Abstract
Objective
To quantify cancer risk in opioid dependence and the association with infection by the oncogenic blood-borne viruses (BBVs) hepatitis C (HCV), hepatitis B (HBV) and HIV.
Design
Cohort study.
Setting
New South Wales, Australia.
Participants
All 45 412 adults aged 16 years or over registered for opioid substitution therapy (OST) between 1985 and 2007. Notifications of cancer, death and infection with HCV, HBV and HIV were ascertained by record linkage with registries.
Main outcome measures
The ratios of observed to expected number of cancers, standardised incidence ratios (SIRs), and the average annual per cent change (AAPC) in overall age and sex-standardised cancer incidence.
Results
Overall cancer risk was modestly increased compared to the general population (SIR 1.15, 95% CI 1.07 to 1.23). Excess risk was observed for 11 cancers, particularly lung (4.02, 95% CI 3.32 to 4.82), non-Hodgkin's lymphoma (1.51, 95% CI 1.20 to 1.88) and liver (8.04, 95% CI 6.18 to 10.3). Reduced risk was observed for six cancers, including prostate (0.16, 95% CI 0.06 to 0.32) and breast (0.48, 95% CI 0.35 to 0.62). Individuals notified with HCV or HBV had a markedly increased risk of liver cancer; lung cancer risk was also increased in those with HCV. HIV was associated with an elevated risk of liver, anus and kidney cancer, non-Hodgkin lymphoma and Kaposi sarcoma. Cancer risk was not increased in individuals without a BBV notification, apart from pancreatic cancer (3.92, 95% CI 1.07 to 10.0). Cancer incidence increased significantly over time (AAPC 9.4%, 4.2% to 15%, p=0.001).
Conclusions
BBVs play a major role in the cancer risk profile of opioid-dependent individuals registered for OST. To address the dramatic increasing trend in cancer incidence, the OST setting could be utilised for cancer prevention strategies.
Keywords: Public Health
Article summary.
Article focus
While opioids and opioid substitution therapies themselves are not known to be carcinogenic, opioid dependence is associated with exposure to a number of carcinogenic agents, including infection by the blood-borne viruses hepatitis B, hepatitis C and HIV.
The risk of cancer in opioid dependence has been examined in two studies with insufficient power to address risk for all cancer types.
There is no prior evidence on the association between blood-borne virus infection and cancer risk in people who are opioid dependent.
Key messages
People who are opioid dependent have an excess risk of a range of cancers compared with the general population.
The excess cancer risk is predominantly restricted to those with blood-borne virus infection.
Cancer incidence rates have increased dramatically over time, supporting use of the opioid substitution therapy (OST) setting to opportunistically implement targeted cancer prevention strategies.
Strengths and limitations of this study
The study is based on a large population-based cohort with infections and outcomes obtained from population-based registries.
Misclassification of infection by blood-borne viruses is possible because not all OST recipients will have been routinely tested.
Data on smoking, alcohol use, blood-borne virus treatment and vaccination are not available.
Introduction
Opioid-dependent individuals are exposed to a multitude of carcinogens. People who inject drugs are more likely to have hepatitis C (HCV), hepatitis B (HBV) and HIV.1 Tobacco-smoking and hazardous alcohol use are extremely common,2 as are risky sexual practices, resulting in high rates of infection with human papillomavirus (HPV).3 Although it is an effective treatment for heroin and other opioid dependence,4 opioid substitution therapy (OST) use is typically cyclic,5 with relapse to drug injection a common occurrence.
In Australia, the long-standing and widespread availability of OST and public health initiatives such as needle and syringe programmes (NSPs) have extended the life expectancy of opioid-dependent people.4 6 In injecting drug users, the prevalence of HIV is low (<1% in heterosexuals), whereas the prevalence of hepatitis C is high (50–60%).7 However, one of the consequences of the increased longevity of opioid-dependent people is the attendant risk of age-related health conditions, including cancer.8 The OST agents themselves are not considered risk factors for cancer as murine studies indicate they are not carcinogenic.9 10
Despite evidence of exposure to multiple cancer risk factors and the public health impact that this may have upon ageing cohorts of opioid-dependent people, there has been no population-based study of the spectrum of cancer risk in this group and no study of this risk in relation to blood-borne virus (BBV) infection. We examined these associations among 45 412 people who entered OST in New South Wales (NSW), Australia over a 23-year period, considering (1) overall and site-specific cancer incidence, (2) the cancer risk profiles of those infected with HCV, HBV and HIV and (3) trends in cancer incidence over time.
Methods
Study population
We conducted a cohort study using record linkage between existing population-based health datasets. The study population comprised individuals registered on the Pharmaceutical Drugs of Addiction System, a record of all NSW Health Department authorities that administer methadone or buprenorphine to opioid-dependent people. Included were all adults (≥16 years) registered for OST between 1 January 1985, the date of inception of the system, and 31 December 2007, the latest date for which cancer diagnoses were available (n=45 483). Individuals were excluded from the cohort if information essential for record linkage was incomplete (n=10, 0.02%) or if the OST end date preceded the start date (n=61, 0.13%). Registrants diagnosed with cancer prior to the start of OST (n=183, 0.40%) were not excluded; however, they did not contribute time at risk for that cancer.
HBV and HCV notification data were available for NSW residents only from 1993. Therefore our analyses of cancer risk in those with hepatitis notifications and those with no BBVs (no HCV, HBV or HIV notification) were restricted to a subcohort of individuals registered for OST from 1 January 1993 (n=32 075). To limit the potential for underascertainment of hepatitis notifications, interstate OST registrants (n=2 462, 7.68%) were excluded and other OST registrants where censored when they moved interstate OST (n=2 297, 7.76%).
