Abstract
Objectives
Childhood adversities may be important determinants of later illnesses and poor health behaviour. However, large-scale prospective studies on the associations between childhood adversities and the onset of asthma in adulthood are lacking.
Design
Prospective cohort study with 7-year follow-up.
Setting
Nationally representative study. Data were collected from the Health and Social Support (HeSSup) survey and national registers.
Participants
The participants represent the Finnish population from the following age groups: 20–24, 30–34, 40–44, and 50–54 years at baseline in 1998 (24 057 survey participants formed the final cohort of this study). The occurrence of childhood adversities was assessed at baseline with a six-item survey scale. The analyses were adjusted for sociodemographic characteristics, behavioural health risks and common mental disorders.
Primary and secondary outcomes
The survey data were linked to data from national health registers on incident asthma during a 7-year follow-up to define new-onset asthma cases with verified diagnoses.
Results
A total of 12 126 (59%) participants reported that they encountered a childhood adversity. Of them 3677 (18% of all) endured three to six adversities. During a follow-up of 7 years, 593 (2.9%) participants were diagnosed with incident asthma. Those who reported three or more childhood adversities had a 1.6-fold (95% CI 1.31 to 2.01) greater risk of asthma compared to those without childhood adversities. This hazard attenuated but remained statistically significant after adjustment for conventional risk factors (HR 1.33; 95% CI 1.06 to 1.67).
Conclusions
Adults who report having encountered adversities in childhood may have an increased risk of developing asthma.
Keywords: Psychiatry, Epidemiology
Article summary.
Article focus
This study investigated, whether self-reported childhood adversities measured at adulthood are associated with the risk of incident asthma.
And whether this risk was attributable to common mental disorders or behavioural health risk factors in a nationally representative sample with a longitudinal setting.
Key messages
Self-reported childhood adversities were significantly associated to onset of asthma in adulthood. Psychiatric morbidity and other risk factors attenuated the association by 47%.
Strengths and limitations of this study
The study was based on a nationally representative sample in a longitudinal setting. National registers with good data coverage and reliability were used to assess asthma diagnoses and psychiatric morbidity. The limitations of study are that assessment of adversities was based on self-reports, which, additionally, did not include items on abuse.
Introduction
It is increasingly recognised that childhood adversities may contribute to risk of various types of adult morbidity as well as poor health behaviour.1–11 Most of the studies in this field of research have focused on exposures such as childhood sexual or physical abuse, or severe neglect. Several,2–5 12 13 but not all7 studies have found support for a dose–response relationship: the greater the number and severity of childhood adversities, the more unfavourable health outcomes. In the National Comorbidity Survey, childhood abuse was associated with increased physical health problems but controlling for the presence of subject's psychiatric illness substantially decreased the strength of this association.7
Many previous studies have found an association of anxiety disorders and also major depression with asthma,14–21 and, additionally, with allergies and atopia.22–26 Furthermore, this association seems to be generalisable across different cultural settings.17 Mental disorders may influence the experience of asthma, quality of life related to asthma and adherence to treatment, hospitalisation rates and outcome in asthma.19 27 28 To our knowledge, only two previous longitudinal studies exist on the relation of mental disorders and asthma, supporting especially panic disorder as a risk factor for later the onset of asthma.20 21 26
The association between mental disorders and asthma could possibly be explained by shared risk factors, such as childhood adversity. Anxiety and depressive disorders are associated with childhood adversities.29 30 There is some evidence linking asthma to childhood adversities. The studies have either been cross-sectional31–37 or used childhood social adversity as exposure in a longitudinal setting.36 38 The outcome has been measured using self-reports of physician-diagnosed asthma36 or symptoms or use of services in adolescence as outcome measure.20 One prospective study on early-life financial adversity and respiratory function in middle age used objective measurement of lung function.38 However, we are aware of no previous large-scale studies with a prospective design that would have used register-based data of incident asthma in adulthood and taken into account factors underlying the association between childhood adversities and the onset of asthma, such as common mental disorders and health risk behaviours. It is so far uncertain, whether the association between preceding mental disorder and subsequent asthma could be accounted for by mental disorders, and whether childhood adversities could have an independent influence on incident asthma.
In this study, we aimed to investigate whether self-reported childhood adversities measured at adulthood are associated with the risk of incident asthma and whether this risk was attributable to mental disorders or behavioural health risk factors at baseline in a large prospective population sample.
Methods
Population
Data were collected from the Health and Social Support (HeSSup) study, a longitudinal study on a population sample representative of the Finnish population in the following four age groups: 20–24, 30–34, 40–44 and 50–54 years at baseline in 1998. The initial response rate of the postal survey was 40%. According to the non-response analysis, no significant selective health-related factors could be identified.39 Of all 25 901 respondents, 24 057 (93%) gave their written consent for health register linkages. Out of that pool, we excluded all participants with asthma (n=3446) and deceased (n=4) before the beginning of the follow-up. Thus, the final sample consisted of 20607 participants (8622 males and 11 985 females). They were followed for a 7-year period from national health registers in order to detect the onset of new asthma cases (1 January, 1999–31 December 2005). The Turku University Hospital Ethics Committee considered that a statement of approval was not required. The subjects originated from a random population sample. While agreeing to participate in the study, the subjects filled up an informed consent form.
