Abstract
Objectives
To determine the prevalence of autonomic dysfunction (dysautonomia) among patients with primary Sjögren's syndrome (PSS) and the relationships between dysautonomia and other clinical features of PSS.
Methods
Multicentre, prospective, cross-sectional study of a UK cohort of 317 patients with clinically well-characterised PSS. Symptoms of autonomic dysfunction were assessed using a validated instrument, the Composite Autonomic Symptom Scale (COMPASS). The data were compared with an age- and sex-matched cohort of 317 community controls. The relationships between symptoms of dysautonomia and various clinical features of PSS were analysed using regression analysis.
Results
COMPASS scores were significantly higher in patients with PSS than in age- and sex-matched community controls (median (IQR) 35.5 (20.9–46.0) vs 14.8 (4.4–30.2), p<0.0001). Nearly 55% of patients (vs 20% of community controls, p<0.0001) had a COMPASS score >32.5, a cut-off value indicative of autonomic dysfunction. Furthermore, the COMPASS total score correlated independently with EULAR Sjögren's Syndrome Patient Reported Index (a composite measure of the overall burden of symptoms experienced by patients with PSS) (β=0.38, p<0.001) and disease activity measured using the EULAR Sjögren's Syndrome Disease Activity Index (β=0.13, p<0.009).
Conclusions
Autonomic symptoms are common among patients with PSS and may contribute to the overall burden of symptoms and link with systemic disease activity.
Introduction
Primary Sjögren's syndrome (PSS) affects 0.05–0.5% of the adult population depending on selection bias and the classification criteria used.1 It is estimated that ∼70% of patients with PSS complain of significant fatigue, with many stating it as the most disabling symptom of the disease.2 We and others have reported several biological associations of fatigue in chronic and autoimmune diseases, with dysfunction of the autonomic nervous system being one of the most notable biological factors.3–5
Several studies have demonstrated an association between autonomic dysfunction (dysautonomia) and PSS,6–11 but conflicting data have also been reported.12–14 However, the relationship between autonomic dysfunction and fatigue has been examined in just two studies with 27 patients each.6 9 Interestingly, data from both of these studies suggest a link between autonomic dysfunction and fatigue. However, the predictors of dysautonomia have not been fully investigated.
In this study, we determined the prevalence of autonomic symptoms in a large, multicentre cohort of patients with clinically well-characterised PSS in the UK using a well-validated comprehensive autonomic symptom-assessment tool. In addition, we explored the relationship between symptoms of autonomic dysfunction and disease activity, patient-reported outcome measures, as well as other potentially relevant clinical and laboratory variables.
Materials and methods
Patient groups
All patients with PSS in this study are participants of the UK Primary Sjögren's Syndrome Registry (UKPSSR, www.sjogrensregistry.org).15 The UKPSSR is an on-going cohort of patients with PSS funded by the Medical Research Council, UK, which aims to facilitate research and clinical trials. All participants fulfil the American European Consensus Group (AECG) classification criteria.16 Informed consent was obtained from all patients according to the principles of the Helsinki Declaration. All clinical and laboratory data were collected prospectively at the time of recruitment as previously described.15 Embedded within the design of the UKPSSR are several sub-studies aiming to address different clinical questions, one of which is the prevalence of autonomic dysfunction using the Composite Autonomic Symptom Scale (COMPASS).17 Participation in this sub-study is optional. At the time of analysis, 474 patients had been recruited to the UKPSSR, of which 396 (83.5%) participated in the COMPASS assessment. Complete datasets for COMPASS were available for 317 patients. Only those with complete datasets for COMPASS were subjected to a full analysis because the COMPASS total score cannot be accurately determined with incomplete data.
Each PSS participant with complete COMPASS data was matched case by case by age (within 2 years) and sex from an existing community control cohort of 596 subjects who had completed COMPASS assessments established by one of the investigators (JLN).18 19
Assessment of autonomic function
Composite Autonomic Symptom Scale (COMPASS)
The severity of autonomic symptoms was assessed using COMPASS,17 which consists of 73 questions grouped into domains describing specific autonomic nervous system symptoms. Each domain is scored on the basis of the presence, severity, distribution, frequency and progression of symptoms. The 10 domains are: orthostatic intolerance, vasomotor, secretomotor, gastroparesis, autonomic diarrhoea, constipation, bladder, pupil and focusing, sleep disorder and syncope. COMPASS also includes an optional male erectile dysfunction domain, which was not included in this study because PSS predominantly affects females. The individual domain scores are then weighted according to clinical relevance as described in the original derivation and validation of the questionnaire.17 The sum of the individual scores provides an indicator of overall symptom burden (COMPASS total score). In all domains, a higher score indicates increased severity of the autonomic symptom. Two domains (‘understatement’ and ‘overstatement’ scales) are incorporated into the assessment tool to detect over- or under-reporting of symptoms between individuals and are scored independently.