Data collection
In Australia, all deaths, newly diagnosed cancers (excluding basal and squamous cell carcinoma of the skin), and HCV, HBV and HIV infections must be reported to government agencies by statute. Dates of death in OST registrants were ascertained by linkage with the National Death Index (1980–2007). The date of diagnosis, topography and morphology of incident cancers were identified by linkage with the Australian Cancer Database, a register of incident primary invasive neoplasms (1982–2007). Solid cancers were classified according to the International Classification of Diseases (ICD), 10th revision while haematopoietic neoplasms and Kaposi sarcomas were classified according to the ICD for Oncology, 3rd edition. Dates of HIV and AIDS notifications were ascertained by linkage with the National HIV Database (1985–2007) and the National AIDS Register (1982–2007), respectively. Linkage with the NSW Health Notifiable Conditions Information Management System (1993–2007) identified dates of HBV and HCV notifications for the subcohort, based on detection of HBV surface antigen or HBV DNA and anti-HCV antibody or HCV RNA, respectively.
The name, sex, date of birth, date of death and state of residence of registrants were used for record linkage. All linkages used probabilistic matching techniques except the HIV/AIDS linkage which used deterministic methods because only the first two letters of the given name and surname, not full name, were recorded on these registers.
Cancer incidence rates for the Australian population were obtained from the Australian Cancer Database by 5-year age group, sex, calendar year and state/territory, for 1985–2007.
Data analysis
Cancer incidence
Person-years of follow-up accumulated from the date of first OST registration and terminated at the first occurrence of cancer diagnosis, death, age 80, interstate transfer (for BBV analyses only) or 31 December 2007.
Crude and age/sex-standardised cancer incidence rates, standardised to the 1996 Australian population, and Poisson 95% CIs were calculated using annual Australian population estimates from the Australian Bureau of Statistics. To describe overall cancer incidence trends over time the average annual per cent change (AAPC) was estimated using Poisson regression.11
Relative risk of cancer
Risk of cancer overall and for each cancer type was examined in the full cohort (1985–2007) and the subcohort (1993–2007) using the standardised incidence ratio (SIR), the ratio of the observed and the expected number of cancers. The expected number of incident cancers was calculated by multiplying cohort person-years at risk by 5-year age-specific, sex-specific, state-specific and calendar year-specific population cancer incidence rates. The exception was Kaposi sarcoma, where 1982 population rates were used to avoid the impact of AIDS on the incidence of this cancer. The SIRs were computed for cancer overall and for the most frequently occurring cancers by sex and age at follow-up (<40, 40–49 and ≥50 years). In addition, SIRs were calculated by BBV notification status; HIV with or without HBV or HCV; HBV monoinfection; HCV monoinfection; HBV/HCV co-infection and HBV/HCV/HIV uninfected. BBV infection was examined in a time-dependent manner, accounting for change in notification status over time.
For registrants with an AIDS notification only or an HIV notification occurring less than 5 years before the AIDS notification (n=59, 0.13%), the date of HIV infection was backdated to 5 years prior to the AIDS notification or to the date of cohort entry, whichever occurred later, to more accurately estimate the date of HIV infection. The resultant retrospectively defined person-years were survival-adjusted by applying period-specific, all-age, sex-specific and site-specific cancer survival rates to account for those individuals with HIV infection who may have developed cancer and subsequently died before being diagnosed with HIV.12 A similar adjustment was performed as a sensitivity analysis for liver cancer risk in those notified with HCV, assuming a median age at infection of 25 years for injecting drug user (IDU)-acquired infection13 and backdating HCV infection up to 15 years.
We compared patterns of cancer risk for the BBV subgroups but could not compare SIRs statistically because of the heterogeneity in subgroup age and sex distributions.14
Within-cohort risk factors were examined for the most frequently occurring cancers in the subcohort (1993–2007); liver, lung and non-Hodgkin's lymphoma. Sex and the time-dependent factors—current age, calendar year and HBV, HCV and HIV notification—were a priori included in all multivariable models. Poisson regression was used to determine incidence rate ratios (IRRs) with 95% CIs.
Analyses were performed using SAS software V.9.2 (SAS Institute Inc, Cary, North Carolina, USA) and Joinpoint Regression Program, V.3.5 (Statistical Methodology and Applications Branch and Data Modeling Branch, Surveillance Research Program, National Cancer Institute). Person-years were calculated using the %stratify macro.15
Results
After applying exclusion criteria, 45 412 individuals registered for OST between 1985 and 2007 were included in the study cohort (table 1). This cohort accumulated 481 936 person-years of follow-up for cancer, a median of 9.9 person-years per registrant (IQR 5.61–15.2; table 1). Two-thirds of the cohort was male. The median age at OST registration was 27 years and the median cumulative time on OST was 2.6 years (IQR 0.6–6.5). A total of 423 (0.8%) registrants were notified with an HIV/AIDS diagnosis prior to or after OST registration.
Table 1.
