Abstract
Background:
Individuals who focused on calorie counting lost more weight than those who focused on increasing vegetable and fruit (V&F) intake in a weight loss program. We now present serum carotenoid data (biomarkers of V&F intake) from both groups and test whether these biomarkers correlate with changes in weight and body fat.
Design:
Sixty obese volunteers were randomized to one of the following weight loss programs: 500 kcal per day reduction (Reduction) or a focus on consuming eight vegetables per day and 2–3 fruits per day (HiVeg). Volunteers in the Reduction group were 36.8±10.3 years with a body mass index of 33.5; 83% were white, 17% chose not to report race; 70% were not Hispanic or Latino, 13% were Hispanic or Latino and 17% chose not to report ethnicity. Volunteers in the HiVeg group were 30.4±6.6 years with a body mass index of 33.2: 74% white, 11% Asian, 5% black or African American, 5% multiracial and 5% chose not to report race; 89% were not Hispanic or Latino, 5% were Hispanic or Latino and 5% chose not to report ethnicity. Subjects were taught basic nutrition principles, received breakfast and lunch 5 days per week for 3 months, meals 2 days per week during month 4, then regular phone calls to month 12.
Results:
Total serum carotenoid concentrations increased from baseline to 3 months and remained elevated at 12 months, but there was no difference between groups. Changes in weight, fat and % fat correlated negatively with serum carotenoid concentrations.
Conclusion:
Increased serum carotenoids (a biomarker for V&F intake) correlated with improved weight and fat loss indicating that increased V&F consumption is an appropriate strategy for weight loss. However, in light of the fact that the Reduction group lost more weight, the consumption of increased V&F for the purpose of weight loss should happen within the context of reducing total caloric intake.
Keywords: obesity, weight loss, vegetable, fruit, body composition, body fat
Introduction
With the current worldwide epidemic of obesity, the need for effective weight loss and weight maintenance strategies has never been higher. Although as many as 75% of Americans are attempting to lose or maintain weight,1, 2, 3, 4 weight-loss and weight-maintenance strategies are clearly not working as the prevalence of obesity continues to increase.
Reduction in the energy density (kcal g−1 food) of the diet can be an effective mechanism for weight loss.5, 6 Results from several studies indicate that fat content, independent of energy density and palatability, does not affect the amount of energy intake by individuals,7, 8, 9 whereas energy density of the diet is an important determinant of total energy intake.10, 11 To achieve reduced energy density, foods high in water and fiber, such as vegetables and fruits, need to replace high-fat and high-calorie foods. These low-energy density foods provide bulk to the diet, resulting in a better sense of satiety.12, 13 For these reasons, advice to increase vegetable and fruit (V&F) consumption often accompanies guidelines for weight loss. We tested the hypothesis that a high-vegetable (eight servings per day) and moderate fruit (2–3 servings per day) intervention would result in greater long-term weight loss compared with a calorie and fat reduction intervention.14 Although the calorie reduction group lost more weight, many individuals in that group did so while increasing vegetable intake. We now present serum carotenoid data (biomarkers of V&F intake) from both groups and test whether these biomarkers correlate with changes in weight and body fat. Some epidemiological data indicate a correlation between increased serum carotenoids and attempts to lose weight.15 We hypothesize that individuals who ate more vegetables as indicated by increased serum carotenoids would lose more weight.
Materials and methods
The study was approved by the Health Sciences Institutional Review Board at the University of Wisconsin. Subjects signed informed consent forms, and the study was in compliance with privacy-act guidelines. Details of subject recruitment and criteria have been previously published.14 Briefly, 60 subjects were randomized for the intervention. Inclusion criteria were age 19–50 years with a body mass index between 30 and 40 kg m−2. Exclusion criteria were consumption of ⩾5 servings per day vegetables and fruits at screening, aerobic exercise exceeding 90 min per week at baseline, pregnancy or lactation, serious medical or psychiatric illness, use of drugs (including obesity drugs) that might interfere with the protocol, a history of insulin treatment, history of drug or alcohol abuse and weight change greater than 3% of body weight in the three months before recruitment. A subset of 42 subjects, for whom serum carotenoid values were available up to 12 months, has been included in these analyses. Baseline characteristics are presented in Table 1. Of the included subjects, 78% were Caucasian, 5% were African American, 5% were Asian, 2% were more than one race and 10% chose not to report race. Ten percent were Hispanic or Latino, 78% were not Hispanic or Latino and 12% chose not to report ethnicity.
