Abstract
A cDNA clone encoding a homolog of the yeast (Saccharomyces cerevisiae) gene Anti-oxidant 1 (ATX1) has been identified from Arabidopsis. This gene, referred to as Copper CHaperone (CCH), encodes a protein that is 36% identical to the amino acid sequence of ATX1 and has a 48-amino acid extension at the C-terminal end, which is absent from ATX1 homologs identified in animals. ATX1-deficient yeast (atx1) displayed a loss of high-affinity iron uptake. Expression of CCH in the atx1 strain restored high-affinity iron uptake, demonstrating that CCH is a functional homolog of ATX1. When overexpressed in yeast lacking the superoxide dismutase gene SOD1, both ATX1 and CCH protected the cell from the reactive oxygen toxicity that results from superoxide dismutase deficiency. CCH was unable to rescue the sod1 phenotype in the absence of copper, indicating that CCH function is copper dependent. In Arabidopsis CCH mRNA is present in the root, leaf, and inflorescence and is up-regulated 7-fold in leaves undergoing senescence. In plants treated with 800 nL/L ozone for 30 min, CCH mRNA levels increased by 30%. In excised leaves and whole plants treated with high levels of exogenous CuSO4, CCH mRNA levels decreased, indicating that CCH is regulated differently than characterized metallothionein proteins in Arabidopsis.
Copper, a plant micronutrient, acts as an effective electron acceptor and donor in the active sites of many proteins involved in oxidation and reduction reactions (for review, see Marschner, 1995). These include electron-transport proteins such as Cyt oxidase and proteins involved in the detoxification of oxygen radicals such as copper/zinc SOD. A variety of enzymes with oxidase function, including ascorbate oxidase, diamine oxidase, and phenol oxidase, require copper for their activity.
Despite its importance to plant metabolism, copper is toxic at high concentrations. Copper toxicity can be oxygen dependent through the Haber-Weiss reaction, which generates reactive oxygen intermediates (Halliwell and Gutteridge, 1989), or oxygen independent by inappropriate binding to biomolecules (Kalstrom and Levine, 1991). Organisms have evolved various metal homeostasis factors to control the cellular accumulation, distribution, and sequestration of the metal (Vulpe and Packman, 1995; Koch et al., 1997). In the yeast Saccharomyces cerevisiae there is an overlap between systems controlling copper-ion homeostasis and oxygen radical metabolism. In yeast, copper-binding metallothioneins protect not only against copper toxicity but also detoxify the superoxide anion (Tamai et al., 1993). Similarly, the copper/zinc SOD1 contributes to both the maintenance of copper homeostasis and to superoxide scavenging (Culotta et al., 1995).
The product of the yeast gene Anti-oxidant 1 (ATX1) shows a similar overlap between copper homeostasis and oxygen radical metabolism. The ATX1 gene was originally isolated in strains lacking SOD1 by its ability to suppress oxygen toxicity in a copper-dependent manner (Lin and Culotta, 1995). ATX1 encodes a soluble copper chaperone. ATX1 binds Cu(I) in the cytoplasm and delivers it to a copper transporter in the membrane of a post-Golgi vesicle. In the vesicle, the copper is inserted into a multicopper oxidase essential for high-affinity iron uptake (Lin et al., 1997; Pufahl et al., 1997). Thus, ATX1 may be involved in both copper transport and defense against oxidative stress. HAH1, the human homolog of ATX1, demonstrates both of these functions, with the amino acid residues involved in antioxidant function separate from the copper-binding region (Hung et al., 1998).
Here we describe the identification of the gene Copper CHaperone (CCH) from Arabidopsis encoding a 121-amino acid protein with sequence similarity to ATX1. By expressing CCH in yeast, we show that CCH, like ATX1, can protect SOD1-deficient yeast from active oxygen toxicity. Moreover, CCH expression can also restore high-affinity iron uptake to ATX1-deficient yeast, indicating that CCH and ATX1 are functional homologs. Thus, the gene product of CCH may be involved in both the detoxification of active oxygen and the delivery of copper. Whereas there is basal CCH mRNA expression in many Arabidopsis tissues, CCH mRNA levels increase during leaf senescence, suggesting a role for the CCH gene product in that process.
MATERIALS AND METHODS
Plant Growth and Determination of Nutrient Content
Seedlings of Arabidopsis ecotype Columbia (Col) and Landsberg erecta (Ler) were transplanted to a commercial potting mix (Fafard Germination Mix, Agawam, MA) and grown at 24°C. Ler plants were grown with continuous illumination of approximately 100 μmol/m2 of cool-white fluorescent light. Col plants were grown with a photoperiod of 16 h of light/8 h of dark in growth cabinets with 65 μmol/m2 of cool-white fluorescent light.
Sequence Analysis
A partial amino acid sequence of SAM45 (Crowell and Amasino, 1991) was entered into the database search program BLASTN (National Institutes of Health, Bethesda, MD). The Arabidopsis cDNA 31B12T7 (DBEST accession no. T04721; isolated by the Arabidopsis Expressed Sequence Tag Project Group [Newman et al., 1994]) revealed high homology to SAM45 and was ultimately named Copper CHaperone (CCH). The nucleotide sequence of the CCH cDNA clone was determined by cycle sequencing on an automated sequencer (Applied Biosystems). The clone was sequenced completely from both the 5′ and 3′ ends. Sequences related to CCH were identified using the program BLASTN. Alignments were generated by the Genetics Computer Group software package (program BESTFIT, Madison, WI). Predictions of secondary structure were determined using the EMBL-Heidelberg data bank and the Genetics Computer Group software.