Characteristics of all New South Wales opioid substitution therapy registrants and those with notified HIV infection, 1985–2007
| Entire cohort |
HIV infection |
|||||
|---|---|---|---|---|---|---|
| Person-years |
Person-years |
|||||
| No. (%) of registrants | Total | Median (IQR) | No. (%) of registrants | Total | Median (IQR) | |
| Total | 45 412 (100) | 481 936 | 9.94 (5.61–15.2) | 426 (100) | 3511 | 7.60 (3.66–12.4) |
| Sex | ||||||
| Men | 30 147 (66.4) | 310 632 | 9.67 (5.31–14.8) | 346 (81.2) | 2796 | 7.58 (3.63–11.8) |
| Women | 15 265 (33.6) | 171 304 | 10.6 (6.30–16.2) | 80 (18.8) | 715 | 7.68 (3.72–14.6) |
| Age at cohort entry (years) | ||||||
| <25 | 16 266 (35.8) | 166 503 | 9.48 (5.83–14.0) | 139 (32.6) | 1176 | 7.38 (3.87–13.0) |
| 25–30 | 14 680 (32.3) | 171 073 | 11.3 (6.26–17.4) | 120 (28.2) | 1027 | 7.96 (3.75–11.7) |
| ≥31 | 14 466 (31.9) | 144 361 | 9.46 (4.86–14.4) | 167 (39.2) | 1308 | 7.25 (2.83–12.7) |
| Year of cohort entry | ||||||
| 1985–1989 | 8476 (18.7) | 161 566 | 20.4 (18.7–21.8) | 75 (17.6) | 887 | 10.2 (6.02–19.2) |
| 1990–1995 | 11 220 (24.7) | 152 938 | 14.1 (12.7–15.7) | 140 (32.9) | 1479 | 12.8 (5.99–14.8) |
| 1996–2001 | 15 302 (33.7) | 133 232 | 8.84 (7.35–10.4) | 122 (28.6) | 908 | 8.00 (6.33–9.65) |
| 2002–2007 | 10 414 (22.9) | 34 200 | 3.44 (1.88–4.81) | 89 (20.9) | 237 | 2.72 (1.22–3.89) |
We observed 819 (1.8%) incident primary cancers (803 first, 16 second cancers) after OST registration and the median age at diagnosis of first cancer was 43 (IQR 37–49) years.
After applying further exclusion criteria, the subcohort with assessable hepatitis data comprised 29 613 participants entering OST between 1993 and 2007 (table 2). They were of similar age and sex as the full cohort (median age at OST registration, 26 years; 69% male). A total of 14 892 (50%) registrants were notified with HCV alone, 598 (2%) with HBV alone, and 898 (3%) with HBV and HCV. Over the 213 008 person-years of follow-up in the subcohort, 240 (0.8%) incident cancers were observed.
Table 2.
Characteristics of New South Wales opioid substitution therapy registrants, by BBV notification status, 1993–2007
| Entire subcohort |
No BBV infection |
HBV monoinfection |
HCV monoinfection |
HBV/HCV co-infection |
HIV infection† |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Person-years |
Person-years |
Person-years |
Person-years |
Person-years |
Person-years |
|||||||||||||
| Number (%) of registrants* | Total | Median | Number (%) of registrants | Total | Median | Number (%) of registrants | Total | Median | Number (%) of registrants | Total | Median | Number (%) of registrants | Total | Median | Number (%) of registrants | Total | Median | |
| Total | 29 613 (100) | 213 008 | 7.28 | 23 650 (100) | 109 313 | 3.53 | 598 (100) | 2608 | 3.56 | 14 892 (100) | 94 331 | 6.36 | 898 (100) | 5249 | 6.02 | 234 (100) | 1504 | 6.20 |
| Sex | ||||||||||||||||||
| Men | 20 348 (68.7) | 144 932 | 7.19 | 16 352 (69.1) | 76 495 | 3.64 | 436 (72.9) | 1918 | 3.60 | 9793 (65.8) | 61 378 | 6.29 | 643 (71.6) | 3782 | 6.10 | 204 (87.2) | 1355 | 6.55 |
| Women | 9265 (31.3) | 68 076 | 7.51 | 7298 (30.9) | 32 818 | 3.25 | 162 (27.1) | 691 | 3.50 | 5099 (34.2) | 32 953 | 6.47 | 255 (28.4) | 1466 | 5.94 | 30 (12.8) | 148 | 4.35 |
| Age at cohort entry (years) | ||||||||||||||||||
| <25 | 11 674 (39.4) | 87 088 | 7.60 | 9915 (41.9) | 47 340 | 3.81 | 278 (46.5) | 1282 | 3.96 | 5706 (38.3) | 35 963 | 6.41 | 351 (39.1) | 2130 | 6.27 | 61 (26.1) | 373 | 6.08 |
| 25–30 | 8427 (28.5) | 60 907 | 7.32 | 6771 (28.6) | 31 905 | 3.56 | 169 (28.3) | 770 | 3.18 | 4106 (27.6) | 26 201 | 6.42 | 266 (29.6) | 1534 | 5.79 | 68 (29.1) | 494 | 7.73 |
| ≥31 | 9512 (32.1) | 65 013 | 6.74 | 6964 (29.4) | 30 068 | 3.06 | 151 (25.3) | 555 | 3.39 | 5080 (34.1) | 32 167 | 6.23 | 281 (31.3) | 1584 | 5.71 | 105 (44.9) | 637 | 5.17 |
| Year of cohort entry | ||||||||||||||||||
| 1993–1997 | 10 212 (34.5) | 111 454 | 11.7 | 8944 (37.8) | 54 619 | 4.76 | 158 (26.4) | 981 | 5.08 | 3563 (23.9) | 36 132 | 11.2 | 129 (14.4) | 1298 | 10.9 | 84 (35.9) | 754 | 10.7 |
| 1998–2002 | 11 655 (39.4) | 81 399 | 7.36 | 8962 (37.9) | 42 095 | 5.35 | 290 (48.5) | 1304 | 5.12 | 6958 (46.7) | 47 049 | 7.05 | 475 (52.9) | 3167 | 6.80 | 82 (35.0) | 573 | 7.55 |
| 2003–2007 | 7746 (26.2) | 20 155 | 2.70 | 5744 (24.3) | 12 599 | 2.11 | 150 (25.1) | 323 | 2.01 | 4371 (29.4) | 11 150 | 2.55 | 294 (32.7) | 784 | 2.75 | 68 (29.1) | 177 | 2.71 |
*Category numbers will not sum to total numbers for the subcohort as individuals were followed up in a time-dependent manner, allowing an individual to contribute person-years to multiple groups as their BBV notification status changed over the period of observation.
