Abstract
Objectives
To confirm the downregulation of PTGER4 mRNA in the conjunctiva of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) and ocular cicatricial pemphigoid (OCP) patients and to examine the expression of its EP4 protein in the conjunctival epithelium of patients with various ocular surface disorders.
Design
Case-control study.
Setting and participants
We performed quantitative reverse transcription-PCR (RT-PCR) analysis of PTGER4 mRNA in conjunctival tissue sections from patients with SJS/TEN and OCP to confirm the downregulation of PTGER4 mRNA expression. We also analysed EP4 immunohistologically in other ocular surface disorders. Conjunctival tissues were obtained from patients undergoing surgical reconstruction of the ocular surface due to chemical eye burns, subacute SJS/TEN or chronic SJS/TEN, chronic OCP, severe graft versus host disease (GVHD) and from patients with Mooren's ulcers treated by resection of the inflammatory conjunctiva.
Primary and secondary outcome measures
The expression of PTGER4 mRNA and EP4 protein assessed by quantitative RT-PCR assay and immunohistological methods.
Results
PTGER4 mRNA was significantly lower in conjunctival tissues from SJS and OCP patients than in the control conjunctivochalasis samples. EP4 protein was detected in conjunctival epithelium from patients with chemical eye burn and in control conjunctival epithelium from patients with conjunctivochalasis. Its expression varied in conjunctival epithelium from patients with Mooren's ulcer. We did not detect EP4 immunoreactivity in conjunctival epithelium from patients with subacute SJS/TEN, severe GVHD, chronic SJS/TEN or OCP.
Conclusions
The strong downregulation of EP4 expression in conjunctival epithelium from patients with OCP or SJS/TEN may be attributable to ocular surface inflammation.
Keywords: Ophthalmology
Article summary.
Article focus
We previously reported that EP4 protein was down-regulated in devastating ocular surface inflammatory disorders such as chronic Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) and chronic ocular cicatricial pemphigoid (OCP). Article focus of this study are to confirm the downregulation of PTGER4 mRNA, which protein is EP4, in the conjunctiva of SJS/TEN and OCP patients and to examine the expression of its EP4 protein in the conjunctival epithelium of patients with other various ocular surface disorders in addition chronic SJS/TEN and OCP.
Key messages
EP4 is expressed not only in normal conjunctival epithelium but also in conjunctival epithelium from patients with chemical eye burns and some patients with Mooren's ulcer. On the contrary, it is strongly downregulated in conjunctival epithelium from patients with OCP and chronic SJS/TEN and subacute SJS/TEN.
Strengths and limitations of this study
The function of EP4 in conjunctival epithelial cells is not elucidated.
Introduction
The prostanoids PGD2, PGE2, PGF2α, PGI2 and TXA2 are lipid mediators that form in response to various stimuli. They are released extracellularly immediately after their synthesis and they act by binding to a G protein-coupled rhodopsin-type receptor on the surface of target cells.1 PGE2 is produced during inflammatory responses and it suppresses the production of cytokines and chemokines induced by lipopolysaccharide-stimulated macrophages2 3 and dendritic cells.4 Elsewhere we reported that PGE2 modulates the expression of polyI:C-induced proinflammatory genes in human conjunctival epithelial cells.5
There are four PGE receptor subtypes, EP1, EP2, EP3 and EP4. The intestinal epithelium has been reported to express EP4 mRNA,6 and intestinal homeostasis was maintained and the immune response downregulated by EP4.7 The ocular surface is also one of the mucosa that is in contact with commensal bacteria like the intestine. Therefore, we focused on the expression of EP4 in human conjunctival epithelium and the difference of its expression between various ocular surface diseases.