Assessment of other clinical features of PSS
The following data are collected for all UKPSSR subjects regardless of their participation in the COMPASS assessments: EULAR Sjögren's Syndrome Disease Activity Index (ESSDAI),20 EULAR Sjögren's Syndrome Patient Reported Index (ESSPRI)21 (measurement of the overall burden of symptoms), EULAR Sicca Scale21 (measurement of overall severity of dryness), Profile of Fatigue (Pro-F)22–24 (measurement of fatigue), Epworth Sleepiness Scale (ESS)25 (measurement of daytime sleepiness), Hospital Anxiety and Depression Scale (HAD)26 (assessment of anxiety and depression), EuroQol-5 Dimension (EQ-5D)27 (www.euroqol.org) (measurement of health-related quality of life). Further details on these instruments are provided in online supplementary file 1.
Autoantibodies
Autoantibodies against Ro (SSA) or La (SSB) antigens were measured in a single laboratory using Euroassay anti-ENA Profile Plus according to manufacturer's protocol (Euroimmun AG, Lubeck, Germany).
Ethics approval
Ethics approval was granted by the North West Research ethics committee for the establishment of the UKPSSR and analysis of the data and samples.
Statistical analysis
All data were analysed using GraphPad or SPSS software. Not all patients in the UKPSSR cohort completed the COMPASS questionnaire. Given the large number of variables, differences between the three groups were first tested using multivariate analysis of variance. If Wilks' lambda was statistically significant (p<0.05), univariate analyses of variance were performed to identify which variables contributed to the difference. Where these were significant (p<0.05), comparisons were made between the COMPASS group and the incomplete-COMPASS and non-COMPASS groups. Raw p values unadjusted for multiplicity are reported throughout, permitting the application of preferred adjustments.
PSS and control groups were compared using Wilcoxon matched pairs test (comparison of medians) or Fisher's exact test (comparison of percentages). Pearson's and Spearman's tests were used for univariate correlation analysis for non-parametric and parametric data, respectively. To identify independent predictors for autonomic symptoms, multivariate stepwise regression analysis was performed using COMPASS total score as the dependent variable, and stepwise logistic regression analysis was performed using the COMPASS total score of 32.5 as cut-off value for the presence or absence of dysautonomia.3
Results
Patient characteristics
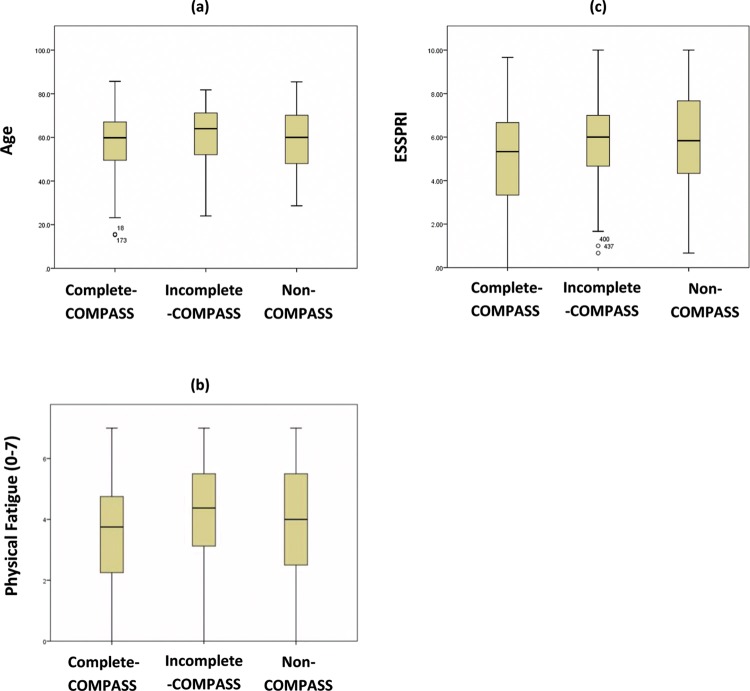
The median age of the group of 317 (298 female, 19 male) participants with PSS (95.6% Caucasian) who had complete datasets on COMPASS was 59.9 years (IQR 49.5–67.1), with median disease duration of 6.1 years (IQR 2.5–11.9). Disease duration was calculated as time from diagnosis using the AECG criteria. Most of these patients (267; 84.2%) had autoantibodies against Ro (SSA) and/or La (SSB). The median (IQR) age of the community control group was 60.0 years (49.5–67.0). To determine whether those completing the COMPASS (‘complete- COMPASS’ group) differed clinically from those with incomplete COMPASS (‘incomplete-COMPASS’ group) or no COMPASS datasets (‘non-COMPASS’ group), multivariate analysis of variance was performed, which revealed significant differences between the three groups (p=0.002). Univariate analyses confirmed several significant differences, in particular age, physical fatigue and ESSPRI scores (p<0.01), with the complete-COMPASS group being younger, with lower levels of fatigue and ESSPRI scores (online supplementary table S1(a)–(c)). Box plots of the age, physical fatigue and ESSPRI of the three groups are shown in figure 1.
Figure 1.
Box plots showing the median, interquartile ranges, extreme ranges and extreme values for (A) age, (B) physical fatigue and (C) ESSPRI of the complete-COMPASS group and the remaining groups of the cohort. COMPASS, Composite Autonomic Symptom Scale; ESSPRI, EULAR Sjögren's Syndrome Patient Reported Index.