†With or without HBV or HCV notification.
BBV, blood-borne viruses; HBV, hepatitis B virus; HCV, hepatitis C virus.
Cancer incidence
For the period 1985–2007 the crude cancer incidence rate was 170/100 000 person-years (95% CI 159 to 182). The age-standardised rate was 349/100 000 person-years (95% CI 337 to 361) and the annual age-standardised rate increased significantly between 1985 and 2007 (AAPC=9.4%, 95% CI 4.2% to 15%; p=0.001).
Cancer risk
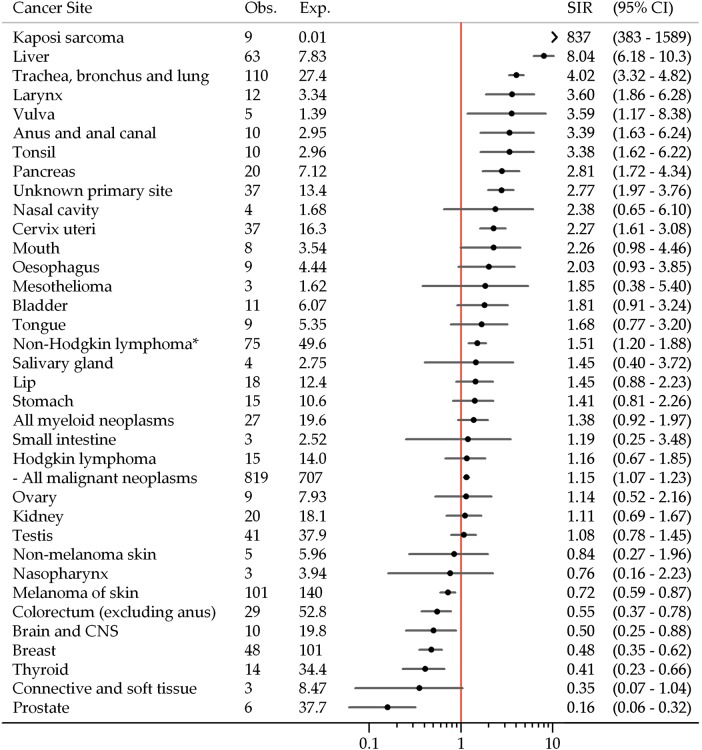
Risk of cancer overall was slightly higher in OST registrants compared to the Australian population (SIR=1.15, 95% CI 1.07 to 1.23). SIRs were significantly greater than unity for cancers of the tonsil, anus and anal canal, liver, pancreas, larynx, trachea bronchus and lung, vulva and cervix, Kaposi sarcoma, non-Hodgkin's lymphoma and cancer of unknown primary site (figure 1). Conversely, SIRs were significantly less than unity for melanoma and cancers of the colorectum, breast, prostate, brain and central nervous system and thyroid.
Figure 1.
Risk of cancer among New South Wales opioid substitution therapy registrants, 1985–2007. CNS, central nervous system; Exp, expected number of cancers; Obs, observed number of cancers; SIR, standardised incidence ratio. *Includes non-Hodgkin's lymphoma not otherwise specified (International Classification of Diseases for Oncology, 3rd edition: 9590). Non-melanoma skin cancer excludes diagnoses of basal cell and squamous cell carcinoma.
Risk of any cancer was significantly increased in men and in those more than 40 years of age (table 3). Liver and lung cancer risk was increased in men and women; liver cancer risk was significantly increased only for those more than 40 years of age, while lung cancer risk was increased regardless of attained age. Similarly, women of all ages experienced half the risk of breast cancer.
Table 3.
Risk of any cancer and the most frequently occurring cancers in New South Wales opioid substitution therapy registrants, by sex and current age (1985–2007)
| Cancer type | Obs | Exp | SIR | 95% CI |
| All cancer | ||||
| Men | 536 | 431 | 1.24 | 1.14–1.35 |
| Women | 283 | 285 | 0.99 | 0.88–1.11 |
| <40 years | 282 | 295 | 0.96 | 0.85–1.07 |
| 40–49 years | 357 | 282 | 1.27 | 1.14–1.40 |
| ≥50 years | 180 | 139 | 1.30 | 1.12–1.50 |
| Liver | ||||
| Men | 51 | 6.92 | 7.37 | 5.49–9.69 |
| Women | 12 | 0.91 | 13.2 | 6.80–23.0 |
| <40 years | 4 | 1.62 | 2.47 | 0.67–6.33 |
| 40–49 years | 26 | 3.96 | 6.57 | 4.29–9.63 |
| ≥50 years | 33 | 2.26 | 14.6 | 10.1–20.6 |
| Trachea, bronchus and lung | ||||
| Men | 82 | 19.8 | 4.15 | 3.32–5.11 |
| Women | 28 | 7.59 | 3.69 | 2.45–5.33 |
| <40 years | 15 | 4.11 | 3.65 | 2.04–6.02 |
| 40–49 years | 58 | 13.0 | 4.46 | 3.41–5.71 |
| ≥50 years | 37 | 10.2 | 3.61 | 2.57–4.90 |
| Melanoma | ||||
| Men | 64 | 91.2 | 0.70 | 0.54–0.89 |
| Women | 37 | 48.7 | 0.76 | 0.54–1.03 |
| <40 years | 48 | 71.6 | 0.67 | 0.50–0.88 |
| 40–49 years | 37 | 51.4 | 0.72 | 0.51–0.98 |
| ≥50 years | 16 | 16.9 | 0.95 | 0.56–1.49 |
| Female breast | ||||
| <40 years | 15 | 32.5 | 0.46 | 0.27–0.74 |
| 40–49 years | 27 | 54.3 | 0.48 | 0.32–0.69 |
| ≥50 years | 6 | 14.1 | 0.43 | 0.17–0.86 |
| Cervical | ||||
| <40 years | 21 | 11.0 | 1.90 | 1.20–2.84 |
| 40–49 years | 14 | 4.67 | 3.00 | 1.64–5.03 |
| ≥50 years | 2 | 0.63 | 3.19 | 0.39–11.5 |
| Non-Hodgkin's lymphoma | ||||
| Men | 62 | 38.1 | 1.63 | 1.26–2.07 |
| Women | 13 | 11.5 | 1.13 | 0.62–1.86 |
| <40 years | 25 | 20.9 | 1.20 | 0.79–1.73 |
| 40–49 years | 37 | 19.8 | 1.87 | 1.33–2.54 |
| ≥50 years | 13 | 8.94 | 1.46 | 0.78–2.49 |
Exp, expected number of cancers; Obs, observed number of cancers; SIR, standardised incidence ratio.