Measures
Childhood adversities
The occurrence of childhood adversities was assessed at baseline with a six-item survey scale with questions on long-term financial difficulties, divorce or separation of the parents, serious conflicts in the family, severe illness of a family member, frequent fear of a family member and alcohol problem of a family member.40 The subjects were asked to respond either ‘yes’, ‘no’ or ‘cannot say’ to each item. The items were analysed as a summary variable with three values (0, 1–2 or 3–6 adversities).
Case definition for asthma
Using the unified personal identification code system, which covers all Finnish citizens, we linked the survey responses to records from three independent and comprehensive Finnish national health registers in order to identify incident cases of asthma. The identification of cases was based on the clinical diagnosis from the Drug Reimbursement Register or the Hospital Discharge Register or detailed information about purchases of the prescribed medication for asthma from the Drug Prescription Register. A participant was classified as having incident asthma when the event was verified for the first time from any of the three data sources between 1 January 1999 and 31 December 2005.
First, we used the Drug Reimbursement Register of the Social Insurance Institution of Finland, which contains information on persons entitled to special reimbursement for certain chronic diseases, such as asthma. Patients who apply for special reimbursement must attach a detailed medical certificate prepared by the treating physician, who also provides data to confirm the diagnosis. The application is then reviewed by a physician in the Social Insurance Institution to determine whether the uniformly defined requirements for the disease are met. From this register, participants were defined as incident asthma cases if they were recorded in the Central Drug Register as eligible for asthma treatment for the first time during the follow-up.
Second, we used prescription data to assess the beginning of medical treatment for asthma. In Finland, the National Social Insurance Scheme at the Social Insurance Institution provides basic reimbursement (currently 42%) for all filled outpatient prescriptions that are recorded in the Drug Prescription Register according to the WHO's Anatomical Therapeutic Chemical (ATC) Classification41 and by date of purchase. We identified all participants with two or more prescriptions for drugs for obstructive airway diseases (ATC code R03) in any year during the follow-up by using the day of the first purchase as an indicator of the onset of asthma.
Third, we obtained data from the Hospital Discharge Register of National Institute for Health and Welfare, which includes records on all inpatient hospital admissions. This register is comprised of countrywide information on virtually all hospitalisations. We obtained the discharge dates and the corresponding main diagnoses for hospitalisation due to asthma (ICD-10 J45). All individuals who were identified as having asthma in any of these registers at baseline in 1998 were excluded from the analysis, as well as all those who reported a lifetime diagnosis of asthma in the baseline survey.
Background variables
All background variables were measured at baseline in 1998. Gender, age group, level of education (basic/vocational/college/university) and marital status (single, divorced or widowed vs married or cohabiting) were included in the analysis as sociodemographic variables.
We assessed four behavioural health risks using standard questionnaire measurements. Smoking status was measured with a variable describing current regular smoking (current/never/ex-smoker). High alcohol intake was present with a weekly self-reported consumption of beer, wine, and spirits exceeding ≥175 g of alcohol for women and ≥263 g of alcohol for men.42 Survey reports on height and weight were used to calculate the average body mass index (BMI) to identify underweight (BMI<20 kg/m2), normal-weight (BMI 20−<25 kg/m2), overweight (25−<30 kg/m2), and obese (BMI ≥30 kg/m2) participants. Physical activity was calculated by the Metabolic Equivalent Task index to measure sedentary life style (<2 MET-hours/day).43
Psychological factors were measured from the questionnaire responses. To measure general feelings of stressfulness in daily life we used the Reeder stress inventory, a four-item questionnaire instrument with a five-point Likert format.44 It consist of four statements: (1) ‘In general, I am usually tense or nervous’; (2) ‘There is a great amount of nervous strain connected with my daily activities’; (3) ‘At the end of the day, I am completely exhausted mentally and physically’ and (4) ‘My daily activities are extremely trying and stressful’. The mean score of the four statements was divided into quartiles (low/medium low/medium high/high general feelings of stressfulness).
Symptoms of sympathetic nervous system (SNS) hyperactivity were measured using an eight-item scale.45 This measure requests the occurrence of the following eight symptoms within the past month: (1) palpitation without exercise; (2) irregular heartbeat; (3) chest pain upon anger or emotion; (4) sweating without exercise; (5) flushing; (6) tremor of hands; (7) tremor of voice and (8) muscle twitching. The following four alternatives were given for each item: daily or almost daily, weekly, less often and never. The mean score of the eight statements was divided into quartiles (low/medium low/medium high/high symptoms of SNS hyperactivity).
Common mental disorders were measured from the questionnaire responses and the medication records. Symptoms of depression were measured with the Beck Depression Inventory (BDI). Depression (no/yes) was indicated by a BDI sum score of more than 18.46 Using data from the National Drug Reimbursement Register we assessed antidepressant or antianxiety purchases in 1998 to identify individuals with more severe depressive and anxiety symptoms (no/yes). To identify participants with clinically significant depression we collected data on hospitalisations from the Hospital Discharge Register. We obtained the discharge dates before 1 January 1999 (no/yes) for the main diagnoses determined by ICD-10 codes F00–F99.