Symptoms of autonomic dysfunction
The COMPASS domain and total scores for the patients with PSS and community controls are shown in figure 2 and table 1. The median (IQR) COMPASS total score for the subjects with PSS was 2.4-fold higher (PSS vs control: 35.5 (20.9–46.0) vs 14.8 (4.4–30.2), p<0.0001). Using the COMPASS total score of >32.5 as the diagnostic criterion for autonomic dysfunction,3 we found that 173 of the 317 (54.6%) patients with PSS compared with 20% of the community controls had evidence of autonomic dysfunction (p<0.0001). Of the 10 COMPASS domains, significantly higher scores were observed among the PSS group than among the controls in seven domains. The domain scores for autonomic diarrhoea, constipation and syncope were comparable between patients with PSS and controls, suggesting that patients with PSS experience many, but not all, symptoms that are related to autonomic dysfunction.
Figure 2.
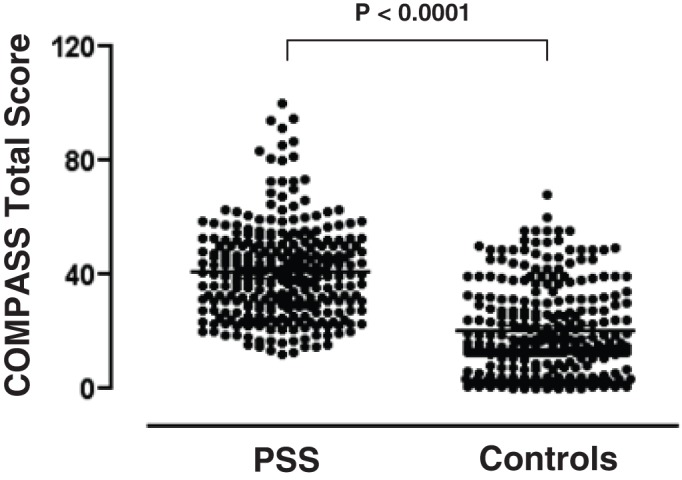
The COMPASS total scores of the patients with primary Sjögren's syndrome (PSS) and the age- and sex-matched community controls. Each dot represents the individual COMPASS total score of the groups. COMPASS, Composite Autonomic Symptom Scale.
Table 1.
Data on COMPASS total and individual domain scores of patients with PSS and community controls
| COMPASS score | |||
|---|---|---|---|
| COMPASS domain | PSS | Controls | PSS vs controls* |
| Orthostatic intolerance | 12.5 (2.5–17.5) | 0.0 (0.0–12.5) | <0.0001 |
| Vasomotor | 0.0 (0.0–5.0) | 0.0 (0.0–0.0) | <0.0001 |
| Secretomotor | 7.5 (7.5–9.0) | 1.5 (0.0–4.5) | <0.0001 |
| Gastroparesis | 0.0 (0.0–1.7) | 0.0 (0.0–0.0) | 0.0001 |
| Autonomic diarrhoea | 0.0 (0.0–8.0) | 2.0 (0.0–8.0) | 0.21 |
| Constipation | 1.5 (0.0–3.0) | 1.5 (0.0–3.0) | 0.25 |
| Bladder control | 2.0 (0.0–4.0) | 2.0 (0.0–2.0) | 0.0002 |
| Pupil and focusing | 2.0 (1.0–3.0) | 0.5 (0.0–1.5) | <0.0001 |
| Sleep disorders | 1.5 (0.0–2.3) | 0.0 (0.0–1.5) | <0.0001 |
| Syncope | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.35 |
| COMPASS total | 35.5 (20.9–46.0) | 14.8 (4.4–30.2) | <0.0001 |
| Understatement | 1.7 (0.0–3.3) | 6.0 (2.0–8.0) | <0.0001 |
| Overstatement | 0.0 (0.0–1.8) | 0.0 (0.0–7.0) | <0.0001 |
Values are median (IQR).
Wilcoxon matched pairs test.
COMPASS, Composite Autonomic Symptom Scale; PSS, primary Sjögren's syndrome.
Two of the ten questions in the secretomotor domain assess symptoms of oral and ocular dryness, which are also key symptoms of PSS. Therefore, patients with PSS may give positive responses to these questions regardless of the underlying aetiology of their sicca symptoms. To investigate how these questions might bias the secretomotor domain and COMPASS total scores, we compared the secretomotor domain and COMPASS total scores between patients with PSS and controls by scoring these two items as ‘negative’. With this approach, the median (IQR) secretomotor scores were 1.5 (1.5–4.5) and 1.5 (0–3.0) for patients with PSS and controls, respectively (p<0.0001), and the median (IQR) COMPASS total score was 29.2 (16.2–40.4) for patients with PSS and 14.8 (4.4–29.6) for controls (p<0.0001). In addition, we compared the COMPASS total scores between patients with PSS and controls without the entire secretomotor domain. With this approach, the median (IQR) COMPASS total scores were 26.6 (13.7–36.7) and 13 (4.3–26.3), respectively, for patients with PSS and controls (p<0.0001). These additional analyses suggest that the difference in the COMPASS total score between patients with PSS and controls cannot be explained by potential bias in the secretomotor domain alone.
