Abstract
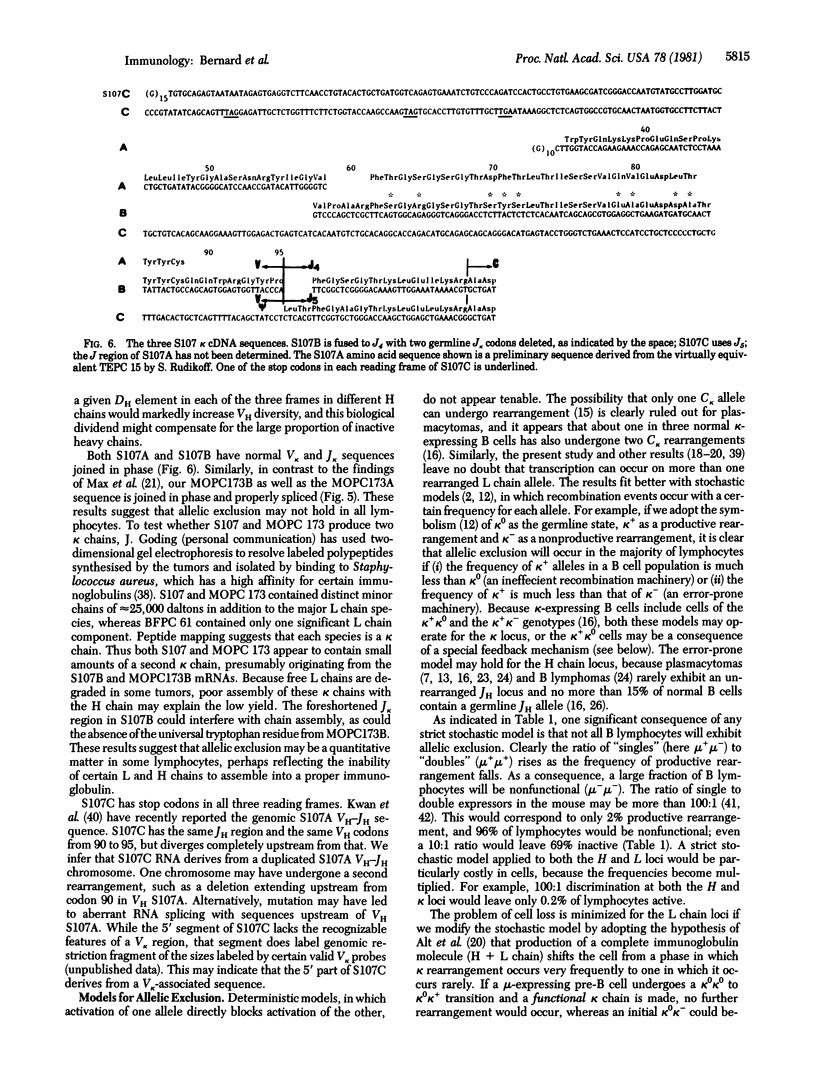
Although only one allele of an immunoglobulin gene is thought to be expressed as a polypeptide by a given lymphocyte ("allelic exclusion"), three murine plasmacytomas were found to contain more than one kappa light chain mRNA species, as evidenced by the sequences of distinct kappa cDNA clones. Two different kappa cDNA sequences were cloned from BFPC 61 microsomal mRNA, two from MOPC 173, and three from S107. One cDNA sequence from each tumor matches the known secreted polypeptide, while the variant sequences differ in the variable (V) region. Hence fusion of a V kappa gene to a joining (J kappa) gene has occurred independently on separate homologous chromosomes and each allele is transcriptionally competent. The BFPC 61 variant sequence contained a normal V kappa sequence linked out of phase to J kappa 2; hence allelic exclusion in this line is accounted for by an error in DNA rearrangement. One S107 variant cDNA has an untranslatable sequence linked to J kappa--C kappa and may derive from a non-V kappa or pseudo-V kappa gene fused to J kappa. Another S107 variant cDNA, however, has a proper V kappa linked in phase to J kappa (albeit missing the first two germ-line J kappa codons) and the MOPC 173 variant sequence also contains a proper V kappa--J kappa join, although it does not encode a tryptophan residue common to all immunoglobulin chains. The presence of two potentially expressible kappa mRNAs in both S107 and MOPC 173 suggests that allelic exclusion does not hold in all lymphocytes, or that it sometimes reflects events subsequent to mRNA production, such as inability of certain kappa chains to assemble properly with the heavy chain. These observations are compatible with a stochastic model for allelic exclusion in which productive and nonproductive V--J recombination events occur at a certain frequency for each allele.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Enea V., Bothwell A. L., Baltimore D. Activity of multiple light chain genes in murine myeloma cells producing a single, functional light chain. Cell. 1980 Aug;21(1):1–12. doi: 10.1016/0092-8674(80)90109-9. [DOI] [PubMed] [Google Scholar]
- Altenburger W., Steinmetz M., Zachau H. G. Functional and non-functional joining in immunoglobulin light chain genes of a mouse myeloma. Nature. 1980 Oct 16;287(5783):603–607. doi: 10.1038/287603a0. [DOI] [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard O., Gough N. M. Nucleotide sequence of immunoglobulin heavy chain joining segments between translocated VH and mu constant regions genes. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3630–3634. doi: 10.1073/pnas.77.6.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Schwartz R. C., Sonenshein G. E., Gefter M. L., Baltimore D. Dual expression of lambda genes in the MOPC-315 plasmacytoma. Nature. 1981 Mar 5;290(5801):65–67. doi: 10.1038/290065a0. [DOI] [PubMed] [Google Scholar]
- Brack C., Hirama M., Lenhard-Schuller R., Tonegawa S. A complete immunoglobulin gene is created by somatic recombination. Cell. 1978 Sep;15(1):1–14. doi: 10.1016/0092-8674(78)90078-8. [DOI] [PubMed] [Google Scholar]
