of β-catenin (Figure 5B) Moreover, the ability of Wnt3a to increase Axin2 mRNA was abolished in these cells (Figure 5A). Increases in α-SMA protein in cells exposed to Wnt3a were reduced after β-catenin siRNA treatment (Figure 5C), consistent with decreases in mRNA (Figure 5A). Interestingly, the observed increases in JNK phosphorylation after Wnt3a stimulation were not affected by the ablation of β-catenin (Figure 5C). Collectively, these findings demonstrate that the ability of Wnt3a to induce the mesenchymal transition of C10 epithelial cells requires β-catenin.
Figure 5.
A causal role for β-catenin in the activation of EMT transcriptome genes in C10 lung epithelial cells after stimulation with Wnt3a. C10 cells were transfected with scrambled or β-catenin–specific siRNA before stimulation with Wnt3a (10 ng/ml). Cells were harvested for the analysis of EMT transcriptome genes (A) or epithelial genes (B) via real-time PCR analysis. Data were normalized to the housekeeping gene cyclophilin, and all results are expressed as fold change compared with scrambled siRNA–transfected control cells. *P < 0.05 (ANOVA), compared with scrambled-siRNA transfected controls (con). †P < 0.05 (ANOVA), compared with Wnt3a-treated siRNA control cells. (C, top) Cells were harvested, and total β-catenin (conformation of siRNA-mediated ablation), β-actin, and α-SMA content was assessed by Western blot analysis. Band intensity was determined and expressed as fold increase of α-SMA over β-actin. (C, bottom) Assessment of JNK phosphorylation in C10 cells transfected with scrambled or β-catenin–specific siRNA before stimulation with Wnt3a (10 ng/ml). Cells were harvested, and phospho-JNK and total JNK content was assessed by Western blot analysis. Band intensity was determined and expressed as fold increase of phospho-JNK over total JNK. * P < 0.05 (ANOVA), compared with control samples. Replicate lanes reflect independent samples.
Attenuation of Wnt3a-Induced Expression of Mesenchymal Markers and Proteins in Primary Tracheal Epithelial Cells Lacking JNK1
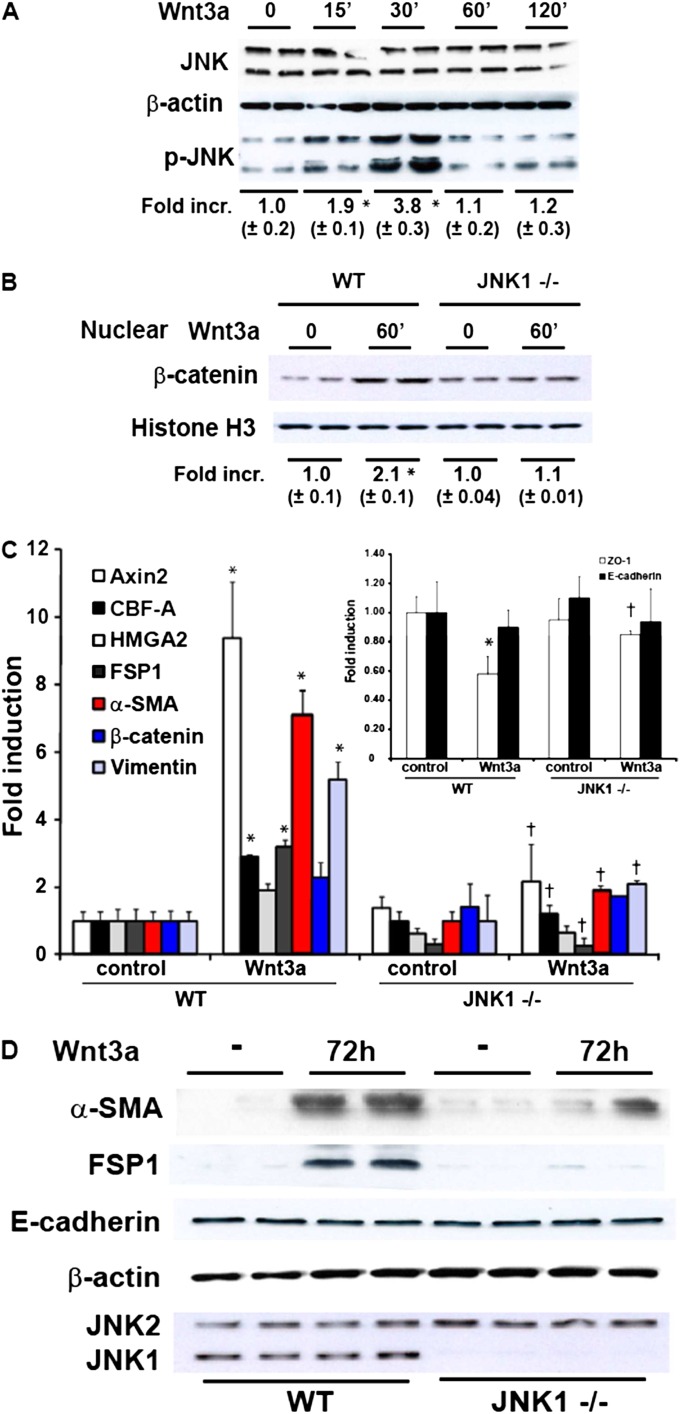
