Abstract
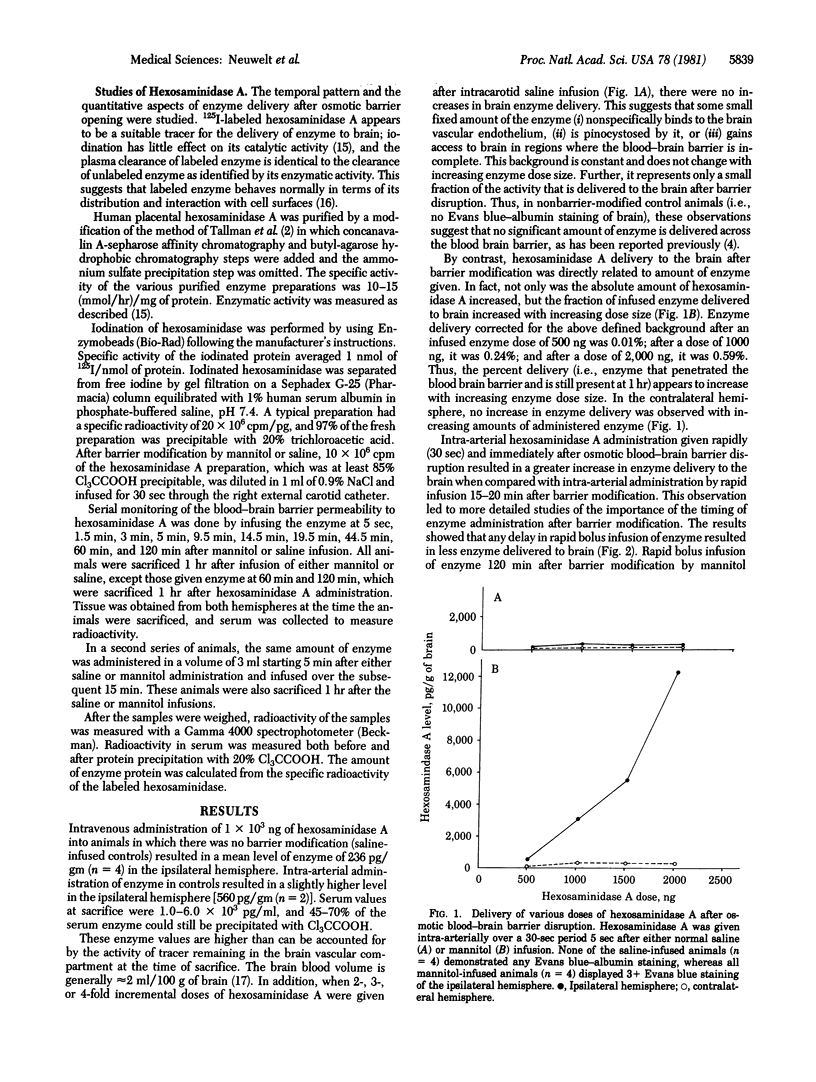
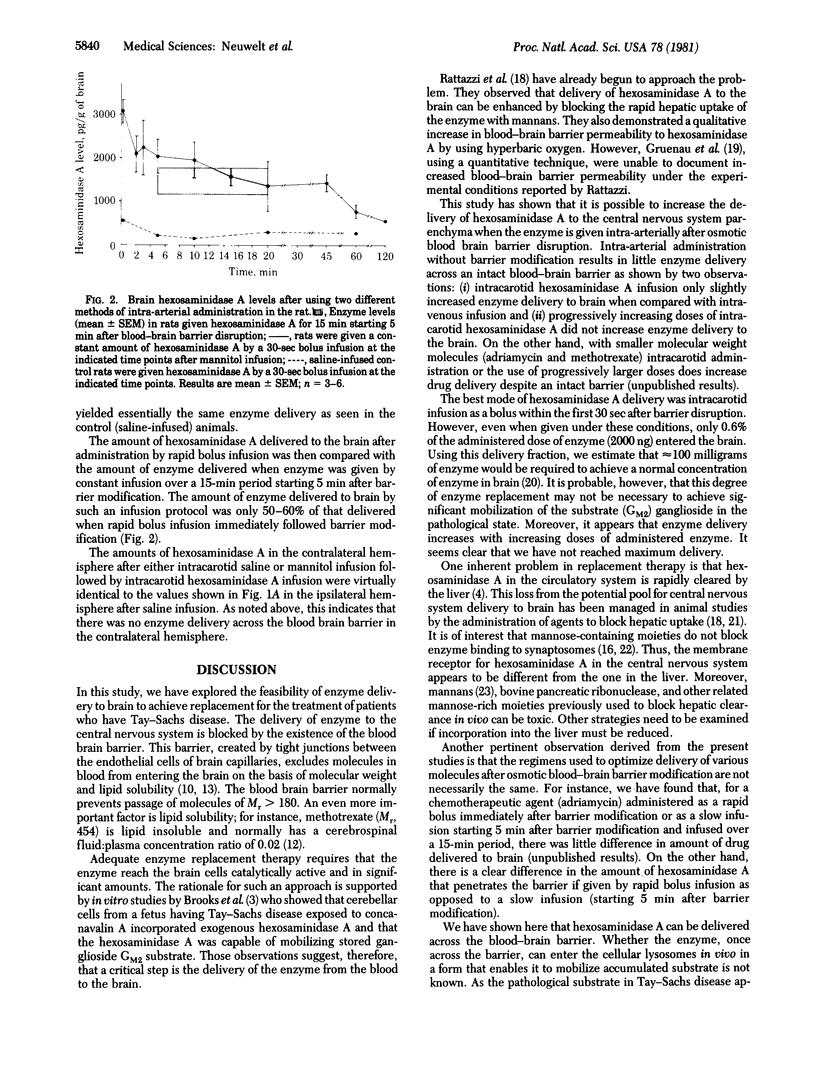
The present studies were undertaken to evaluate the possibility that hexosaminidase A, the enzyme deficient in Tay--Sachs disease, could be effectively delivered to brain. Previous studies from our laboratory have shown that hypertonic mannitol can be used to osmotically produce reversible disruption of the blood--brain barrier in animals (rat and dog) and man without significant neurotoxicity and that such barrier modification significantly increases the delivery of cytoreductive chemotherapy agents to selected areas of brain. By using the rat model of blood--brain barrier modification and radiolabeled enzyme, increased hexosaminidase A delivery to brain has been demonstrated in more than 85 animals. The time of injection of hexosaminidase A after blood--brain barrier disruption is critical for maximum delivery. Rapid (over 30 sec) intra-arterial administration of hexosaminidase A immediately after blood--brain barrier disruption resulted in a marked increase in enzyme delivery to the brain when compared with controls without prior barrier disruption. When the enzyme was administered 15-20 min after barrier disruption, approximately 50% less hexosaminidase A was delivered; when given 60-120 min after barrier modification, the amount delivered was the same as in control animals. This critical time course is very different than that seen in trials of low molecular weight chemotherapeutic agents (methotrexate and adriamycin). These preliminary studies suggest that hexosaminidase A can be delivered to the brain by blood--brain barrier modification and may be indicative of the potential for enzyme replacement in patients who hae Tay--Sachs disease.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barranger J. A., Rapoport S. I., Fredericks W. R., Pentchev P. G., MacDermot K. D., Steusing J. K., Brady R. O. Modification of the blood-brain barrier: increased concentration and fate of enzymes entering the brain. Proc Natl Acad Sci U S A. 1979 Jan;76(1):481–485. doi: 10.1073/pnas.76.1.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S. E., Hoffman L. M., Adachi M., Amsterdam D., Schneck L. Enzyme replacement treatment for Tay-Sachs disease brain cells in culture utilizing concanavalin A-mediated hexosaminidase A uptake: biochemical and morphological evidence of GM2 mobilization. Acta Neuropathol. 1980;50(1):9–17. doi: 10.1007/BF00688529. [DOI] [PubMed] [Google Scholar]
