Abstract
OBJECTIVES:
We aimed to test the hypotheses that (i) plasma choline metabolites differ between normal (body mass index (BMI)<25 kg m−2) and overweight (BMI ⩾25 kg m−2) men, and (ii) an elevated BMI alters associations between plasma choline metabolites and indicators of metabolic stress.
DESIGN:
This was a cross-sectional study. A one-time fasting blood sample was obtained for measurements of the choline metabolites and metabolic stress indicators (that is, serum alanine aminotransferase (ALT), glucose, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides and homocysteine), and for genotype determination.
SUBJECTS:
The analysis was conducted with 237 Mexican American men with a median age of 22 years.
RESULTS:
Compared with men with a normal BMI (n=98), those with an elevated BMI (n=139) had 6% lower (P=0.049) plasma betaine and an 11% lower (P=0.002) plasma betaine to choline ratio. Among men with an elevated BMI, plasma betaine and the plasma betaine to choline ratio positively associated (P⩽0.044) with a favorable serum cholesterol profile, and inversely associated (P=0.001) with serum ALT, a marker of liver dysfunction. The phosphatidylethanolamine N-methyltransferase (PEMT) 5465G→A (rs7946) genotype interacted (P⩽0.007) with the plasma betaine to choline ratio to modulate indicators of metabolic stress with stronger inverse associations observed among overweight men with the PEMT 5465GG genotype.
CONCLUSIONS:
Plasma choline metabolites predict metabolic stress among overweight men often in a genotype-specific manner. The diminished betaine among overweight men coupled with the inverse association between betaine and metabolic stress suggest that betaine supplementation may be effective in mitigating some of the metabolic insults arising from lipid overload.
Keywords: choline, betaine, body mass index, metabolic stress, phosphatidylethanolamine N-methyltransferase 5465G→A (rs7946), methylenetetrahydrofolate dehydrogenase 1 1958G→A (rs2236225)
Introduction
Obesity can lead to a number of metabolic disturbances and increase the risk of metabolic syndrome, type 2 diabetes and cardiovascular diseases.1, 2, 3, 4, 5, 6 The metabolic stress imposed by obesity is often indicated by alterations in circulating lipoproteins, triglycerides, glucose and markers of liver function. Obesity also manifests as excess lipid deposition in adipose tissue and frequently the liver.7, 8 Studies in rodent models of obesity suggest that hepatic lipid overload results in perturbations in choline-related one-carbon metabolism.9, 10
Choline, an essential nutrient, is a major lipotrophe (that is, nutrient that is involved in the mobilization of fat from liver).11 Its derivative phosphatidylcholine (Figure 1), which is synthesized from choline via the cytidine diphosphate–choline pathway, is required for the production of hepatic very-low-density lipoprotein and the subsequent export of fat from liver. Choline deficiency results in fatty liver and liver dysfunction, which leads to elevations in serum concentrations of the liver enzyme, alanine aminotransferase (ALT).12, 13 Phosphatidylcholine can also be produced via the phosphatidylethanolamine N-methyltransferase (PEMT) pathway where methyl groups associated with S-adenosylmethionine are used to sequentially methylate phosphatidylethanolamine. Like the cytidine diphosphate–choline pathway, PEMT-mediated phosphatidylcholine biosynthesis is required for the hepatic exportation of triglycerides.14
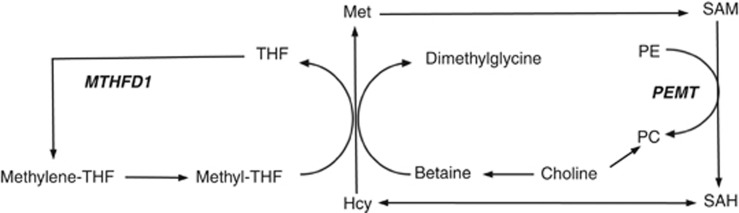
Figure 1.
Simplified diagram of choline-related one-carbon metabolism. Choline is the precursor of betaine, which serves as a source of methyl groups for homocysteine (Hcy) remethylation to methionine (Met). Alternatively, homocysteine can be remethylated with methyl groups from methyl-tetrahydrofolate (methyl-THF), which is derived from methylenetetrahydrofolate (methylene-THF). MTHFD1 (methylenetetrahydrofolate dehydrogenase 1), a trifunctional enzyme catalyzes the production of methylene-THF from THF. Methionine is used in the synthesis of S-adenosylmethionine, a methyl donor for more than 50 methyltransferases, including phosphatidylethanolamine N-methyltransferase (PEMT). PEMT catalyzes the synthesis of phosphatidylcholine (PC) from phosphatidylethanolamine (PE) and produces S-adenosylhomocysteine (SAH). In addition, PC can be synthesized from choline via the cytidine diphosphate–choline pathway.
Choline can also be oxidized to betaine, which serves as a source of methyl groups for the synthesis of phosphatidylcholine through the PEMT pathway (Figure 1). Betaine depletion and fatty liver develop in rodents consuming a high-fat/high-energy diet. Remarkably, betaine supplementation restores betaine status, improves insulin sensitivity and ameliorates fatty liver in these animals.10, 15, 16 Betaine supplementation has also been shown to reduce animal fat mass in livestock and is routinely used in pig and poultry farming to reduce the fat content of the product.17, 18
In humans, plasma choline metabolites were associated with components of metabolic syndrome among older adults19 and with plasma lipids in a population with acute coronary syndrome.20 However, no studies have investigated the relationships between circulating choline metabolites, lipid load (indicated by body mass index (BMI)) and metabolic stress in a young, disease-free population.
