Abstract
Background
In this study the level of IL-23 and IL-27 produced by macrophages derived from peripheral blood mononuclear cell culture collected from patients with healing or non-healing form of cutaneous leishmaniasis lesion were compared before and after treatment with live Leishmania to explore whether IL-23 or IL-27 plays any role in healing process of cutaneous lesions induced by L. major.
Methods
Twenty patients resident in Isfahan Province, with healing or non-healing form of cutaneous leishmaniasis lesion caused by Leishmania major participated in this study. In vitro productions of IL-23 and IL-27 by peripheral blood derived macrophages, before and after stimulation with live L. major (MRHO/IR/75/ER) promastigotes were evaluated using ELISA method. Patient with healing form of lesion received no treatment and patient with non-healing form of lesion received at least 2 courses of glucantime.
Results
The mean production of IL-23 and IL-27 from macrophages of patients with healing form of lesion was significantly higher than patients with non-healing form of lesion. The levels of IL-23 and IL-27 in culture supernatants before and after stimulation in healing form of CL was significantly higher than non- healing form of CL (P < 0.001).
Conclusion
IL-23 and IL-27 might play a role in human leishmaniasis and further studies are needed to understand the role of IL-23 and IL-27 in leishmaniasis.
Keywords: IL-23, IL-27, Macrophages, Promastigote, Cutaneous leishmaniasis
Introduction
Cutaneous leishmaniasis (CL) is a self-healing lesion which heals spontaneously but rarely might develop to a non-healing form of disease refractory to various types of remedies (1). It seems that clinical manifestation of leishmaniasis depends upon the type of immune response generated and the species of Leishmania (2, 3). Although tremendous data is available in murine model of leishmaniasis which indicate generation of Th1 type of response in healing form of disease and Th2 type of response for progressive infection (4–7) but immunological surrogate marker(s) of healing and protection in human is yet not well defined (8–11). Studies on various cytokine profile of Th1/Th2 are published with controversy results (8–12). Cytokines have fundamental roles in development and regulation of immune responses against various infectious and non-infectious diseases (13–16). Recently, IL-23 and IL-27 were identified as heterodimeric cytokines which are structurally and functionally related to IL-12 family which promote IFN-γ production and Th1 development (17, 18). IL-12 composed of two subunits: p35 and p40, and IL-23 consisted of the p40 subunit of IL-12 and p19 subunit (19). IL-27 is another heterodimeric cytokine composed of a p40-related molecule, and p28 (20). There is evidence that IL-27 promotes Th1 differentiation and IL-23 plays an important role in proliferation of memory-type Th1 cells. IL-23 and IL-27 are involved in the regulation of Th17 response (21), while IL-23 induces differentiation of Th17 cells; IL-27 inhibits Th17 differentiation and IL-17 production by GATA-3 expression through the Stat1-dependent pathway (22). As it is well established that successful control of infection against intracellular pathogens requires the production of IFN-γ, thus presence of IL-12 family as an inducer of Th1 phenotype is important (17, 23).
In this study the level of IL-23 and IL-27 produced by macrophages derived from peripheral blood mononuclear cell culture collected from patients with healing or non-healing form of lesion were compared before and after treatment with live Leishmania to explore whether IL-23 or IL-27 plays any role in healing process of cutaneous lesions induced by L. major.
Materials and Methods
This study was approved by the Ethical Committee on Human Research, Isfahan University of Medical Sciences. The volunteers were informed about the objective and procedure of the study and those who were willing to participate, donate blood sample and sign an informed consent were recruited.
Study groups
Two groups of CL patients were selected from the patients referred to the Skin Disease and CL Research Center, Isfahan University of Medical Sciences.
Ten parasitologically proven CL patients with healing form of lesion with onset less than 6 months and no history of treatment for CL and 10 parasitologically proven CL patients with non-healing form of lesion with duration of lesion more than 1 year and history of at least 2 courses of Glucantime treatment were included in this study. Diagnosis was based on observation of Leishmania using Giemsa stained smear and/or growth of promastigotes in NNN culture. Identification of Leishmania causative agent of the lesions was done using PCR method.
DNA extraction
In order to identify the Leishmania species, PCR method was used. The promastigotes isolated from the culture of each patient's lesion were centrifuged at 700×g, 4°C for 10min, and then were washed 3 times with PBS. DNA was extracted using High Pure PCR Template Preparation Kit (Roche, Germany). Purified DNA was eluted in 200µl of elution buffer and stored in −20°C until use. DNA from L. major (MRHO/IR/75/ER) was used as a control.