Other possible risk factors for the development of asthma that were considered in this study included exposure to pets, parents’ smoking and allergy. Information about a pet was obtained by asking whether the participant has a pet (having a pet at home vs not having one). The participants reported whether their parents had smoked at home during the school-age (no/yes). Allergy (no/yes) was determined using prescriptions for drugs for allergy diseases (ATC-codes R01AC, R03BC).
Statistical analysis
All analyses were performed by using SAS release 9.2/2008. Descriptive statistics included the associations between the various background variables (demographics, psychological factors, asthma risk factors and health-related factors) and childhood adversities; differences were studied by using logistic regression.
The Cox proportional hazard models were used to analyse the association between the baseline childhood adversities and the onset of asthma. The time-dependent interaction terms between any childhood adversity and the logarithm of the follow-up period were all non-significant confirming that the proportional hazard assumptions were justified. Follow-up began on 1 January 1999, and ended upon the first occurrence of the outcome measure or censoring event death (the date of death was obtained from the Statistics Finland register) or the end of follow-up (31 December 2005), whichever came first. We calculated HRs and their 95% CI. The models were adjusted for background variables; first the various background variables separately and finally all background variables in the same model. The gender difference in the association of childhood adversities with the onset of asthma was assessed by entering the interaction term sex × childhood adversities into the model. Because no interaction was found (p=0.12), we analysed men and women together.
Results
The sample included 8556 (41.7%) men and 11 946 (58.3%) women. Altogether, 12 126 (59%) participants in our study reported that they encountered a childhood adversity. Of these participants, 8449 (41% of all participants) reported 1–2 adversities and 3677 (18% of all participants) 3–6 adversities. Table 1 shows the characteristics of the participants and the associations between childhood adversities by the baseline characteristics. Except for marital status, physical activity and allergy, the characteristics of the participants were associated with childhood adversities. In this cohort, women, older subjects, those with basic level education, who smoked, had higher alcohol intake and BMI, who reported symptoms of SNS hyperactivity, had a higher score on stressfulness measure and any indication of psychiatric morbidity reported an increased number of childhood adversities (p<0.001).
Table 1.
Baseline characteristics by childhood adversities
| Childhood adversities |
|||||
|---|---|---|---|---|---|
| 0 | 1–2 | >2 | |||
| (n=8376) | (n=8449) | (n=3677) | |||
| Characteristic | Number | Per cent | Per cent | Per cent | Significance |
| Gender | <0.001 | ||||
| Men | 8556 | 45 | 42 | 35 | |
| Women | 11 946 | 55 | 58 | 65 | |
| Age group (years) | <0.001 | ||||
| 20–24 | 5771 | 32 | 26 | 24 | |
| 30–34 | 4866 | 24 | 22 | 27 | |
| 40–44 | 4832 | 22 | 24 | 26 | |
| 50–54 | 5033 | 22 | 28 | 23 | |
| Marital status | <0.001 | ||||
| Single/divorced/widowed | 6781 | 34 | 32 | 34 | |
| Married/cohabiting | 13 707 | 66 | 68 | 66 | |
| Occupational education | <0.001 | ||||
| Basic | 6433 | 30 | 32 | 35 | |
| Vocational school | 4666 | 22 | 24 | 24 | |
| College | 6417 | 32 | 31 | 31 | |
| University | 2781 | 16 | 13 | 10 | |
| Smoking | <0.001 | ||||
| Never | 8667 | 53 | 44 | 35 | |
| Ex-smoker | 5214 | 25 | 29 | 33 | |
| Current | 4938 | 22 | 27 | 32 | |
| Physical activity | 0.667 | ||||
| No | 4352 | 22 | 22 | 21 | |
| Yes | 15 718 | 78 | 78 | 79 | |
| High alcohol intake (>16 drinks/week) | <0.001 | ||||
| No | 19 294 | 95 | 95 | 92 | |
| Yes | 1179 | 5 | 5 | 8 | |
| BMI (kg/m2) | <0.001 | ||||
| <20 | 2055 | 11 | 9 | 10 | |
| 20–25 | 10 606 | 53 | 51 | 52 | |
| 25–30 | 5906 | 28 | 30 | 28 | |
| >30 | 1823 | 8 | 10 | 10 | |
| Pet | <0.001 | ||||
| No | 11 979 | 60 | 58 | 55 | |
| Yes | 8523 | 40 | 42 | 45 | |
| Parents’ smoking | <0.001 | ||||
| No | 9508 | 57 | 44 | 27 | |
| Yes | 10 994 | 43 | 56 | 73 | |
| Prescribed antiallergy drugs | 0.9669 | ||||
| No | 18 958 | 93 | 92 | 92 | |
| Yes | 1544 | 7 | 9 | 8 | |
| Symptoms of SNS hyperactivity (quartile) | <0.001 | ||||
| Lowest | 4035 | 23 | 18 | 16 | |
| Second | 5288 | 28 | 26 | 22 | |
| Third | 6302 | 30 | 31 | 31 | |
| Highest | 4823 | 19 | 25 | 31 | |
| General feeling of stressfulness (quartile) Reeder | <0.001 | ||||
| Low | 5747 | 32 | 27 | 23 | |
| Medium low | 6333 | 33 | 31 | 29 | |
| Medium high | 4010 | 19 | 20 | 20 | |
| High | 4204 | 16 | 22 | 28 | |
| Depression (BDI) | <0.001 | ||||
| No | 19 534 | 98 | 95 | 92 | |
| Yes | 837 | 2 | 5 | 8 | |
| Psychotropic medication | <0.001 | ||||
| No | 19 421 | 96 | 95 | 92 | |
| Yes | 1081 | 4 | 5 | 8 | |
| Hospitalisation due to psychiatric disorder | |||||
| No | 20 382 | 99.7 | 99.3 | 99 | <0.001 |
| Yes | 120 | 0.3 | 0.7 | 1 | |
BDI, Beck Depression Inventory; BMI, body mass index; SNS, sympathetic nervous system.