BBVs and cancer risk
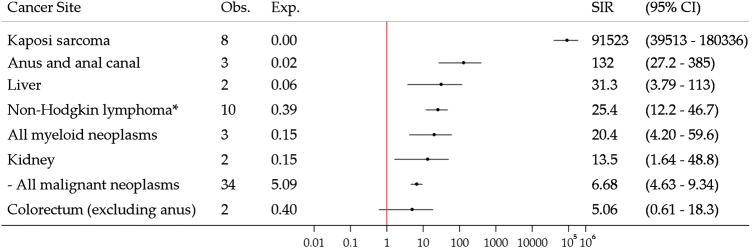
Thirty-four cancers were observed in registrants notified with HIV (irrespective of infection with other BBVs), with an SIR of 6.68 (95% CI 4.63 to 9.34; figure 2). SIRs were significantly greater than unity for several cancers, including Kaposi sarcoma, non-Hodgkin's lymphoma and anal cancer.
Figure 2.
Risk of cancer in New South Wales opioid substitution therapy registrants with notified HIV infection, 1985–2007. Exp, expected number of cancers; Obs, observed number of cancers; SIR, standardised incidence ratio. *Includes non-Hodgkin's lymphoma not otherwise specified (International Classification of Diseases for Oncology, 3rd edition: 9590).
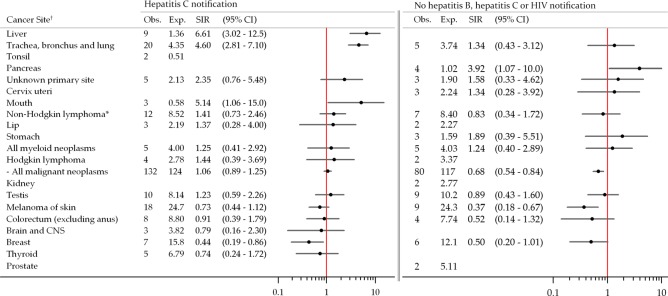
In those notified with HCV monoinfection, the overall risk of cancer was not significantly different from that of the general population (SIR=1.06, 95% CI 0.89 to 1.25; figure 3) but the SIR for liver cancer was 6.61 (95% CI 3.02 to 12.5). After adjusting for an assumed age of HCV infection of 25 years and for survival, the SIR for liver cancer increased to 13.1 (95% CI 6.00 to 24.9). Risk of lung cancer and mouth cancer were also elevated. On the contrary, breast cancer risk was decreased and while six prostate cancers were expected, none were observed. Few cancers were observed in those notified with HBV (one case) or HBV-HCV co-infection (18 cases). The risk of liver cancer in individuals notified with HBV-HCV co-infection was markedly elevated (SIR=35.9, 95% CI 7.41 to 105).
Figure 3.
Risk of cancer among New South Wales opioid substitution therapy registrants with notified hepatitis C infection and registrants without notified hepatitis B, hepatitis C or HIV infection, 1993–2007. *Includes non-Hodgkin lymphoma not otherwise specified (International Classification of Diseases for Oncology, 3rd edition: 9590). †SIRs not estimated for cancers with less than 3 observed cases.
Registrants without a notification of BBV infection were at decreased risk of cancer overall (SIR=0.68, 95% CI 0.54 to 0.84; figure 3) and melanoma. In this subgroup, no infection-related cancers occurred at rates significantly different to the general population; however, the risk of pancreatic cancer was significantly elevated.
In multivariable analyses adjusting for age, calendar year, sex and HCV, HBV and HIV notification status, age was an independent risk factor for the most frequently occurring cancers (table 4). Notification of HBV and HCV infection predicted risk of liver cancer, and notification of HIV infection predicted risk of non-Hodgkin's lymphoma. Notification of HCV infection independently predicted risk of lung cancer.
Table 4.