Yeast Strains and Vectors
The yeast (Saccharomyces cerevisiae) strains used in this study were: KS107 (sod1Δ) (Culotta et al., 1995) and SL215 (atx1Δ derivative of YPH250) (Lin et al., 1997). SL201 (sod1Δ,ctr1Δ) was constructed by introducing a ctr1Δ::LEU2 deletion (Dancis et al., 1994) into KS107.
To create a vector to express CCH in yeast, the CCH coding sequence was amplified from expressed sequence tag cDNA clone 31B12T7 (Newman et al., 1994); the upstream primer for amplification was designed such that an EcoRI site was generated 3 bases upstream of the CCH start codon (5′-AAGAATTCGCCATGGCTCAGACCG-3′). The downstream primer for amplification was the SP6 primer (Promega). The 3′ end of the amplified fragment contained a BamHI site downstream of the poly(A+) tail of the CCH cDNA. The CCH coding region was directionally subcloned into the EcoRI and BamHI sites of the yeast expression vector pSM703 (Culotta et al., 1995) to create vector pB-038 for CCH expression in yeast. Other plasmids used were p413-A1 (expression of ATX1) (Lin et al., 1997) and pRS413 (the expression vector used to create p413-A1 with no insert) (Sikorski and Hieter, 1989).
Complementation of Yeast by CCH
The atx1Δ strain SL215Δ was transformed with p413-A1, pRS413, pSM703, or pB-038 as described previously (Lin et al., 1997). To test for restoration of iron uptake in this strain, transformants were grown on complete synthetic dextrose medium (Rose et al., 1991) or synthetic dextrose medium buffered with 50 mm Na-Mes, pH 6.1, and 3 mm ferrozine (ferrozine(3-(2-pyridyl)-5,6-bis(4-phenylsulfonic acid)-1,2,4-triazine); Sigma) with or without 50 μm ferrous ammonium sulfate (Sigma) for 5 d at 30°C.
pB-038 and p413-A1 were transformed separately into KS107. To test for reversal of Lys and Met auxotrophy of this SOD1-deficient yeast strain, transformants were plated on complete synthetic dextrose medium, synthetic dextrose medium without Lys, and synthetic dextrose medium without Met, and grown in air for 3 d at 30°.
To determine whether CCH action was copper dependent, strain KS107 was transformed with either pSM703 (an empty expression vector) or pB-038 (a CCH expression vector). These were tested for oxygen tolerance by aerobic growth in yeast extract-peptone-dextrose liquid medium as described previously (Lin and Culotta, 1995). Anaerobic growth was tested as described (Liu et al., 1992). Where indicated, cultures were supplemented with 150 μm BCS or 150 μm BCS plus 50 μm CuSO4. After 18 h, A600 was determined for each culture.
Ozone Treatment
Three- to 4-week-old plants (ecotype Col) grown under described conditions were moved into 500-L ozone fumigation chambers located inside growth cabinets. Ozone (800 nL/L) was generated by an ozonifier (model Eco-Lab.ppm, Eco Ozono, Valencia, Spain) and monitored continuously using an ozone analyzer (model 1180, Dabisi, Environment Corp., Glendale, CA). Rosettes were harvested and frozen in liquid nitrogen at the indicated times and stored at −80°C until RNA isolation.
Metal Treatment
The fifth and sixth leaves of Arabidopsis were removed 16 DAG and placed in Murashige and Skoog liquid medium (Murashige and Skoog, 1962) with or without 50 μm CuSO4·5H2O (Mallinckrodt, Chesterfield, MO). Leaves were incubated in continuous light at 24°C for 18 h with gentle shaking. Total RNA was extracted from the leaves as described below.
Three- to 4-week-old Arabidopsis plants (ecotype Col) were removed from soil and their roots were submerged in 1 mm CuSO4 solutions for 0.25, 0.5, 1, 2, 4, or 8 h. After treatment, shoots (primarily rosette tissue, no roots) were harvested for nutrient analysis or for mRNA extraction. Copper uptake was determined by atomic absorption spectroscopy on 10 mg of lyophilized Arabidopsis plants treated with nitric acid at 80°C overnight and diluted one-third with water.
RNA Isolation and Gel-Blot Analyses
Total RNA was isolated from Arabidopsis plants (ecotype Col) as described by Prescott and Martin (1987). Total RNA was isolated from the fifth and sixth leaves (excluding cotyledons) of Arabidopsis (ecotype Ler). Total RNA was purified from leaf samples using an RNA isolator (Genosys Biotechnologies, The Woodlands, TX) following the manufacturer's instructions. Total RNA was quantified by spectrophotometry and by 18S rRNA abundance as visualized by ethidium bromide staining on agarose gels and by hybridization to a radiolabeled 18S rRNA probe. For RNA blots, equal amounts of RNA were separated by denaturing-agarose gel electrophoresis and transferred to a nylon membrane as described previously (Sambrook et al., 1989). RNA blots were probed with [32P]ATP-labeled cDNA probes and analyzed using a phosphor imager (Molecular Dynamics, Sunnyvale, CA), a radioanalytical imaging system (InstantImager 2024, Packard, Canberra, Australia), or by exposure to x-ray film at −80°C with an intensifying screen.