During a follow-up of 7 years, 593 participants were diagnosed with incident asthma. As shown in figure 1, the risk of adult onset asthma increased with increasing number of childhood adversities. The difference between the groups of no adversities, 1–2 adversities and 3–6 adversities became evident after 3 years of follow-up and widened during the course of time.
Figure 1.
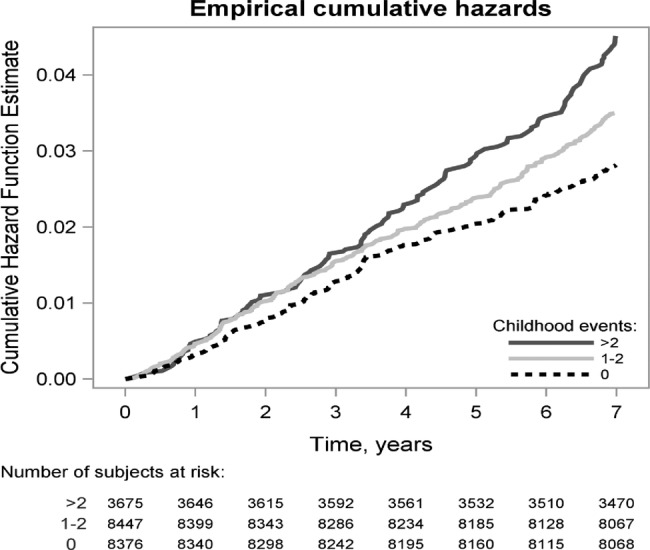
Cumulative hazard function curves according to the Cox model with adult onset asthma during the course of the follow-up (n=20 502).
Exposure to 1–2 childhood adversities associated with 1.20-fold (95% CI 1.00 to 1.45) greater risk of asthma compared to participants who did not report any childhood adversities. However, after adjustments for the background variables this association became nonsignificant (table 2). Exposure to 3–6 childhood adversities associated with 1.6-fold (95% CI 1.31 to 2.01) greater risk of asthma. This excess hazard was attenuated by 19.4% to 1.50 (95% CI 1.21 to 1.86), when controlling for demographic information. Adjustment for psychiatric disorders attenuated the excess hazard by 22.6% to 1.48 (95% CI 1.19 to 1.84), for asthma risk factors by 8% to 1.57 (95% CI 1.26 to 1.95) and for health behaviours by 11.3% to 1.55 (95% CI 1.25 to 1.92). When all the different factors were concurrently adjusted for, the excess hazard was attenuated by 47% to 1.33 (95% CI 1.06 to 1.67) (table 2). To test the linear trend in the association between childhood adversities and incident asthma, we treated childhood adversities as a count variable; in the unadjusted model the HR was 1.12 (95% CI 1.07 to 1.19, p<0.001) and in the fully adjusted model 1.06 (95% CI 1.01 to 1.13, p=0.032).
Table 2.
Childhood adversities as the predictors of incident asthma
| Childhood adversities |
|||||
|---|---|---|---|---|---|
| Number of subjects in the analysis | Number of subjects with the event | 0 | 1–2 Childhood adversities | >2 | |
| Crude | 17 894 | 593 | 1 | 1.20 (1.0–1.45) | 1.62 (1.31–2.01) |
| Adjusted for demographics* | 17 894 | 593 | 1 | 1.16 (0.96–1.40) | 1.50 (1.21–1.86) |
| Adjusted for psychiatric disorders† | 17 894 | 593 | 1 | 1.16 (0.96–1.40) | 1.48 (1.19–1.84) |
| Adjusted for asthma risk factors‡ | 17 894 | 593 | 1 | 1.19 (0.98–1.43) | 1.57 (1.26–1.95) |
| Adjusted for health behaviours§ | 17 894 | 593 | 1 | 1.17 (0.97–1.41) | 1.55 (1.25–1.92) |
| Adjusted for all aforementioned | 17 894 | 593 | 1 | 1.11 (0.92–1.34) | 1.33 (1.06–1.67) |
*Sex, age group, marital status, education.