Table 1. Baseline characteristics of subjects (n=42; mean±s.d.).
| Reduction | HiVeg | |
|---|---|---|
| Age (years) | 36.78±10.31 | 30.42±6.56 |
| Body weight (kg) | 97.76±16.86 | 94.05±15.59 |
| BMI (kg m−2) | 33.48±3.43 | 33.19±3.52 |
| F/M | 16/7 | 13/6 |
Abbreviation: BMI, body mass index.
Weight, height (baseline only), body mass index, body composition and serum carotenoid concentrations were measured at baseline, 3 and 12 months. Body composition was measured by air displacement plethysmography (BOD POD, Life Measurements, Concord, CA, USA).16, 17 Weight was measured using the BOD POD scale, which was tested with calibration weights each day of use. Height was measured using a wall-mounted stadiometer. Serum samples were drawn by vacuum into serum separator tubes and kept at room temperature for 10–20 min. Samples were centrifuged at 2200 g for 10 min at 4 °C. Serum was transferred to cryovials and stored under argon at −80 °C until further analysis. Serum carotenoids were analyzed by high-performance liquid chromatography as previously described.18
Details of the dietary intervention have been previously described.14 In brief, subjects were randomly assigned to the high vegetable/moderate fruit consumption group (HiVeg, n=19) or to the calorie/fat reduction group (Reduction, n=23). The goal of the former was to consume eight servings of vegetables and 2–3 servings of fruits daily. The goal of the latter was to reduce total intake by 500 kcal per day and keep fat intake at or below 25% of total calories. Both groups were provided with 10 meals per week for the first 3 months in an effort to help them reach their dietary goals. Subjects also attended educational sessions twice weekly during the first 3 months to learn healthy eating and exercise strategies. Sessions were taught by LDW and consisted of short lessons designed to address healthy eating and exercise strategies (for example, strategies regarding meal planning and preparation, grocery shopping, eating in restaurants and incorporating exercise daily). All sessions were the same for both groups except for the initial session that explained tracking individual dietary goals. All nutrition advice was based on Dietary Guidelines for Americans (fifth edition).19 The HiVeg subjects were instructed to increase vegetable intake to eight servings per day. The fourth month of the study was a transition in which two meals were provided 2 days per week. There were no educational sessions, but subjects followed individual dietary goals independently for the final 8 months while receiving phone calls from investigators with decreasing frequency. Subjects completed 3-day diet records (two weekdays and one weekend day) at baseline, 3 and 12 months that were analyzed for energy, fat, protein, fiber, vegetables and fruits using Nutritionist Pro Version 3.1.0 (Axxya Systems, Stafford, TX, USA).
Statistical analyses were performed using SAS software (version 9.3; SAS Institute, Cary, NC, USA). Fruit and vegetable intakes and serum carotenoid measurements are presented as means±s.d. Differences from baseline to 3 and 12 months in the two groups were tested using a 2 × 3 repeated measures analysis of variance followed by Tukey contrasts when appropriate. At each follow-up time point, correlation coefficients were calculated between serum carotenoid changes and weight loss and body composition changes. Multiple regression analysis was used to partial out differences at baseline. α<0.05 was considered statistically significant.
Results
Serum carotenoid concentrations at baseline, 3 and 12 months are shown in Table 2. Serum concentrations of retinol, lutein and zeaxanthin did not change over time. In the reduction group, lycopene was elevated at 12 months compared with baseline and 3 months (P<0.05), but there was no difference across time in the HiVeg group. Serum concentrations of α-carotene and β-carotene were elevated at 3 months compared with baseline and 12 months (P<0.05), with no difference between groups. Total carotenoids in both dietary groups were elevated at 3 and 12 months compared with baseline (P<0.05) with no differences between groups.