RESULTS
Identification of the CCH Gene
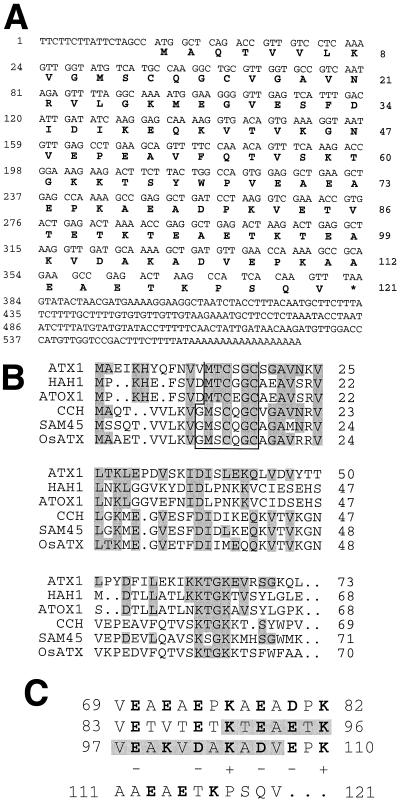
We have previously isolated a gene, SAM45, which is up-regulated at the mRNA level in soybean cell cultures that are cytokinin depleted (Crowell and Amasino, 1991). Cytokinins are known to prevent leaf senescence, and removal of cytokinin from cell cultures causes some symptoms of senescence. We identified an Arabidopsis homolog of SAM45 in the expressed sequence tag collection (accession no. 21536) (Newman et al., 1994) with 97% identity to SAM45 at the amino acid level. The 585-bp cDNA clone contained an open reading frame capable of encoding a 121-amino acid polypeptide with a molecular mass of 13 kD and a pI of 4.9 (Fig. 1A). The reading frame shown was the only possible open reading frame in the cDNA and had both a start and stop codon, indicating that the sequence contained the complete coding region. Similar sequences were identified from S. cerevisiae (ATX1; Lin and Culotta, 1995) and from rice (OsATX1; accession no. DBEST 70798). ATX1 is involved in copper trafficking (Lin et al., 1997), and because the Arabidopsis gene corresponding to cDNA 31B12T7 has a similar function (see below), we refer to it as Copper Chaperone. DNA-blot analysis indicated that there was a single copy of CCH in the Arabidopsis genome (data not shown).
Figure 1.
CCH sequence analysis. A, Nucleotide and predicted amino acid sequences for the CCH cDNA. B, Alignment of yeast ATX1 with the predicted amino acid sequence of HAH1 (human), ATOX1 (mouse; accession no. AF004591), and the 69 N-terminal amino acids of CCH (Arabidopsis), SAM45 (soybean), and rice ATX1 (OsATX1; accession no. DBEST 70798). Conserved residues in the alignment are shaded. The putative metal-binding domain is boxed. C, Amino acid sequence features of the C-terminal region of CCH. Charged residues are shown in boldface and their charges are indicated below them. Secondary-structure prediction suggested that an α-helix could be formed in the shaded region.
The alignment of ATX1 with the predicted sequence of CCH and other similar amino acid sequences is shown in Figure 1B. The amino acid sequence of CCH and ATX1 are 36% identical and 54% similar across the length of ATX1. CCH exhibits the same degree of similarity to ATX1 homologs from mouse (ATOX1) and human (HAH1). CCH is 67% identical to a potential ATX1 homolog from rice (OsATX1). Both the CCH and OsATX1 polypeptides conserve the motif GMXCXXC (boxed in Fig. 1B), which is also described at the N-terminal region of copper-pumping ATPases such as CopA from Enterococcus hirae and the human Menkes and Wilson disease gene products (Solioz et al., 1994). This sequence is similar to the more general heavy-metal-binding motif (MTCXXC) found in ATX1 and other metal-binding proteins (Lin and Culotta, 1995). This motif has been shown to bind copper (Pufahl et al., 1997).
CCH extended 48 amino acids beyond the C-terminal end of ATX1; this domain contained 44% charged amino acids, many of which were separated by a single nonpolar amino acid (Fig. 1C). The alternating opposite-charged amino acids observed within the CCH C-terminal region suggests that an α-helix could be formed from residues 83 to 100, with a spatial distribution of basic amino acids on one side and acidic amino acids on the other. Thus, CCH may be composed of two different domains, an N-terminal region involved in copper binding and the highly charged C-terminal region, which may be involved in interactions with other molecules.
Complementation of atx1 and sod1 Mutant Yeast Strains
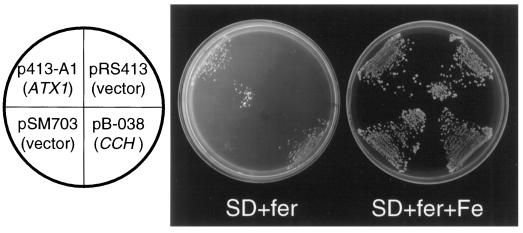
ATX1 is involved in the transport of copper ions to the secretory pathway, where the copper is made available to a copper-dependent oxidase essential for high-affinity iron uptake at the plasma membrane (Pufahl et al., 1997; Valentine and Gralla, 1997). Thus, the atx1 mutant strain is deficient in high-affinity iron uptake. In the presence of the iron chelator ferrozine, yeast cells acquire iron through the high-affinity uptake pathway. Thus, atx1 mutants cannot grow on ferrozine-containing medium unless supplemented with excess iron (Lin et al., 1997). To determine if CCH could replace the function of ATX1 in high-affinity iron uptake, CCH was expressed in an atx1 mutant strain. The complete open reading frame of CCH was inserted into a multicopy vector, allowing constitutive expression of CCH in yeast. Transformants were plated on medium with and without supplemental iron (Fig. 2). Strains expressing either ATX1 or CCH grew in the absence of supplemental iron, demonstrating that high-affinity iron uptake was restored by expression of either ATX1 or CCH. Although restored growth on ferrozine was slow, the growth rate was comparable to that of the wild type (data not shown).
Figure 2.