We documented that while normal human conjunctival epithelium expressed EP4 protein, it was down-regulated in devastating ocular surface inflammatory disorders such as chronic Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) and chronic ocular cicatricial pemphigoid (OCP).8 Here we examined the mRNA expression of PTGER4, which is the gene of EP4 protein, in the conjunctiva of SJS/TEN and OCP patients in the chronic stage to confirm that PTGER4 mRNA EP4 is down-regulated in their conjunctiva. We also examined the expression of PTGER4 mRNA protein in the conjunctival epithelium of patients with various ocular surface disorders such as chemical eye burn, Mooren's ulcer, severe graft versus host disease (GVHD) and of patients in the subacute stage of SJS/TEN.
Materials and methods
Human conjunctival tissues
This study was approved by the Institutional Review Board of Kyoto Prefectural University of Medicine, Kyoto, Japan. All experiments were conducted in accordance with the principles set forth in the Helsinki Declaration.
For quantitative reverse transcription-PCR (RT-PCR) the controls were nearly normal conjunctival tissues obtained at surgery for conjunctivochalasis, a disease in which the conjunctiva relaxes due to aging, resulting in a foreign body sensation on the ocular surface. We also prepared human conjunctival tissues from samples obtained during surgery to reconstruct the ocular surface in four patients in the chronic stage of SJS/TEN and four patients in the chronic stage of OCP.
The controls for immunohistochemical analyses were nearly normal conjunctival tissues obtained during surgery for conjunctivochalasis. We also prepared human conjunctival tissues from samples obtained during surgery to reconstruct the ocular surface in three patients with chemical (alkali) eye burn (two in the chronic stage and one in the subacute stage), two patients with subacute SJS/TEN, one patient with severe GVHD and from four patients with Mooren's ulcer undergoing resection of inflammatory conjunctiva. SJS/TEN, OCP, Mooren's ulcer, chemical burn and GVHD are all ocular surface inflammatory diseases with persistent inflammation on the ocular surface not only in the acute stage but also in the chronic stage.
Quantitative RT-PCR
Total RNA was isolated from conjunctival tissue sections using the RNeasy mini kit (Qiagen, Valencia, California, USA) according to the manufacturer's instructions. The RT reaction was with the SuperScript preamplification kit (Invitrogen, Carlsbad, California, USA). Quantitative RT-PCR was on an ABI-prism 7700 instrument (Applied Biosystems, Foster City, California, USA). The probes for human PTGER4 and human GAPDH were from Applied Biosystems. For cDNA amplification we performed PCR in a 25 μl total volume that contained a 1 μl cDNA template in 2×TaqMan universal PCR master mix (Applied Biosystems) at 50°C for 2 min and 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. The results were analysed with sequence detection software (Applied Biosystems). The quantification data were normalised to the expression of the housekeeping gene GAPDH.
Immunohistochemistry
For EP4 staining we used rabbit polyclonal antibody to EP4 (Cayman Chemical Co, Ann Arbor, Michigan, USA). The secondary antibody (Biotin-SP-conjugated AffiniPure F(ab’)2 fragment donkey antirabbit IgG (H+L), 1 : 500 dilution; Jackson Immuno Research, Baltimore, Maryland, USA) was applied for 30 min. The VECTASTAIN ABC reagent (Vector Laboratories, Inc, Burlingame, California, USA) was used for increased sensitivity with peroxidase substrate solution (DAB substrate kit; Vector) as a chromogenic substrate.
Data analysis
Data were expressed as the mean±SEM and evaluated by the Student's t test using the Microsoft Excel software program.