In a cohort of 237 Mexican American men, we aimed to test the hypotheses that (i) plasma choline metabolites differ between normal (BMI<25 kg m−2) and overweight (BMI⩾25 kg m−2) men, and (ii) an elevated BMI alters associations between plasma choline metabolites and indicators of metabolic stress. The indicators of metabolic stress examined in the present study included serum concentrations of ALT, glucose, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), triglycerides and homocysteine. As genetic variants in one-carbon metabolic genes (that is, MTHFD1 1958G→A [rs2236225] and PEMT 5465G→A [rs7946]) affect choline metabolism,21, 22, 23 we examined the potential modifying effects of these two genetic variants on associations between the plasma choline metabolites and indicators of metabolic stress.
Methods
Study participants and study design
A cohort of self-defined healthy, non-smoking Mexican American men (n=237) aged 18–48 years was included in the study. All participants completed a study questionnaire, which queried the men about their age, height, weight, education, medical history, medication and supplement use, activity level, and consumption of alcohol, soda and caffeine. Recruiting and enrollment for this study occurred from June 2005 through September 2006 in Pomona, California and surrounding areas. Approval for conducting this study was obtained by the Institutional Review Board at Cal Poly Pomona University and informed consent was obtained from all study participants.
This was a cross-sectional study in which a one-time fasting venous blood sample was collected and processed for serum, plasma and leukocytes as previously described.24 The fasting blood was collected into ethylenediaminetetraacetic acid-coated tubes for the separation of plasma.
Analytical measurements
Choline metabolites, homocysteine and folate
Plasma concentrations of free choline and betaine were measured by liquid chromatography–tandem mass spectrometry according to the method of Holm et al.25 with modifications based on our instrumentation.26 Plasma total homocysteine was quantified using a modified high-performance liquid chromatography procedure with fluorescent detection as described previously.27 Folate concentrations of serum and red blood cells were determined microbiologically using microtiter plate adaptation with Lactobacillus rhamnosis.27
Biochemical analytes
Serum creatinine, total cholesterol, HDL-C, LDL-C, glucose, triglycerides, ALT and blood urea nitrogen were measured on MODULAR System (Roche/Hitachi, Tokyo, Japan) at Cedars-Sinai Medical Center, Department of Pathology and Laboratory Medicine (Los Angeles, CA, USA).
Genotyping
DNA for genotyping was extracted from blood leukocytes using a commercially available kit (DNeasy Tissue Kit, Qiagen Science, Germantown, MD, USA). PEMT 5465G→A (rs7946) genotype was determined using a fluorescent TaqMan probe commercially available kit (Applied Biosystems, Foster City, CA, USA) and real-time polymerase chain reaction (PCR) Chromo 4 (Bio-Rad, Hercules, CA, USA) following the manufacturer's protocol. A restriction fragment length polymorphism assay as described by Hol et al.28 was used for the determination of the MTHFD1 1958G→A (rs2236225).
Statistical analyses
Two hundred and thirty-seven apparently healthy Mexican American men were included in the analysis. Participants were stratified into a normal weight group (BMI<25 kg m−2, n=98) and an overweight group (BMI⩾25.0 kg m−2, n=139). In the final analysis, data were available for ⩾210 participants for each variable. Normality distributions of variables were tested with Kolmogorov–Smirnov test; log transformations of plasma homocysteine, serum glucose, serum triglycerides, the ratio of plasma betaine to choline, serum total cholesterol, total cholesterol to HDL-C ratio (TC/HDL-C) and ALT were performed and log-transformed data were used in the one-way analysis of variance, age- and BMI-adjusted bivariate partial correlation tests and general linear regression models.
To compare plasma choline metabolites between normal and overweight men, a one-way analysis of variance was conducted for each dependent variable. In addition, a χ2 test for independence was used to compare the distribution of the genetic polymorphism (for example, MTHFD1 1958GG, 1958GA and 1958AA) between normal and overweight men.
To assess the relationships between plasma choline-related metaoblites (for example, plasma betaine) and indicators of metabolic stress (for example, serum ALT), age- and BMI-adjusted bivariate partial correlation tests were conducted for normal weight and overweight men, separately. The relationships were further tested with general linear models, in which age, BMI, education level, physical activity level as well as the consumptions of alcohol, soda and caffeine were controlled as covariates if they were significant predictors of the dependent variable (P<0.1). The influence of the genetic variants (that is, PEMT 5465G→A (rs7946) and MTHFD1 1958G→A (rs2236225)) was also examined by adding each variant separately to the final regression model. When interactions were detected between the genetic variants and the plasma choline metabolites, the data were further stratified to delineate the nature of these interactions.
As the number and age of study participants differed between the overweight and normal weight groups, the analysis was repeated with 98 of the youngest overweight men (median age=22 years) and 98 of the normal weight men (median age=21 years). The results of this analysis were similar to the analysis including all overweight men and did not alter the study findings or conclusions.