CCH restored high-affinity iron uptake to atx1 mutant yeast. The atx1 mutant strain SL215 was transformed with vector p413-A1 (ATX1 expression), pRS413 (expression vector, negative control), pB-038 (CCH expression), or pSM703 (expression vector, negative control) and streaked on plates in the positions indicated. All plates contained ferrozine, an iron chelator. Plates contained synthetic dextrose medium plus 3 mm ferrozine (SD+fer) or synthetic dextrose medium plus 3 mm ferrozine plus 50 μm ferrous ammonium sulfate (SD+fer+Fe). Plates were photographed after incubation at 30°C for 4 d.
ATX1 was identified by its ability to protect against oxygen toxicity when expressed in a yeast strain lacking the SOD genes SOD1 and SOD2 (Lin and Culotta, 1995). Phenotypes of SOD1 deficiency in aerobically grown yeast include retarded growth rate and auxotrophy for Lys and Met (Liu et al., 1992). The synthetic pathways for these amino acids contain steps that are hypersensitive to reactive oxygen (Liu et al., 1992; Slekar et al., 1996). In the sod1 mutant, ATX1 expressed from a multicopy expression vector can restore Lys and Met biosynthesis, allowing the transformed strain to grow without supplemental Lys or Met (Lin and Culotta, 1995).
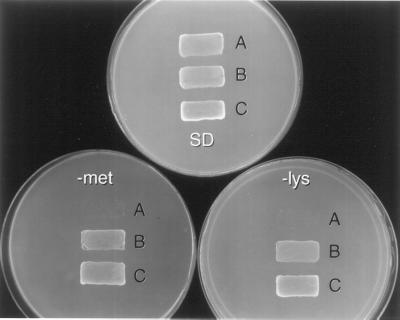
To determine whether CCH could restore Lys and Met synthesis to aerobically grown, SOD-deficient yeast, we expressed CCH in the mutant strain (Fig. 3). The untransformed SOD-deficient yeast could grow on complete medium but failed to grow in the absence of Lys or Met. The same strain expressing either ATX1 or CCH could grow with Lys and Met absent from the medium, indicating that CCH expression in aerobically grown SOD-deficient yeast protects the cells from oxygen toxicity to the extent that Lys and Met synthesis are restored. Thus, CCH function can replace ATX1 function in this strain.
Figure 3.
CCH can restore Lys and Met synthesis in aerobically grown sod1 mutant yeast. Strain KS107 (sod1) was plated after transformation with no expression vector (A, negative control), pRS-A1 (B, ATX1 expression), or pB-038 (C, CCH expression). Plates contained complete medium (SD) or medium lacking Met (−met) or Lys (−lys). Plates were photographed after aerobic growth at 30°C for 2 d.
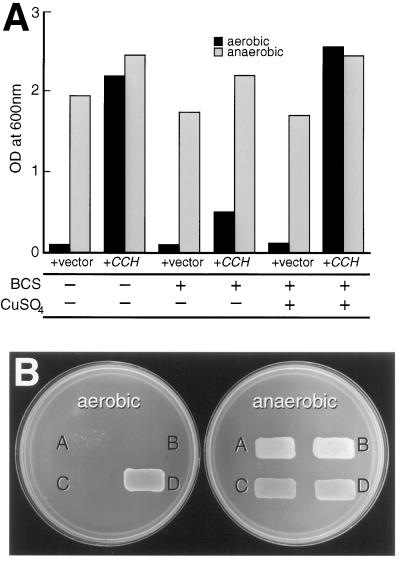
To determine whether CCH protects sod1 yeast in a copper-dependent manner, a sod1 strain transformed with the CCH expression construct was grown in the presence of BCS, a copper chelator (Fig. 4A). BCS chelated copper in the growth medium, making it unavailable to the growing cells. In the absence of BCS, CCH expression allowed sod1 yeast to grow in aerobic conditions at a rate similar to that of sod1 yeast grown in anaerobic conditions. When free copper was chelated by BCS, the growth of the CCH-expressing sod1 cells in aerobic conditions was inhibited and was similar to that of sod1 cells transformed with the vector only. When excess CuSO4 was added to medium containing BCS, copper became available to the cells; under these conditions, growth was restored to CCH-expressing sod1 yeast in aerobic conditions. As additional evidence for the requirement of copper in CCH function, CCH was expressed in sod1,ctr1 double mutants. In these mutants the absence of CTR1, a copper transporter at the plasma membrane, prevents uptake of copper to the cytoplasm (Dancis et al., 1994). CCH expression was unable to restore Lys synthesis in sod1,ctr1 double mutants (Fig. 4B). These results indicate that the ability of CCH to protect sod1 yeast from reactive oxygen toxicity is dependent on copper availability.
Figure 4.
CCH rescued sod1 mutant yeast in a copper-dependent manner. A, Strain KS107 (sod1Δ) was transformed with the vector pSM703 (+vector) as a negative control or pB-038 (+CCH) as the CCH expression vector. Yeast was grown in yeast extract-peptone-dextrose medium alone or yeast extract-peptone-dextrose plus 150 μm BCS with or without 50 μm CuSO4. A600 was determined after yeast was grown in either aerobic or anaerobic conditions for 18 h beginning at A600 = 0.01. B, A, SL201 (sod1Δ,ctr1Δ) plus pSM703 (empty expression vector); B, KS107 (sod1Δ) plus pSM703; C, SL201 plus pB-038 (CCH expression); and D, KS107 plus pB-038 yeast were grown in aerobic or anaerobic conditions for 2 d on synthetic dextrose medium lacking Lys.
