Abstract
Objectives
Exposure to trichloramine (NCl3) in indoor swimming-pool environments is known to cause mucous membrane irritation, but if it gives rise to changes in lung function or asthma in adults is not known. (1) We determined lung function in volunteers before and after exposure to indoor pool environments. (2) We studied the occurrence of respiratory symptoms and asthma in a cohort of pool workers.
Design/methods/participants
(1) We studied two groups of volunteers, 37 previously non-exposed healthy persons and 14 pool workers, who performed exercise for 2 h in an indoor pool environment. NCl3 in air was measured during pool exposures and in 10 other pool environments. Filtered air exposures were used as controls. Lung function and biomarkers of pulmonary epithelial integrity were measured before and after exposure. (2) We mailed a questionnaire to 1741 persons who indicated in the Swedish census 1990 that they worked at indoor swimming-pools.
Results
(1) In previously non-exposed volunteers, statistically significant decreases in FEV1 (forced expiratory volume) and FEV% (p=0.01 and 0.05, respectively) were found after exposure to pool air (0.23 mg/m3 of NCl3). In pool workers, a statistically significant decrease in FEV% (p=0.003) was seen (but no significant change of FEV1). In the 10 other pool environments the median NCl3 concentration was 0.18 mg/m3. (2) Our nested case/control study in pool workers found an OR for asthma of 2.31 (95% CI 0.79 to 6.74) among those with the highest exposure. Exposure-related acute mucous membrane and respiratory symptoms were also found.
Conclusions
This is the first study in adults showing statistically significant decreases in lung function after exposure to NCl3. An increased OR for asthma among highly exposed pool workers did not reach statistical significance, but the combined evidence supports the notion that current workroom exposures may contribute to asthma development. Further research on sensitive groups is warranted.
Keywords: Public Health, Respiratory Medicine (See Thoracic Medicine), Occupational & Industrial Medicine
Article summary.
Article focus
Exposure to trichloramine (NCl3) in swimming-pool air is known to cause mucous membrane and pulmonary effects, but statistically significant changes in lung function among adults have not been reported.
Epidemiological studies of asthma among pool workers are not available.
Key messages
In this study we found for the first time, statistically significant decreases in lung function in volunteers after exposure to pool air with commonly occurring levels of NCl3.
We found a tendency towards a higher OR for asthma in a nested case reference study within a cohort of 1102 pool workers.
Our findings support the notion that current workroom exposures of NCl3 may contribute to asthma development.
Strengths and limitations of this study
This is the first study showing small but statistically significant decreases in lung function after exposure to pool air. This is the first nested case/control study in pool workers. It reports an OR for asthma of 2.31 (95% CI 0.79 to 6.74) among pool workers with the highest exposure (after correction for heredity), but this finding did not reach statistical significance.
Introduction and objectives
Monochloramines, dichloramines and trichloramines are formed following a reaction between ammonia (NH3) or other nitrogen-containing substances present in swimming-pool water when hypochlorite is used as a disinfectant. Trichloramine (NCl3) is the most volatile chloramine and is emitted into the air of indoor swimming-pools. Exposure to this substance was the suspected cause of outbreaks of short-incubation ocular and respiratory illness,1 2 but concentrations of NCl3 in pool environments were not known in these outbreaks. It is known, however, that acute respiratory and eye symptoms may occur among recreational swimmers in relation to measured levels of NCl3 in pool environments3 and NCl3 is considered to be the causative agent.
Only few and inconclusive studies have been performed on lung function among adults after exposures to measured levels of NCl3 in pool environments4 5 and additional studies are required.
Clara cell protein 16 (CC16) is an epithelial protective protein in peripheral lung tissue and changes in its serum levels are used as a biomarker of epithelial integrity.6 It has been shown to be decreased in relation to the frequency of pool attendance.7 However, changes in serum levels of CC16 have not been studied after short-term exposure to NCl3.
Thickett et al8 reported three cases of occupational asthma among British pool workers exposed to NCl3. There is a lack of epidemiological studies on asthma among those working in swimming-pool environments.
The objectives of the present study were (1) to perform a controlled human exposure study of lung function and biomarkers of pulmonary epithelial integrity in volunteers before and after exposure to indoor swimming-pool environments. (2) To perform an epidemiological study of self-reported asthma and subjective symptoms in a cohort of indoor swimming-pool workers.
Design, materials and methods
Air sampling and determination of NCl3
Exposure measurements in human exposure study
In the two pool environments where our study of volunteers and pool workers took place hypochlorite was used as disinfectant. Air samples were collected in the breathing zone: one sample for each 2 h exposure, in total 51 samples.