Multivariable analysis of risk factors for common cancers among New South Wales opioid substitution therapy registrants, 1993–2007
| Lung cancer |
Non-Hodgkin's lymphoma |
Liver cancer |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | IRR* | 95% CI | p Value | n | IRR* | 95% CI | p Value | n | IRR* | 95% CI | p Value | |
| Current age† | 27 | 1.19 | 1.15–1.24 | <0.0001 | 23 | 1.06 | 1.01–1.11 | 0.025 | 13 | 1.20 | 1.13–1.27 | <0.0001 |
| Calendar year† | 27 | 0.91 | 0.81–1.03 | 0.131 | 23 | 1.16 | 1.01–1.37 | 0.054 | 13 | 1.13 | 0.91–1.49 | 0.317 |
| Sex | ||||||||||||
| Men | 22 | 1.55 | 0.63–4.65 | 0.378 | 17 | 1.03 | 0.42–2.90 | 0.954 | 12 | 3.94 | 0.76–72.2 | 0.190 |
| Women (ref) | 5 | 1.00 | 6 | 1.00 | 1 | 1.00 | ||||||
| Notified HCV infection | ||||||||||||
| Yes | 21 | 3.29 | 1.39–9.07 | 0.011 | 13 | 1.12 | 0.49–2.65 | 0.792 | 12 | 9.05 | 1.73–166 | 0.036 |
| No (ref) | 6 | 1.00 | 10 | 1.00 | 1 | 1.00 | ||||||
| Notified HBV infection‡ | ||||||||||||
| Yes | 2 | 1.59 | 0.25–5.42 | 0.531 | 0 | – | 3 | 4.63 | 1.03–15.4 | 0.021 | ||
| No (ref) | 25 | 1.00 | 23 | 10 | 1.00 | |||||||
| Notified HIV infection‡ | ||||||||||||
| Yes | 0 | – | 4 | 26.0 | 7.40–71.3 | <0.0001 | 0 | - | ||||
| No (ref) | 27 | 19 | 1.00 | 13 | ||||||||
HBV, hepatitis B virus; HCV, hepatitis C virus; IRR, incidence rate ratio; ref, reference group in analysis.
*Adjusted for current age (years), calendar year, sex and time-dependent HCV notification status, HBV notification status and HIV notification status.
†IRR refers to 1 year increase.
‡IRRs could not be calculated if a cancer case did not occur in one of the binary groupings.
Note: Infection groupings are not mutually exclusive.
Discussion
We found that cancer risk in opioid-dependent people registered for OST was significantly increased for a number of cancers causally related to infection with oncogenic viruses, smoking and alcohol consumption. The excess cancer risk was almost entirely restricted to those notified with a BBV infection, except for pancreatic cancer. The most common cancers for which there was an excess risk were independently associated with increasing age and infection by one or more BBVs; liver cancer (HCV and HBV), lung cancer (HCV) and non-Hodgkin's lymphoma (HIV). Cancer incidence also increased significantly over time, highlighting cancer as an emerging public health concern for this population.
Strengths and weaknesses
The strengths of this study include the large population size and the lengthy follow-up, which provided the statistical power to improve upon sparse knowledge of the cancer risk faced by the opioid-dependent population. Additionally, use of national, population-based registers for ascertainment of cancer diagnoses, HIV/AIDS diagnoses and deaths enabled unbiased and comprehensive follow-up of the cohort. On the contrary, although routine testing for BBV infection is recommended, it is likely that not all OST recipients were routinely tested, and use of the notification date in analysis underestimates the timing of the infection. We also lacked data on BBV treatment and HBV vaccination. Furthermore, it was not possible to determine serological clearance of HCV. Thus, it is possible that we have misclassification with respect to BBV infection, meaning that we have underestimated the association of chronic HCV infection with cancer in OST registrants.
In addition, this study was retrospective, relying upon linkage with routinely collected administrative data, and some false-positive and false-negative linkages do occur. Nevertheless, quality assurance practices performed by the data linkage units can result in high sensitivity and specificity (eg, hepatitis linkage sensitivity >99.9%, specificity 99.8%).16 The lack of data on smoking and alcohol use of the OST recipients meant that we could not examine the contribution by these agents to the excess risk of liver and lung cancer in those with BBV infection. Given it is likely that around 50% of Australians with opioid dependence enter OST at some point,17 our findings are likely to be generalisable to the broader opioid-dependent population. Our cohort being a representative sample is supported by the similarity of BBV prevalence with NSP survey results.7 With respect to other OST populations, our data may represent a conservative estimate of the public health burden given the markedly lower incidence of HIV infection in comparison to most other countries.18
Context
This is the first population-based study of cancer incidence among people who are opioid dependent, and the first study to examine cancer incidence in relation to BBV infection. Of the two prior studies measuring cancer incidence in opioid dependent individuals, one had very limited statistical power and examined a US cohort of mostly Hispanic injecting drug users with a high prevalence of HIV,19 while the other studied Israeli OST recipients but did not link with a population-based death registry and examined risk for only eight cancer types.20
Explanations
Apart from Kaposi sarcoma, the strongest excess cancer risk in those registered for OST was observed for liver cancer. Stratification by BBV notification and within-cohort risk factor analyses showed that this excess risk occurred only in those with BBV infection, especially HCV alone or HCV/HBV co-infection. Surveillance bias did not strongly affect our risk estimates, as only one liver cancer was diagnosed within 6 months of the start of OST. Lung cancer risk was elevated in the cohort overall and in the subgroup with HCV. While HCV notification independently predicted lung cancer risk, there is no evidence of a biological link and this result may indicate those with HCV smoke more heavily than those without HCV. Tobacco exposure may also explain the excess risk of mouth cancer in this subgroup. The excess risk of liver20 and lung19 20 cancer is consistent with prior evidence.