EMT has been predominantly studied in Type II alveolar epithelial cells. However, previous studies also demonstrated EMT in upper airway epithelial cells (1). Because of findings that demonstrated increases in nuclear β-catenin in both bronchiolar and Type II epithelial cells in patients with IPF (4, 5), we extended our observations by using WT and Jnk1−/− primary upper airway epithelial cells isolated from tracheas (i.e., MTE cells) (1) because of the feasibility of establishing tracheal cultures compared with bronchial cultures. First we evaluated whether Wnt3a stimulation can activate JNK in MTE cells. The results in Figure 6A demonstrate a rapid significant increase in JNK phosphorylation that peaked at 30 minutes and decreased to baseline by 60 minutes, similar to observations in C10 cells (Figure 2A). The nuclear β-catenin content was increased in WT MTE cells stimulated with Wnt3a. In contrast, Jnk1−/− MTE cells did not demonstrate increases in nuclear β-catenin in response to Wnt3a (Figure 6B). Next we analyzed the mRNA expression of EMT transcriptional regulators and markers comparatively in WT and Jnk1−/− MTE cells. The results in Figure 6C demonstrate that the Wnt3a-induced expression of Axin2 was decreased in Jnk1−/− MTE cells. Similarly, the Wnt3a-induced expression of CBF-A, FSP1, α-SMA, and vimentin was also decreased in Jnk1−/− MTE cells compared with WT counterparts. Although Wnt3a failed to increase the high mobility group AT-hook 2 (HMGA2) content in C10 cells, small increases in HMGA2 mRNA were observed in MTE cells upon the stimulation of WT cells with Wnt3a, whereas Jnk1−/− MTE cells were refractory to Wnt3a-mediated increases in HMGA2 mRNA. In agreement with our findings in C10 cells, the stimulation of WT MTE cells with Wnt3a resulted in a decrease in ZO-1 mRNA concentrations, which was not observed in Jnk1−/− MTE cells. E-cadherin mRNA was unaffected in cells stimulated with Wnt3a (Figure 6C, inset). Lastly, we confirmed the ability of the activation of the Wnt/-β-catenin signaling pathway to induce EMT in the MTE cells by monitoring the content of mesenchymal and epithelial proteins. The results in Figure 6D demonstrate increases in the α-SMA and FSP1 content of WT MTE cells after stimulation with Wnt3a. In contrast, the Wnt3a-induced expression of α-SMA and FSP1 was substantially decreased in Jnk1−/− MTE cells compared with WT counterparts, consistent with the modulation of mRNA expression. E-cadherin concentrations in MTE cells were not affected upon the exposure of cells to Wnt3a, consistent with findings in C10 cells (Figure 3B). In aggregate, these findings demonstrate that the ability of Wnt3a to induce a mesenchymal expression profile in lung epithelial cells requires the presence of JNK1.