PCR amplification
Specific oligonucleotide primer was designed based on GenBank accession no. AJ300485, (Forward: CAA CAC GCC GCC TCC TCT CT, Reverse: CCT CTC TTT TTT CGC TGT GC) (Bioneer). Amplifications were performed in 25 µl containing, 2.5µl PCR buffer(10xbuffer), 0.75mM MgCl2 (50mM), 0.5 mM dNTP (10mM), 5pmol of each primer, 0.25unit of Taq polymerase (5u/ µl) and 1µl of DNA template. DNA was amplified using thermal cycler (Corbett) under the following conditions: at 94°C for 5min followed by 25 repetitive cycles of 30s at 94°C, 30s at 54°C, 30s at 72 °C and a final elongation at 72°C for 10min.
PCR products were run on 1.5% agarose gel in TBE buffer. The bands were visualized under UV illumination and the size of the products was determined.
Isolation of mononuclear cells
Peripheral Blood Mononuclear Cells (PBMCs) were isolated using Ficoll-hypaque density gradient (Lymphodex, Germany). PBMCs were washed 3 times with PBS and the pellet was resuspended in 4 ml of RPMI 1640 medium (Gibco, Germany), supplemented with 2mM L-glutamine, penicillin (100u/ml), gentamicin (100µg/ml) and 10% heat-inactivated Fetal Bovine Serum (Gibco). The cell number was estimated using light microscopy and the viability of the cells was checked by trypan blue (0.4%). PBMCs were distributed in two 3.5 cm tissue culture plates with a concentration of 5x106 cell/ml in each plate and incubated at 37°C with 5% CO2 for 2 hours and then non adherent cells were removed by washing with PBS. Adherent cells were incubated at 37°C with 5% CO2 for additional 6 days in order to differentiate to macrophages (24) and the medium was changed every 72h. The size of the cells was morphologically increased and numerous short pseudopods were formed. The cellular phenotype was analyzed by flowcytometry using anti CD14 monoclonal antibody (Abcam, United Kingdom) (data not shown).
Parasite
L. major (MRHO/IR/75/ER), was used for leishmanization and preparation of experimental Leishmania vaccine and leishmanin was used in this experiment (25). Parasite was grown on biphasic NNN medium and sub passaged in RPMI 1640 supplemented with 10% FBS. Promastigotes were harvested at stationary phase.
Macrophage and parasite interaction
After 6 days monocyte derived macrophages were infected with L. major promastigotes harvested at stationary phase at a ratio of 5:1 parasite/macrophage. After 6h, non-internalized free promastigotes were removed by washing. Then, the infected cells were incubated in complete RPMI 1640 for additional 18h. The culture supernatants were collected and stored at -70°C for IL-23 and IL-27 titration using ELISA assay.
Cytokines measurement
The frozen culture supernatants were thawed and cytokine levels of IL-23 and IL-27 were determined using commercial kits (Abcam, United Kingdom) according to the manufacturer's instructions. Briefly during the first incubation IL-23or IL-27 antigen was added to the wells. After washing, biotinylated monoclonal antibody specific for IL-23or IL-27 was incubated. Then the enzyme (streptavidin-peroxydase) was added. After incubation and washing to remove all unbounded enzyme, a substrate solution which acts on the bound enzyme was added. The absorbance of each well was read at 450nm. The concentration of IL-23 and IL-27 in the treated and untreated supernatants was determined using standard curve. Cytokine values were expressed as picogram/ml (pg/ml).
Statistical analysis
Statistical analysis was performed using SPSS version 16. Student's paired and independent t test were used to determine whether significant difference exists between the cytokine levels.
Results
Among 20 patients recruited with proven CL, 18 were male and 2 were female. The basic information of the study is summarized in Table 1.
Table 1.