†Sympathetic nervous system, Reeder, Beck, prescribed antidepressant or antianxiety, National Hospital Discharge Register F-diagnoses.
‡Pet, parents’ smoking, prescribed antiallergy drugs.
§Smoking, body mass index, physical activity, high alcohol intake.
Of the specific types of childhood adversities in the family, economical difficulties, severe conflicts and severe and long term illness were associated with 1.2–1.4 times higher hazard of asthma onset while the corresponding association of divorce, fear of family member and alcohol problem with asthma, although slightly elevated, remained non-significant (table 3).
Table 3.
Associations (HR) between individual types of childhood adversities and adult onset asthma*
| Childhood adversity | HR | 95% CI | Significance |
|---|---|---|---|
| Parents’ divorce | 1.11 | 0.89 to 1.38 | 0.346 |
| Economical difficulties | 1.41 | 1.17 to 1.70 | <0.001 |
| Severe conflicts between parents | 1.20 | 1.00 to 1.45 | 0.05 |
| Fear of family member | 1.21 | 0.96 to 1.51 | 0.104 |
| Severe and chronic illness | 1.29 | 1.05 to 1.51 | 0.013 |
| Alcohol problem | 1.19 | 0.99 to 1.43 | 0.066 |
*Adjusted for age, education, marital status and gender.
Discussion
In this study we found self-reported childhood adversities to be associated with register-verified asthma diagnoses. Our results suggest that about half of this association is mediated by several factors. Psychiatric morbidity attenuated the relative risk most, almost a quarter, and demographic factors by about a fifth, whereas asthma risk factors accounted for a smaller portion of the attenuation of the risk after adjustment. Our analyses suggest that psychiatric disorder, having no relationship, being female, belonging to an older age group, low level of education, having allergy or atopia, health behaviours contribute to the excess risk of adult onset asthma associated with childhood adversity. The fact that circa half of this association was not accounted for by the variables measured in this study may reflect an independent relation between childhood adversities and adult asthma or some other unaccounted factors.
This is to our knowledge the first large-scale prospective, population-based study using various forms of childhood adversity as the exposure and comprehensive measures of asthma as the outcome, while controlling for sociodemographic factors, common risk factors, psychological distress and psychiatric morbidity. Our findings are consistent with previous longitudinal studies that reported an association between adverse childhood events and asthma.20 36 38 However, the EPIC-Norfolk study used incident hospitalisation as outcome, but did not adjust for mental disorders.36 The adversities measured in our study covered equally heterogeneous20 or a more comprehensive36 38 set of childhood adversities and the outcome measures of the present study were derived from reliable national registers comprehensively covering new asthma cases.
The study by Bartley et al38 focused on lung function and found that financial adversity was associated with poor lung function partly through poor housing and partly through pathways involving continuities in social disadvantage and the associated environmental exposures and behaviours. In our cohort roughly a fifth of the association between adversities and asthma was mediated by sociodemographic factors. Additionally, socioeconomic status (SES) has been found to associate with the risk of mental disorders and poor health behaviours.47 48 Hence, socioeconomic factors may operate on the micro level through these risk factors.
Likewise, our study is in line with the 10 cross-sectional population surveys conducted as part of the World Mental Health (WMH) surveys. In that particular study childhood adversities predicted adult-onset asthma with risk increasing with the number of adversities experienced (HR 1.49–1.71). However, the researchers of the WMH surveys also found that early-onset depression and anxiety disorders and childhood adversities both predicted adult-onset asthma after mutual adjustment.37 In our study, psychiatric disorder was only used as a covariate. It was not studied as a risk factor for asthma.
Interestingly, low maternal childhood SES was found to associate with increased cord blood immunoglobulin E (IgE) levels and repeated wheeze through both direct and indirect effects.49 Additionally, maternal cumulative interpersonal trauma was also associated with increased cord blood IgE levels. Psychoneuroimmunological pathway may be one key mechanism between adversity and asthma. Parenting difficulties have, likewise, been linked to childhood asthma and the risk seemed to be highest among those, who also had elevated IgE levels.32 Adults who have exposed to childhood maltreatment have been found to have elevated levels of inflammation biomarkers.10 50 Also anxiety disorders have been linked to inflammation.51 It has been suggested that stress-related elevation in clinically relevant inflammation proteins could contribute to the biological embedding of childhood stress.50
Methodological issues
In this study, the incidence of asthma was 2.9%, providing sufficient statistical power to adequately study the association between childhood adversities and adult-onset asthma. The strengths of our study are, first, reliable register-based information on the outcomes, and second, a large nationwide population sample with a prospective study design. The Finnish hospital discharge and mortality registers provide virtually complete population-wide data on hospital discharge and mortality. In Finland, the validity of the national registers has been found to be high, reasonably accurate and highly reliable for epidemiological study purposes.52 53
The limitations of the present study include a relatively low baseline questionnaire response rate as well as a retrospective assessment of the childhood adversities based on self-reports. The non-response analysis of the cohort was based on two strategies: (1) comparisons made between early and late responders and (2) comparisons made between all responders and routine statistical data of the general population. The first analysis showed no significant differences in self-reports of physician-diagnosed illnesses including depression, panic disorder and eating disorder, and the second that there were no indications of selective physical health-related factors. Additionally, the subjects reporting physician-diagnosed panic disorder, heavy alcohol use and use of tranquillizers gave significantly more commonly than others (94.5% vs 90.9%, respectively) consent to use register-based data.39 We deemed that a significant bias on important health-related was unlikely. However, it is known from other studies that persons with mental disorders are more likely to be among non-responders. As in the cohort of this study, consent to use register data was likely to compensate for mental disorder bias. As the linkage to register data on outcomes was almost complete (93%) we believe selection bias is an unlikely explanation for our findings.