Table 2. Serum carotenoid concentrations (μmol l−1)a.
| Month | Retinol | Lutein | Zeaxanthin | Lycopeneb | α-Carotenec | β-Carotenec | Totald |
|---|---|---|---|---|---|---|---|
| Reduction | |||||||
| 0 | 2.07±0.53 | 0.33±0.14 | 0.09±0.04 | 0.54±0.18a | 0.08±0.06 | 0.57±0.68 | 1.60±0.89 |
| 3 | 2.18±0.74 | 0.34±0.11 | 0.09±0.03 | 0.50±0.12a | 0.27±0.13 | 1.14±0.63 | 2.34±0.83 |
| 12 | 2.39±0.72 | 0.46±0.20 | 0.08±0.03 | 0.80±0.18b | 0.14±0.09 | 0.81±0.48 | 2.29±0.80 |
| HiVeg | |||||||
| 0 | 2.29±0.46 | 0.36±0.13 | 0.07±0.03 | 0.81±0.27b | 0.05±0.02 | 0.39±0.22 | 1.68±0.44 |
| 3 | 2.19±0.50 | 0.44±0.29 | 0.07±0.02 | 0.65±0.24ab | 0.34±0.21 | 1.21±0.84 | 2.70±1.16 |
| 12 | 2.41±0.35 | 0.40±0.12 | 0.08±0.02e | 0.82±0.23b | 0.10±0.07 | 0.63±0.41 | 2.02±0.55 |
| P-values | |||||||
| Diet | 0.43 | 0.41 | 0.02 | 0.0001 | 1.00 | 0.34 | 0.69 |
| Time | 0.14 | 0.10 | 0.83 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| DietxTime | 0.65 | 0.10 | 0.19 | 0.02 | 0.06 | 0.49 | 0.22 |
All values are means±s.d.'s. n=23 for Reduction and n=19 for HiVeg, except where noted.
Means within a column not followed by a common alphabet are significantly different, P<0.05 by Tukey contrast.
Regardless of treatment group, values at 3 months were significantly greater than at 0 or 12 months, P<0.05 by Tukey contrast.
Regardless of treatment group, values at 0 months were significantly lower than at 3 or 12 months, P<0.05 by Tukey contrast.
n=21.
By self-report on diet records, the Reduction group had no change over time in vegetable or fruit intake, but the HiVeg group had a significant increase in vegetable intake at 3 months; 12 month vegetable intake did not differ from baseline or 3 months in the HiVeg group (Table 3).
Table 3. Vegetable and fruit intake from 3-day diet recordsa.
| Month | Veg. cupsb | Fruit cups |
|---|---|---|
| Reduction | ||
| 0 | 1.3±0.7a | 0.7±0.6 |
| 3 | 1.8±0.7ab | 1.2±1.1 |
| 12 | 1.8±1.0ab | 1.0±0.8 |
| HiVeg | ||
| 0 | 1.6±0.9ab | 0.6±0.7 |
| 3 | 3.2±1.1c | 0.8±0.5 |
| 12 | 2.4±0.9bc | 1.2±1.6 |
| P-values | ||
| Diet | <0.0001 | 0.56 |
| Time | <0.0001 | 0.07 |
| Diet × time | 0.03 | 0.42 |
Abbreviation: veg., vegetable.
n=19–22 for Reduction and 15–17 for HiVeg.
Means within a column not followed by a common alphabet are significantly different, P<0.05 by Tukey contrast.