Relationship between autonomic symptoms and clinical features of PSS
To explore the relationship between autonomic symptoms and clinical features of PSS, we performed univariate correlation analysis between COMPASS total score and a range of prespecified variables including demographics (age, sex), measures of disease activity and severity (disease duration, ESSDAI, erythrocyte sedimentation rate, C-reactive protein, physician's assessment of disease damage, autoantibody status), patient reported outcomes (ESSPRI, dryness (EULAR Sicca Score, overall dryness), pain, fatigue (physical fatigue, mental fatigue, overall fatigue), anxiety, depression, daytime somnolence, health-related quality of life) and other potentially relevant factors (systolic and diastolic blood pressure). These variables were chosen on the basis of potential biological links and data from previous studies.
There was no correlation between COMPASS total score and age, disease duration, blood pressure, erythrocyte sedimentation rate or C-reactive protein. The median COMPASS total scores were not significantly different between patients with or without autoantibodies against Ro/La or between female and male patients (data not shown). There was a significant correlation between the COMPASS total score and ESSDAI, but not with the physician-assessed severity of end-organ damage measured using a Likert scale. Total autonomic symptom burden was associated most strongly with ESSPRI, followed by fatigue and pain. The presence of autonomic symptoms was also associated with dryness, daytime sleepiness, anxiety, depression and reduced quality of life (online supplementary table S2).
To identify multivariate predictors of COMPASS total scores, stepwise multiple regression analysis was used. Gender and anti-Ro/La status were entered as dummy variables, and goodness of fit was assessed through residual analysis. Stepwise multiple regression identified three important predictors of COMPASS total scores: ESSPRI (β=0.38, p<0.001), anxiety score (HAD-A) (β=0.23, p<0.001) and ESSDAI (β=0.13, p<0.009). As these scores increased, COMPASS total scores increased. These three predictors accounted for 41% of the variability in COMPASS scores. Mental fatigue also independently predicted COMPASS total score, but the effect was small and only marginally significant (β=0.12, p=0.037) (table 2). Furthermore, logistic regression analysis using COMPASS total score of >32.5 as cut-off for the presence or absence of dysautonomia identified ESSPRI and HAD-A as the two most important predictors (figure 3).
Table 2.
Key statistics from stepwise multiple regression analysis using COMPASS total score as dependent variable to identify independent predictors
| Unstandardised coefficients | Standardised coefficients | ||||
|---|---|---|---|---|---|
| Model | B | se | β | t | Significance |
| Constant | 7.066 | 2.202 | 3.209 | 0.001 | |
| ESSPRI total | 3.070 | 0.484 | 0.383 | 6.343 | 0.000 |
| HAD-A | 0.905 | 0.212 | 0.227 | 4.262 | 0.000 |
| ESSDAI | 0.589 | 0.225 | 0.128 | 2.620 | 0.009 |
| Mental fatigue | 1.223 | 0.584 | 0.125 | 2.094 | 0.037 |
| COMPASS total=7.066+3.070 (ESSPRI total)+0.905 (HAD-A)+0.589 (ESSDAI)+1.223 (Mental fatigue)+error | |||||
| R2=0.420 | |||||
COMPASS, Composite Autonomic Symptom Scale; ESSDAI, EULAR Sjögren's Syndrome Disease Activity Index; ESSPRI, EULAR Sjögren's Syndrome Patient Reported Index; HAD, Hospital Anxiety and Depression Scale.
Figure 3.
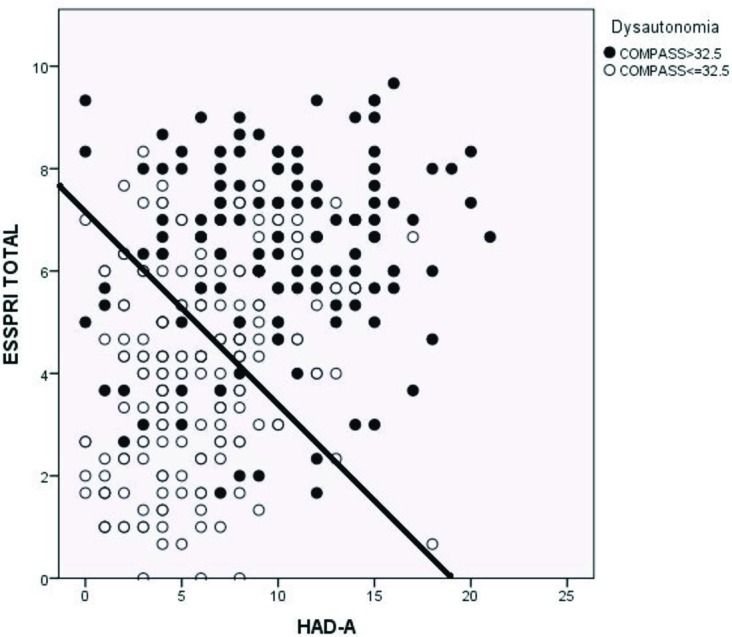
Observed membership of the dysautonomic group as a function of ESSPRI and HAD-A scores in the logistic regression model. ESSPRI scores and HAD-A scores were significant predictors of group membership (p<0.001 for both predictors). The solid line indicates the 50% predicted probability for membership of the Dysautonomic Group. The filled circles indicate those patients with COMPASS scores >32.5. If the model were a perfect predictor of group membership then all points to the right and above would be filled (indicating COMPASS scores >32.5) and all those to the left and below would be unfilled (indicating COMPASS scores <32.5). COMPASS, Composite Autonomic Symptom Scale; ESSPRI, EULAR Sjögren's Syndrome Patient Reported Index; HAD, Hospital Anxiety and Depression Scale.