The associations between asthma and mental disorder symptoms and disorders seem to be bidirectional.20 Including psychiatric risk factors during the follow-up into our model could have attenuated further the association between asthma and mental health risk factors, but made it more cumbersome to settle on their temporal relationships. The same goes for behavioural risk factors as well.
There is some evidence to suggest that the childhood abuse reports by different informants such as children as victims, their parents and teachers may differ.54 However, severe abuse is most often well recalled and false-positive reports are rather rare.55 56 Less severe adversities may well be under-reported.56 Furthermore, adjustment for adulthood depression may partly lead to over-control, as prospective cohort studies have shown that childhood adversities may increase the risk of depression, in particular when combined with genetic vulnerability.57 Research on documented adverse childhood experiences indicates that their consequences are not merely artefacts of retrospective recall.58 The reliability of the measure on childhood adversities has been previously assessed and found good, the κ-values of responses between 1998 and 2003 ranging from 0.56 (‘severe illness of a family member’) to 0.90 (‘parents divorce’).59 Further prospective studies starting from childhood are needed to examine this issue in detail. Our findings on the relationships between adversities and incident asthma are not likely to be over-estimates.
Conclusions
In this prospective population-based study of Finnish adults, self-report of childhood adversities was associated with increased incidence of diagnosed asthma. Although further studies are needed to confirm this finding, the results emphasise the importance of early risk factors in the identification and treatment of risk groups for poor health outcomes. If confirmed, these findings suggest it would be of importance to provide resources to face or avoid psychosocial risk factors in the prevention of asthma at population level.
Supplementary Material
Footnotes
Competing interests: JK has received honoraria for lectures from Eli Lilly, Lundbeck, Wyeth, Pfizer (related to the subject matter of depression and psychoses), and has participated three international congresses or symposia with Janssen-Cilag, Lundbeck, and Pfizer. All other authors: none.
Contributors: All authors have contributed substantially in the conception and design, acquisition of data or analysis and interpretation of data, in drafting the article and revising it critically for important intellectual content and final approval of the version published according to the uniform requirements.
Funding: The HeSSup Study has been supported by grants from the Finnish Academy, the Yrjö Jahnsson Foundation and the Heart Research Foundation Foundation, Finland. Markku Koskenvuo, Jussi Vahtera and Mika Kivimäki are supported by the Academy of Finland (grants #117604, #124271, #124322 and #129262).
Ethics approval: The Turku University Hospital Ethics Committee considered that a statement of approval was not required. The subjects originated from a random population sample. While agreeing to participate the study, the subjects filled-in an informed consent form.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Orth-Gomér K. Psychosocial and behavioral aspects of cardiovascular disease prevention in men and women. Curr Opin Psychiatry 2007;20:147–51 [DOI] [PubMed] [Google Scholar]
- 2.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 1998;14:245–58 [DOI] [PubMed] [Google Scholar]
- 3.Edwards VJ, Holden GW, Felitti VJ, et al. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry 2003;160:1453–60 [DOI] [PubMed] [Google Scholar]
- 4.Chapman DP, Whitfield CL, Felitti VJ, et al. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Dis 2004;82:217–25 [DOI] [PubMed] [Google Scholar]
- 5.Arnow BA. Relationships between childhood maltreatment, adult health and psychiatric outcomes, and medical utilization. J Clin Psychiatry 2004;65(Suppl 12):10–15 [PubMed] [Google Scholar]
- 6.Rodgers CS, Lang AJ, Laffaye C, et al. The impact of individual forms of childhood maltreatment on health behavior. Child Abuse Negl 2004;28:575–86 [DOI] [PubMed] [Google Scholar]
- 7.Sachs-Ericsson N, Blazer D, Plant EA, et al. Childhood sexual and physical abuse and the 1-year prevalence of medical problems in the National Comorbidity Survey. Health Psychol Med 2005;24:32–40 [DOI] [PubMed] [Google Scholar]
- 8.Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatry 2007;64:49–56 [DOI] [PubMed] [Google Scholar]
- 9.Chartier MJ, Walker JR, Naimark B. Childhood abuse, adult health and health care utilization: results from a representative community sample. Am J Epidemiol 2007;165:1031–8 [DOI] [PubMed] [Google Scholar]