Figure 6.
Attenuation of Wnt3-induced nuclear β-catenin content, EMT transcriptome gene expression, and mesenchymal proteins in murine tracheal epithelial (MTE) cells lacking Jnk1. (A) Confirmation of Wnt3a-induced JNK phosphorylation in MTE cells. Cells were stimulated with 10 ng/ml Wnt3a, and at the indicated times, lysates were prepared for Western blot analysis of phosphorylated (p)–JNK and total JNK. Band intensity was determined and expressed as fold increase of phospho-JNK over total JNK. *P < 0.05 (ANOVA), compared with control samples. Replicate lanes reflect independent samples. (B) Assessment of nuclear β-catenin content in WT or Jnk1−/− MTECs stimulated with 10 ng/ml of Wnt3a for 1 hour before the isolation of nuclear extracts. Band intensity was determined and expressed as fold increase of β-catenin over histone H3. *P < 0.05 (ANOVA), compared with control samples. (C) Attenuation of Wnt3a-induced expression of EMT transcriptome genes in MTE cells lacking Jnk1. WT or Jnk1−/− MTECs were stimulated with Wnt3a for 72 hours, and cells were lysed for the assessment of mRNA expression via real-time PCR analysis. Data were normalized to the housekeeping gene cyclophilin, and all results are expressed as fold change compared with WT control cells. *P < 0.05 (ANOVA), compared with WT control cells. †P < 0.05 (ANOVA), compared with Wnt3a-treated WT cells. Inset: E-cadherin and ZO-1 mRNA content in WT or Jnk1−/− MTE cells, assessed via real-time PCR analysis. (D) Assessment of content of FSP1, α-SMA, and E-cadherin in WT or Jnk1−/− cells exposed to 10 ng/ml of Wnt3a for 72 hours. Lysates were prepared for Western blot analysis. β-actin, loading control. JNK1 and JNK2 expression was assessed to confirm the Jnk1−/− genotype.
Discussion
The notion that epithelial cells are important contributors to the pathogenesis of pulmonary fibrosis has emerged (26), potentially through the process of epithelial-to-mesenchymal transition (EMT) (2, 22, 27, 28). Early lineage tracing studies suggested that in murine models of fibrosis, a substantial portion (up to 30%) of expanding fibroblasts are derived from epithelial cells (28, 29), although a recent study based on lineage tracing and analysis with confocal laser scanning cytometry failed to confirm a role for EMT in bleomycin-induced fibrosis (30). Alveolar Type II epithelial cells isolated from patients with IPF were shown to express mesenchymal proteins (31), and some evidence of EMT in patients with IPF exists (22, 28). The profibrogenic mediator TGF-β1 has been identified as one of the key players in the process of EMT (1, 22, 28). However, recent studies also suggest a role of the Wnt/β-catenin signaling pathway in EMT (32). Activated Wnt/β-catenin signaling has been demonstrated in lung epithelial cells in IPF (4, 5), and in experimental models of fibrosis, the Wnt/β-catenin pathway was shown to be up-regulated (7, 8). However, the exact role of Wnt/β-catenin in fibrosis remains to be elucidated.
In the present study, we demonstrate that the stimulation of lung epithelial cells with Wnt3a results in the activation of JNK, consistent with previous observations (17). We also demonstrate here that the activation of the Wnt/β-catenin pathway induces a variety of EMT signature genes and proteins in primary lung epithelial cells, and that JNK1 plays an important role in this process. The ablation of JNK1 attenuated Wnt3a-induced TCF/LEF transcriptional activity and EMT-related mRNAs and proteins, in association with a loss of accumulation of nuclear β-catenin.
Originally, studies in Drosophila suggested that JNK plays an important role in noncanonical Wnt signaling, specifically in planar cell polarity signaling (16). However, recent studies revealed a role for JNK in canonical Wnt signaling. In support of this, we demonstrated here that the genetic ablation of JNK1 specifically attenuates Wnt/β-catenin–activated TCF/LEF signaling (Figure 2). The present study confirms the requirement for JNK1 in the facilitation of Wnt/β-catenin signaling in lung epithelial cells, and suggests that JNK activation may also provide an indication of canonical Wnt signaling.