Determination of NCl3 at other indoor swimming-pools
Additional determinations of NCl3 were performed 2004–2008 at 10 different pool establishments (7 conventional ones and 3 ‘adventure water lands’) in northern Sweden with totally 30 indoor pools. Hypochlorite was used as disinfectant. At each swimming-pool, air was sampled during 3 h at three to four different locations in close vicinity of the pool. The equipment was mounted on a stand with the filter at a height of approximately 1.5 m. Sampling was performed on three different days during winter and three different days during summer.
Air collection and analysis
One litre/min of air was pumped through a filter (quartz filter QM-A 37 mm Whatman International Ltd, Maidstone, England). The filter was soaked in a solution of sodium carbonate and arsenic trioxide (AsO3) and dried as presented earlier.9 When NCl3 is collected on the filter it is reduced to chloride ion (Cl−).9 After sampling, the filters were extracted with 10 ml of ultrapure water, shaken for 30 min and filtered through a 13 mm syringe filter (IC Acrodisc, PALL). The chlorides were analysed in a suppressed ion chromatography system (Triatlon 900 autosampler, Spark, The Netherlands); ICSep AN1, Anion column (CETAC, Omaha, USA); SCX membrane suppressor column (Sequant, Umeå, Sweden); JD-21 conductivity detector (Costech Microanalytical Ltd, Tallin, Estonia). The eluent was 7.5 mM NaOH and the suppressor 5 mM H2SO4. Control samples of two known chloride concentrations (0.5 and 3 mg/l) and at least two blanks were run together with the samples in each run. The chloride concentrations in the blanks were subtracted from the concentration in the samples. The detection limits of NCl3 (1.78 and 1.18 µg/m3 for 2 and 3 h samplings, respectively) were determined as three times the mean SD of the amount collected on filters of 10 blanks. The limits of quantification (5.9 and 3.9 µg/m3 for 2 and 3 h samplings, respectively) were determined as 10 times the mean SD for the same blanks.
Human exposure study
Study groups
Group A: 37 healthy subjects (20 men and 17 women, mean age 24.5 years). They were not regular swimming-pool visitors and they had not visited a swimming-pool within 4 weeks before study start.
Group B: 14 workers at swimming-pools (5 men, 9 women, mean age 39.9 years).
All participants were non-smokers with normal lung function and had no history of allergy or pre-existing lung disease. Subjects were free of airway infection for ≥4 weeks prior to the first exposure and throughout the remainder of the study.
Study design
The study was conducted in a crossover control manner. Each volunteer was exposed to filtered air in an exposure chamber and on another occasion to an indoor pool environment. In the exposure chamber, located in a separate building away from swimming-pools, incoming air was adjusted to room temperature and filtered through a particle filter. The exposures were performed in random order. Successive exposures were separated by ≥2 weeks. The exposures were performed either between 8:00 h and 10:00 h or between 10:00 h and 12:00 h. All exposures (pool environment or filtered air) lasted for 2 h. The study subject was exercising on a bicycle ergometer with moderate exercise (minute ventilation 20 l/min/m2), during 15 min followed by 15 min of rest, that is, four periods of exercise and four periods of rest.
Lung function
Forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) was determined using a portable spirometer connected to a computer (KoKo Spirometer and KoKo DigiDoser; Pulmonary Data Service Instrumentation, Inc, Louisville, Kentucky, USA), calibrated in the morning and after every 10th measurement. FEV% was calculated as a percentage of FVC (FEV%=FEV1×100/FVC). Lung function was measured immediately before and after exposure in a room with non-detectable levels of NCl3 (<0.002 mg NCl3/m3) or in a room adjacent to the exposure chamber.
Blood sampling and determination of biomarkers
We obtained blood samples from the antecubital vein at 0 and 2 h, that is, before and after exposure, and at 4, 6 and 8 h. Peripheral blood was collected into BD Vacutainer tubes (BD, Plymouth, UK). Each sample was allowed to clot for 1–2 h at room temperature, centrifuged at 3000×g and serum was transferred to cryotubes and frozen at –80°C. These samples were sent to the Industrial Toxicology Unit at the Catholic University of Louvain in Brussels (IUTUCL), Belgium for determination of Clara Cell protein 16 (CC16) and surfactant protein D (SPD). CC16 was determined by latex immunoassay using a rabbit anti-CC16 antibody (Dakopatts, Glostrup, Denmark) and CC16 purified at (IUTUCL) as standards.10 11 All samples were run in duplicate at two different dilutions. The between-run and within-run coefficients of variation range 5–10% and results are comparable with ELISA methods.11 SPD determinations were performed using the Biovendor ELISA kit (Biovendor, Heidelberg, Germany). Analyses were done in duplicate as recommended by the manufacturer.