- 10.Danese A, Moffitt TE, Pariante CM, et al. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry 2008;65:409–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harkonmäki K, Korkeila K, Vahtera J, et al. Childhood adversities as a predictor of disability retirement. J Epidemiol Community Health 2007;61:479–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussey J, Chang JJ, Kotch JB. Child maltreatment in the United States: prevalence, risk factors, and adolescent health consequences. Pediatrics 2006;118:933–42 [DOI] [PubMed] [Google Scholar]
- 13.Springer KW, Sheridan J, Kuo D, et al. Long-term physical and mental health consequences of childhood physical abuse: results from a large population-based sample of men and women. Child Abuse Negl 2007;31:517–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodwin RD, Jacobi F, Thefeld W. Mental disorders and asthma in the community. Arch Gen Psychiatry 2003;60:1125–30 [DOI] [PubMed] [Google Scholar]
- 15.Richardson L, Lozano P, Russo J, et al. Asthma symptom burden: relationship to asthma severity and anxiety and depressive symptoms. Pediatrics 2006;118:1042–51 [DOI] [PubMed] [Google Scholar]
- 16.Katon W, Lin EHB, Kroenke K. The association of depression and anxiety with medical symptom burden with chornic medical illness. Gen Hosp Psychiatry 2007;29:147–55 [DOI] [PubMed] [Google Scholar]
- 17.Scott KM, von Korff M, Ormel J, et al. Mental disorders smong adults with asthma: results from the World Mental Health Surveys. Gen Hosp Psychiatry 2007;29:123–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lev-Tzion R, Friedman T, Shochat T, et al. Asthma and psychiatric disorders in male army recruits and soldiers. IMAJ 2007;9:361–4 [PubMed] [Google Scholar]
- 19.Di Marco F, Santus P, Centanni S. Anxiety and depression in asthma. Curr Opin Pulm Med 2011;17:39–44 [DOI] [PubMed] [Google Scholar]
- 20.Goodwin RD, Fergusson DM, Horwood LJ. Asthma and depressive and anxiety disorders among young persons in the community. Psychol Med 2004;34:1465–74 [DOI] [PubMed] [Google Scholar]
- 21.Hasler G, Gergen PJ, Kleinbaum DG, et al. Asthma and panic in young adults: a 20-year prospective community study. Am J Respir Crit Care Med 2005;171:1224–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stauder A, Kovacs M. Anxiety symptoms in allergic patients: identification and risk factors. Psychosom Med 2003;65:615–23 [DOI] [PubMed] [Google Scholar]
- 23.Infante M, Slattery MJ, Klein MH, et al. Association of internalizing disorders and allergies in a child and adolescent psychiatric clinical sample. J Clin Psychiatry 2007;68:1419–25 [DOI] [PubMed] [Google Scholar]
- 24.Chida Y, Hamer M, Steptoe A. A bidirectional relationship between psychosocial factors and atopic disorders: a systematic review and meta-analysis. Psychosom Med 2008;70:102–16 [DOI] [PubMed] [Google Scholar]
- 25.Lien L, Green K, Thoresen M, et al. Atopic conditions and mental health problems: a 3-year follow-up study. Eur Child Adolesc Psychiatry 2010;19:705–13 [DOI] [PubMed] [Google Scholar]
- 26.Slattery MJ, Essex MJ. Secificty in the association of anxiety, depression, and anxiety disorders in a community sample of adolescents. J Psychiatr 2011;45:788–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ten Thoren C, Peterman F. Reviewing asthma and anxiety. Respir Med 2000;94:409–15 [DOI] [PubMed] [Google Scholar]
- 28.Manusco C, Peterso C, Charlson M. Effects of depressive symptoms in health-related quality of life in asthma patients. J Geriatr Intern Med 2000;94:566–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levitan RD, Rector NA, Sheldon T, et al. Childhood adversities associated with major depression and/or anxiety disorders in a community sample of Ontario: issues of co-morbidity and specificity. Depress Anxiety 2003;17:34–42 [DOI] [PubMed] [Google Scholar]
- 30.Korkeila J, Vahtera J, Nabi H, et al. Childhood adversities, adulthood life events and depression. J Affect Disord 2010;127:130–8 [DOI] [PubMed] [Google Scholar]
- 31.Cassibba R, van IJzendoorn MH, Bruno S, et al. Attachment of mothers and children with recurrent asthmatic bronchitis. J Asthma 2004;41:419–31 [DOI] [PubMed] [Google Scholar]
- 32.Klinnert MD, Nelson HS, Price MR, et al. Onset and persistence of childhood asthma: predictors from infancy. Pediatrics 2001;108:E69. [DOI] [PubMed] [Google Scholar]
- 33.Lau JTF, Liu JLY, Cheung JCK, et al. Prevalence and correlates of physical abuse in Hong Kong Chinese adolescents: a population-based approach. Child Abuse Negl 1999;23:549–57 [DOI] [PubMed] [Google Scholar]
- 34.Romans S, Belaise C, Martin J, et al. Childhood abuse and later medical disorders in women. An epidemiological study. Psychother Psychosom 2002;71:141–50 [DOI] [PubMed] [Google Scholar]