- Coleclough C., Perry R. P., Karjalainen K., Weigert M. Aberrant rearrangements contribute significantly to the allelic exclusion of immunoglobulin gene expression. Nature. 1981 Apr 2;290(5805):372–378. doi: 10.1038/290372a0. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J. M. Deletions are associated with somatic rearrangement of immunoglobulin heavy chain genes. Cell. 1980 Jan;19(1):37–51. doi: 10.1016/0092-8674(80)90386-4. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J. M., Kemp D. J. Somatic rearrangements forming active immunoglobulin mu genes in B and T lymphoid cell lines. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4943–4947. doi: 10.1073/pnas.77.8.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Webb E., Gough J., Adams J. M. Recombination events near the immunoglobulin Cmu gene join variable and constant region genes, switch heavy-chain expression, or inactivate the locus. Biochemistry. 1981 Apr 28;20(9):2662–2671. doi: 10.1021/bi00512a047. [DOI] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Bernard O. Sequences of the joining region genes for immunoglobulin heavy chains and their role in generation of antibody diversity. Proc Natl Acad Sci U S A. 1981 Jan;78(1):509–513. doi: 10.1073/pnas.78.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough N. M., Webb E. A., Cory S., Adams J. M. Molecular cloning of seven mouse immunoglobulin kappa chain messenger ribonucleic acids. Biochemistry. 1980 Jun 10;19(12):2702–2710. doi: 10.1021/bi00553a026. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Korsmeyer S. J., Waldmann T. A., Leder P. Human immunoglobulin kappa light-chain genes are deleted or rearranged in lambda-producing B cells. Nature. 1981 Apr 2;290(5805):368–372. doi: 10.1038/290368a0. [DOI] [PubMed] [Google Scholar]
- Hurwitz J. L., Coleclough C., Cebra J. J. CH gene rearrangements in IgM-bearing B cells and in the normal splenic DNA component of hybridomas making different isotypes of antibody. Cell. 1980 Nov;22(2 Pt 2):349–359. doi: 10.1016/0092-8674(80)90345-1. [DOI] [PubMed] [Google Scholar]
- Joho R., Weissman I. L. V-J joining of immunoglobulin kappa genes only occurs on one homologous chromosome. Nature. 1980 Mar 13;284(5752):179–181. doi: 10.1038/284179a0. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kurosawa Y., von Boehmer H., Haas W., Sakano H., Trauneker A., Tonegawa S. Identification of D segments of immunoglobulin heavy-chain genes and their rearrangement in T lymphocytes. Nature. 1981 Apr 16;290(5807):565–570. doi: 10.1038/290565a0. [DOI] [PubMed] [Google Scholar]
- Kwan S. P., Rudikoff S., Seidman J. G., Leder P., Scharff M. D. Nucleic acid and protein sequences of phosphocholine-binding light chains. J Exp Med. 1981 May 1;153(5):1366–1370. doi: 10.1084/jem.153.5.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. doi: 10.1073/pnas.76.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Miller H., Leder P. Variation in the crossover point of kappa immunoglobulin gene V-J recombination: evidence from a cryptic gene. Cell. 1980 Oct;21(3):793–799. doi: 10.1016/0092-8674(80)90442-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKean D. J., Bell M., Potter M. Mechanisms of antibody diversity: multiple genes encode structurally related mouse kappa variable regions. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3913–3917. doi: 10.1073/pnas.75.8.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse H. C., 3rd, Pumphrey J. G., Potter M., Asofsky R. Murine plasma cells secreting more than one class of immunoglobulin heavy chain. I. Frequency of two or more M-components in ascitic fluids from 788 primary plasmacytomas. J Immunol. 1976 Aug;117(2):541–547. [PubMed] [Google Scholar]
- Nottenburg C., Weissman I. L. Cmu gene rearrangement of mouse immunoglobulin genes in normal B cells occurs on both the expressed and nonexpressed chromosomes. Proc Natl Acad Sci U S A. 1981 Jan;78(1):484–488. doi: 10.1073/pnas.78.1.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernis B., Chiappino G., Kelus A. S., Gell P. G. Cellular localization of immunoglobulins with different allotypic specificities in rabbit lymphoid tissues. J Exp Med. 1965 Nov 1;122(5):853–876. doi: 10.1084/jem.122.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Coleclough C., Kearney J. F. Organization and expression of immunoglobulin genes in fetal liver hybridomas. Proc Natl Acad Sci U S A. 1981 Jan;78(1):247–251. doi: 10.1073/pnas.78.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Coleclough C., Seidman J. G., Leder P., Tonegawa S., Matthyssens G., Weigert M. Transcription of mouse kappa chain genes: implications for allelic exclusion. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1937–1941. doi: 10.1073/pnas.77.4.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schiff C., Fougereau M. Determination of the primary structure of a mouse IgG2a immunoglobulin. Amino-acid sequence of the light chain. Eur J Biochem. 1975 Nov 15;59(2):525–537. doi: 10.1111/j.1432-1033.1975.tb02479.x. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Leder P. A mutant immunoglobulin light chain is formed by aberrant DNA- and RNA-splicing events. Nature. 1980 Aug 21;286(5775):779–783. doi: 10.1038/286779a0. [DOI] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. A rapid enzymatic DNA sequencing technique: determination of sequence alterations in early simian virus 40 temperature sensitive and deletion mutants. Nucleic Acids Res. 1980 May 24;8(10):2225–2240. doi: 10.1093/nar/8.10.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E., Krolick K. Allelic exclusion of IgD allotypes on murine B cells. J Immunol. 1980 Jun;124(6):2988–2990. [PubMed] [Google Scholar]
- Walfield A., Selsing E., Arp B., Storb U. Misalignment of V and J gene segments resulting in a nonfunctional immunoglobulin gene. Nucleic Acids Res. 1981 Mar 11;9(5):1101–1109. doi: 10.1093/nar/9.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert M., Gatmaitan L., Loh E., Schilling J., Hood L. Rearrangement of genetic information may produce immunoglobulin diversity. Nature. 1978 Dec 21;276(5690):785–790. doi: 10.1038/276785a0. [DOI] [PubMed] [Google Scholar]
- Wilson R., Miller J., Storb U. Rearrangement of immunoglobulin genes. Biochemistry. 1979 Oct 30;18(22):5013–5021. doi: 10.1021/bi00589a032. [DOI] [PubMed] [Google Scholar]