Total IgE was determined in human serum by a double-antibody sandwich ELISA method (Human IgE ELISA kit, Immunology Consultants Lab; Inc, Newberg, Oregon, USA). The quantity of IgE in the samples was interpolated from a standard curve.
Statistical analyses
All data from CC16 measurements were corrected for diurnal variation according to Helleday et al12 and recalculated to correspond to 7:00 h. CC16(corr)=CC16+0.582*T−0.032*T2. T is the time after 7:00 h when the blood sample was taken. Because CC16 values are highest in the morning,12 corrected CC16 values were somewhat greater than measured values.
Statistics: We used repeated measures analyses of variance (Huynh-Feldt corrected) with time and exposure as within-subject factors and group as between-subject factor. Paired t test or Wilcoxon signed rank test was used when comparing exposures to filtered air and pool environment at baseline (0 h) and after exercise (2 h). SPSS V.17.0 was used to perform the statistical analyses. A p value of 0.05 was considered statistically significant.
Epidemiological study
Population
The epidemiological study group included 1741 persons in the Swedish Census of Population and Housing 1990 who had indicated that they worked at swimming-pools. Early 2007 a questionnaire was mailed to them. There was one reminder.
Questionnaire
Questions dealt with: year hired as a pool worker, time periods in various jobs, time spent in swimming-pool environments, various symptoms from the respiratory tract and mucous membranes of the eyes and possible use of medication for asthma 589 women and 513 men, age 30−>80 years (mean age 51.2 years, SD 12) responded (63%). Among 50 non-responders, interviews were performed via telephone. There was a lower prevalence of asthma and respiratory symptoms among the non-responders, not statistically significant.
‘Self reported asthma’ was derived from a positive answer to the following question: “Do you suffer from asthma or have you suffered from asthma?” Whether a person's asthma started before or after he/she was hired as a pool worker was derived from the combination of questions about year hired as pool worker and when the first symptoms of asthma occurred. Under the general heading ‘Acute symptoms when working in a swimming-pool environment’ there was a question “How large a part of a working day did you usually spend in the swimming-pool environment Hours?”
In a nested case–control study within this cohort, 44 cases of self-reported asthma occurred after the person was hired as a pool worker. In total 128 age-matched and sex-matched controls were selected within the cohort (mean age 50.5 years, SD 10.7).
Exposure assessment
On the basis of information on work titles given by each individual, exposure was classified into three different categories; 0, 1 or 2; where 0 stands for no exposure, 1 for low exposure and 2 for high exposure. The exposure level is not an estimate of the concentration of NCl3 in air but is based on the average time during a workday the individual spent in the pool area. Those within category 0 did not spend any time in a pool area, for example, a cashier. A person within category 1 did occasionally spend some time in the pool area. A manager of a swimming-pool or a technician belongs to this category. Individuals belonging to category 2 were those spending most of the workday in the pool area, for example, a swimming teacher, or a swimming-pool worker.
Statistics
Fisher's test was used to test differences between proportions. Conditional logistic regression was used for analyses in the nested case–control study and logistic regression for analyses of asthma in relation to years worked in swimming-pool environments. All statistical analyses were performed using the statistical package R, V.2.9.0 (www.r-project.org). p Values equal to or less than 0.05 were considered statistically significant.
Ethics
The project was approved by the Regional ethical review board in Umea, Sweden (Dnr 05-044M) and volunteers provided written informed consent. The study was carried out according to the declaration of Helsinki.
Results
Air sampling
Experimental exposure (human exposure study)
The NCl3 levels during the experimental exposures were
Group A: mean 0.23 mg/m3 (SD 0.09)
Group B: mean 0.15 mg/m3 (SD 0.04)
Other swimming-pools
NCl3 concentrations in air at the 10 different indoor swimming-pool establishments were between 0.001 and 0.77 mg/m3, median 0.18 mg/m3, arithmetic mean (AM) 0.21 mg/m3 (n=129). The AM concentrations of NCl3 in each of the 10 different pool establishments were between 0.09 and 0.32 mg/m3. There was no difference in NCl3 concentrations during summer compared with winter conditions (results not shown).
Human exposure study
Lung function
Group A
Measured FEV1 volumes among healthy volunteers as well as the difference before and after 2 h of exposure to pool environment or filtered air are summarised in table 1. There was a small, statistically significant decrease (p=0.01) in FEV1 (mean decrease=0.05 litre) after exposure to swimming-pool air. After exposure to filtered air there was a slight, not statistically significant increase in FEV1 (mean increase 0.01 litre). When comparing the differences (Δ-values) in FEV1 before and after exposure to pool environment with the Δ-values for exposure to filtered air in the same individuals, the difference between Δ-values was statistically significant (p=0.01).
Table 1.