Major characteristics of study groups
| Characteristic | Study group | |
|---|---|---|
| Healing | Non-healing | |
| No | 10 | 10 |
| Age(range)/ years | 18-60 | 22-65 |
| M/F ratio | 8/2 | 10/0 |
| Average duration of lesion | 3.1 month | 2.3 year |
Molecular analysis
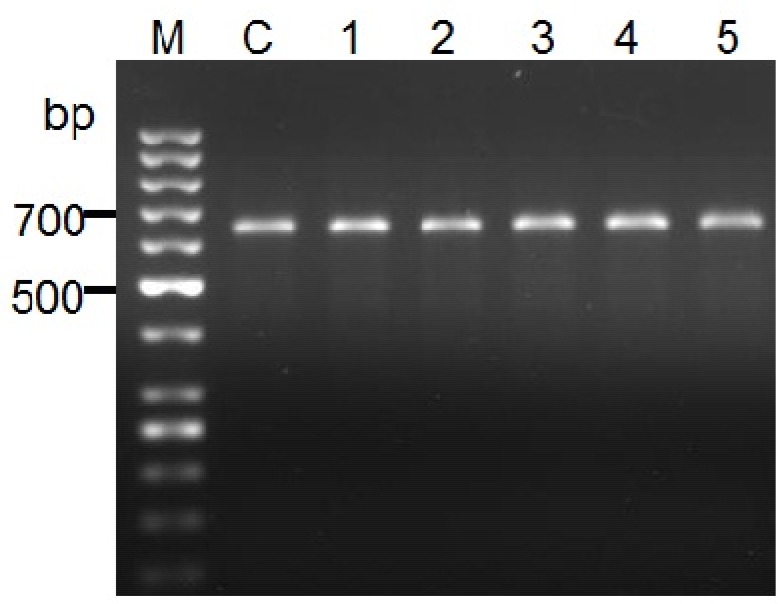
All samples were undertaken microscopic examinations and PCR assay. PCR experiment revealed a single 625bp DNA fragment was seen on the gel which is accordance with DNA fragment of L. major reference (Fig. 1).
Fig. 1.
PCR product of the internal transcribed spacer1 (ITS1) region of genomic DNA samples from healing and non-healing patients’ lesions. DNA from L.major (MRHO/IR/75/ER) used as control (C). No.1, 2 3 are samples from healing patients and No.4, 5 are obtained from non-healing individuals. M, is DNA ladder 50 bp (Fermentas)
Cytokine production
IL-23 levels
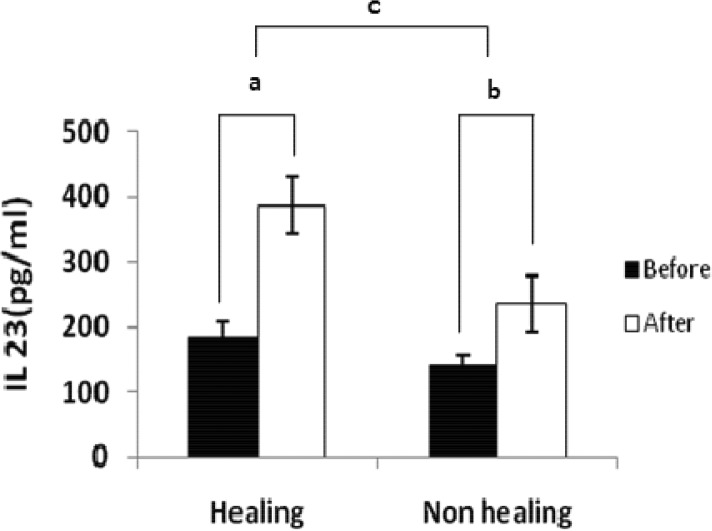
The level of IL-23 in healing and non-healing form of CL before stimulation was 182.9±23.9 pg/ml and 141.3±13.5 pg/ml respectively which was significantly different (P < 0.001). The level of IL-23 after stimulation in supernatants of macrophages in healing and non-healing form of CL was 386 ±43.9 pg/ml vs. 235 ±43.9 pg/ml which was significantly different (P < 0.001). The level of IL-23 before and after stimulation in supernatants of macrophages in healing form of CL was 182.9±23.9pg/ml and 386.9±43.9 pg/ml, respectively, whereas the level of IL-23 in non-healing form was 141.3±13.5pg/ml and 235.5±43.9pg/ml, respectively. The differences between IL-23 level in supernatant of the macrophage culture before and after stimulation in patients with healing and non-healing form of CL were 203.9±38.3 vs. 94.1±36.5 respectively.The data were shown the difference in healing form of CL was significantly higher than non-healing form of CL (P < 0.001).
Fig. 2.