CCH Gene Expression
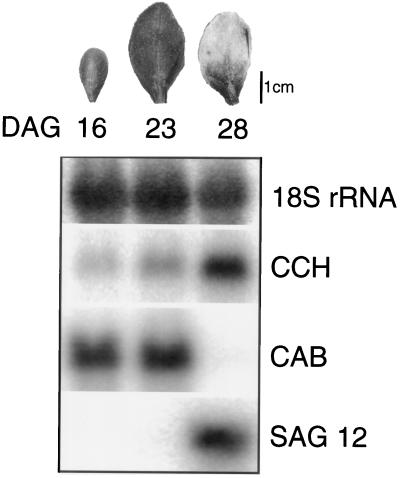
RNA-blot analysis established that CCH mRNA was expressed in the root, stem, leaf, inflorescence, and silique (not shown). To determine the expression of CCH during leaf senescence, total RNA was isolated from the fifth and sixth leaves of Arabidopsis at 16, 23, and 28 DAG (Fig. 5). In this population, leaves at 16 DAG were not fully expanded, leaves at 23 DAG were fully expanded but showed no yellowing, and leaves at 28 DAG were, by visual estimation, 50% yellow and clearly undergoing senescence. In these experiments, CCH mRNA levels increased by more than 7-fold after the onset of leaf senescence. The senescence-associated gene SAG12, which is expressed in a highly senescence-specific manner, and the chlorophyll a/b-binding protein CAB, which is rapidly down-regulated during senescence, serve as positive and negative controls for leaf senescence (Lohman et al., 1994).
Figure 5.
CCH mRNA was up-regulated during leaf senescence. The fifth and sixth leaves of Arabidopsis plants (ecotype Ler) were removed at 16, 23, or 28 DAG, and total RNA was extracted and used for RNA-blot analysis (leaf photographs are representative of leaves at each time point). RNA blots were created with equal amounts of each RNA sample, as determined by spectrophotometry and ethidium bromide staining. Equal loading was confirmed by detection of 18S rRNA with a radiolabeled probe. RNA blots were probed with [32P]ATP-labeled cDNA probes for 18S rRNA (control for equal loading on the RNA blot), CCH cDNA, chlorophyll a/b-binding protein (CAB), and the senescence-associated gene (SAG 12).
Response to Ozone
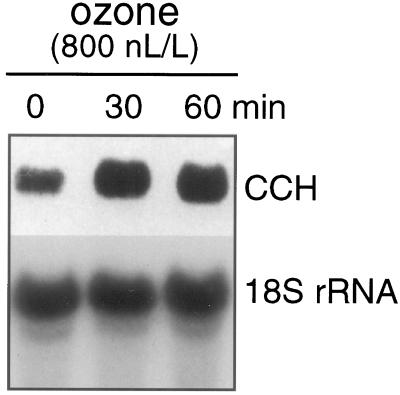
The expression of ATX1 is induced by oxidative stress (Lin and Culotta, 1995). To determine whether CCH was similarly induced, 3- to 4-week-old Arabidopsis plants were treated with high ozone concentrations (800 nL/L) for 30 or 60 min and total RNA from the aerial tissues was analyzed for CCH expression by RNA-blot analysis (Fig. 6). After 30 min of treatment a more than 30% increase in CCH mRNA was observed. Longer exposures did not increase the level of CCH transcripts. This modest induction of the CCH message by ozone treatment was observed in three separate trials.
Figure 6.
CCH mRNA levels increased after treatment with ozone. Arabidopsis plants (3 to 4 weeks old) were subjected to 800 nL/L ozone treatment for 0, 30, or 60 min in ozone fumigation chambers. Ten micrograms of total RNA was used for RNA-blot analysis and probed with either CCH cDNA or 18S rRNA.
Response to Copper Treatment
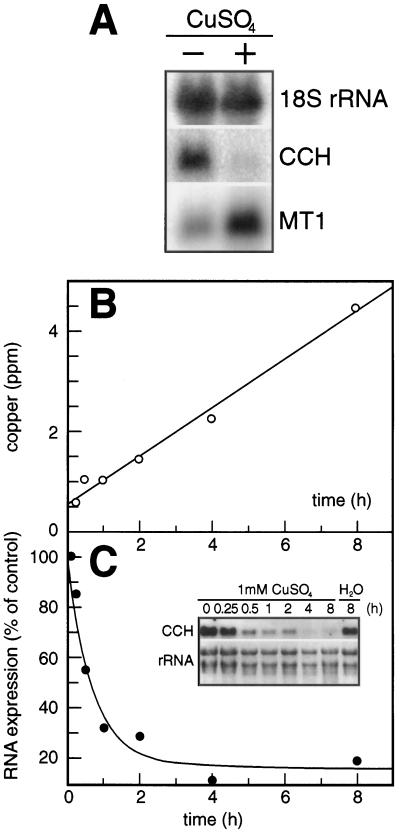
To determine whether CCH is regulated by copper, the fifth and sixth leaves of Arabidopsis plants at 16 DAG were removed and incubated in liquid medium with or without 50 μm CuSO4 for 18 h. Total RNA isolated from these leaves was used to generate RNA blots (Fig. 7A). CCH mRNA levels decreased by more than 5-fold during this treatment, suggesting that CCH is down-regulated at the mRNA level by high levels of exogenous copper. Separately, as a control for copper treatment, mRNA levels of MT1 increased in copper-treated leaves, as has been observed previously (Zhou and Goldsbrough, 1994). These results indicate that the CCH message is regulated differently than the message of a copper-binding metallothionein, at least when nonphysiologically high levels of copper are applied.
Figure 7.