Discussion
This study aimed to determine the prevalence of autonomic symptoms in a large cohort of patients with PSS and to investigate whether there is a relationship between autonomic symptoms and biological and psychosocial variables commonly found in PSS. We have demonstrated that symptoms of autonomic dysfunction are common among patients with PSS, with 55% fulfilling the criterion of dysautonomia. Furthermore, autonomic dysfunction is independently associated with ESSPRI, disease activity (ESSDAI) and symptoms of anxiety, and possibly mental fatigue.
Many case reports, case series and other studies have reported a link between autonomic dysfunction and PSS.6–11 13 14 28–32 However, the sample sizes of these studies were relatively small, rendering estimation of the prevalence and analysis of the relationship between autonomic dysfunction and other clinical features of PSS less reliable. Analysis of the data of over 300 patients with clinically well-characterised PSS in this study enables a more robust interrogation of the relationship between autonomic symptoms and other clinical features of PSS. The multicentre design also increases the ecological validity of our data compared with those derived from single-centre studies. To our knowledge, this is the first large, multicentre study to examine the prevalence, severity and predictors of autonomic symptoms in PSS.
ESSPRI consists of three components: fatigue, pain and dryness. Since the secretomotor domain score is among the best predictors of COMPASS total score,17 the correlation between ESSPRI and autonomic symptoms could be a consequence of commonalities between these instruments. Indeed, all three individual components of ESSPRI independently predict COMPASS total score, although fatigue and pain are better predictors than dryness (online supplementary table S3). On the other hand, as there is no obvious commonality between the ESSDAI and COMPASS instruments, factors (eg, common pathogenetic mechanisms) other than commonalities between the instruments may be responsible for the correlation between ESSDAI and COMPASS total score. Although an association does not equate to causal relationship, it is tempting to speculate that autonomic dysfunction may play a key role in PSS pathogenesis. It is noteworthy that antibodies against muscarinic receptor have been implicated in PSS pathogenesis.33–39 Therefore, investigating the relationship between such antibodies and autonomic dysfunction in PSS is warranted. Previous studies did not identify links between autonomic dysfunction and PSS disease activity. However, disease activity was defined using different variables such as inflammatory markers, immunoglobulin or complement levels and white cell counts. Our study is the first to examine the relationship between autonomic symptoms and PSS disease activity using a validated instrument, the ESSDAI.
The positive correlation between symptoms of autonomic dysfunction and anxiety score is consistent with a previous report.9 However, the mechanistic basis for such an association remains unclear. Many symptoms of anxiety such as palpitations, dizziness and sweats are also symptoms of dysautonomia, and this may provide a possible explanation for the association between the COMPASS total and the anxiety scores. As the average anxiety score among the PSS cohort is low, it is unlikely that the increased COMPASS total score is a consequence of anxiety disorder in these patients.
Associations between autonomic symptoms and fatigue6 9 and symptoms of depression9 in patients with PSS have also been reported. In this study, the COMPASS total scores correlated significantly with fatigue and depression in univariate analyses. Although mental fatigue was independently associated with COMPASS total score, the effect was small and was only marginally significant statistically. Further study is needed to explore the relationship between dysautonomia and fatigue and depression.
The COMPASS assesses different components of autonomic function, grouped according to individual domains. Two other groups of investigators have also used this instrument to study patients with PSS.6 8 9 Interestingly, significantly higher scores in the secretomotor, orthostatic intolerance, pupil and focusing, and vasomotor domains among patients with PSS compared with healthy controls were observed in all four studies (including a follow-up study). The tendency for these autonomic symptoms may provide insight for understanding the mechanisms of autonomic dysfunction in PSS. Furthermore, symptoms of orthostatic intolerance can be effectively treated using a combination of non-pharmacological and pharmacological interventions. Thus, our observations suggest that patients with PSS should be assessed for symptoms of orthostatic intolerance, and managed appropriately in clinics.
Two groups have shown that impaired gastric emptying is more common in patients with PSS than in controls.40 41 However, the gastroparesis domain score was not increased among patients with PSS and did not correlate with the objective signs of gastroparesis,40 and clinically significant gastroparesis symptoms were rarely reported.41 Consistently, the median gastroparesis domain scores were zero for both patients with PSS and controls in this study.