Cytokine production from macrophages in patients’ with healing and non healing lesions. Macrophages were stimulated with or without live L. major promastigotes. After 24 hours supernatants were assayed for IL-23 production. Results were obtained by an ELISA assay. Difference between cytokine production in stimulated and unstimulated macrophages in healing group (a), difference between cytokine production in stimulated and unstimulated macrophages in non- healing group(b)and difference between a and b(c). Data are expressed as the mean±standard deviation of the mean. Significant differences were shown between these groups (P < 0.001)
Fig. 3.
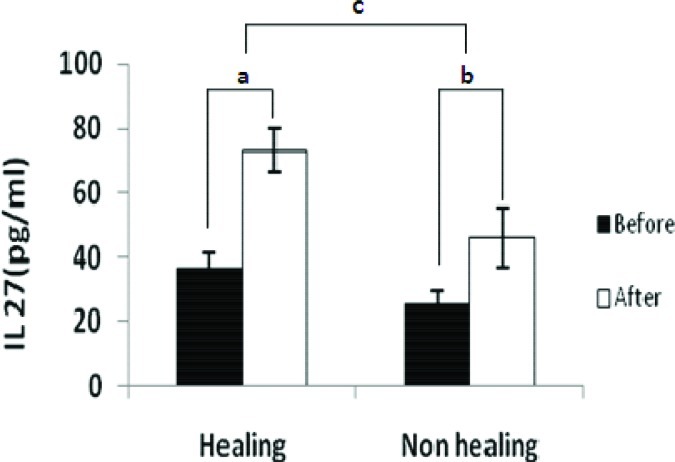
Cytokine production from Macrophages in patients’ with healing and non healing lesions. Macrophages were stimulated with or without live L. major promastigotes. After 24hours supernatants were assayed for IL-27 production. Results were obtained by an ELISA assay. Difference between cytokine production in stimulated and unstimulated macrophages in healing group (a), difference between cytokine production in stimulated and unstimulated macrophages in non- healing group(b)and difference between a and b(c). Data are expressed as the mean ± standard deviation of the mean. Significant differences were shown between these groups (P < 0.001)
IL-27 level
The concentration of IL-27 in healing and non-healing form of CL before stimulation was 36.6±4.5 pg/ml and 25.4±4.2 pg/ml respectively which was significantly different (P < 0.001). The level of IL-27 after stimulation in supernatants of macrophages in healing and non-healing form of CL was 73.3 ±6.8 pg/ml vs. 46.2±9.2 pg/ml which was significantly different (P < 0.001). The concentration of IL-27 before and after stimulation in supernatants of macrophages in healing form of CL was 36.6±4.5pg/ml and 73.3±6.8pg/ml, respectively, whereas the level of IL-27 in non-healing form was 25.4±4.2pg/ml vs. 46.2±9.2pg/ml. There was a significant difference (P < 0.001) in IL-27 level before and after stimulation in healing and non-healing form of CL.
The differences between IL-27 concentration in supernatant of the macrophage culture before and after stimulation in patients with healing form and non-healing form of CL was 36.7±6.48pg/ml vs. 20.8±7.78pg/ml respectively. The data were shown the difference of IL-27 in healing form of CL was significantly higher than non- healing form of CL (P < 0.001).
Discussion
Although immune responses in leishmaniasis have been studied but still no surrogate marker(s) of healing and protection in human is identified (8-12). IL-12, IL-23 and IL-27 are related to the family which stimulates Th1 type of immune response. Effective immune response in intracellular parasites such as Trypanosoma cruzi, Toxoplasma gondii and Leishmania major is Th1 type of response (11, 26). In this study the level of IL-23 and IL-27 were studied to explore possible relationship between IL-23 and IL-27 and healing process in patients with CL caused by L. major. The results showed that the level of IL-23 and IL-27 in both groups of patient were elevated after the macrophages were stimulated with L. major. Moreover, the difference of IL- 23 and IL- 27 levels before and after stimulation was significantly (P<0.001) different between macrophages collected from patients with healing and non-healing form of the lesion. The differences was seen in IL-23 and IL-27 production in these two groups might be due to different interaction between the structures referred to pathogen-associated molecular patterns (PAMPs) that are recognized by pathogen recognition receptors (PRRs) of innate immune system (27). Another possibility is that different intracellular signaling pathway or adaptor molecules might be involved (28). This alteration may result from a difference in regulatory factors, enhancers and inhibitor molecules due to primary expression of cytokines (29). In the past few years immune responses against Leishmania infection and cytokine profile in human and animal leishmaniasis have been deeply studied (7, 8, 10, 11, 30). It is reported that IL-27 showed in vitro capacity to inhibit Th2 cell differentiation (31) and in another study it is shown that daily in vivo treatment of Leishmania major infected BALB/c mice by IL-27 induced lower parasite burden via up regulation of Th1 responses (22). It also shown that although IL-27 and its receptor are promoters of Th1 differentiation, but new finding showed that IL-27R is not required for the generation of IFN-γ mediated immunity to intracellular pathogens such as Toxoplasma infection (32). Almost all studies indicated that IL-12, plays a major role in resistance against toxoplasmosis but in the absence of IL-12, IL-23 can provide a limited mechanism of resistance to this infection (33). On the other hand, it is shown that IL-23 plays a key role in Helicobacter hepaticus–induced T cell–dependent colitis (34). It is also reported that Th1 development induced by Gram negative bacteria-primed macrophages is likely to be mediated by the joint action of different IL-12 family members and Th1 polarization might be driven by the action of the novel IL-12 family members IL-27 and/or IL-23 (35).