Our present study also demonstrates that Wnt/β-catenin signaling in lung epithelial cells induces a mesenchymal gene and protein expression profile and partly represses the epithelial profile, and those effects are dependent on the presence of JNK1. Both the overexpression of WT and S37A β-catenin and stimulation with Wnt3a induce mesenchymal markers such as CBF-A (23), HMGA2 (33), FSP1 (23), α-SMA (24), and vimentin (25). In contrast to these observed increases in the expression of mesenchymal markers that reflect EMT, mRNA expression and the content of E-cadherin were not affected in C10 lung epithelial cells or primary tracheal epithelial cells stimulated with Wnt3a (Figures 3 and 5). Additional experiments will be required to assess the relocalization of E-cadherin from the membrane. Furthermore, in primary tracheal epithelial cells, a loss of transepithelial resistance was not observed after stimulation with Wnt3a (data not shown). However, the mRNA expression and protein abundance of the epithelial tight junction protein ZO-1 were markedly diminished after Wnt activation. The delocalization of ZO-1 from the cell membrane during epithelial cell migration has been described (34), although we did not observe the nuclear translocation of ZO-1. These findings suggest that Wnt3a induces a mesenchymal expression profile and a partial loss of epithelial expression in lung epithelial cells, along with morphological changes that exemplify EMT. These observations are in contrast to findings after stimulation of the same cell types under the same experimental conditions with TGF-β1 (1), and suggest that TGF-β1 and Wnt3a induce JNK1-dependent but distinct transcriptional programs that regulate unique facets of EMT. In support of that notion, Wnt3a led to no or minor increases in HMGA2 mRNA expression (Figures 4A and 5A), whereas more robust increases in HMGA2 mRNA were observed in response to TGF-β1 (3). In contrast to Wnt3a, which resulted in robust increases of TCF/LEF transcriptional activity, the stimulation of C10 lung epithelial cells with TGF-β1 did not result in an increase of TCF/LEF transcriptional activity (Figure 1), consistent with previous studies (35). However, additional experiments are needed to compare fully the EMT transcriptome in response to the stimulation of cells with Wnt3 or TGF-β1. In the present study, we found that stimulation with Wnt3a also led to a rapid increase in TGF-β1 expression (data not shown), consistent with previous studies in which TGF-β was identified as one of the genes up-regulated after Wnt3a stimulation (36, 37). Despite the up-regulation of TGF-β1 mRNA, we could not detect any increases in the phosphorylation of Receptor (R)-Smad 2 and 3 after Wnt3a stimulation (Figure E1 in the online supplement). Additional studies will be required to elucidate the contributions of TGF-β1 in mediating the Wnt3a-induced mesenchymal expression profile in lung epithelial cells. Nonetheless, these findings raise the possibility that Wnt3a-induced or TGF-β1–induced pathways may act synergistically. Indeed, the stable expression of a dominant-negative β-catenin in kidney epithelial cells exposed to TGF-β1 was shown to block increases in α-SMA expression (38). Similarly, interactions between SMAD2, which is activated after TGF-β1 signaling, and β-catenin occur in the bleomycin model of fibrosis in lungs of patients with IPF, and have been linked to EMT (38). A recent study demonstrated interactions between β-catenin and SMAD3, in response to TGF-β1, which were shown to be essential for the transcriptional activation of α-SMA (39).
In agreement with our observation that Wnt3a induces α-SMA expression (Figures 3–6), Wnt3a was shown to induce α-SMA expression in myofibroblasts through a β-catenin–dependent mechanism (40). However, some discrepancy exists, based on another report that failed to demonstrate increases in α-SMA expression upon the stimulation of fibroblasts with Wnt3a, despite increased migration (41). Moreover, another study showed decreased expression of smooth muscle α-actin after stimulation with Wnt3a (42). The causes of these disparate results are not clear, but seem to suggest that the functional outcome of the activation of the Wnt/β-catenin signaling pathway may be cell type–specific and context-specific.