CCH mRNA was down-regulated in response to copper treatment. A, The fifth and sixth leaves were removed from Arabidopsis plants (ecotype Ler) at 16 DAG. Leaves were incubated in light for 18 h with gentle shaking in either Murashige and Skoog medium (−) or Murashige and Skoog medium plus 50 μm CuSO4 (+). Total mRNA was extracted from leaves and used for RNA-blot analysis. Blots were probed with either 18S rRNA (control for equal loading on RNA blot), CCH, or MT1 cDNA. B, Arabidopsis plants (ecotype Col) were removed from soil and their roots submerged in 1 mm CuSO4. Kinetics of copper uptake to the aboveground parts of the plant were measured by atomic absorption at various time points. C, Total RNA was isolated from plants after the treatment described above, as well as from plants treated in parallel with water. RNA blots made from these samples were probed with [32P]ATP-labeled CCH (see inset). The graph plots the change in CCH mRNA levels (expressed in cpm on the RNA blot probed with the CCH probe) over time of treatment. The 0-h time point was set to 100% and subsequent values were normalized to the 0-h value.
To determine if copper could down-regulate CCH expression in intact plants, 3- to 4-week-old Arabidopsis plants were removed from soil and their roots were submerged in 1 mm CuSO4 solutions for different times. Copper transport into the shoot was followed by atomic absorption of nitric acid-digested samples (Fig. 7B). Associated with the accumulation of copper was a decrease in CCH mRNA (Fig. 7C). After 30 min, RNA blotting revealed that mRNA levels of CCH decreased by 50%. Longer copper treatment further lowered CCH levels.
DISCUSSION
We describe the characterization of CCH from Arabidopsis. CCH encodes a predicted protein of 121 amino acids that, in the N-terminal region, is similar in sequence to ATX1 from yeast and contains the heavy-metal-binding sequence MXCXXC that is common to metal-binding proteins in bacteria, fungi, animals, and plants. The predicted amino acid sequence of CCH includes a highly charged carboxyl domain that could interact with other molecules, perhaps in a copper-dependent manner. The homologs of ATX1, HAH1 (human), and ATOX1 (mouse) encode proteins of similar length to ATX1, i.e. they do not bear a C-terminal extension (Klomp et al., 1997).
CCH is a functional homolog of ATX1. Both CCH and ATX1 can restore Met and Lys biosynthesis to a SOD1-deficient strain, indicating that CCH and ATX1 share the ability to protect yeast from some effects of the oxidative damage produced by SOD deficiency. The observation that ATX1 levels increase in yeast cells challenged with reactive oxygen intermediates is consistent with ATX1 being involved in a response to reactive oxygen (Lin and Culotta, 1995).
It will be interesting to determine whether the CCH gene product has the ability to suppress oxygen toxicity in Arabidopsis mutants lacking SOD in a copper-dependent manner, as has been described for yeast (Culotta et al., 1995; Lin and Culotta, 1995). Also, in the wild type, it would be interesting to determine whether CCH overexpression might protect against the pernicious effects of ozone common in industrialized areas. Levels of CCH mRNA increase moderately after ozone exposure, yet it remains to be determined whether this up-regulation is part of a defense against oxidative stress. Because little information exists regarding the interactions between copper metabolism and protection from oxidative stress in plants, the study of CCH could clarify the relationship between these two processes. The human homolog of CCH, HAH1, has distinct regions mediating copper delivery and oxidative defense (Hung et al., 1998). CCH shows high homology to HAH in these regions and may retain the same functions.
In the yeast S. cerevisiae, ATX1 is part of pathway that links copper transport to iron uptake at the cell surface (Yaun et al., 1995; Stearman et al., 1996; Lin et al., 1997; Valentine and Gralla, 1997). ATX1 interacts with the membrane-bound copper transporter CCC2 to deliver copper to the interior of a post-Golgi vesicle (Pufahl et al., 1997). Ultimately, copper becomes incorporated into a complex capable of reducing iron outside the cell (Stearman et al., 1996). An extracellular mechanism for iron reduction serves to reduce Fe(III), the common extracellular form of iron, to Fe(II) to facilitate uptake. A homologous copper transport pathway has been identified in humans; defects in this pathway are the cause of Menkes and Wilson disease, the symptoms of which result from deficiencies in copper loading of copper-containing proteins such as ceruloplasmin (Yaun et al., 1995; Klomp et al., 1997).
The discovery that the CCH gene product can replace ATX1 in the high-affinity iron uptake pathway indicates that CCH can interact with CCC2 to deliver copper to the post-Golgi vesicles of yeast cells. It remains to be determined whether plants express genes encoding functions homologous to those of other members of the yeast high-affinity iron-uptake pathway. Nevertheless, the physiology of iron uptake in plants is consistent with the existence of such a pathway. In many plants uptake of iron requires reduction of Fe(III), the common form of iron in aerated soils, to Fe(II) in the root zone (Marschner, 1995). CCH mRNA is expressed at a basal level in the roots, stems, flowers, siliques, and leaves of Arabidopsis. It is not known whether iron reduction is taking place in all of these tissues or, if so, whether CCH is needed for that reduction.
Several metallothioneins that are up-regulated at the mRNA level during leaf senescence have been identified from plants (Buchanan-Wollaston, 1994; Weaver et al., 1997). These metallothioneins are believed to protect the cell by binding free copper ions in the cytoplasm. Their up-regulation during leaf senescence suggests that a copper detoxification function is important in senescing leaf cells. The CCH gene product may serve a similar function. However, CCH is regulated differently than one senescence-induced metallothionein, MT1, in nonsenescent leaves treated with exogenous copper; copper treatment sufficient to induce MT1 mRNA expression eliminates CCH expression at the mRNA level. Thus, if CCH functions as a copper chelator, it does not appear to do so in all situations in which copper toxicity threatens the leaf cell. The yeast ATX1 message is also not induced in cells treated with exogenous copper (S.-J. Lin and V.C. Culotta, unpublished data).