Taken together, seems that IL-23 and IL-27 may play a possible and complementary role with Th1 cytokines in human protection against Leishmania infection. Further studies are required to establish the role of these cytokines as surrogate markers in healing process of cutaneous leishmaniasis.
Acknowledgements
The main part of this work was supplied by Isfahan University of Medical Sciences (Grant No. 188051). We wish to thanks Miss Maryam Peymani and Miss Leila Shirani for their kindly collaborations and excellent helps. The authors declare that there is no conflict of interests.
References
- 1.Dowlati Y. Cutaneous leishmaniasis: clinical aspect. Clin Dermatol. 1996;14(5):425–31. doi: 10.1016/0738-081x(96)00058-2. [DOI] [PubMed] [Google Scholar]
- 2.Herwaldt BL. Leishmaniasis. Lancet. 1999;354(9185):1191–9. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 3.Salman SM, Rubeiz NG, Kibbi AG. Cutaneous leishmaniasis: clinical features and diagnosis. Clin Dermatol. 1999;17(3):291–6. doi: 10.1016/s0738-081x(99)00047-4. [DOI] [PubMed] [Google Scholar]
- 4.Lohoff M, Gessner A, Bogdan C, Rollinghoff M. Experimental murine leishmaniasis and the Th1/Th2 cell concept. Tokai J Exp Clin Med. 1998;23(6):347–50. [PubMed] [Google Scholar]
- 5.Milon G, Del Giudice G, Louis JA. Immunobiology of experimental cutaneous leishmaniasis. Parasitol Today. 1995;11(7):244–7. doi: 10.1016/0169-4758(95)80200-2. [DOI] [PubMed] [Google Scholar]
- 6.Muller I, Fruth U, Louis JA. Immunobiology of experimental leishmaniasis. Med Microbiol Immunol. 1992;181(1):1–12. doi: 10.1007/BF00193391. [DOI] [PubMed] [Google Scholar]
- 7.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2(11):845–58. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 8.Ajdary S, Alimohammadian MH, Eslami MB, Kemp K, Kharazmi A. Comparison of the immune profile of nonhealing cutaneous leishmaniasis patients with those with active lesions and those who have recovered from infection. Infect Immun. 2000;68(4):1760–4. doi: 10.1128/iai.68.4.1760-1764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ajdary S, Jafari-Shakib R, Riazi-Rad F, Khamesipour A. Soluble CD26 and CD30 levels in patients with anthroponotic cutaneous leishmaniasis. J Infect. 2007;55(1):75–8. doi: 10.1016/j.jinf.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Habibi GR, Khamesipour A, McMaster WR, Mahboudi F. Cytokine gene expression in healing and non-healing cases of cutaneous leishmaniasis in response to in vitro stimulation with recombinant gp63 using semi-quantitative RT-PCR. Scand J Immunol. 2001;54(4):414–20. doi: 10.1046/j.1365-3083.2001.00990.x. [DOI] [PubMed] [Google Scholar]
- 11.Mahmoodi M, Rajabalian S, Fekri A, Esfandiarpour I. Evaluation of in vitro production of ifn-gamma, il-10, il-12 and il-13 by blood cells in patients with cutaneous leishmaniasis lesions. Iran J Allergy Asthma Immunol. 2005;4(1):15–21. [PubMed] [Google Scholar]
- 12.Jafari-Shakib R, Shokrgozar MA, Nassiri-Kashani M, et al. Plasma sCD26 and sCD30 levels in cutaneous leishmaniasis. Acta Trop. 2009;109(1):61–3. doi: 10.1016/j.actatropica.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Bogdan C, Gessner A, Rollinghoff M. Cytokines in leishmaniasis: a complex network of stimulatory and inhibitory interactions. Immunobiology. 1993;189(3-4):356–96. doi: 10.1016/S0171-2985(11)80366-9. [DOI] [PubMed] [Google Scholar]
- 14.Cutler A, Brombacher F. Cytokine therapy. Ann N Y Acad Sci. 2005 Nov;1056:16–29. doi: 10.1196/annals.1352.002. [DOI] [PubMed] [Google Scholar]
- 15.Calandra T, Bochud PY, Heumann D. Cytokines in septic shock. Curr Clin Top Infect Dis. 2002;22:1–23. [PubMed] [Google Scholar]