An excess risk of Kaposi sarcoma, non-Hodgkin's lymphoma and anal cancer was observed in OST registrants overall, particularly those with HIV. These cancers have an established causal association with HIV-related immunosuppression and are likely to result from impaired immune surveillance in people with infection by Kaposi sarcoma-associated herpesvirus, Epstein-Barr virus and HPV, respectively.21 An excess risk of five non-AIDS-defining malignancies was also observed, supporting evidence that these cancers are becoming increasingly important for those with HIV infection.22
A number of cancers occurred at rates significantly lower than in the matched general population. For melanoma, two explanations are suggested. First, Aboriginal Australians, who experience one-tenth of the risk of melanoma compared to non-Aboriginal Australians, are over-represented in the OST population compared to the general population (11% vs 1.5–2%).23 Second, 69% of a recent sample (n=154) of active IDUs in NSW reported a history of incarceration,17 which limits sun exposure, an established risk factor for melanoma. Underparticipation in population-based and other cancer screening programmes may explain the reduced risk of breast, colorectal and prostate cancer in OST recipients. However, only 4% of the total follow-up time was contributed by individuals who were age-eligible for these programmes, substantially weakening the case for attenuation in risk due to underparticipation in screening. A reduced risk of colorectal and breast cancer was observed in Israeli OST recipients,20 and there is some evidence that opioid use may impart a decreased risk of certain cancers. For example, a side effect of chronic opioid use is hypogonadism, resulting in low oestrogen (women) and testosterone (men).24 Low levels of these hormones may decrease premenopausal breast cancer and prostate cancer risk; however, the evidence is inconclusive.25 26 Characteristics common to OST registrants, high parity and early age at first pregnancy,17 27 both considered protective for breast cancer,28 as well as reduced prevalence of overweight and obesity,29 may also contribute to the observed risk reduction.
Implications
The observed cancer risk profile strongly supports the implementation of targeted cancer prevention strategies in the OST setting. Given the concentration of excess risk among those with BBV notifications, and the high prevalence of HCV among this population, there is a clear need for interventions that reduce HCV incidence4 and that treat people who have developed chronic infections.1 NSPs are highly effective at reducing the rate of acquisition of BBVs.4 Antiviral treatments for HCV and HBV infection induce regression of fibrosis and decrease liver cancer risk.30 People with a history of injecting drug use respond positively to HCV therapies, with acceptable levels of sustained virological response31 and antiviral therapy is also not contraindicated in people with HIV receiving OST. The issue, however, is one of coverage. Many physicians remain unwilling to provide such treatments to people who use illicit drugs in the face of evidence that they have similar levels of adherence to treatment as other patient groups.32 Thus, despite HCV being one of the strongest risk factors for cancer in OST, very few people in OST have received treatment to address this risk.1
As the risks for cancers with established causal links to tobacco-smoking and alcohol use were elevated, namely lung, larynx and pancreatic cancer (smoking), and liver, oral, larynx and oesophageal cancer (alcohol), a reduction in these behaviours would similarly mitigate cancer risk. OST clients show strong interest in smoking cessation programmes,33 and they do not adversely impact OST,34 but again few are offered such treatments in routine healthcare settings. The excess risk of cancer of the cervix, vulva and anus, cancers caused by chronic infection with HPV, may be reduced by safer sexual practices. The increased risk of cervical cancer also supports the need for greater participation in cervical screening.
Conclusions
Opioid-dependent people registered for OST face an excess risk of a variety of cancers compared to the general population, in particular cancers associated with infection by BBVs. The implementation of harm reduction strategies in the OST setting represents an evidently underutilised opportunity to respond to the escalating cancer burden facing this marginalised population.
Supplementary Material
Acknowledgments
We thank the NSW Ministry of Health for providing the Pharmaceutical Drugs of Addiction System data, and Pia Salmelainen of the Pharmaceutical Services Branch, NSW Health, for her assistance with data extraction and interpretation. We are also grateful to the staff of the state and territory cancer registries and Health Departments for the use of their data on cancer and notifiable diseases, respectively. We thank the Australian Institute of Health and Welfare and the Centre for Health Record Linkage for conducting the data linkage. We are indebted to Preeyaporn Srasuebkul, Michael Falster and Sadaf Marashi-Pour for biostatistical advice and assistance.
Footnotes
Contributors: The study was conceived and designed by CMV, LB and LD. The data were obtained from data custodians by LM, NSM and CMV. All authors contributed to the analytical plans. The data were prepared and analysed by AS. AS, CMV and LD drafted the paper. All authors reviewed, revised and approved the final draft. CMV is the guarantor.
Funding: National Health and Medical Research Council (NHMRC; ID630531) and Faculty of Medicine, University of New South Wales. AEG is supported by an NHMRC principal research fellowship (ID568819). LD is supported by an NHMRC Senior Research Fellowship (ID510279). CMV is supported by an NHMRC Career Development Fellowship (ID1023159) and a Cancer Institute New South Wales Career Development Fellowship (ID10/CDF/2-42). The funding bodies played no role in the study design or conduct, data collection, analysis or interpretation of data, in the writing of the article or the decision to submit the article for publication.
Competing interests: None.
Ethics approval: The study was reviewed and approved by all relevant ethics committees and the requirement for informed consent was waived because the researchers received only de-identified data.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no additional data available.