Healthy volunteers(n=37): FEV1 (forced expiratory volume, litre during 1 s) and FEV% (FEV1×100/forced vital capacity) measured before and after 2 h exercise in filtered air and pool air, respectively. Mean±SD. Mean differences (before-after) within parentheses.
| Expiratory volume | Exposure in filtered air |
Exposure in pool air |
Difference in changes≠ | ||||
|---|---|---|---|---|---|---|---|
| before | after | mean difference Δ-values | before | after | mean difference Δ-values | ||
| FEV1 | 4.10±0.85 | 4.11±0.87 | (−0.01)° | 4.14±0.87 | 4.09±0.86 | (0.05)** | p=0.01 |
| FEV% | 80.5±5.8 | 80.9±5.2 | (−0.4)° | 80.7±5.3 | 79.9±5.3 | (0.8)* | p=0.004 |
**FEV1 significantly lower after exposure in pool air, p=0.01
*FEV% lower after exposure to pool air, p=0.05.
°Indicates no statistically significant difference.
≠Statistical significance of difference between Δ-values in filtered air and in pool air.
FEV% values among healthy volunteers are also given in table 1. After exposure to pool air, there was a small decrease (0.8 FEV%) that was marginally statistically significant (p=0.05). After exposure to filtered air, there was a small (statistically non-significant) increase in FEV% values. When the individual differences (Δ-values) of FEV% before and after exposure to pool air were compared with the corresponding Δ-values in filtered air, a statistically significant difference was demonstrated (p=0.004, paired t test). Airway obstruction is usually defined as FEV% below 70 (www.goldcopd.com). Only one value was below 70 (after exposure) among the healthy volunteers.
Group B
In table 2, FEV1 values for the swimming-pool workers are summarised. After exposure to pool air there was a small and not statistically significant decrease in FEV1, 0.01 litre. There was also a small decrease in FEV1 after exposure to filtered air (0.05 litre, p=0.054). When considering the FEV% values for the workers (table 2) before and after exposure to pool air, there was a statistically significant decrease of 1.36% (p=0.003). After exposure to filtered air the small decrease in FEV% of 0.43% was not statistically significant. Only two FEV% values among the pool workers (one before and one after exposure) were below 70. When comparing the Δ-values in filtered air with those in pool air no statistically significant differences were found. The lack of such differences may be partly related to the lower exposure level in group B compared to Group A.
Table 2.
Swimming-pool workers (n=14): FEV1 (forced expiratory volume, litre during 1 s) and FEV% (FEV1×100/forced vital capacity) measured before and after 2 h exercise in filtered air and pool air, respectively. Mean±SD. Mean differences (before-after) within parentheses.
| Expiratory volume | Exposure in filtered air |
Exposure in pool air |
Difference in changes≠ | ||||
|---|---|---|---|---|---|---|---|
| before | after | mean difference Δ-values | before | after | mean difference Δ-values | ||
| FEV1 | 3.56±0.99 | 3.51±0.91 | (0.05)° | 3.59±0.93 | 3.57±0.92 | (0.014)° | Non-significant |
| FEV% | 78.86±6.3 | 78.43±5.42 | (0.43)° | 79.1±4.1 | 77.8±5.1 | (1.36)* | Non-significant |
*FEV% lower after exposure to pool air, p=0.003 (Wilcoxon signed rank test).
°Indicates no statistically significant difference.
≠Statistical significance of difference between Δ-values in filtered air and in pool air.
Biomarkers of pulmonary epithelial integrity:
CC16, Group A
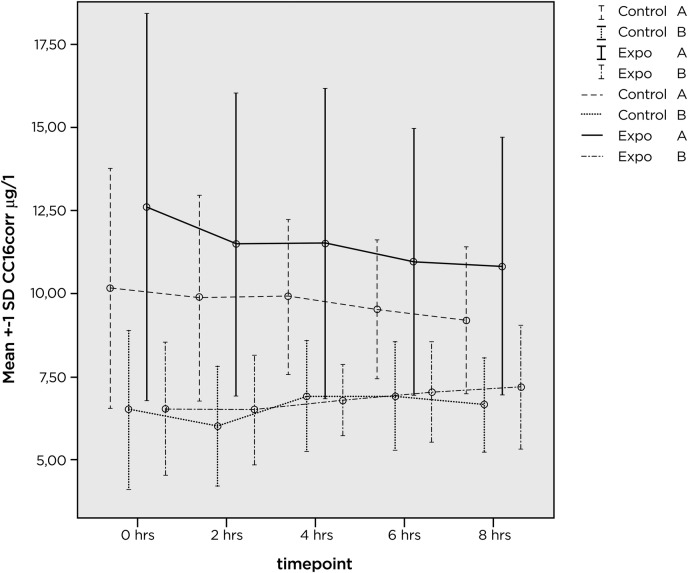
Mean CC16corr values and related SDs in previously unexposed healthy volunteers, are shown in figure 1 for 33 of the participants in group A. For the remaining four persons, values were missing and they were therefore excluded from analysis.