- Gruenau S. P., Folker M. T., Rapoport S. I. Lack of hyperbaric O2 effect on blood-brain barrier permeability in conscious rats. Aviat Space Environ Med. 1981 Mar;52(3):162–165. [PubMed] [Google Scholar]
- Johnson W. G., Desnick R. J., Long D. M., Sharp H. L., Krivit W., Brady B., Brady R. O. Intravenous injection of purified hexosaminidase A into a patient with Tay-Sachs disease. Birth Defects Orig Artic Ser. 1973 Mar;9(2):120–124. [PubMed] [Google Scholar]
- Kusiak J. W., Barranger J. A. 125Iodine labeling of beta-hexosaminidase A without modifying its properties. Clin Chim Acta. 1979 Oct 1;97(2-3):155–158. doi: 10.1016/0009-8981(79)90411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusiak J. W., Toney J. H., Quirk J. M., Brady R. O. Specific binding of 125I-labeled beta-hexosaminidase A to rat brain synaptosomes. Proc Natl Acad Sci U S A. 1979 Feb;76(2):982–985. doi: 10.1073/pnas.76.2.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld E. F. The uptake of enzymes into lysosomes: an overview. Birth Defects Orig Artic Ser. 1980;16(1):77–84. [PubMed] [Google Scholar]
- Neuwelt E. A., Diehl J. T., Vu L. H., Hill S. A., Michael A. J., Frenkel E. P. Monitoring of methotrexate delivery in patients with malignant brain tumors after osmotic blood-brain barrier disruption. Ann Intern Med. 1981 Apr;94(4 Pt 1):449–454. doi: 10.7326/0003-4819-94-4-449. [DOI] [PubMed] [Google Scholar]
- Neuwelt E. A., Frenkel E. P., Diehl J., Vu L. H., Rapoport S., Hill S. Reversible osmotic blood-brain barrier disruption in humans: implications for the chemotherapy of malignant brain tumors. Neurosurgery. 1980 Jul;7(1):44–52. doi: 10.1227/00006123-198007000-00007. [DOI] [PubMed] [Google Scholar]
- Neuwelt E. A., Frenkel E. P. Is there a therapeutic role for blood-brain barrier disruption? Ann Intern Med. 1980 Jul;93(1):137–139. doi: 10.7326/0003-4819-93-1-137. [DOI] [PubMed] [Google Scholar]