There are several potential weaknesses in the design of this study. First, not all patients underwent the COMPASS assessment, and some did not complete the questionnaire. Furthermore, the group completing the COMPASS questionnaire was possibly less affected by their condition, showing lower levels of physical fatigue and ESSPRI scores. If true, given the positive correlation between COMPASS total and ESSPRI scores, our data probably underestimate the prevalence/severity of autonomic symptoms in PSS. It also raises an intriguing possibility that the incomplete-COMPASS and non-COMPASS groups may be ‘too unwell’ to complete/participate in the COMPASS assessment. However, since the p values of these univariate comparisons were unadjusted for multiplicity, caution is needed when interpreting these differences between the complete-COMPASS group and the remaining groups of the cohort. Second, since the recruitment for the UKPSSR cohort is ongoing, it is possible that patients with PSS with autonomic symptoms had been preferentially recruited before those without dysautonomia or vice versa, although we consider it unlikely. Furthermore, the design of this study was embedded in the UKPSSR set-up, with analysis undertaken according to a predetermined protocol based on the estimated sample size needed. Therefore, we believe that 317 patients is a sufficiently large sample size, and, given the statistically highly significant results, we consider it unlikely that our conclusion would have been different even if we had analysed more patients from the UKPSSR cohort. Third, the key findings of this study are based on self-reported symptoms of dysautonomia, which may be susceptible to bias among the respondents. However, the COMPASS has been validated and shown to correlate well with objective autonomic assessments.17 42 Furthermore, the scores for the overstatement domain among patients with PSS were low, suggesting that their autonomic symptoms are unlikely to be the result of psychosomatic overscoring. Finally, oral and ocular dryness are cardinal symptoms of PSS. Therefore, patients with PSS may give positive responses to two of the 10 questions in the secretomotor domain of the COMPASS questionnaire regardless of the underlying aetiology of their symptoms, leading to potential overestimation of the severity of autonomic dysfunction in PSS. However, the COMPASS total score for patients with PSS remained significantly higher than that of the controls even with these two items scored as negative or with the entire secretomotor domain score removed. These observations suggest that factors other than potential bias in the secretomotor domain alone are responsible for the increased prevalence and severity of autonomic symptoms in PSS.
In conclusion, autonomic symptoms are common among patients with PSS, with a preponderance of certain symptoms such as orthostatic intolerance, which may be amenable to treatment. Symptoms of dysautonomia should therefore be sought when assessing patients with PSS. Moreover, autonomic dysfunction correlates with patient-reported outcomes and PSS disease activity, indicating that dysautonomia may be a key element of the pathological processes of PSS. Additional work investigating the biological and psychosocial factors in PSS-associated dysautonomia would be worthwhile.
Acknowledgments
This study received grant support from the Medical Research Council (G0800629 to WFN, SB, BG), British Sjögren's Syndrome Association and the Newcastle University for the sub-study. This work also received infrastructure support from the Newcastle NIHR Biomedical Research Centre, Newcastle and North Tyneside Comprehensive Local Research Network and the Comprehensive Local Research Network to the recruiting centres. The authors would also like to thank all the patients who have participated in the UKPSSR and the autonomic function assessment sub-study.
Footnotes
Contributors: WFN and JLN designed the study and wrote the manuscript. JLN, WFN, DL, JF, DP, KH, KW analysed and interpreted the data. All other individual authors were involved in data collection, data interpretation and writing of the manuscript.
Collaborators: WFN, SJB and BG are investigators of the UKPSSR. JLN and WFN are investigators of the UKPSSR autonomic function sub-study. The other UKPSSR members (as of 1 July 2011) include, in alphabetical order of their affiliations: Elalaine C Bacabac, Robert Moots (Aintree University Hospitals); Kuntal Chadravarty, Shamin Lamabadusuriya (Barking, Havering and Redbridge NHS Trust); Michele Bombardieri, Constantino Pitzalis, Nurhan Sutcliffe (Bart and the London NHS Trust); Nagui Gendi, Rashidat Adeniba (Basildon Hospital); John Hamburger, Andrea Richards (Birmingham Dental Hospital); Saaeha Rauz (Birmingham & Midland Eye Centre); Sue Brailsford (Birmingham University Hospital); Joanne Logan, Diarmuid Mulherin (Cannock Chase Hospital); Jacqueline Andrews, Paul Emery, Alison McManus, Colin Pease (Chapel Allerton Hospital, Leeds); Alison Booth, Marian Regan (Derbyshire Royal Infirmary); Theodoros Dimitroulas, Lucy Kadiki, Daljit Kaur, George Kitas (Dudley Group of Hospitals NHS Foundation Trust); Mark Lloyd, Lisa Moore (Frimley Park Hospital); Esther Gordon, Cathy Lawson (Harrogate District Foundation Trust Hospital); Monica Gupta, John Hunter, Lesley Stirton (Gartnavel General Hospital, Glasgow); Gill Ortiz, Elizabeth Price (Great Western Hospital); Gavin Clunie, Ginny Rose, Sue Cuckow (Ipswich Hospital NHS Trust); Susan Knight, Deborah Symmons, Beverley Jones (Macclesfield District General Hospital & Arthritis Research UK Epidemiology Unit, Manchester); Andrew Carr, Suzanne Edgar, Marco Carrozzo, Francisco Figuereido, Heather Foggo, Colin Gillespie, Dennis Lendrem, Iain Macleod, Sheryl Mitchell, Jessica Tarn (Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University); Adrian Jones, Peter Lanyon, Alice Muir (Nottingham University Hospital); Paula White, Steven Young-Min (Portsmouth Hospitals NHS Trust); Susan Pugmire, Saravanan Vadivelu (Queen's Elizabeth Hospital, Gateshead); Annie Cooper, Marianne Watkins (Royal Hampshire County Hospital); Anne Field, Stephen Kaye, Devesh Mewar, Patricia Medcalf, Pamela Tomlinson, Debbie Whiteside (Royal Liverpool University Hospital); Neil McHugh, John Pauling, Julie James, Nike Olaitan (Royal National Hospital for Rheumatic Diseases); Mohammed Akil, Jayne McDermott, Olivia Godia (Royal Sheffield Hospital); David Coady, Elizabeth Kidd, Lynne Palmer (Royal Sunderland Hospital); Bhaskar Dasgupta, Victoria Katsande, Pamela Long (Southend University Hospital); Usha Chandra, Kirsten MacKay (Torbay Hospital); Stefano Fedele, Ada Ferenkeh-Koroma, Ian Giles, David Isenberg, Helena Marconnell, Stephen Porter (University College Hospital & Eastman Dental Institute); Paul Allcoat, John McLaren (Whyteman's Brae Hospital, Kirkaldy).