All analyses were performed using SPSS (v.20.0; Chicago, IL, USA) and a P⩽0.05 was considered significant.
Results
Study participant characteristics
Table 1 displays the characteristics and biochemical measurements of the study population after dividing the cohort into a normal weight group (BMI<25 kg m−2; n=98) and an overweight group (BMI⩾25 kg m−2; n=139). The median BMI of the normal weight group was 22 kg m−2 with 97% of the participants having a BMI⩾18.5 and <24.9 kg m−2. The median BMI of the overweight group was 28 kg m−2 with 64% (n=89) of the participants having a BMI⩾25.0 and <30.0 kg m−2. Overall, the study cohort was comprised of young men with the normal weight group having a lower median age (21 years) than the overweight group (23 years). As expected in a population exposed to folic acid fortification, folate status was elevated and measurements of folate did not differ between the weight categories. Overall, the frequencies of the genetic variants were 20% GG, 50% GA and 30% AA for MTHFD1 1958G→A (rs2236225) and 16% GG, 51% GA and 33% AA for PEMT 5465G→A (rs7946). For each gene, the alleles of the polymorphism were in Hardy–Weinberg equilibrium.
Table 1. Age, BMI, circulating concentrations of choline and one-carbon metabolites, and metabolic stress indicators among normal (n=98) and overweight (n=139) mena,b.
| Normal weight BMI<25 kg m−2 | Overweight BMI⩾25 kg m−2 | P-values | |
|---|---|---|---|
| Age (years) | 21 (17, 41) | 23 (18, 56) | 0.001 |
| BMI (kg m−2) | 22.7 (16.3, 24.8) | 28.3 (25.0, 39.3) | <0.001 |
| Plasma choline (μmol l−1) | 7.1 (3.7, 12.8) | 7.5 (3.1, 11.3) | 0.15 |
| Plasma betaine (μmol l−1) | 40.1 (24.4, 58.2) | 37.3 (21.2, 79.4) | 0.049 |
| Plasma betaine:choline ratio | 5.6 (3.0, 11.0) | 4.9 (2.4, 12.5) | 0.002 |
| Serum folate (ng ml−1) | 14.3 (6.0, 38.4) | 14.9 (3.8, 48.9) | 0.848 |
| RBC folate (ng ml−1) | 758 (424, 1402) | 751 (397, 1162) | 0.819 |
| Plasma Hcy (μmol l−1) | 7.6 (4.2, 23.3) | 7.4 (4.5, 28.6) | 0.69 |
| Serum ALT (U l−1) | 19 (8, 95) | 26 (7, 173) | <0.001 |
| Serum BUN (mg dl−1) | 14 (7, 23) | 14 (7, 26) | 0.895 |
| Serum creatinine (mg dl−1) | 1.0 (0.7, 1.3) | 1.0 (0.7, 1.4) | 0.981 |
| Serum glucose (mg dl−1) | 89 (67, 116) | 91 (60, 135) | 0.004 |
| Serum HDL-C (mg dl−1) | 48 (29, 82) | 43 (27, 88) | 0.018 |
| Serum LDL-C (mg dl−1) | 89 (43, 161) | 106 (52, 168) | <0.001 |
| Serum TC (mg dl−1) | 157 (85, 236) | 176 (104, 256) | <0.001 |
| Serum TC/HDL-C | 3.2 (2.0, 6.9) | 4.1 (0.9, 8.2) | <0.001 |
| Serum triglycerides (mg dl−1) | 88 (22, 335) | 118 (32, 135) | <0.001 |
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; Hcy, total homocysteine; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; RBC, red blood cell; TC, total cholesterol; TC/HDL-C, total cholesterol to HDL-C ratio.
Data are presented as median (range).
Data were analyzed by using one-way analysis of variance; n=89∼98 for BMI<25 kg m−2 group, n=129∼139 for BMI⩾25 kg m−2; log-transformed plasma betaine:choline ratio, Hcy, ALT, glucose, TC/HDL-C and triglycerides were used in the statistical test.
Choline metabolites and genotype frequencies differed between normal weight and overweight men
Overweight men had 6% lower (P=0.049) plasma betaine and an 11% lower (P=0.002) plasma betaine:choline ratio than normal weight men (Table 1). Overweight men also had lower serum HDL-C but higher serum concentrations of TC, LDL-C, TC/HDL-C, glucose, triglycerides and ALT (Table 1).
In addition, the distribution of PEMT 5465G→A (rs7946) genotype differed between the overweight and normal weight groups with a higher (P=0.03) percentage of the GA and AA in the overweight (89%) versus the normal weight (77%) group. The distribution of MTHFD1 1958G→A (rs2236225) genotype also tended to differ (P=0.073) with greater representation of the MTHFD1 1958AA genotype in the overweight men.