Results
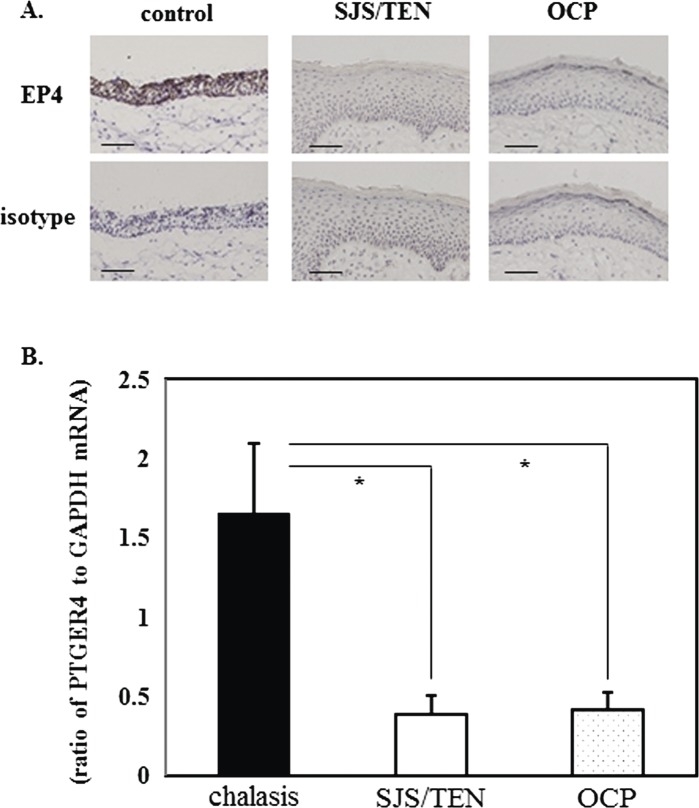
We previously documented that EP4 protein expression was down-regulated in conjunctival epithelium of devastating ocular surface inflammatory disorders such as chronic SJS/TEN and chronic OCP.8 In this study, to confirm the down-regulation of EP4 in the ocular surface of SJS/TEN and OCP patients we examined the expression of PTGER4 mRNA in control conjunctival tissues from six conjunctival chalasis patients and in conjunctival tissues from four SJS/TEN patients and four OCP patients. Representative findings of EP4 immunoreactivity in each of these groups are shown in figure 1A. Although EP4 protein was detected in the control tissues, conjunctival epithelium from SJS patients and OCP patients did not manifest EP4 immunoreactivity. PTGER4 mRNA was significantly lower in conjunctival tissues from SJS/TEN and OCP patients than in the control conjunctivochalasis samples (figure 1B).
Figure 1.
The expression of PTGER4 mRNA in conjunctival tissues from patients with Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN), ocular cicatricial pemphigoid (OCP) and the controls. (A) Representative findings of EP4 immunoreactivity in each group (control, SJS/TEN, OCP). (B) Expression of PTGER4 mRNA in human conjunctival tissues (*p<0.05).
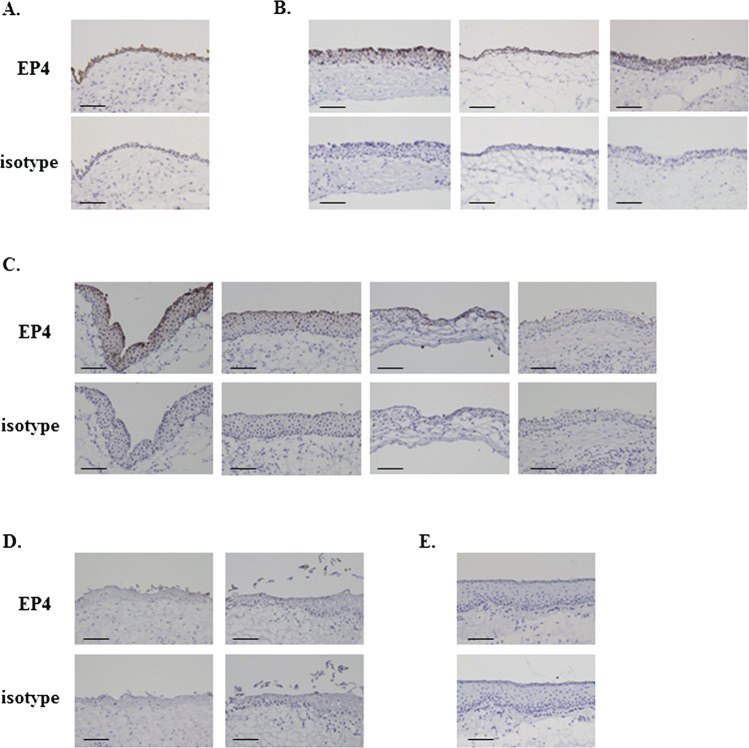
Moreover, we examined the expression of EP4 protein in the conjunctival epithelium of patients with other various ocular surface disorders. EP4 protein was detected in nearly normal conjunctival epithelium from patients with conjunctivochalasis (figure 2A) and in conjunctival tissues from three patients with chemical eye burn (figure 2B). Its expression varied in conjunctival epithelium from four patients with Mooren's ulcer (figure 2C): in one patient is was similar to the control, in two it was slightly lower than in the control and in the remaining patient it was not detected. There was no EP4 immunoreactivity in conjunctival epithelium from two patients with subacute SJS/TEN (figure 2D), a patient with severe GVHD (figure 2E) as same as patients with chronic SJS/TEN or OCP.8
Figure 2.