Funding: Medical Research Council, UK.
Competing interests: None.
Patient Consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The anonymised raw data of this study concerning the patients may be made available upon request to the corresponding author WFN. Informed consent on data sharing has been obtained from patients participating in this study. Otherwise, no additional data are available.
References
- 1.Gabriel SE, Michaud K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther 2009;11:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng WF, Bowman SJ. Primary Sjogren's syndrome: too dry and too tired. Rheumatology (Oxford) 2010;49:844–53 [DOI] [PubMed] [Google Scholar]
- 3.Newton JL, Okonkwo O, Sutcliffe K, et al. Symptoms of autonomic dysfunction in chronic fatigue syndrome. QJM 2007;100:519–26 [DOI] [PubMed] [Google Scholar]
- 4.Freeman R, Komaroff AL. Does the chronic fatigue syndrome involve the autonomic nervous system? Am J Med 1997;102:357–64 [DOI] [PubMed] [Google Scholar]
- 5.Rowe PC, Bou-Holaigah I, Kan JS, et al. Is neurally mediated hypotension an unrecognised cause of chronic fatigue? Lancet 1995;345:623–4 [DOI] [PubMed] [Google Scholar]
- 6.Cai FZ, Lester S, Lu T, et al. Mild autonomic dysfunction in primary Sjögren's syndrome: a controlled study. Arthritis Res Ther 2008;10:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovács L, Paprika D, Tákacs R, et al. Cardiovascular autonomic dysfunction in primary Sjögren's syndrome. Rheumatology (Oxford) 2004;43:95–9 [DOI] [PubMed] [Google Scholar]
- 8.Mandl T, Wollmer P, Manthorpe R, et al. Autonomic and orthostatic dysfunction in primary Sjögren's syndrome. J Rheumatol 2007;34:1869–74 [PubMed] [Google Scholar]
- 9.Mandl T, Hammar O, Theander E, et al. Autonomic nervous dysfunction development in patients with primary Sjogren's syndrome: a follow-up study. Rheumatology (Oxford) 2010;49:1101–6 [DOI] [PubMed] [Google Scholar]
- 10.Andonopoulos AP, Christodoulou J, Ballas C, et al. Autonomic cardiovascular neuropathy in Sjögren's syndrome. A controlled study. J Rheumatol 1998;25:2385–8 [PubMed] [Google Scholar]
- 11.Mandl T, Granberg V, Apelqvist J, et al. Autonomic nervous symptoms in primary Sjogren's syndrome. Rheumatology (Oxford) 2008;47:914–9 [DOI] [PubMed] [Google Scholar]
- 12.Niemelä RK, Pikkujämsä SM, Hakala M, et al. No signs of autonomic nervous system dysfunction in primary Sjörgen's syndrome evaluated by 24 hour heart rate variability. J Rheumatol 2000;27:2605–10 [PubMed] [Google Scholar]
- 13.Barendregt PJ, Tulen JH, van den Meiracker AH, et al. Spectral analysis of heart rate and blood pressure variability in primary Sjögren's syndrome. Ann Rheum Dis 2002;61:232–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tumiati B, Perazzoli F, Negro A, et al. Heart rate variability in patients with Sjögren's syndrome. Clin Rheumatol 2000;19:477–80 [DOI] [PubMed] [Google Scholar]
- 15.Ng WF, Bowman SJ, Griffiths B. United Kingdom Primary Sjogren's Syndrome Registry–a united effort to tackle an orphan rheumatic disease. Rheumatology (Oxford) 2011;50:32–9 [DOI] [PubMed] [Google Scholar]
- 16.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002;61:554–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suarez GA, Opfer-Gehrking TL, Offord KP, et al. The Autonomic Symptom Profile: a new instrument to assess autonomic symptoms. Neurology 1999;52:523–8 [DOI] [PubMed] [Google Scholar]
- 18.Costigan A, Elliott C, McDonald C, et al. Orthostatic symptoms predict functional capacity in chronic fatigue syndrome: implications for management. QJM 2010;103:589–95 [DOI] [PubMed] [Google Scholar]
- 19.Newton JL, Elliott C, Frith J, et al. Functional capacity is significantly impaired in primary biliary cirrhosis and is related to orthostatic symptoms. Eur J Gastroenterol Hepatol 2011;23:566–72 [DOI] [PubMed] [Google Scholar]
- 20.Seror R, Ravaud P, Bowman SJ, et al. EULAR Sjogren's syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren's syndrome. Ann Rheum Dis 2010;69:1103–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seror R, Ravaud P, Mariette X, et al. EULAR Sjogren's Syndrome Patient Reported Index (ESSPRI): development of a consensus patient index for primary Sjogren's syndrome. Ann Rheum Dis 2011;70:968–72 [DOI] [PubMed] [Google Scholar]