Functional cooperation between JNK and β-catenin has been reported (43). Specifically, it was shown that after exposure to Wnt3a, Ras-related C3 botulinum toxin substrate 1 (Rac-1) leads to the activation of JNK2, which in turn phosphorylates β-catenin on serine 191 and 605, leading to its nuclear translocation (44). Although that study (44) failed to demonstrate a robust role for JNK1 in the phosphorylation and nuclear translocation of β-catenin, our present findings demonstrate that the nuclear translocation of β-catenin after stimulation with Wnt3a was decreased in JNK1−/− MTE cells, compared with their WT control cells (Figure 6). Moreover, β-catenin–induced or Wnt3a-induced TCF/LEF transcriptional activity was substantially decreased after the ablation of JNK1, demonstrating a critical role for JNK1 in canonical Wnt signaling in lung epithelial cells. After the siRNA ablation of β-catenin, the Wnt3a-induced phosphorylation of JNK was not diminished. These observations suggest that the Wnt3a-induced phosphorylation of JNK1 occurs upstream of β-catenin. Additional studies are required to address formally the role of Rac-1 in the regulation of JNK1 phosphorylation in lung epithelial cells in response to Wnt3a. The JNK-mediated phosphorylation of β-catenin at serine 37 and threonine 41, the sites phosphorylated by GSK-3β, has also been reported, in association with a disruption of cell contacts (45). In addition, several reports also recognized a role for c-Jun in TCF-dependent transcription (43, 46, 47). An interaction between phosphorylated c-Jun and TCF4 has been reported, and both of these were found in a complex with β-catenin on the c-jun promoter (43). The interaction between c-Jun and TCF4 was dependent on JNK activity, and a model was proposed whereby c-Jun bound to an activator protein-1 site in the promoter, interacted with TCF4, and bound to an upstream TCF site. Although the present study demonstrates that JNK1 is important in the augmentation of the transcriptional expression of EMT regulators after Wnt/β-catenin activation, the exact mechanisms, including the JNK1 phosphorylation targets that are responsible for these events, require further investigation.
In conclusion, our present study demonstrates that Wnt3a induces a mesenchymal expression profile in lung epithelial cells that requires JNK1. Additional studies will be required to address formally the exact role of JNK1 and β-catenin signaling in cells of epithelial origin in the pathogenesis of tissue fibrosis. The present study corroborates the importance of JNK1 in promoting EMT in response to diverse agonists, and strongly suggests that JNK1 is a relevant therapeutic target in patients with tissue fibrosis.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health grants T32 HL076122 and R01 HL085464 (to Y.M.W.J.-H.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0297OC on March 29, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Alcorn JF, Guala AS, van der Velden J, McElhinney B, Irvin CG, Davis RJ, Janssen-Heininger YM. C-Jun N-terminal kinase 1 regulates epithelial-to-mesenchymal transition induced by TGF-beta1. J Cell Sci 2008;121:1036–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thiery JP. Epithelial–mesenchymal transitions in development and pathologies. Curr Opin Cell Biol 2003;15:740–746 [DOI] [PubMed] [Google Scholar]
- 3.Velden JL, Alcorn JF, Guala AS, Badura EC, Janssen-Heininger YM. C-Jun N-terminal kinase 1 promotes transforming growth factor–beta1–induced epithelial-to-mesenchymal transition via control of linker phosphorylation and transcriptional activity of SMAD3. Am J Respir Cell Mol Biol 2010;44:571–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, et al. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol 2003;162:1495–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konigshoff M, Balsara N, Pfaff EM, Kramer M, Chrobak I, Seeger W, Eickelberg O. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS ONE 2008;3:e2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med 2008;5:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim TH, Kim SH, Seo JY, Chung H, Kwak HJ, Lee SK, Yoon HJ, Shin DH, Park SS, Sohn JW. Blockade of the Wnt/beta-catenin pathway attenuates bleomycin-induced pulmonary fibrosis. Tohoku J Exp Med 2011;223:45–54 [DOI] [PubMed] [Google Scholar]