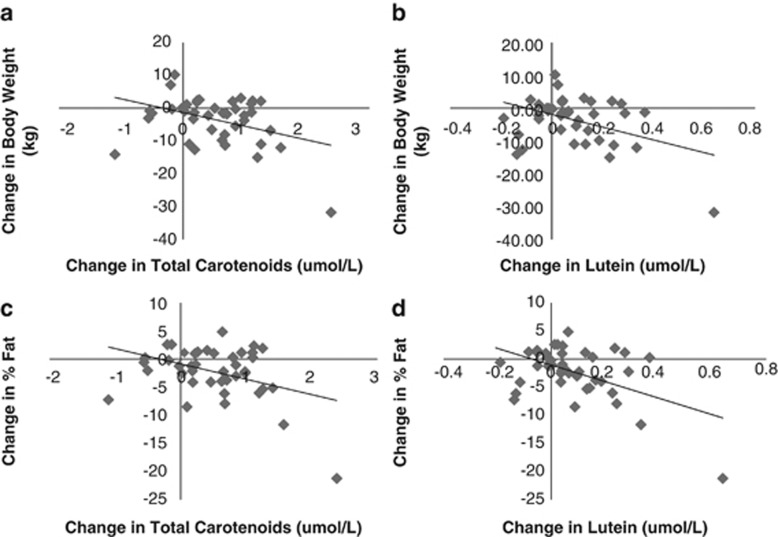
As total carotenoid levels increased and remained elevated in both groups regardless of dietary treatment, and both groups lost weight at 3 months,14 we wanted to see if elevated serum carotenoid levels were correlated with weight loss. Scatter plots and regression analyses of changes from baseline to 12 months in weight and % body fat vs changes in total serum carotenoid concentrations and serum lutein over the same time period are shown in Figure 1. Changes in weight, fat weight and % fat were negatively correlated with changes in total serum carotenoid concentrations (respective correlation coefficients: −0.38, −0.40, −0.39; P=0.01 for all) and serum lutein (respective correlation coefficients: −0.42, −0.46, −0.50; P⩽0.002; Table 4). There was a significant negative correlation between change in weight and change in α-carotene (correlation coefficient=−0.34; P<0.04) as well as with change in fat weight and change in β-carotene (correlation coefficient=−0.31; P<0.05). There were no significant correlations between changes in retinol, zeaxanthin and lycopene with changes in weight, fat weight and percent body fat. Changes in weight, fat weight and % fat also correlated with total carotenoids at 12 months (respective correlation coefficients: −0.61, −0.62, −0.60; P<0.0001 for all) but not at 3 months (respective correlation coefficients: −0.21, −0.23, −0.18; P not significant for all). These changes showed similar trends when intervention groups were analyzed separately (data not shown). Additional analyses adjusting for baseline measures of body weight, fat weight and % body fat did not change correlations (data not shown). Of particular interest, the negative correlations between changes in weight, fat and % fat and changes in total carotenoids and lutein were the same or more significant in the Reduction group alone (compared with all subjects combined) and not significant when the high-vegetable group was analyzed alone. Changes in weight did not correlate with changes in self-reported vegetable or fruit intake (data not shown).
Figure 1.
Scatter plots of changes from baseline to 12 months in weight (a and b) and % fat (c and d) vs changes in total serum carotenoid concentrations (a and c) and serum lutein (b and d). (a) r=−0.38, P=0.01; (b) r=−0.42, P=0.006; (c) r=−0.39, P=0.01; (d) r=−0.50, P=0.0007.
Table 4. Change from baseline to 12 months in serum carotenoid concentrations (μmol l−1) and corresponding correlation coefficients for changes in body weight, fat weight and % fat.
| Carotenoid changea,b | Correlation coefficient | |||
|---|---|---|---|---|
| |
|
Body weight change |
Fat weight change |
% Fat change |
| Retinol | 0.23±0.51 | −0.06 | −0.06 | −0.04 |
| Lutein | 0.09±0.16 | −0.42** | −0.46** | −0.50** |
| Zeaxanthin | −0.004±0.03c | −0.18 | −0.18 | −0.22 |
| Lycopene | 0.15±0.27 | −0.15 | −0.15 | −0.14 |
| α-Carotene | 0.06±0.08 | −0.34* | −0.26 | −0.21 |
| β-Carotene | 0.24±0.42 | −0.28 | −0.31* | −0.30* |
| Total | 0.52±0.67d | −0.38** | −0.40** | −0.39** |
*P⩽0.05.