Immunohistological analysis of prostaglandin E receptor subtype EP4 in conjunctival epithelium of patients with ocular surface diseases. (A) Nearly normal conjunctival tissues from patients with conjunctivochalasis. (B) Conjunctival tissues from patients with chemical eye burn requiring ocular surface reconstruction. (C) Inflammatory conjunctival tissues from patients with active Mooren's ulcer requiring resection of the inflammatory conjunctiva. (D) Conjunctival tissues from Stevens-Johnson syndrome/toxic epidermal necrolysis patients in the subacute stage. (E) Conjunctival tissues from a patient with severe graft versus host disease. Each scale bar represents 100 μm.
We found that, as in normal human conjunctival epithelium, EP4 is expressed in conjunctival epithelium from patients with chemical eye burn. On the other hand, EP4 immunoreactivity was not detected in conjunctival epithelium from patients with SJS/TEN, OCP or severe GVHD. We did not detect EP4 protein in cells infiltrating subconjunctival tissues in any of the human conjunctival tissues we examined.
Discussion
Elsewhere we reported the expression of EP4 in normal human conjunctival epithelium and its down-regulation in conjunctival epithelium from patients with SJS/TEN and OCP.8 Here we confirmed that in conjunctival tissues from SJS/TEN and OCP patients its mRNA expression was significantly down-regulated, and we also document that EP4 is expressed normally in conjunctival epithelium from patients with severe chemical eye burn which, like SJS/TEN and OCP, is a devastating ocular surface disorder.
On the ocular surface of patients with severe chemical eye burn, conjunctival invasion into the cornea may occur due to the stem cell deficiency of corneal epithelial cells. This results in devastating ocular surface disorders similar to OCP and SJS/TEN. However, in the conjunctiva of patients with severe chemical eye burns, EP4 expression was not down-regulated.
In patients with Mooren's ulcer, an ocular surface inflammatory disease, the expression of EP4 protein varied; in some patients it was down-regulated. In patients in the subacute stage of SJS/TEN with ocular surface inflammation, the expression of EP4 protein was remarkably down-regulated.
Our results suggest that it is possible that EP4 in conjunctival epithelium might contribute the ocular surface homeostasis, while the EP4 may not necessarily be down-regulated in all devastating ocular surface disorders.
Kabashima et al7 reported that in mice, EP4 deficiency impaired mucosal barrier function and induced the aggregation of lymphocytes and neutrophils in the colon, and that the administration of an EP4-selective agonist to wild-type mice ameliorated severe colitis. In mice treated with an EP4-selective antagonist the recovery from colitis was suppressed, leading them to conclude that EP4 maintains intestinal homeostasis by preserving mucosal integrity and down-regulating the immune response. On the other hand, Yao et al9 found that PGE2 acting on its receptor EP4 on T cells and dendritic cells not only facilitated T helper 1 (TH1) cell differentiation but also amplified interleukin-23-mediated TH17-cell expansion in vitro. The administration of an EP4-selective antagonist to mice with experimental autoimmune encephalomyelitis or contact hypersensitivity decreased the accumulation of both TH1 and TH17 cells in regional lymph nodes and suppressed disease progression. Based on these observations they concluded that PGE2-EP4 signalling promotes immune inflammation.