Figure 1.
Mean values (µg/l) and SD for CC16corr at various time points before (0 h), immediately after exposure (2h) and the following 2 (4 h), 4 (6 h) and 6 h (8 h). Values are shown for the previously unexposed group of healthy volunteers (A) after exposure in a pool environment, after exposure to filtered air (two upper set of lines and bars). The two lower lines and related bars represent exposure in pool environment and filtered air for group B, recruited among pool workers with several years exposure to pool environments.
At baseline (0 h), mean CC16corr=12.6 µg/l before pool exp (0 h) and 10.3 µg/l immediately before (0 h) exposure to filtered air. This difference (p=0.018, paired t test) is difficult to explain because the same volunteers were exposed to both pool environment and filtered air and they were randomly assigned to either exposure.
CC16, Group B
Results are shown in figure 1. The mean CC16corr was 6.5 µg/l before both pool and filtered air exposures.
The difference between groups A and B persisted during and after exposure (0–8 h) and is statistically significant (p<0.001 repeated measures analysis of variance on log transformed data). There is also a different change with time. Group A decreases with time and group B increases with time. The difference in trend is statistically significant p=0.038.
The decrease with time in group A during and after exposure to pool environment as well as filtered air is statistically significant (p<0.05, GLM repeated analysis model). In groups A and B there is no statistically significant difference in change with time between pool exposure and filtered air. For improved analysis, values were converted to their natural logarithms, SDs decreased, providing improved statistical conditions, but no statistically significant effect of exposure could be shown (data not shown).
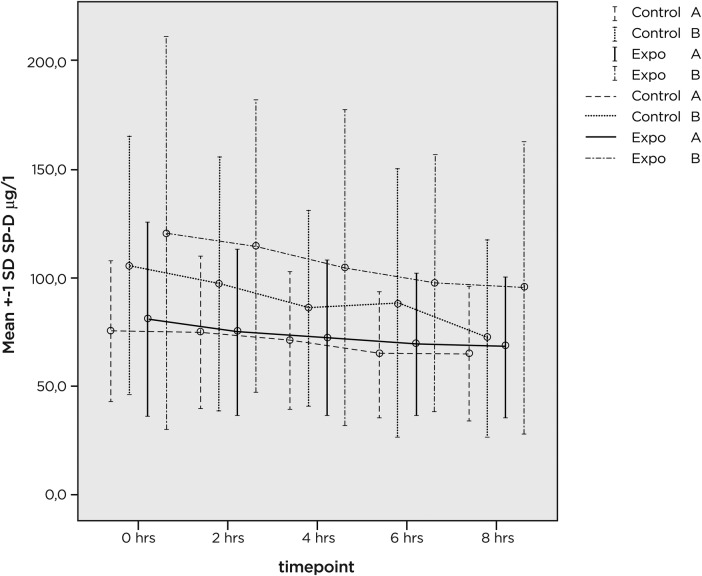
SPD values, shown in figure 2, also display a change with time, with lower values with increasing time intervals from initiation of exposure. Considering the log-transformed SPD variable, there was a difference (p<0.05) before and after exposure (ie, SPD values were higher at 0 than at 2 h) and there was a further decrease (p<0.01) with time at 2–8 h (figure 2). This decrease was similar for exposure to pool air and filtered air. In groups A and B we found no statistically significant changes in SPD values in relation to exposurex.
Figure 2.
Mean and SD for measured surfactant protein D values (μg/l) at various time points (0–8 h) of the study. Exposure to pool environment or filtered air took place for 2 h (between 0 and 2 h). Group A: previously unexposed healthy volunteers. Group B: pool workers.
IgE
The median IgE value was low 1 mg/l in group A and 0.0 in group B.
Epidemiological study
There was a statistically significant relationship between the number of hours, during an average day, spent in the swimming-pool environment and the percentage of workers reporting acute symptoms during work (p<0.01; logistic regression). Frequent symptoms were: dyspnoea (13%), cough (23%), nose irritation (29%), throat irritation (24%) and eye irritation (37%).
In the nested case–control study, the OR for asthma was 2.53 (95% CI 0.89 to 7.19) for persons with exposure level 2 (114 controls and 42 cases) compared with persons exposed to level 0 or 1 (14 controls and 2 cases). After correction for heredity, the corresponding numbers were: OR 2.31 (95% CI 0.79 to 6.74).