**P⩽0.01.
All values are means±s.d.'s.
n=42 except where noted.
n=39.
n=41.
Discussion
Increased consumption of vegetables and fruits has multiple health benefits, including lower blood pressure, as well as reduction in risk of cardiovascular disease, certain types of cancer and some vision problems.20, 21, 22, 23, 24 Use of this strategy for weight loss is gaining increasing attention.25, 26, 27 While our study to compare high vegetable intake with caloric reduction found that subjects in the Reduction group lost more weight and fat,14 the analyses presented here indicate that individuals who ate more vegetables and fruits (based on a serum biomarker of V&F intake), regardless of group assignment, lost more weight and fat. Similar relationships were found for changes in fat and % fat. In fact, the individual who lost the most weight and fat during the study also increased serum carotenoids the most and was in the calorie reduction group.
Of particular relevance, the analyses presented here are based on serum carotenoid concentrations rather than self-reported dietary intake. Use of serum carotenoid concentrations as a biomarker of vegetable intake is considerably more reliable than self-report,28, 29, 30, 31, 32, 33, 34, 35 especially in an intervention with an emphasis on increasing vegetable intake that may raise reporting bias. In fact, the Institute of Medicine concluded that ‘Blood concentrations of carotenoids are the best biological markers for consumption of fruits and vegetables'.36 Indeed, we found no correlation between self-reported intake and weight or fat loss, likely owing to poor compliance with dietary record keeping and inaccuracies inherent with these methods. Future obesity-related research will benefit from using serum carotenoids as a measure of dietary compliance to vegetable intake recommendations rather than relying on self-reported intake.
Nevertheless, an important factor to consider is that weight loss itself could contribute to increased serum carotenoids during periods of rapid weight loss. Adipose tissue is a major storage site for carotenoids.37, 38, 39 During weight loss, carotenoids stored in adipose could become mobilized and increase serum carotenoid levels as shown in an animal model.40 However, it is unlikely this contributed to the high correlation of serum carotenoids and weight loss seen in the current study because the majority of the weight loss occurred between 0 and 3 months,14 and the strongest correlations were seen at 12 months. Furthermore, subjects who routinely consumed high levels of vegetables were not enrolled in this study. Therefore, adipose storage was likely low in these subjects at the beginning of the study. Nonetheless, the impact of adipose sources of carotenoids on serum carotenoid levels during weight loss is an important area for future research as an increasing number of studies are relying on serum carotenoids as biomarkers of V&F intake during weight loss.15, 41, 42, 43
The correlation between increased serum carotenoids (a biomarker of V&F intake) and improved weight and fat loss add support to the advice to increase V&F intake in an effort to reduce weight. However, in combination with our previous results14 in which the V&F group did not lose as much weight as the Reduction group, simply advising increased vegetable intake, even in the context of weight loss, without an emphasis on maintaining reduced calorie intake is likely to be less effective than a combined effort to increase vegetable intake while maintaining reduced caloric intake. A notable challenge is getting individuals to increase V&F intake sufficiently and sustainably. With fewer than 24% of Americans consuming fruits and vegetables five or more times per day,44 there is still a need to find better ways to increase fruit and vegetable consumption.
Acknowledgments
Research was supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant number 2003-35200-05377 and the Wisconsin Cranberry Board. The authors thank Maria Lanasa, Katy Mijal, Jessica Nehring, Kelly O'Heron, Abby Small and Lisa Stanek for their devoted assistance with food preparation and data gathering; Rachel Staffeldt and Sara Kroes for organizing the phlebotomy; Karla Horsfall, Emily Nuss and Amy Petersen for assistance with data entry; and Dr Earl Shrago for serving as backup physician. LDW, RLA and SAT designed the research; LDW and ARV conducted the research; LDW, ZZ and LKJ analyzed the data; and LDW, RLA and SAT wrote the manuscript. All authors read and approved the final manuscript.