- 8.Henderson WR, Jr, Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, Borok Z, Knight DA, Kahn M. Inhibition of Wnt/beta-catenin/CREB binding protein (CPB) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci USA 2010;107:14309–14314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science 2004;303:1483–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huelsken J, Behrens J. The Wnt signalling pathway. J Cell Sci 2002;115:3977–3978 [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 2002;108:837–847 [DOI] [PubMed] [Google Scholar]
- 12.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature 2000;407:530–535 [DOI] [PubMed] [Google Scholar]
- 13.Li L, Yuan H, Weaver CD, Mao J, Farr GH, III, Sussman DJ, Jonkers J, Kimelman D, Wu D. Axin and FRAT1 interact with Dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J 1999;18:4233–4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nusse R. Cell biology: relays at the membrane. Nature 2005;438:747–749 [DOI] [PubMed] [Google Scholar]
- 15.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004;20:781–810 [DOI] [PubMed] [Google Scholar]
- 16.Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and Wingless signaling. Cell 1998;94:109–118 [DOI] [PubMed] [Google Scholar]
- 17.Bikkavilli RK, Feigin ME, Malbon CC. G alpha O mediates Wnt–JNK signaling through dishevelled 1 and 3, RhoA family members, and MEKK 1 and 4 in mammalian cells. J Cell Sci 2008;121:234–245 [DOI] [PubMed] [Google Scholar]
- 18.Alcorn JF, van der Velden J, Brown AL, McElhinney B, Irvin CG, Janssen-Heininger YM. C-Jun N-terminal kinase 1 is required for the development of pulmonary fibrosis. Am J Respir Cell Mol Biol 2009;40:422–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu R, Smith D. Continuous multiplication of rabbit tracheal epithelial cells in a defined, hormone-supplemented medium. In Vitro 1982;18:800–812 [DOI] [PubMed] [Google Scholar]
- 20.Malkinson AM, Dwyer-Nield LD, Rice PL, Dinsdale D. Mouse lung epithelial cell lines: tools for the study of differentiation and the neoplastic phenotype. Toxicology 1997;123:53–100 [DOI] [PubMed] [Google Scholar]
- 21.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol 2003;13:680–685 [DOI] [PubMed] [Google Scholar]
- 22.Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial–mesenchymal transition in alveolar epithelial cells by transforming growth factor–beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol 2005;166:1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venkov CD, Link AJ, Jennings JL, Plieth D, Inoue T, Nagai K, Xu C, Dimitrova YN, Rauscher FJ, Neilson EG. A proximal activator of transcription in epithelial–mesenchymal transition. J Clin Invest 2007;117:482–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masszi A, Di Ciano C, Sirokmany G, Arthur WT, Rotstein OD, Wang J, McCulloch CA, Rosivall L, Mucsi I, Kapus A. Central role for Rho in TGF-beta1–induced alpha–smooth muscle actin expression during epithelial–mesenchymal transition. Am J Physiol Renal Physiol 2003;284:F911–F924 [DOI] [PubMed] [Google Scholar]
- 25.Korsching E, Packeisen J, Liedtke C, Hungermann D, Wulfing P, van Diest PJ, Brandt B, Boecker W, Buerger H. The origin of vimentin expression in invasive breast cancer: epithelial–mesenchymal transition, myoepithelial histogenesis or histogenesis from progenitor cells with bilinear differentiation potential? J Pathol 2005;206:451–457 [DOI] [PubMed] [Google Scholar]
- 26.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc 2006;3:364–372 [DOI] [PubMed] [Google Scholar]