These values refer to cases of self-reported asthma occurring after they started pool work, compared with controls without asthma.
Among individuals who worked more than 1 year, there was a tendency to a reduced risk of developing asthma in relation to the number of years of work in swimming-pool environments. Only asthma cases that occurred after they started to work as pool workers were considered. This tendency was, however not, statistically significant p=0.07.
Discussion
Our observations of statistically significant decreases in FEV1 and FEV% in previously non-exposed volunteers and in FEV% in pool workers after exposure to pool air are the first such observations in adults. Carbonelle et al4 reported an increase in FEV1/VC among children and a non-statistically significant decrease in adults (n=13) after they had attended a chlorinated pool. Carbonelle et al4 found FEV1/VC to be unchanged in 11 young adults after swimming in a non-chlorinated pool and slightly, but not statistically significantly decreased after swimming in a chlorinated pool. The lack of statistically significant decrease may be related to the fact that only 11 adults were studied,4 while the statistically significant decrease in our study was based on 37 previously unexposed healthy volunteers. The findings in volunteers were further supported by statistically significant differences in Δ-values. In the 14-pool workers, only one measurement of lung function (FEV%) was statistically significantly decreased and no statistically significant difference was seen when Δ-values were compared. A possible effect in pool workers at the exposure level of our study (0.15 mg/m3) may be considered uncertain. Very few FEV% values were below 70 (indicating no clinically significant airway obstruction within the study group). The reduction in FEV% seen after exposure in pool air here, albeit small, may be a sign of an obstructive airway effect. In children, Bernard et al13 found a statistically highly significant relationship between cumulative pool attendance during kindergarten and PEF 15 (post-exercise reduction of peak expiratory flow by 15%), providing supportive evidence of airway effects of exposure to chlorinated pool environments.
CC16 levels in serum increase when lung epithelium permeability is adversely affected by air pollutants or other lung toxicants.6 10 14 15 On the contrary, reduced levels of CC16 in lung lavage fluid occur in several lung disorders, probably due to a decrease in the production of CC16 as a consequence of a depletion of Clara cells.16 We found a statistically significant difference in the serum level of CC16 between pool workers compared to volunteers. This finding is consistent with our previous finding of a lower CC16 value in school children frequently attending indoor swimming-pools than in those with a low attendance at such pools.7 The difference between workers and previously unexposed healthy volunteers may be due to the older age of the workers but is more likely due to repeated exposures because a similar difference occurred among school children and all these differences may be due to a depletion of Clara cells. We did not find any statistically significant exposure-related changes in concentrations of the biomarkers of pulmonary epithelial integrity (CC16 and SPD) after exposure to pool air for 2 h. The lack of such an exposure-related change was probably due to the relatively short exposure duration and low exposure level of NCl3. Another possible explanation is that NCl3 acts preferentially in the more proximal parts of the respiratory tract, inducing a mild constriction of the central airways, but with less interference in the terminal bronchioles, where the Clara cells are located. In previous studies of volunteers exposed to ozone,6 we found both a decrease in FEV1 and an increase in serum CC16 concentrations after exposure.
deally, all exposures should have been performed at the same hour, because it is known that CC16 has diurnal variation.12 However, for practical reasons exposures were started at somewhat different times during the day and all CC16 values in the present study were corrected for diurnal variation.12 Such correction is essential, but introduces a certain element of uncertainty. In spite of such correction, there was a statistically significant decrease with time of experiment from 0 to 8 h in group A (regardless of exposure to NCl3). This indicates that the real diurnal variation exceeded the one assumed in the employed correction calculation. For group B there is an opposite trend with time, possibly related to an inadequate correction of the values in this group. The pool workers were older and had been more exposed to NCl3 during many years of work in pool environments. Our data on SPD, with a statistically significant decrease with time between 0 and 8 h, confirm previously reported17 diurnal variation.
The absence of exposure-related effects (after 2 h exposure) on serum concentrations of CC16 and SPD in combination with small, statistically significant decreases in FEV1 and FEV% show that the 2 h exposure level in this experiment can be regarded as the Lowest-Observed-Adverse-Effect-Level on the lung for this group of volunteers. It should be borne in mind that individuals with increased sensitivity to adverse respiratory effects, like those with pre-existing asthma, were not included in the present study. Our observation may be of use in relation to administrative action in setting exposure limits for NCl3. To our knowledge, no health-based limit values for occupational or environmental exposures have yet been set for NCl3. A technical value of 0.2 mg/m3 was recently recommended in Germany.18
Bernard et al19 showed that serum total IgE was a factor determining the risk of adverse pulmonary effects after exposure to pool environments. Serum levels of total IgE in the volunteers and workers of our study were low. The absence of an increased level of total serum IgE among the present volunteers indicates that individuals with possibly increased sensitivity due to increased IgE had been successfully excluded. Further studies on persons with elevated serum IgE would be of interest. Another group that may suffer respiratory effects at lower air concentrations of NCl3 is competitive swimmers because their breathing volumes exceed those of the volunteers in the present study. Helenius et al20 found increased respiratory symptoms and bronchial responsiveness in elite swimmers.