The authors declare no conflict of interest.
References
- Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- Bish CL, Blanck HM, Serdula MK, Marcus M, Kohl HW, Khan LK. Diet and physical activity behaviors among Americans trying to lose weight: 2000 Behavioral Risk Factor Surveillance System. Obes Res. 2005;13:596–607. doi: 10.1038/oby.2005.64. [DOI] [PubMed] [Google Scholar]
- Kruger J, Galuska DA, Serdula MK, Jones DA. Attempting to lose weight: specific practices among U.S. adults. Am J Prev Med. 2004;26:402–406. doi: 10.1016/j.amepre.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Andreyeva T, Long MW, Henderson KE, Grode GM. Trying to lose weight: diet strategies among Americans with overweight or obesity in 1996 and 2003. J Am Diet Assoc. 2010;110:535–542. doi: 10.1016/j.jada.2009.12.029. [DOI] [PubMed] [Google Scholar]
- Hill AM, Kris-Etherton PM. Contemporary strategies for weight loss and cardiovascular disease risk factor modification. Curr Atheroscler Rep. 2008;10:486–496. doi: 10.1007/s11883-008-0076-1. [DOI] [PubMed] [Google Scholar]
- Rolls BJ. The relationship between dietary energy density and energy intake. Physiol Behav. 2009;97:609–615. doi: 10.1016/j.physbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Stratum P, Lussenburg RN, van Wezel LA, Vergroesen AJ, Cremer HD. The effect of dietary carbohydrate:fat ratio on energy intake by adult women. Am J Clin Nutr. 1978;31:206–212. doi: 10.1093/ajcn/31.2.206. [DOI] [PubMed] [Google Scholar]
- Saltzman E, Dallal GE, Roberts SB. Effect of high-fat and low-fat diets on voluntary energy intake and substrate oxidation: studies in identical twins consuming diets matched for energy density, fiber, and palatability. Am J Clin Nutr. 1997;66:1332–1339. doi: 10.1093/ajcn/66.6.1332. [DOI] [PubMed] [Google Scholar]
- Stubbs RJ, Ritz P, Coward WA, Prentice AM. Covert manipulation of the ratio of dietary fat to carbohydrate and energy density: effect on food intake and energy balance in free-living men eating ad libitum. Am J Clin Nutr. 1995;62:330–337. doi: 10.1093/ajcn/62.2.330. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Bell EA. Dietary approaches to the treatment of obesity. Med Clin North Am. 2000;84:401–418. doi: 10.1016/s0025-7125(05)70228-5. [DOI] [PubMed] [Google Scholar]
- Ello-Martin JA, Roe LS, Ledikwe JH, Beach AM, Rolls BJ. Dietary energy density in the treatment of obesity: a year-long trial comparing 2 weight-loss diets. Am J Clin Nutr. 2007;85:1465–1477. doi: 10.1093/ajcn/85.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EA, Castellanos VH, Pelkman CL, Thorwart ML, Rolls BJ. Energy density of foods affects energy intake in normal-weight women. Am J Clin Nutr. 1998;67:412–420. doi: 10.1093/ajcn/67.3.412. [DOI] [PubMed] [Google Scholar]
- Stubbs RJ, Johnstone AM, O'Reilly LM, Barton K, Reid C. The effect of covertly manipulating the energy density of mixed diets on ad libitum food intake in ‘pseudo free-living' humans. Int J Obes Relat Metab Disord. 1998;22:980–987. doi: 10.1038/sj.ijo.0800715. [DOI] [PubMed] [Google Scholar]
- Tanumihardjo SA, Valentine AR, Zhang Z, Whigham LD, Lai HJ, Atkinson RL. Strategies to increase vegetable or reduce energy and fat intake induce weight loss in adults. Exp Biol Med. 2009;234:542–552. doi: 10.3181/0810-RM-293. [DOI] [PubMed] [Google Scholar]