In human conjunctival tissues EP4 protein was expressed in epithelial cells but not in cells infiltrating subconjunctival tissues. We posit that the down-regulation of EP4 in conjunctival epithelium is associated with the ocular surface inflammation seen in patients with OCP, SJS/TEN and Mooren's ulcer.
On the other hand, elsewhere we reported that although EP3 and EP2 agonists suppressed the production of CCL5, CXCL11 and CCL20 in response to polyI:C stimulation, these chemokines were not suppressed by the EP4 agonist in human conjunctival epithelial cells.5 Studies are underway in our laboratory to elucidate the function of EP4 in conjunctival epithelial cells.
In summary, EP4 is expressed not only in normal conjunctival epithelium but also in conjunctival epithelium from patients with chemical eye burns and some patients with Mooren's ulcer. On the other hand, it is strongly down-regulated in conjunctival epithelium from patients with OCP and chronic SJS/TEN and subacute SJS/TEN.
Supplementary Material
Acknowledgments
The authors thank Chikako Endo for technical assistance. This work was supported in part by grants-in-aid for scientific research from the Japanese Ministry of Health, Labour and Welfare, the Japanese Ministry of Education, Culture, Sports, Science and Technology, CREST from JST, a research grant from the Kyoto Foundation for the Promotion of Medical Science, the Intramural Research Fund of Kyoto Prefectural University of Medicine and an Immunological Research Grant from the Shimizu Foundation.
Footnotes
Contributors: All the authors substantially contributed to the conception and design, acquisition of data, analysis and interpretation of data, drafting the article or revising it critically for important intellectual content and final approval of the version to be published.
Competing interests: None.
Ethics approval: Ethics—Human Subjects.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no additional data available.
References
- 1.Matsuoka T, Narumiya S. Prostaglandin receptor signaling in disease. ScientificWorldJournal 2007;7:1329–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takayama K, Garcia-Cardena G, Sukhova GK, et al. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J Biol Chem 2002;277:44147–54 [DOI] [PubMed] [Google Scholar]
- 3.Xu XJ, Reichner JS, Mastrofrancesco B, et al. Prostaglandin E2 suppresses lipopolysaccharide-stimulated IFN-beta production. J Immunol 2008;180:2125–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiraishi H, Yoshida H, Saeki K, et al. Prostaglandin E2 is a major soluble factor produced by stromal cells for preventing inflammatory cytokine production from dendritic cells. Int Immunol 2008;20:1219–29 [DOI] [PubMed] [Google Scholar]
- 5.Ueta M, Matsuoka T, Yokoi N, et al. Prostaglandin E2 suppresses polyinosine-polycytidylic acid (polyI:C)-stimulated cytokine production via prostaglandin E2 receptor (EP) 2 and 3 in human conjunctival epithelial cells. Br J Ophthalmol 2011;95:859–63 [DOI] [PubMed] [Google Scholar]
- 6.Morimoto K, Sugimoto Y, Katsuyama M, et al. Cellular localization of mRNAs for prostaglandin E receptor subtypes in mouse gastrointestinal tract. Am J Physiol 1997;272:G681–7 [DOI] [PubMed] [Google Scholar]
- 7.Kabashima K, Saji T, Murata T, et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J Clin Invest 2002;109:883–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueta M, Sotozono C, Yokoi N, et al. Prostaglandin E receptor 4 expression in human conjunctival epithelium and its downregulation in devastating ocular surface inflammatory disorders. Arch Ophthalmol 2010;128:1369–71 [DOI] [PubMed] [Google Scholar]
- 9.Yao C, Sakata D, Esaki Y, et al. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med 2009;15:633–40 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.