Our study indicates that employees in Swedish indoor pools are exposed to approximately the same level of NCl3 as employees in France and Belgium. We found median NCl3 concentrations of 0.18 mg/m3 (mean 0.21 mg/m3) in 10 different premises, while Hery et al9 reported 0.14–0.91 mg/m3 and Massin et al3 reported a mean of 0.24 mg/m3 in Public pool environments and 0.67 mg/m3 in establishments with private owners. There are no previous published data on NCl3 exposure in Swedish indoor pools. The work environment, that is, ventilation and the use of sodium hypochlorite as disinfectant has probably not changed during the past few decades. This makes it reasonable to estimate that pool workers have been exposed to NCl3 at approximately the same levels as reported in this study.
In the epidemiological part of the present study, we found a statistically significant relationship between the number of hours spent in swimming-pool environments and the percentage of workers reporting acute symptoms when working. The percentage varied from 13% for dyspnoea to 37% for eye irritation. These findings are in accordance with previous observations in France3 and Holland.1 These are subjective symptoms reported in a questionnaire also collecting exposure information and there is a possibility for recall bias. However, similar clear outcomes have been reported also in other studies.1 3
Our nested case-referent study found an OR for asthma of 2.53 (95% CI 0.89 to 7.19) for workers with more extensive exposure in pool areas (exposure level 2 compared to persons with exposure level 0 or 1). After correction for heredity the corresponding numbers were: OR 2.31 (95% CI 0.79 to 6.74). These values refer to cases of self-reported asthma occurring after they started to work in swimming-pool environments, compared to controls without asthma.
Cases of asthma in pool workers have been reported in the UK,8 but no epidemiological evidence has been reported. The findings of the present study did not reach statistical significance and provide only limited support for a causal relationship between asthma and work at indoor swimming–pools. Individuals who are fit for these types of jobs tend to exercise more regularly and may notice respiratory symptoms; this may contribute to confounding. The fact that there was a tendency towards a decreasing risk of asthma in workers with longer work history may indicate a healthy worker effect due to the irritating properties of NCl3 in pool environments. A recent study21 reported a higher prevalence (4.5%) of new-onset asthma among recreational swimmers with >320 h of cumulative pool attendance compared to 0.4% among swimmers with <320 h of pool attendance, thus supporting a role for exposure at chlorinated pools for development of asthma. In children engaged in recreational swimming, a statistically significant relationship was shown between cumulative attendance at indoor swimming-pools and the probability of developing asthma in those with increased total IgE in serum.13 19 Attendance at chlorinated pools before the age of 2 y increased the risk of bronchiolitis and asthma.22
The present findings support the previously advanced hypothesis7 13 19 21 that exposures to NCl3 levels commonly occurring in indoor swimming-pool environments can cause acute airway and mucosal symptoms as well as changes in lung function and deterioration of asthma.
Conclusions
For the first time in adults, statistically significant but small decreases in lung function were found in previously unexposed subjects after exposure to pool air containing 0.23 mg/m3 of NCl3 compared to filtered air. The changes in lung function occurred in adults without any signs of allergy and with low IgE values. In a cohort of pool workers we found exposure-related acute mucous membrane and respiratory symptoms. An increased OR for asthma (OR 2.31, 95% CI 0.79 to 6.74) was indicated in workers in the highest exposure category compared to lower exposures. Our observations give support to a previously advanced hypothesis that current exposures to NCl3 can cause adverse effects on mucous membranes and lungs of humans and contribute to the development of asthma. Further research in sensitive groups is warranted.
Supplementary Material
Footnotes
Contributors: GN, N-GL, BF, AH, BJ-sL, JN, MS, AB, LN, AB, XD, HB and KE contributed as follows: (1) substantial contributions to conception and design, acquisition of data or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content and (3) final approval of the version to be published.
Funding: The Swedish Council for Working Life and Social Research (FAS) and Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS).
Competing interests: None.
Ethics approval: Regional ethical review board in Umea, Sweden, Dnr 05-044M.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no additional data available.