- Kant AK. Interaction of body mass index and attempt to lose weight in a national sample of US adults: association with reported food and nutrient intake, and biomarkers. Eur J Clin Nutr. 2003;57:249–259. doi: 10.1038/sj.ejcn.1601549. [DOI] [PubMed] [Google Scholar]
- Dempster P, Aitkens S. A new air displacement method for the determination of human body composition. Med Sci Sports Exerc. 1995;27:1692–1697. [PubMed] [Google Scholar]
- Ginde SR, Geliebter A, Rubiano F, Silva AM, Wang J, Heshka S, et al. Air displacement plethysmography: validation in overweight and obese subjects. Obes Res. 2005;13:1232–1237. doi: 10.1038/oby.2005.146. [DOI] [PubMed] [Google Scholar]
- Howe JA, Valentine AR, Hull AK, Tanumihardjo SA. 13C natural abundance in serum retinol acts as a biomarker for increases in dietary provitamin A. Exp Biol Med. 2009;234:140–147. doi: 10.3181/0806-RM-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services & US Department of Agriculture Dietary Guidelines for Americans5th edn.US Government Printing Office: Washington, DC; 2002 [Google Scholar]
- Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- Retelny VS, Neuendorf A, Roth JL. Nutrition protocols for the prevention of cardiovascular disease. Nutr Clin Pract. 2008;23:468–476. doi: 10.1177/0884533608323425. [DOI] [PubMed] [Google Scholar]
- Steinmetz KA, Potter JD. Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc. 1996;96:1027–1039. doi: 10.1016/S0002-8223(96)00273-8. [DOI] [PubMed] [Google Scholar]
- Cho E, Seddon JM, Rosner B, Willett WC, Hankinson SE. Prospective study of intake of fruits, vegetables, vitamins, and carotenoids and risk of age-related maculopathy. Arch Ophthalmol. 2004;122:883–892. doi: 10.1001/archopht.122.6.883. [DOI] [PubMed] [Google Scholar]
- Christen WG, Liu S, Glynn RJ, Gaziano JM, Buring JE. Dietary carotenoids, vitamins C and E, and risk of cataract in women: a prospective study. Arch Ophthalmol. 2008;126:102–109. doi: 10.1001/archopht.126.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services & US Department of Agriculture Dietary Guidelines for Americans6th edn.US Government Printing Office: Washington, DC; 2005 [Google Scholar]
- Rolls BJ, Ello-Martin JA, Tohill BC. What can intervention studies tell us about the relationship between fruit and vegetable consumption and weight management. Nutr Rev. 2004;62:1–17. doi: 10.1111/j.1753-4887.2004.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Riccardi G, Capaldo B, Vaccaro O. Functional foods in the management of obesity and type 2 diabetes. Curr Opin Clin Nutr Metab Care. 2005;8:630–635. doi: 10.1097/01.mco.0000171126.98783.0c. [DOI] [PubMed] [Google Scholar]
- Yeum KJ, Booth SL, Sadowski JA, Liu C, Tang G, Krinsky NI, et al. Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am J Clin Nutr. 1996;64:594–602. doi: 10.1093/ajcn/64.4.594. [DOI] [PubMed] [Google Scholar]
- Smith-Warner SA, Elmer PJ, Tharp TM, Fosdick L, Randall B, Gross M, et al. Increasing vegetable and fruit intake: randomized intervention and monitoring in an at-risk population. Cancer Epidemiol Biomarkers Prev. 2000;9:307–317. [PubMed] [Google Scholar]
- Hann CS, Rock CL, King I, Drewnowski A. Validation of the Healthy Eating Index with use of plasma biomarkers in a clinical sample of women. Am J Clin Nutr. 2001;74:479–486. doi: 10.1093/ajcn/74.4.479. [DOI] [PubMed] [Google Scholar]
- El-Sohemy A, Baylin A, Kabagambe E, Ascherio A, Spiegelman D, Campos H. Individual carotenoid concentrations in adipose tissue and plasma as biomarkers of dietary intake. Am J Clin Nutr. 2002;76:172–179. doi: 10.1093/ajcn/76.1.172. [DOI] [PubMed] [Google Scholar]