Associations between plasma choline metabolites and metabolic stress differed between normal weight and overweight men
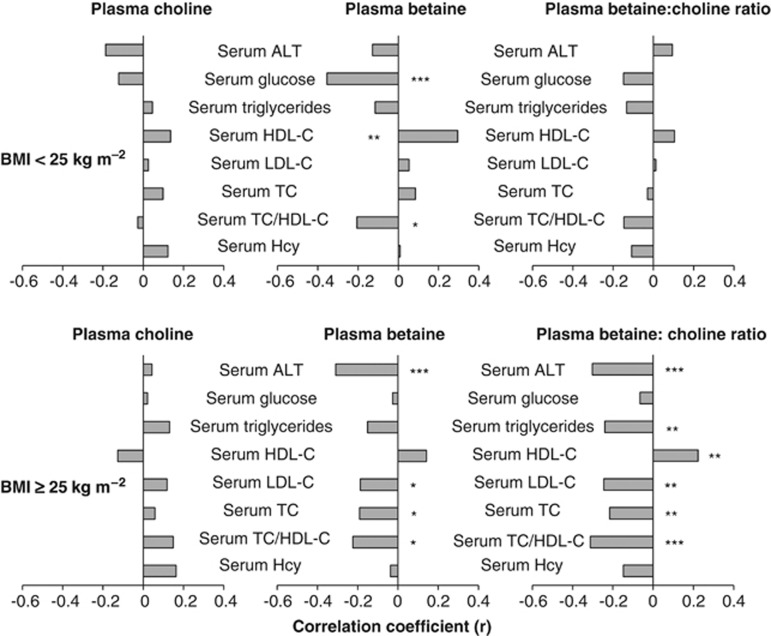
The associations between plasma choline metabolites and metabolic stress indicators (serum concentrations of ALT, glucose, HDL-C, LDL-C, total cholesterol, triglycerides and homocysteine) were initially tested by bivariate partial analysis (Figure 2). General linear regression models were used to confirm the results of the bivariate partial analysis (confounding factors were controlled) and to examine the modifying effect of genotype. Only results that achieved a statistical significance of P⩽0.05 are described.
Figure 2.
Age- and BMI-adjusted bivariate associations between plasma choline, betaine and the plasma betaine:choline ratio with metabolic stress among normal weight (BMI<25 kg m−2; n=89∼98) and overweight (BMI⩾25 kg m−2, n=129∼139) men, separately. Log-transformed plasma betaine:choline ratio, Hcy, ALT, glucose, TC/HDL-C and triglycerides were used in the bivariate partial correlation test. *P⩽0.05, **P⩽0.01 and ***P⩽0.001. TC, total cholesterol; Hcy, total homocysteine.
Normal weight men
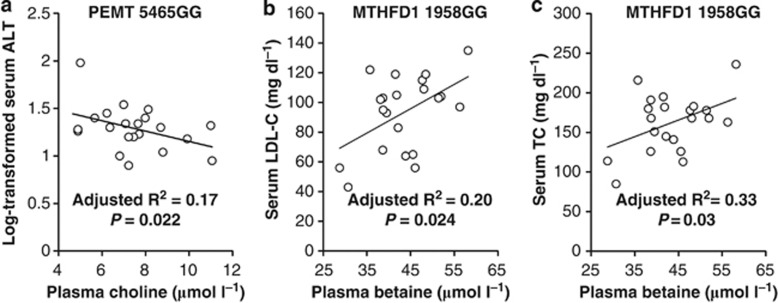
Plasma choline and the PEMT 5465G→A (rs7946) genotype interacted (P=0.01) to modify serum ALT. An inverse relationship between plasma choline and serum ALT was detected (P=0.022) only among men with the PEMT 5465GG genotype (Figure 3a).
Figure 3.
Plasma choline was inversely associated with the log-transformed serum ALT (a) among normal weight men with the PEMT 5465GG genotype. Plasma betaine was positively associated with serum LDL-C (b) and serum TC (c) among normal weight men with MTHFD1 1958GG genotype. TC, total cholesterol; MTHFD1, methylenetetrahydrofolate dehydrogenase 1.
Plasma betaine was inversely associated with serum glucose (P=0.001, Figure 2), positively associated with serum HDL-C (P=0.004, Figure 2) and inversely associated with TC/HDL-C (P=0.004, Figure 2); inclusion of covariates in the regression analysis did not alter these relationships. In addition, plasma betaine and MTHFD1 1958G→A (rs2236225) genotype interacted (P⩽0.031) to influence LDL-C and TC. In men with MTHFD1 1958GG genotype, plasma betaine was positively associated with serum LDL-C (P=0.024, Figure 3b) and serum TC (P=0.03, Figure 3c).
A main effect of the PEMT 5465G→A (rs7946) genotype on serum HDL-C was also detected. Men with at least one copy of the variant A allele had lower serum HDL-C compared with men with the 5465GG genotype (P=0.004; 47 versus 55 mg dl−1).