- Neuwelt E. A., Frenkel E. P., Rapoport S., Barnett P. Effect of osmotic blood-brain barrier disruption on methotrexate pharmacokinetics in the dog. Neurosurgery. 1980 Jul;7(1):36–43. doi: 10.1227/00006123-198007000-00006. [DOI] [PubMed] [Google Scholar]
- Neuwelt E. A., Maravilla K. R., Frenkel E. P., Barnett P., Hill S., Moore R. J. Use of enhanced computerized tomography to evaluate osmotic blood-brain barrier disruption. Neurosurgery. 1980 Jan;6(1):49–56. doi: 10.1227/00006123-198001000-00007. [DOI] [PubMed] [Google Scholar]
- Neuwelt E. A., Maravilla K. R., Frenkel E. P., Rapaport S. I., Hill S. A., Barnett P. A. Osmotic blood-brain barrier disruption. Computerized tomographic monitoring of chemotherapeutic agent delivery. J Clin Invest. 1979 Aug;64(2):684–688. doi: 10.1172/JCI109509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport S. I., Bachman D. S., Thompson H. K. Chronic effects of osmotic opening of the blood-brain barrier in the monkey. Science. 1972 Jun 16;176(4040):1243–1245. doi: 10.1126/science.176.4040.1243. [DOI] [PubMed] [Google Scholar]
- Rapoport S. I., Ohno K., Pettigrew K. D. Drug entry into the brain. Brain Res. 1979 Aug 24;172(2):354–359. doi: 10.1016/0006-8993(79)90546-8. [DOI] [PubMed] [Google Scholar]
- Rattazzi M. C., Lanse S. B., McCullough R. A., Nester J. A., Jacobs E. A. Towards enzyme replacement in GM2 gangliosidosis: organ disposition and induced central nervous system uptake of human beta-hexosaminidase in the cat. Birth Defects Orig Artic Ser. 1980;16(1):179–193. [PubMed] [Google Scholar]
- Tallman J. F., Brady R. O., Quirk J. M., Villalba M., Gal A. E. Isolation and relationship of human hexosaminidases. J Biol Chem. 1974 Jun 10;249(11):3489–3499. [PubMed] [Google Scholar]
- Tallman J. F., Johnson W. G., Brady R. O. The metabolism of Tay-Sachs ganglioside: catabolic studies with lysosomal enzymes from normal and Tay-Sachs brain tissue. J Clin Invest. 1972 Sep;51(9):2339–2345. doi: 10.1172/JCI107045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Specht B. U., Geiger B., Arnon R., Passwell J., Keren G., Goldman B., Padeh B. Enzyme replacement in Tay-Sachs disease. Neurology. 1979 Jun;29(6):848–854. doi: 10.1212/wnl.29.6.848. [DOI] [PubMed] [Google Scholar]