References
- 1.Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 2011;378:571–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Best D, Lehmann P, Gossop M, et al. Eating too little, smoking and drinking too much: wider lifestyle problems among methadone maintenance patients. Addic Res Theory 1998;6:89–98 [Google Scholar]
- 3.Plitt SS, Sherman SG, Viscidi RP, et al. Human papillomavirus seroprevalence among young male and female drug users. Sex Transm Dis 2007;34:676–80 [DOI] [PubMed] [Google Scholar]
- 4.Degenhardt L, Mathers BM, Vickerman P, et al. Prevention of HIV infection for people who inject drugs: why individual, structural, and combination approaches are needed. Lancet 2010;376:285–301 [DOI] [PubMed] [Google Scholar]
- 5.Bell J, Burrell T, Indig D, et al. Cycling in and out of treatment; participation in methadone treatment in NSW, 1990–2002. Drug Alcohol Depend 2006;81:55–61 [DOI] [PubMed] [Google Scholar]
- 6.Degenhardt L, Bucello C, Mathers B, et al. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction 2011;106:32–51 [DOI] [PubMed] [Google Scholar]
- 7.The Kirby Institute HIV, viral hepatitis and sexually transmissible infections in Australia Annual Surveillance Report 2011. Sydney, NSW: The Kirby Institute, the University of New South Wales, 2011 [Google Scholar]
- 8.Ferreros I, Lumbreras B, Hurtado I, et al. The shifting pattern of cause-specific mortality in a cohort of human immunodeficiency virus-infected and non-infected injecting drug users. Addiction 2008;103:651–9 [DOI] [PubMed] [Google Scholar]
- 9.Brambilla G, Martelli A. Genotoxicity and carcinogenicity studies of analgesics, anti-inflammatory drugs and antipyretics. Pharmacol Res 2009;60:1–17 [DOI] [PubMed] [Google Scholar]
- 10.Rosenkrantz H, Fleischman RW. In vivo carcinogenesis assay of DL-methadone HCl in rodents. Fundam Appl Toxicol 1988;11:640–51 [DOI] [PubMed] [Google Scholar]
- 11.Clegg LX, Hankey BF, Tiwari R, et al. Estimating average annual per cent change in trend analysis. Stat Med 2009;28:3670–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grulich AE, Wan X, Law MG, et al. Risk of cancer in people with AIDS. AIDS 1999;13:839–43 [DOI] [PubMed] [Google Scholar]
- 13.Amin J, Dore GJ, O'Connell DL, et al. Cancer incidence in people with hepatitis B or C infection: a large community-based linkage study. J Hepatol 2006;45:197–203 [DOI] [PubMed] [Google Scholar]
- 14.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd edn Philadelphia: Lippincott Williams & Wilkins, 2008 [Google Scholar]
- 15.Rostgaard K. Methods for stratification of person-time and events–a prerequisite for Poisson regression and SIR estimation. Epidemiol Perspect Innov 2008;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kariminia A, Butler T, Corben S, et al. Mortality among prisoners: how accurate is the Australian National Death Index? Aust NZ J Pub Health 2005;29:572–5 [DOI] [PubMed] [Google Scholar]
- 17.Stafford J, Burns L. Australian Drug Trends 2010. Findings from the Illicit Drug Reporting System (IDRS). Sydney, NSW: National Drug and Alcohol Research Centre, UNSW, 2011 [Google Scholar]
- 18.Mathers BM, Degenhardt L, Phillips B, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet 2008;372:1733–45 [DOI] [PubMed] [Google Scholar]
- 19.Gachupin-Garcia A, Selwyn PA, Budner NS. Population-based study of malignancies and HIV infection among injecting drug users in a New York City methadone treatment program, 1985–1991. AIDS 1992;6:843–8 [DOI] [PubMed] [Google Scholar]
- 20.Grinshpoon A, Barchana M, Lipshitz I, et al. Methadone maintenance and cancer risk: an Israeli case registry study. Drug Alcohol Depend 2011;119:88–92 [DOI] [PubMed] [Google Scholar]
- 21.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens—Part B: biological agents. Lancet Oncol 2009;10:321–2 [DOI] [PubMed] [Google Scholar]
- 22.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011;103:753–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Australian Institute of Health and Welfare National Opioid Pharmacotherapy Statistics Annual Data Collection: 2010 report. Canberra: ACT: Australian Institute of Health and Welfare (AIHW), 2011 [Google Scholar]
- 24.Reddy RG, Aung T, Karavitaki N, et al. Opioid induced hypogonadism. BMJ 2010;341:c4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eliassen A, Spiegelman D, Xu X, et al. Urinary estrogens and estrogen metabolites and subsequent risk of breast cancer among premenopausal women. Cancer Res 2012;72:696–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roddam A, Allen N, Appleby P, et al. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst 2008;100:170–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burns L, Mattick RP, Cooke M. The use of record linkage to examine illicit drug use in pregnancy. Addiction 2006;101:873–82 [DOI] [PubMed] [Google Scholar]
- 28.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev 1993;15:36–47 [DOI] [PubMed] [Google Scholar]
- 29.Hsieh C-C, Trichopoulos D, Katsouyanni K, et al. Age at menarche, age at menopause, height and obesity as risk factors for breast cancer: associations and interactions in an international case-control study. Int J Cancer 1990;46:796–800 [DOI] [PubMed] [Google Scholar]
- 30.Chander G, Sulkowski MS, Jenckes MW, et al. Treatment of chronic hepatitis C: a systematic review. Hepatology 2002;36:s135–44 [DOI] [PubMed] [Google Scholar]
- 31.Novick DM, Kreek MJ. Critical issues in the treatment of hepatitis C virus infection in methadone maintenance patients. Addiction 2008;103:905–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health 2008;33:126–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clarke JG, Stein MD, McGarry KA, et al. Interest in smoking cessation among injection drug users. Am J Addict 2001;10:159–66 [DOI] [PubMed] [Google Scholar]
- 34.Reid MS, Fallon B, Sonne S, et al. Smoking cessation treatment in community-based substance abuse rehabilitation programs. J Subst Abuse Treat 2008;35:68–77 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.