Overweight men
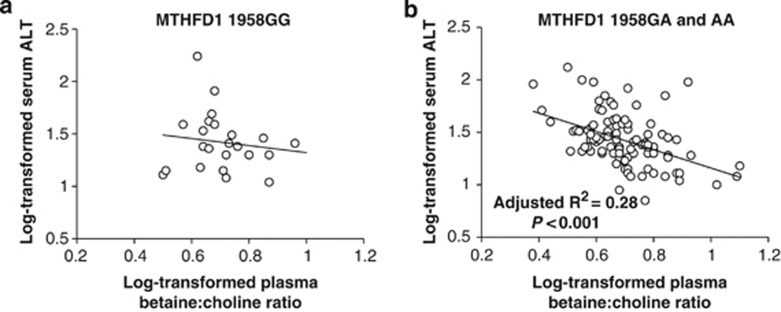
Plasma betaine (P=0.001) and the plasma betaine:choline ratio (P=0.001) were inversely associated with serum ALT (Figure 2). However, the MTHFD1 1958G→A (rs2236225) genotype interacted with the plasma betaine:choline ratio (P=0.027) to influence serum ALT such that an inverse relationship was detected (P<0.001) only among men with at least one copy of the variant MTHFD1 1958A allele (Figure 4).
Figure 4.
The log-transformed plasma betaine:choline ratio was inversely associated with the log-transformed serum ALT among overweight men with at least one copy of the MTHFD1 1958A allele (b) but not among overweight men with the MTHFD1 1958GG genotype (a). MTHFD1, methylenetetrahydrofolate dehydrogenase 1.
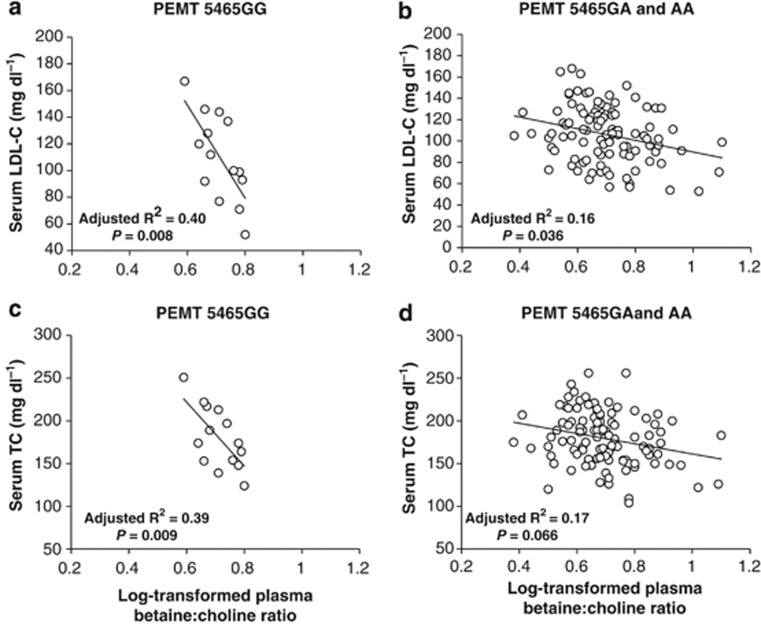
Plasma betaine was inversely associated with serum concentrations of TC (P=0.036), LDL-C (P=0.044) and TC/HDL-C (P=0.014) (Figure 2). Substituting plasma betaine with the plasma betaine:choline ratio yielded similar associations with HDL-C (P=0.014), LDL-C (P=0.008), TC (P=0.018), TC/HDL-C (P<0.001) and triglycerides (P=0.008) (Figure 2). However, an interaction between the plasma betaine:choline ratio and PEMT 5465G→A (rs7946) was detected in the regression models for serum LDL-C (P=0.003) and TC (P=0.005). The inverse associations between the plasma betaine:choline ratio with serum LDL-C and TC were stronger among men with the PEMT 5465GG versus the 5465GA and AA genotype (Figure 5).
Figure 5.
An inverse association between the log-transformed plasma betaine:choline ratio and serum LDL-C (a, b) was stronger among overweight men with the PEMT 5465GG (a) versus the 5465GA and AA genotype (b). Similarly, an inverse association between the log-transformed plasma betaine:choline ratio and serum TC (c, d) was stronger among overweight men with PEMT 5465GG (c) versus the 5465GA and AA genotype (d). TC, total cholesterol.
Finally, the plasma betaine:choline ratio interacted (P=0.042) with the PEMT 5465G→A (rs7946) genotype to affect plasma homocysteine. An inverse association (P=0.032) was detected only among men with PEMT 5465AA genotype (data not shown).
Discussion
In this cohort of young men, we demonstrate that (i) plasma choline metabolites differ between normal and overweight men, (ii) plasma choline metabolites, especially the ratio of plasma betaine to choline, predict metabolic stress among overweight men and (iii) the relationship between plasma choline metabolites and metabolic stress is frequently genotype-specific.