- Bogers RP, Dagnelie PC, Westerterp KR, Kester AD, Van Klaveren JD, Bast A, et al. Using a correction factor to correct for overreporting in a food-frequency questionnaire does not improve biomarker-assessed validity of estimates for fruit and vegetable consumption. J Nutr. 2002;133:1213–1219. doi: 10.1093/jn/133.4.1213. [DOI] [PubMed] [Google Scholar]
- Campbell DR, Gross MD, Martini MC, Grandits GA, Slavin JL, Potter JD. Plasma carotenoids as biomarkers of vegetable and fruit intake. Cancer Epidemiol Biomarkers Prev. 1994;3:493–500. [PubMed] [Google Scholar]
- Le Marchand L, Hankin JH, Carter FS, Essling C, Luffey D, Franke AA, et al. A pilot study on the use of plasma carotenoids and ascorbic acid as markers of compliance to a high fruit and vegetable dietary intervention. Cancer Epidemiol Biomarkers Prev. 1994;3:245–251. [PubMed] [Google Scholar]
- Martini MC, Campbell DR, Gross MD, Grandits GA, Potter JD, Slavin JL. Plasma carotenoids as biomarkers of vegetable intake: the University of Minnesota Cancer Prevention Research Unit Feeding Studies. Cancer Epidemiol Biomarkers Prev. 1995;4:491–496. [PubMed] [Google Scholar]
- Institute of Medicine, National Academy of Sciences, Food and Nutrition Board, Panel on Dietary Antioxidants and Related Compounds β-Carotene and other carotenoids Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids National Academy Press: Washington, DC; 2000. p325 [Google Scholar]
- Dagadu JM. Distribution of carotene and vitamin A in liver, pancreas and body fat of Ghanaians. Br J Nutr. 1997;21:453–456. doi: 10.1079/bjn19670046. [DOI] [PubMed] [Google Scholar]
- Parker RS. Carotenoid and tocopherol composition of human adipose tissue. Am J Clin Nutr. 1988;47:33–36. doi: 10.1093/ajcn/47.1.33. [DOI] [PubMed] [Google Scholar]
- Chung HY, Ferreira AL, Epstein S, Paiva SA, Castaneda-Sceppa C, Johnson EJ. Site-specific concentrations of carotenoids in adipose tissue: relations with dietary and serum carotenoid concentrations in healthy adults. Am J Clin Nutr. 2009;90:533–539. doi: 10.3945/ajcn.2009.27712. [DOI] [PubMed] [Google Scholar]
- Arias E, González A, Shimada A, Varela-Echavarria A, Ruiz-López F, During A, et al. Beta-carotene is incorporated or mobilized along with triglycerides in bovine adipose tissue in response to insulin or epinephrine. J Anim Physiol Anim Nutr. 2009;93:83–93. doi: 10.1111/j.1439-0396.2007.00783.x. [DOI] [PubMed] [Google Scholar]
- Kant AK. Weight-loss attempts and reporting of foods and nutrients, and biomarkers in a national cohort. Int J Obes Relat Metab Disord. 2002;26:1194–1204. doi: 10.1038/sj.ijo.0802024. [DOI] [PubMed] [Google Scholar]
- Noakes M, Foster PR, Keogh JB, Clifton PM. Meal replacements are as effective as structured weight-loss diets for treating obesity in adults with features of metabolic syndrome. J Nutr. 2004;134:1894–1899. doi: 10.1093/jn/134.8.1894. [DOI] [PubMed] [Google Scholar]
- Rock CL, Pakiz B, Flatt SW, Quintana EL. Randomized trial of a multifaceted commercial weight loss program. Obesity. 2007;15:939–949. doi: 10.1038/oby.2007.614. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Behavioral Risk Factor Surveillance System Survey Data. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention Centers for Disease Control and Prevention (CDC): Atlanta, Georgia; 2009 , http://apps.nccd.cdc.gov/brfss/index.asp . Accessed 2 August 2012. [Google Scholar]