- 22.Bowman SJ, Hamburger J, Richards A, et al. Patient-reported outcomes in primary Sjogren's syndrome: comparison of the long and short versions of the Profile of Fatigue and Discomfort–Sicca Symptoms Inventory. Rheumatology (Oxford) 2009;48:140–3 [DOI] [PubMed] [Google Scholar]
- 23.Bowman SJ, Booth DA, Platts RG. Measurement of fatigue and discomfort in primary Sjogren's syndrome using a new questionnaire tool. Rheumatology (Oxford) 2004;43:758–64 [DOI] [PubMed] [Google Scholar]
- 24.Segal B, Thomas W, Rogers T, et al. Prevalence, severity, and predictors of fatigue in subjects with primary Sjögren's syndrome. Arthritis Rheum 2008;59:1780–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540–5 [DOI] [PubMed] [Google Scholar]
- 26.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70 [DOI] [PubMed] [Google Scholar]
- 27.EuroQol – a new facility for the measurement of health-related quality of life. The EuroQol Group Health Policy 1990;16:199–208 [DOI] [PubMed] [Google Scholar]
- 28.Andonopoulos AP, Ballas C. Autonomic cardiovascular neuropathy in primary Sjögren's syndrome. Rheumatol Int 1995;15:127–9 [DOI] [PubMed] [Google Scholar]
- 29.Sakakibara R, Hirano S, Asahina M, et al. Primary Sjogren's syndrome presenting with generalized autonomic failure. Eur J Neurol 2004;11:635–8 [DOI] [PubMed] [Google Scholar]
- 30.Sorajja P, Poirier MK, Bundrick JB, et al. Autonomic failure and proximal skeletal myopathy in a patient with primary Sjögren syndrome. Mayo Clin Proc 1999;74:695–7 [DOI] [PubMed] [Google Scholar]
- 31.Waterschoot MP, Guerit JM, Lambert M, et al. Bilateral tonic pupils and polyneuropathy in Sjögren's syndrome: a common pathophysiological mechanism? Eur Neurol 1991;31:114–6 [DOI] [PubMed] [Google Scholar]
- 32.Mori K, Iijima M, Koike H, et al. The wide spectrum of clinical manifestations in Sjögren's syndrome-associated neuropathy. Brain 2005;128:2518–34 [DOI] [PubMed] [Google Scholar]
- 33.Reina S, Sterin-Borda L, Passafaro D, et al. Anti-M(3) muscarinic cholinergic autoantibodies from patients with primary Sjögren's syndrome trigger production of matrix metalloproteinase-3 (MMP-3) and prostaglandin E(2) (PGE(2)) from the submandibular glands. Arch Oral Biol 2011;56:413–20 [DOI] [PubMed] [Google Scholar]
- 34.Reina S, Sterin-Borda L, Orman B, et al. Autoantibodies against cerebral muscarinic cholinoceptors in Sjögren syndrome: functional and pathological implications. J Neuroimmunol 2004;150:107–15 [DOI] [PubMed] [Google Scholar]
- 35.He J, Guo JP, Ding Y, et al. Diagnostic significance of measuring antibodies to cyclic type 3 muscarinic acetylcholine receptor peptides in primary Sjogren's syndrome. Rheumatology (Oxford) 2011;50:879–84 [DOI] [PubMed] [Google Scholar]
- 36.Dawson LJ, Stanbury J, Venn N, et al. Antimuscarinic antibodies in primary Sjögren's syndrome reversibly inhibit the mechanism of fluid secretion by human submandibular salivary acinar cells. Arthritis Rheum 2006;54:1165–73 [DOI] [PubMed] [Google Scholar]
- 37.Waterman SA, Gordon TP, Rischmueller M. Inhibitory effects of muscarinic receptor autoantibodies on parasympathetic neurotransmission in Sjögren's syndrome. Arthritis Rheum 2000;43:1647–54 [DOI] [PubMed] [Google Scholar]
- 38.Robinson CP, Brayer J, Yamachika S, et al. Transfer of human serum IgG to nonobese diabetic Igmu null mice reveals a role for autoantibodies in the loss of secretory function of exocrine tissues in Sjögren's syndrome. Proc Natl Acad Sci USA 1998;95:7538–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen KH, Brayer J, Cha S, et al. Evidence for antimuscarinic acetylcholine receptor antibody-mediated secretory dysfunction in nod mice. Arthritis Rheum 2000;43:2297–306 [DOI] [PubMed] [Google Scholar]
- 40.Hammar O, Ohlsson B, Wollmer P, et al. Impaired gastric emptying in primary Sjogren's syndrome. J Rheumatol 2010;37:2313–8 [DOI] [PubMed] [Google Scholar]
- 41.Kovács L, Papós M, Takács R, et al. Autonomic nervous system dysfunction involving the gastrointestinal and the urinary tracts in primary Sjögren's syndrome. Clin Exp Rheumatol 2003;21:697–703 [PubMed] [Google Scholar]
- 42.Low PA, Benrud-Larson LM, Sletten DM, et al. Autonomic symptoms and diabetic neuropathy: a population-based study. Diabetes Care 2004;27:2942–7 [DOI] [PubMed] [Google Scholar]