Plasma choline metabolites differ between normal and overweight men
The lower concentrations of betaine and the reduced plasma betaine:choline ratio in men with an elevated BMI is consistent with previous findings of an inverse relationship between betaine and BMI in humans.19, 20 As BMI is a surrogate for lipid load, and phosphatidylcholine is required for hepatic lipid export, the lower plasma betaine concentrations among men with an elevated BMI may arise from the increased use of betaine as a methyl donor for phosphatidylcholine biosynthesis through the PEMT pathway. In this regard, several independent studies have reported that consumption of a high-fat diet by male mice selectively upregulated the PEMT pathway29, 30 and resulted in obesity and diminished hepatic betaine.9, 31
Plasma choline metabolites predict metabolic stress among overweight men
Previous work has reported divergent associations between plasma choline (positive) and plasma betaine (inverse) with metabolic stress among older adults.19 In our cohort of young men with an elevated BMI, plasma betaine was inversely associated with serum concentrations of ALT, LDL-C, TC and the TC/HDL-C ratio but positively associated with the serum concentrations of HDL-C. Although, we did not detect statistically significant associations between plasma choline and metabolic disturbances, the ratio of plasma betaine:choline was a better predictor of metabolic stress than plasma betaine alone (Figure 2). This ratio combines the predictive power of both metabolites with metabolic stress, and its higher sensitivity arises from its ability to capture the divergent associations of choline and betaine with metabolic disturbances. The divergent associations of choline and betaine with metabolic stress may stem from an increase in the PEMT pathway in response to a lipid load (that is, elevated BMI) as demonstrated in mice.9 An upregulated PEMT pathway will increase the use of betaine as a methyl donor and enhance the production of choline thereby yielding a lower plasma betaine: choline ratio (betaine→methionine→S-adenosylmethionine→phosphatidylcholine→choline).
The inverse association between plasma betaine and metabolic stress among men with an elevated BMI may imply that a higher betaine is metabolically advantageous as demonstrated in animal models of obesity.10, 15, 16 As such, supplementation with betaine may mitigate some of the metabolic disturbances arising from an elevated BMI, and associated lipid overload, as previously suggested.20, 32, 33, 34 Potential mechanisms by which betaine may mitigate metabolic stress include its role as a methyl donor and lipotrope (the focus of this study) but also via its osmoprotective properties.35, 36, 37 Nevertheless, data that contradict the beneficial metabolic benefits of supplemental betaine also exist,38, 39, 40, 41, 42 including reports of small, but statistically significant, increases in serum LDL-C in response to large doses of supplemental betaine (that is, ⩾6 g).
The relationship between plasma choline metabolites and metabolic stress is frequently genotype-specific
Many of the associations between plasma choline metabolites and indicators of metabolic stress were modified by genetic variants in one-carbon metabolism. Among overweight men, the inverse association between the plasma betaine:choline ratio and serum LDL-C was stronger in men with the PEMT 5465GG genotype as compared with those with the PEMT 5465GA and AA genotypes. Although these alleles may impact PEMT activity,21 genotype did not influence any of the one-carbon metabolic markers (that is, choline metabolites, homocysteine and folate) in the present study. Thus, the mechanism by which the PEMT 5465G→A (rs7946) genotype modifies the association between the ratio and serum LDL-C is unclear.
MTHFD1 has a critical role in folate metabolism and the variant 1958A allele is associated with increased susceptibility to liver dysfunction.22 Among overweight men in the present study, the inverse association between the plasma betaine:choline ratio and serum ALT was detected only in men with one or two copies of the variant 1958 A allele. The A allele diminishes the pool of folate available for the biosynthesis of S-adenosylmethionine, the substrate for the PEMT pathway. In turn, less phosphatidylcholine would be available for the maintenance of the plasma membrane, which can result in the leakage of the liver enzymes into circulation.14 Under this metabolic scenario in which folate is restricted, betaine becomes a more significant source of methyl groups, and an elevated betaine among men with the variant 1958A allele may be advantageous as it can provide one carbon for the PEMT reaction.
It is also worth noting that genetic variants within one-carbon metabolizing genes may independently predispose to obesity. We observed that the PEMT variant 5465A allele was over represented among overweight versus normal weight men. Others have reported a higher prevalence of the variant A allele among patients with fatty liver,21, 43 and recent studies with PEMT knockout mice suggest that PEMT may be important for whole-body energy metabolism.31 More human studies with independent cohorts are needed to further investigate whether the PEMT 5465 variant A allele is a genetic risk factor for obesity.
Strengths and weaknesses
To the best of our knowledge, this is the first study examining the relationships between markers of choline metabolism (metabolites and genetic variants), lipid load (indicated by elevated BMI) and metabolic stress among a young and ethnically homogenous population exposed to folic acid fortification. The study population was well defined, the potential confounders were considered and controlled in the analysis, and the main study findings were consistent with the results of experimental animal studies. However, this study was not adequately powered to adjust for the multiple comparisons performed in the genotype sub-analyses. In addition, the study findings may not be generalizable to women owing to gender differences in PEMT activity and in choline and betaine metabolism.44, 45 Finally, association does not imply causation and more studies are needed to delineate the mechanisms underlying the observed relationships between markers of choline metabolism (metabolites and genetic variants) and indicators of metabolic stress.
Conclusion
In sum, these data suggest that a lipid overload (indicated by an elevated BMI) can perturb choline metabolism (that is, reduce betaine) among young men. In addition, the inverse association between plasma betaine and metabolic stress among overweight men suggests that supplemental betaine (or choline) may be useful in mitigating some of the metabolic insults induced by lipid overload. Our study findings also suggest that select genetic subgroups (for example, persons with the PEMT 5465GG genotype) may be more responsive to nutritional strategies (that is, betaine supplementation) designed to mitigate metabolic stress and/or attenuate fatty liver.
Acknowledgments
This work was funded in part by National Institute of Health Grant no. S06GM053933 and funds from the California Agricultural Research Institute.