- 16.Santamaria P. Cytokines and chemokines in autoimmune disease: an overview. Adv Exp Med Biol. 2003;520:1–7. doi: 10.1007/978-1-4615-0171-8_1. [DOI] [PubMed] [Google Scholar]
- 17.Hunter CA, Villarino A, Artis D, Scott P. The role of IL-27 in the development of T-cell responses during parasitic infections. Immunol Rev. 2004;202:106–14. doi: 10.1111/j.0105-2896.2004.00213.x. [DOI] [PubMed] [Google Scholar]
- 18.Tan ZY, Bealgey KW, Fang Y, Gong YM, Bao S. Interleukin-23: immunological roles and clinical implications. Int J Biochem Cell Biol. 2009;41(4):733–5. doi: 10.1016/j.biocel.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 19.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13(5):715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 20.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4 (+) T cells. Immunity. 2002;16(6):779–90. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida H, Miyazaki Y. Regulation of immune responses by interleukin-27. Immunol Rev. 2008;226:234–47. doi: 10.1111/j.1600-065X.2008.00710.x. [DOI] [PubMed] [Google Scholar]
- 22.Yoshimoto T, Yasuda K, Mizuguchi J, Nakanishi K. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: a novel therapeutic way for Th2-mediated allergic inflammation. J Immunol. 2007;179(7):4415–23. doi: 10.4049/jimmunol.179.7.4415. [DOI] [PubMed] [Google Scholar]
- 23.Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O'Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139–56. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 24.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 25.Khamesipour A, Rafati S, Davoudi N, Maboudi F, Modabber F. Leishmaniasis vaccine candidates for development: a global overview. Indian J Med Res. 2006;123(3):423–38. [PubMed] [Google Scholar]
- 26.Beadling C, Slifka MK. Regulation of innate and adaptive immune responses by the related cytokines IL-12, IL-23, and IL-27. Arch Immunol Ther Exp (Warsz) 2006;54(1):15–24. doi: 10.1007/s00005-006-0002-6. [DOI] [PubMed] [Google Scholar]
- 27.Kawai T, Akira S. Toll-like receptor downstream signaling. Arthritis Res Ther. 2005;7(1):12–9. doi: 10.1186/ar1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 29.Romagnani S. Regulation of the T cell response. Clin Exp Allergy. 2006;36(11):1357–66. doi: 10.1111/j.1365-2222.2006.02606.x. [DOI] [PubMed] [Google Scholar]
- 30.Rogers KA, DeKrey GK, Mbow ML, Gillespie RD, Brodskyn CI, Titus RG. Type 1 and type 2 responses to Leishmania major . FEMS Microbiol Lett. 2002;209(1):1–7. doi: 10.1111/j.1574-6968.2002.tb11101.x. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida H, Hamano S, Senaldi G, Covey T, Faggioni R, Mu S, et al. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15(4):569–78. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 32.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, et al. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19(5):645–55. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 33.Lieberman LA, Cardillo F, Owyang AM, Rennick DM, Cua DJ, Kastelein RA, et al. IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J Immunol. 2004;173(3):1887–93. doi: 10.4049/jimmunol.173.3.1887. [DOI] [PubMed] [Google Scholar]
- 34.Kullberg MC, Jankovic D, Feng CG, et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203(11):2485–94. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smits HH, van Beelen AJ, Hessle C, et al. Commensal Gram-negative bacteria prime human dendritic cells for enhanced IL-23 and IL-27 expression and enhanced Th1 development. Eur J Immunol. 2004;34(5):1371–80. doi: 10.1002/eji.200324815. [DOI] [PubMed] [Google Scholar]