References
- 1.Jacobs JH, Spaan S, van Rooy GB, et al. Exposure to trichloramine and respiratory symtoms in indoor swimming pool workers. Eur Respir J 2007;29:690–8 [DOI] [PubMed] [Google Scholar]
- 2.Bowen AB, Kile JC, Otto C, et al. Outbreaks of short-incubation ocular and respiratory illness following exposure to indoor swimming pools. Environ Health Perspect 2007;115:267–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massin N, Bohadana AB, Wild P, et al. Respiratory symtoms and bronchial responsiveness in lifeguards exposed to nitrogen trichloride in indoor swimming pools. Occup Environ Med 1998;55:258–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carbonnelle S, Francaux M, Doyle I, et al. Changes in serum pneumoproteins caused by short-term exposures to nitrogen trichloride in indoor chlorinated swimming pools. Biomarkers 2002;7:464–78 [DOI] [PubMed] [Google Scholar]
- 5.Carbonnelle S, Bernard A, Doyle IR, et al. Fractional exhaled NO and serum pneumo-proteins after swimming in a chlorinated pool. Med Sci Sports Exerc 2008;40:1472–6 [DOI] [PubMed] [Google Scholar]
- 6.Blomberg A, Mudway I, Svensson M, et al. Clara cell protein as a biomarker for ozone –induced lung injury in humans. Eur Respir J 2003;22:883–8 [DOI] [PubMed] [Google Scholar]
- 7.Lagerkvist BJ, Bernard A, Blomberg A, et al. Pulmonary epithelial integrity in children: relationship to ambient ozone exposure and swimming pool attendence. Environ Health Perspect 2004;112:1768–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thickett KM, McCord JS, Gerber JM, et al. Occupational asthma caaused by chloramines in indoor swimming-pool air. Eur Respir J 2002;19:827–32 [DOI] [PubMed] [Google Scholar]
- 9.Hery M, Hecht G, Gerber JM, et al. Exposure to chloramines in the atmosphere of indoor swimming pools. Ann Occup Hyg 1995;39:427–39 [Google Scholar]
- 10.Bernard A, Marchandise FX, Depelchin S, et al. Clara cell protein in serum and bronchalveolar lavage. Eur Resp J 1992;5:1231–8 [PubMed] [Google Scholar]
- 11.Hermans C, Aly O, Nyberg BI, et al. Determinants of Clara cell protein (CC16) concentration in serum: a reassessment with two different immunoassays. Clin Chim Acta 1998;272:101–10 [DOI] [PubMed] [Google Scholar]
- 12.Helleday R, Segerstedt B, Forsberg B, et al. Exploring the time dependence of serum Clara cell protein as a biomarker of pulmonary injury in humans. Chest 2006;130:672–5 [DOI] [PubMed] [Google Scholar]
- 13.Bernard A, Carbonelle S, Michel O, et al. Lung hyperpermeability and asthma prevalence in schoolchildren : unexpected associations with the attendence at indoor chlorinated swimming pools. Occup Environ Med 2003;60:385–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermans C, Bernard A. Clara cell protein: characteristics and potential applications as marker of lung toxicity. Biomarkers 1996;1:3–8 [DOI] [PubMed] [Google Scholar]
- 15.Broeckaert F, Arsalane K, Hermans C, et al. Lung epithelial damage at low concentrations of ambient ozone. Lancet 1999;353:900–1 [DOI] [PubMed] [Google Scholar]
- 16.Hermans C, Bernard A. State of the art. Lung epithelium-specific proteins. Characteristics and potential applications as markers. Am J Respir Crit Care Med 1999;159:646–78 [DOI] [PubMed] [Google Scholar]
- 17.Hoegh SV, Sorensen GL, Tornoe I, et al. Long-term stability and circadian variation in circulating levels of surfactant protein D. Immunobiology 2010;215:314–20 [DOI] [PubMed] [Google Scholar]
- 18.German Working Group on Indoor Guide Values of the Federal Environment Agency Risk assessment of trichloramine in the air of indoor swimming pools. Bundesgesundheitsbl 2011;54:997–1004 (in German with abstract in English) [Google Scholar]
- 19.Bernard A, Carbonnelle S, De Burbure C, et al. Chlorinated pool attendence, atopy, and the risk of asthma during childhood. Environ Health Perspect 2006;114:1567–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helenius IJ, Tikkanen HO, Sarna S, et al. Astma and increased bronchial responsiveness in elite athletes: atopy and sports event as risk factors. J Allergy Clin Immunol 1998;101:646–52 [DOI] [PubMed] [Google Scholar]
- 21.Ferrari M, Schenk K, Mantovani W, et al. Attendance at chlorinated indoor pools and risk of asthma in adult recreational swimmers. J Sci Med Sport 2011;14:184–9 [DOI] [PubMed] [Google Scholar]
- 22.Viosin C, Sardella A, Marcucci F, et al. Infant swimming in chlorinated pools and the risk of bronchiolitis, asthma and allergy. Eur Resp J 2010;36 41–7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.