Author contributions
JY and MAC designed the research; JY, LBW and BB-W conducted the research; JY analyzed the data with significant input from FV; and JY and MAC wrote the paper. MAC had primary responsibility for final content. All authors read and approved the final manuscript. The authors are grateful to the study subjects whose participation made the study possible
The authors declare no conflict of interest.
References
- Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link. Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- Goran MI, Ball GDC, Cruz ML. Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. J Clin Endocrinol Metab. 2003;88:1417–1427. doi: 10.1210/jc.2002-021442. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. NEJM. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Mertens IL, Christophe E. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40:S5–S10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- Rubio-Aliaga I, de Roos B, Sailer M, McLoughlin GA, Boekschoten MV, van Erk M, et al. Alterations in hepatic one-carbon metabolism and related pathways following a high-fat dietary intervention. Physiol Genomics. 2011;43:408–416. doi: 10.1152/physiolgenomics.00179.2010. [DOI] [PubMed] [Google Scholar]
- Kathirvel E, Morgan K, Nandgiri G, Sandoval BC, Caudill MA, Bottiglieri T, et al. Betaine improves nonalcoholic fatty liver and associated hepatic insulin resistance: a potential mechanism for hepatoprotection by betaine. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1068–G1077. doi: 10.1152/ajpgi.00249.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu Rev Nutr. 1994;14:269–296. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]
- Buchman AL, Dubin MD, Moukarzel AA, Jenden DJ, Roch M, Rice KM, et al. Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology. 1995;22:1399–1403. [PubMed] [Google Scholar]
- Zeisel SH, Da Costa K, Franklin PD, Alexander EA, Lamont J, Sheard N, et al. Choline, an essential nutrient for humans. FASEB J. 1991;5:2093–2098. [PubMed] [Google Scholar]
- Li Z, Agellon LB, Allen TM, Umeda M, Jewell L, Mason A, et al. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab. 2006;3:321–331. doi: 10.1016/j.cmet.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yao T, Pini M, Zhou Z, Fantuzzi G, Song Z. Betaine improved adipose tissue function in mice fed a high-fat diet: a mechanism for hepatoprotective effect of betaine in nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2010;298:G634–G642. doi: 10.1152/ajpgi.00249.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Deaciuc I, Zhou Z, Song M, Chen T, Hill D, et al. Involvement of AMP-activated protein kinase in beneficial effects of betaine on high-sucrose diet-induced hepatic steatosis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G894–G902. doi: 10.1152/ajpgi.00133.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund M, Bauer E, Wamatu J, Mosenthin R. Potential nutritional and physiological functions of betaine in livestock. Nutr Res Rev. 2005;18:31–48. doi: 10.1079/NRR200493. [DOI] [PubMed] [Google Scholar]
- Ratriyanto A, Mosenthin R, Bauer E, Eklund M. Metabolic, osmoregulatory and nutritional functions of betaine in monogastric animals. Asian-Austral J Animal Sci. 2009;22:1461–1476. [Google Scholar]
- Konstantinova SV, Tell GS, Vollset SE, Nygård O, Bleie Ø, Ueland PM. Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J Nutr. 2008;138:914–920. doi: 10.1093/jn/138.5.914. [DOI] [PubMed] [Google Scholar]
- Lever M, George PM, Atkinson W, Molyneux SL, Elmslie JL, Slow S, et al. Plasma lipids and betaine are related in an acute coronary syndrome cohort. PloS One. 2011;6:e21666. doi: 10.1371/journal.pone.0021666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, da Costa KA, Fischer LM, Kohlmeier M, Kwock L, Wang S, et al. Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD) FASEB J. 2005;19:1266–1271. doi: 10.1096/fj.04-3580com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmeier M, Da Costa KA, Fischer LM, Zeisel SH. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc Natl Acad Sci USA. 2005;102:16025–16030. doi: 10.1073/pnas.0504285102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, Nash-Barboza S, Hinkis S, Caudill MA. Genetic variants in phosphatidylethanolamine N-methyltransferase and methylenetetrahydrofolate dehydrogenase influence biomarkers of choline metabolism when folate intake is restricted. J Am Diet Assoc. 2009;109:313–318. doi: 10.1016/j.jada.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinotte CL, Burns MG, Axume JA, Hata H, Urrutia TF, Alamilla A, et al. Methylenetetrahydrofolate reductase 677C-> T variant modulates folate status response to controlled folate intakes in young women. J Nutr. 2003;133:1272–1280. doi: 10.1093/jn/133.5.1272. [DOI] [PubMed] [Google Scholar]
- Holm PI, Ueland PM, Kvalheim G, Lien EA. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin Chem. 2003;49:286–294. doi: 10.1373/49.2.286. [DOI] [PubMed] [Google Scholar]