- 35.Wainwright NW, Surtees PG, Wareham NJ, et al. Psychosocial factors and asthma in a community sample of older adults. J Psychosom Res 2007;62:357–61 [DOI] [PubMed] [Google Scholar]
- 36.Wainwright NW, Surtees PG, Wareham NJ, et al. Psychosocial factors and incident asthma hospital admissions in the EPIC-Norfolk cohort study. Allergy 2007;62:554–60 [DOI] [PubMed] [Google Scholar]
- 37.Scott KM, Von Korff M, Alonso J, et al. Childhood adversity, early-onset depressive/anxiety disorders, and adult-onsetasthma. Psychosom Med 2008;70:1035–43 [DOI] [PubMed] [Google Scholar]
- 38.Bartley M, Kelly Y, Sacker A. Early life financial adversity and respiratory function in midlife: a prospective birth cohort study. Am J Epidemiol 2012;175:33–42 [DOI] [PubMed] [Google Scholar]
- 39.Korkeila K, Suominen S, Ahvenainen J, et al. Non-response and related factors in a nation-wide health survey. Eur J Epidemiol 2001;17:991–9 [DOI] [PubMed] [Google Scholar]
- 40.Korkeila K, Kivelä SL, Suominen S, et al. Childhood adversities, parent-child relationships and dispositional optimism in adulthood. Soc Psychiatry Psychiatr Epidemiol 2004;39:286–92 [DOI] [PubMed] [Google Scholar]
- 41.WHO Collaborating Centre for Drug Statistics Methodology, Guidelines for ATC-classification and DDD assignment Oslo, Norway: WHO Collaborating Centre for Drug Statistics Methodology, 2003 [Google Scholar]
- 42.Halme JT, Seppa K, Alho H, et al. Hazardous drinking: prevalence and associations in the Finnish general population. Alcohol Clin Exp Res 2008;32:1615–22 [DOI] [PubMed] [Google Scholar]
- 43.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 1993;25:71–80 [DOI] [PubMed] [Google Scholar]
- 44.Metcalfe C, Smith GD, Wadsworth E, et al. A contemporary validation of the Reeder Stress Inventory. Br J Health Psychol 2003;8:83–94 [DOI] [PubMed] [Google Scholar]
- 45.Vahtera J, Kivimäki M, Hublin C, et al. Liability to anxiety and severe life events as predictors of new-onset sleep disturbances. Sleep 2007;30:1537–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev 1988;8:77–100 [Google Scholar]
- 47.Regier DA, Farmer ME, Rae DS, et al. One-month prevalence of mental disorders in the United States and sociodemographic characteristics: the epidemiologic catchment area study. Acta Psychiatr Scand 1993;88:35–47 [DOI] [PubMed] [Google Scholar]
- 48.Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff (Millwood) 2002;21:60–76 [DOI] [PubMed] [Google Scholar]
- 49.Sternthal MJ, Coull BA, Chiu YH, et al. Associations among maternal childhood socioeconomic status, cord blood IgE levels, and repeated wheeze in urban children. J Allergy Clin Immunol 2011;128:337–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Danese A, Caspi A, Williams B, et al. Biological embedding of stress through inflammation processes in childhood. Mol Psychiatry 2011;16:244–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liukkonen T, Räsänen P, Jokelainen J, et al. The association between anxiety and C-reactive protein (CRP) levels: results from the Northern Finland 1966 birth cohort study. Eur Psychiatry 2011;26:363–9 [DOI] [PubMed] [Google Scholar]
- 52.Gissler M, Haukka J. Finnish health and social welfare registers in epidemiological research. Norsk Epidemiologi 2004;14:113–20 [Google Scholar]
- 53.Rapola JM, Virtamo J, Korhonen P, et al. Validity of diagnoses of major coronary events in national registers of hospital diagnoses and deaths in Finland. Eur J Epidemiol 1997;13:133–8 [DOI] [PubMed] [Google Scholar]
- 54.Sternberg KJ, Lamb ME, Guterman E, et al. Effects of early and later family violence on children's behavior problems and depression: a longitudinal, multi-informant perspective. Child Abuse Negl 2006;30:283–306 [DOI] [PubMed] [Google Scholar]
- 55.Goodman GS, Ghetti S, Quas JA, et al. A prospective study of memory for child sexual abuse: new findings relevant to the repressed-memory controversy. Psychol Sci 2003;14:113–18 [DOI] [PubMed] [Google Scholar]
- 56.Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J Child Psychol Psychiatry 2004;45:260–73 [DOI] [PubMed] [Google Scholar]
- 57.Jokela M, Keltikangas-Järvinen L, Kivimäki M, et al. Serotonin receptor 2A gene and the influence of childhood maternal nurturance on adulthood depressive symptoms. Arch Gen Psychiatry 2007;64:356–60 [DOI] [PubMed] [Google Scholar]
- 58.Horwitz AV, Widom CS, McLaughlin J, et al. The impact of childhood abuse and neglect on adult mental health: a prospective study. J Health Soc Behav 2001;42:184–201 [PubMed] [Google Scholar]
- 59.Sumanen M, Koskenvuo M, Sillanmäki L, et al. Childhood adversities experienced by working-aged coronary heart disease patients. J Psychosom Res 2005;59:331–5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