- 27.Kalluri R, Neilson EG. Epithelial–mesenchymal transition and its implications for fibrosis. J Clin Invest 2003;112:1776–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA 2006;103:13180–13185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanjore H, Xu XC, Polosukhin VV, Degryse AL, Li B, Han W, Sherrill TP, Plieth D, Neilson EG, Blackwell TS, et al. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med 2009;180:657–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BL. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA 2011;108:E1475–E1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marmai C, Sutherland RE, Kim KK, Dolganov GM, Fang X, Kim SS, Jiang S, Golden JA, Hoopes CW, Matthay MA, et al. Alveolar epithelial cells express mesenchymal proteins in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2011;301:L71–L78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y. New insights into epithelial–mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 2010;21:212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin CH, Moustakas A. Transforming growth factor–beta employs HMGA2 to elicit epithelial–mesenchymal transition. J Cell Biol 2006;174:175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gottardi CJ, Arpin M, Fanning AS, Louvard D. The junction-associated protein, zonula occludens–1, localizes to the nucleus before the maturation and during the remodeling of cell–cell contacts. Proc Natl Acad Sci USA 1996;93:10779–10784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim SK, Hoffmann FM. SMAD4 cooperates with lymphoid enhancer–binding factor 1/T cell–specific factor to increase c-myc expression in the absence of TGF-beta signaling. Proc Natl Acad Sci USA 2006;103:18580–18585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen S, McLean S, Carter DE, Leask A. The gene expression profile induced by Wnt-3a in NIH 3T3 fibroblasts. J Cell Commun Signal 2007;1:175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carre AL, James AW, MacLeod L, Kong W, Kawai K, Longaker MT, Lorenz HP. Interaction of Wingless protein (Wnt), transforming growth factor–beta1, and hyaluronan production in fetal and postnatal fibroblasts. Plast Reconstr Surg 2010;125:74–88 [DOI] [PubMed] [Google Scholar]
- 38.Kim Y, Kugler MC, Wei Y, Kim KK, Li X, Brumwell AN, Chapman HA. Integrin alpha3beta1–dependent beta-catenin phosphorylation links epithelial SMAD signaling to cell contacts. J Cell Biol 2009;184:309–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou B, Liu Y, Kahn M, Ann DK, Han A, Wang H, Nguyen C, Flodby P, Zhong Q, Krishnaveni MS, et al. Interactions between beta-catenin and transforming growth factor–beta signaling pathways mediate epithelial–mesenchymal transition and are dependent on the transcriptional co-activator cAMP–response element–binding protein (CREB)–binding protein (CBP). J Biol Chem 2012;287:7026–7038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carthy JM, Garmaroudi FS, Luo Z, McManus BM. Wnt3a induces myofibroblast differentiation by upregulating TGF-beta signaling through SMAD2 in a beta-catenin–dependent manner. PLoS ONE 2011;6:e19809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lam AP, Flozak AS, Russell S, Wei J, Jain M, Mutlu GM, Budinger GR, Feghali-Bostwick CA, Varga J, Gottardi CJ. Nuclear {beta}–catenin is increased in SSC pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am J Respir Cell Mol Biol 2011;45:915–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laeremans H, Rensen SS, Ottenheijm HC, Smits JF, Blankesteijn WM. Wnt/Frizzled signalling modulates the migration and differentiation of immortalized cardiac fibroblasts. Cardiovasc Res 2010;87:514–523 [DOI] [PubMed] [Google Scholar]
- 43.Nateri AS, Spencer-Dene B, Behrens A. Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature 2005;437:281–285 [DOI] [PubMed] [Google Scholar]
- 44.Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell 2008;133:340–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee MH, Koria P, Qu J, Andreadis ST. JNK phosphorylates beta-catenin and regulates adherens junctions. FASEB J 2009;23:3874–3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gan XQ, Wang JY, Xi Y, Wu ZL, Li YP, Li L. Nuclear Dvl, c-Jun, beta-catenin, and TCF form a complex leading to stabilization of beta-catenin–TCF interaction. J Cell Biol 2008;180:1087–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toualbi K, Guller MC, Mauriz JL, Labalette C, Buendia MA, Mauviel A, Bernuau D. Physical and functional cooperation between AP-1 and beta-catenin for the regulation of TCF-dependent genes. Oncogene 2007;26:3492–3502 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.