- Yan J, Wang W, Gregory JF, Malysheva O, Brenna JT, Stabler SP, et al. MTHFR C677T genotype influences the isotopic enrichment of one-carbon metabolites in folate-compromised men consuming d9-choline. Am J Clin Nutr. 2011;93:348–355. doi: 10.3945/ajcn.110.005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis C, Veenema K, Ivanov AA, Tran S, Li R, Wang W, et al. Folate intake at RDA levels is inadequate for Mexican American men with the methylenetetrahydrofolate reductase 677TT genotype. J Nutr. 2008;138:67–72. doi: 10.1093/jn/138.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hol FA, Put NMJ, Geurds M, Heil SG, Trijbels FJM, Hamel BCJ, et al. Molecular genetic analysis of the gene encoding the trifunctional enzyme MTHFD (methylenetetrahydrofolate-dehydrogenase, methenyltetrahydrofolate-cyclohydrolase, formyltetrahydrofolate synthetase) in patients with neural tube defects. Clin Genet. 1998;53:119–125. doi: 10.1111/j.1399-0004.1998.tb02658.x. [DOI] [PubMed] [Google Scholar]
- Noga AA, Vance DE. A gender-specific role for phosphatidylethanolamine N-methyltransferase-derived phosphatidylcholine in the regulation of plasma high density and very low density lipoproteins in mice. J Biol Chem. 2003;278:21851–21859. doi: 10.1074/jbc.M301982200. [DOI] [PubMed] [Google Scholar]
- Noga AA, Zhao Y, Vance DE. An unexpected requirement for phosphatidylethanolamine N-methyltransferase in the secretion of very low density lipoproteins. J Biol Chem. 2002;277:42358–42365. doi: 10.1074/jbc.M204542200. [DOI] [PubMed] [Google Scholar]
- Jacobs RL, Zhao Y, Koonen DPY, Sletten T, Su B, Lingrell S, et al. Impaired de novo choline synthesis explains why phosphatidylethanolamine N-methyltransferase-deficient mice are protected from diet-induced obesity. J Biol Chem. 2010;285:22403–22413. doi: 10.1074/jbc.M110.108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever M, Slow S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin Biochem. 2010;43:732–744. doi: 10.1016/j.clinbiochem.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Price RK, Keaveney EM, Hamill LL, Wallace JMW, Ward M, Ueland PM, et al. Consumption of wheat aleurone-rich foods increases fasting plasma betaine and modestly decreases fasting homocysteine and LDL-cholesterol in adults. J Nutr. 2010;140:2153–2157. doi: 10.3945/jn.110.126961. [DOI] [PubMed] [Google Scholar]
- Ueland PM. Choline and betaine in health and disease. J Inherit Metab Dis. 2011;34:3–15. doi: 10.1007/s10545-010-9088-4. [DOI] [PubMed] [Google Scholar]
- Lang F. Mechanisms and significance of cell volume regulation. J Am Coll Nutr. 2007;26 (suppl 5:613S–623S. doi: 10.1080/07315724.2007.10719667. [DOI] [PubMed] [Google Scholar]
- Burg MB, Ferraris JD. Intracellular organic osmolytes: function and regulation. J Biol Chem. 2008;283:7309–7313. doi: 10.1074/jbc.R700042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YW, Ellis JM, Coleman RA, Zeisel SH. Mouse betaine-homocysteine S-Methyltransferase deficiency reduces body fat via increasing energy expenditure and impairing lipid synthesis and enhancing glucose oxidation in white adipose tissue. J Biol Chem. 2012;287:16187–16198. doi: 10.1074/jbc.M111.303255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgregor DO, Dellow WJ, Robson RA, Lever M, George PM, Chambers ST. Betaine supplementation decreases post-methionine hyperhomocysteinemia in chronic renal failure. Kidney Int. 2002;61:1040–1046. doi: 10.1046/j.1523-1755.2002.00199.x. [DOI] [PubMed] [Google Scholar]
- Schwab U, Törrönen A, Toppinen L, Alfthan G, Saarinen M, Aro A, et al. Betaine supplementation decreases plasma homocysteine concentrations but does not affect body weight, body composition, or resting energy expenditure in human subjects. Am J Clin Nutr. 2002;76:961–967. doi: 10.1093/ajcn/76.5.961. [DOI] [PubMed] [Google Scholar]
- Olthof MR, Van Vliet T, Verhoef P, Zock PL, Katan MB. Effect of homocysteine-lowering nutrients on blood lipids: results from four randomised, placebo-controlled studies in healthy humans. PLoS Med. 2005;2:e135. doi: 10.1371/journal.pmed.0020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab U, Alfthan G, Aro A, Uusitupa M. Long-term effect of betaine on risk factors associated with the metabolic syndrome in healthy subjects. Eur J Clin Nutr. 2011;65:70–76. doi: 10.1038/ejcn.2010.230. [DOI] [PubMed] [Google Scholar]
- Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Wang J, Li C, Hirose A, Nozaki Y, Takahashi M, et al. The phosphatidylethanolamine N-methyltransferase gene V175M single nucleotide polymorphism confers the susceptibility to NASH in Japanese population. J Hepatol. 2007;46:915–920. doi: 10.1016/j.jhep.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Resseguie M, Song J, Niculescu MD, da Costa KA, Randall TA, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J. 2007;21:2622–2632. doi: 10.1096/fj.07-8227com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew TW, Jiang X, Yan J, Wang W, Lusa AL, Carrier BJ, et al. Folate intake, Mthfr genotype, and sex modulate choline metabolism in mice. J Nutr. 2011;141:1475–1481. doi: 10.3945/jn.111.138859. [DOI] [PMC free article] [PubMed] [Google Scholar]