We have observed in Arabidopsis that CCH is up-regulated during leaf senescence, suggesting a possible role for CCH function in copper binding or transport during that process. During senescence nutrients are redistributed from senescing tissue, contributing to growth in other parts of the plant (Noodén and Leopold, 1988). An indication of the importance of copper in plant metabolism is that many plants redistribute copper from leaves before abscission, thus avoiding copper loss to the environment (Mauk and Nooden, 1992; Drossopoulos et al., 1994, 1996; Hocking, 1994). Metals exiting the leaves during senescence are likely to do so by way of the phloem. Recent studies of metal transport in castor bean indicate that copper and iron can move through phloem chelated to organic molecules, in particular the amino acid nicotianamine (Schmidke and Stephan, 1995).
As chloroplasts and their constituent proteins are broken down during senescence, copper is released. As discussed above, the metallothionein MT1 is up-regulated at the mRNA level during leaf senescence and may be involved in the sequestration of copper released during senescence. Also, CCH mRNA is up-regulated during senescence and may be involved in copper sequestration during this process. Furthermore, CCH, as a functional homolog of ATX1, may be involved in the delivery of copper to the secretory system in preparation for phloem loading and transport from senescing leaves. In Arabidopsis we have observed that copper levels drop by one-half several days after the onset of senescence (E. Himelblau and R.M. Amasino, unpublished data). It will be interesting to determine if plants in which CCH expression is eliminated or attenuated during senescence can continue to transport copper from senescing tissue. Also, reactive oxygen intermediates accumulate in leaves undergoing senescence (Noodén and Leopold, 1988). As described above, CCH may encode antioxidant function, suggesting an additional role for CCH expression during leaf senescence.
ACKNOWLEDGMENTS
We thank the Arabidopsis Biological Resource Center (Columbus, OH) and the Arabidopsis Expressed Sequence Tag Project (Department of Energy Plant Research Laboratory, Michigan State University, East Lansing) for providing cDNA sequences and the 31B12T7 clone. We are grateful to Dr. Peter Goldsbrough for providing the MT1 cDNA clone. We thank Joaquín Moreno, Pedro Carrasco, Francisco Estruch, Edgar Spalding, and Betania Quirino for critical reading of the manuscript.
Abbreviations:
- BCS
bathocuproinedisulfonic acid
- DAG
days after germination
- SOD
superoxide dismutase
Footnotes
E.H. and R.M.A. were supported by the Consortium for Plant Biotechnology Research (grant no. DE-FG02-97ER20280), E.H. was supported by the National Institutes of Health (NIH) Biotechnology Training Program (grant no. 5 T32 GM08349), H.M. and L.P. were supported by the Direccion General de Investigacion Cientifica y Technica Spain (grant no. PB95-0029-C02-02) and the Conselleria d' Educació y Ciencia de la Generalitat Valenciana, and S.-J.L. and V.C.C. were supported by the Johns Hopkins National Institute of Environmental Health Services Center and NIH (grant no. GM RO1 50016).
The accession number for the nucleotide sequence reported in this article is U88711.
LITERATURE CITED
- Buchanan-Wollaston V. Isolation of cDNA clones for genes that are expressed during leaf senescence in Brassica napus. Plant Physiol. 1994;105:839–846. doi: 10.1104/pp.105.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell D, Amasino RM. Induction of specific mRNAs in cultured soybean cells during cytokinin or auxin starvation. Plant Physiol. 1991;95:711–715. doi: 10.1104/pp.95.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotta VC, Joh HD, Slekar KH, Strain J. A physiological role for Saccharomyces cerevisiae copper/zinc superoxide dismutase in copper buffering. J Biol Chem. 1995;270:29991–29997. doi: 10.1074/jbc.270.50.29991. [DOI] [PubMed] [Google Scholar]
- Dancis A, Haile D, Yaun DS, Klausner RD. The Saccharomyces cerevisiae copper transport protein (Ctr1p) J Biol Chem. 1994;269:25660–25667. [PubMed] [Google Scholar]
- Dancis A, Yaun DS, Haile D, Askwith C, Eide D, Moehle C, Kaplan J, Klausner RD. Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell. 1994;76:393–402. doi: 10.1016/0092-8674(94)90345-x. [DOI] [PubMed] [Google Scholar]
- Drossopoulos JB, Bouranis DL, Bairaktari BD. Patterns of mineral nutrient fluctuations in soybean leaves in relation to their position. J Plant Nutr. 1994;17:1017–1035. [Google Scholar]
- Drossopoulos JB, Kouchaji GG, Bouranis DL. Seasonal dynamics of mineral nutrients by walnut tree reproductive organs. J Plant Nutr. 1996;19:421–434. [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford, UK: Clarendon Press; 1989. [Google Scholar]
- Hocking PJ. Dry-matter production, mineral nutrient concentrations, and nutrient distribution and redistribution in irrigated spring wheat. J Plant Nutr. 1994;17:1289–1308. [Google Scholar]
- Hung IH, Casareno RLB, Labesse G, Mathews FS, Gitlin JD. HAH1 is a copper-binding protein with distinct amino acid residues mediating copper homeostasis and antioxidant defense. J Biol Chem. 1998;273:1749–1754. doi: 10.1074/jbc.273.3.1749. [DOI] [PubMed] [Google Scholar]
- Kalstrom AR, Levine RL. Copper inhibits the protease from human immunodeficiency virus 1 by both cysteine-dependent and cysteine-independent mechanisms. Proc Natl Acad Sci USA. 1991;88:5552–5556. doi: 10.1073/pnas.88.13.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klomp LWJ, Lin SJ, Yaun DS, Klausner RD, Culotta VC, Gitlin JD. Identification and functional expression of HAH1, a novel human gene involved in copper homeostasis. J Biol Chem. 1997;272:9221–9226. doi: 10.1074/jbc.272.14.9221. [DOI] [PubMed] [Google Scholar]
- Koch K, Pena M, Thiele D. Copper-binding motifs in catalysis, transport, detoxification and signaling. Chem Biol. 1997;4:549–560. doi: 10.1016/s1074-5521(97)90241-6. [DOI] [PubMed] [Google Scholar]
- Lin S, Culotta VC. The Atx1 gene of Saccharomyces cerevisiae encodes a small metal homeostasis factor that protects cells against reactive oxygen toxicity. Proc Natl Acad Sci USA. 1995;92:3784–3788. doi: 10.1073/pnas.92.9.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Pufahl RA, Dancis A, O'Halloran TV, Culotta VC. A role for the Saccharomyces cerevisiae ATX1 gene in copper trafficking and iron transport. J Biol Chem. 1997;272:9215–9220. [PubMed] [Google Scholar]
- Liu XF, Elashvili I, Gralla EB, Valentine JS, Lapinskas P, Culotta VC. Yeast lacking superoxide dismutase. J Biol Chem. 1992;267:18298–18302. [PubMed] [Google Scholar]
- Lohman K, Gan S, John M, Amasino RM. Molecular analysis of natural leaf senescence in Arabidopsis thaliana. Physiol Plant. 1994;92:322–328. [Google Scholar]
- Marschner H. Mineral Nutrition of Higher Plants. London: Academic Press; 1995. [Google Scholar]
- Mauk CS, Nooden LD. Regulation of mineral redistribution in pod-bearing soybean explants. J Exp Bot. 1992;43:1429–1440. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Newman T, de Bruijn FJ, Green P, Keegstra K, Kende H, McIntosh L, Ohlrogge J, Raikhel N, Somerville S, Thomashow M and others. Genes galore: a summary of methods for accessing results from large-scale partial sequencing of anonymous Arabidopsis cDNA clones. Plant Physiol. 1994;106:1241–1255. doi: 10.1104/pp.106.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noodén LD, Leopold AC. Senescence and Aging in Plants. San Diego, CA: Academic Press; 1988. [Google Scholar]
- Prescott A, Martin C. A rapid method for the quantitative assessment of levels of specific mRNAs in plants. Plant Mol Biol Rep. 1987;4:219–224. [Google Scholar]
- Pufahl R, Singer C, Peariso K, Lin S-J, Schmidt J, Fahrni C, Cizewski Culotta V, Penner-Hahn J, O'Halloran T. Metal ion chaperone function of the soluble Cu(I) receptor Atx1. Science. 1997;278:853–856. doi: 10.1126/science.278.5339.853. [DOI] [PubMed] [Google Scholar]
- Rose MD, Winston F, Hieter P. Methods in Yeast Genetics: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1991. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schmidke I, Stephan UW. Transport of metal micronutrients in the phloem of castor bean (Ricinus communis) seedlings. Physiol Plant. 1995;95:147–153. [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slekar KH, Kosman DJ, Cizewski Culotta V. The yeast copper/zinc superoxide dismutase and the pentose phosphate pathway play overlapping roles in oxidative stress protection. J Biol Chem. 1996;271:28831–28836. doi: 10.1074/jbc.271.46.28831. [DOI] [PubMed] [Google Scholar]
- Solioz M, Odermatt A, Krapf X. Copper pumping ATPases: common concepts in bacteria and man. FEBS Lett. 1994;346:44–47. doi: 10.1016/0014-5793(94)00316-5. [DOI] [PubMed] [Google Scholar]
- Stearman R, Yaun DS, Yamaguchi-Iwai Y, Klausner RD, Dancis A. A permease-oxidase complex involved in high-affinity iron uptake in yeast. Science. 1996;271:1552–1557. doi: 10.1126/science.271.5255.1552. [DOI] [PubMed] [Google Scholar]
- Tamai KT, Gralla EB, Ellerby LM, Valentine JS, Thiele DJ. Yeast and mammalian metallothioneins functionally substitute for yeast copper-zinc superoxide dismutase. Proc Natl Acad Sci USA. 1993;90:8013–8017. doi: 10.1073/pnas.90.17.8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine J, Gralla EB. Delivering copper inside yeast and human cells. Science. 1997;278:817–818. doi: 10.1126/science.278.5339.817. [DOI] [PubMed] [Google Scholar]
- Vulpe CD, Packman S. Cellular copper transport. Annu Rev Nutr. 1995;15:293–322. doi: 10.1146/annurev.nu.15.070195.001453. [DOI] [PubMed] [Google Scholar]
- Weaver LM, Himelblau E, Amasino RM (1997) Leaf senescence: gene expression and regulation. In JK Setlow, ed, Genetic Engineering, Vol 19. Plenum Press, New York, pp 215–234
- Yaun DS, Stearman R, Dancis A, Dunn T, Beeler T, Klausner RD. The Menkes/Wilson disease gene homologue in yeast provides copper to a ceruloplasmin-like oxidase required for iron uptake. Proc Natl Acad Sci USA. 1995;92:2632–2636. doi: 10.1073/pnas.92.7.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Goldsbrough PB. Functional homologs of fungal metallothionein genes from Arabidopsis. Plant Cell. 1994;6:875–884. doi: 10.1105/tpc.6.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]