Abstract
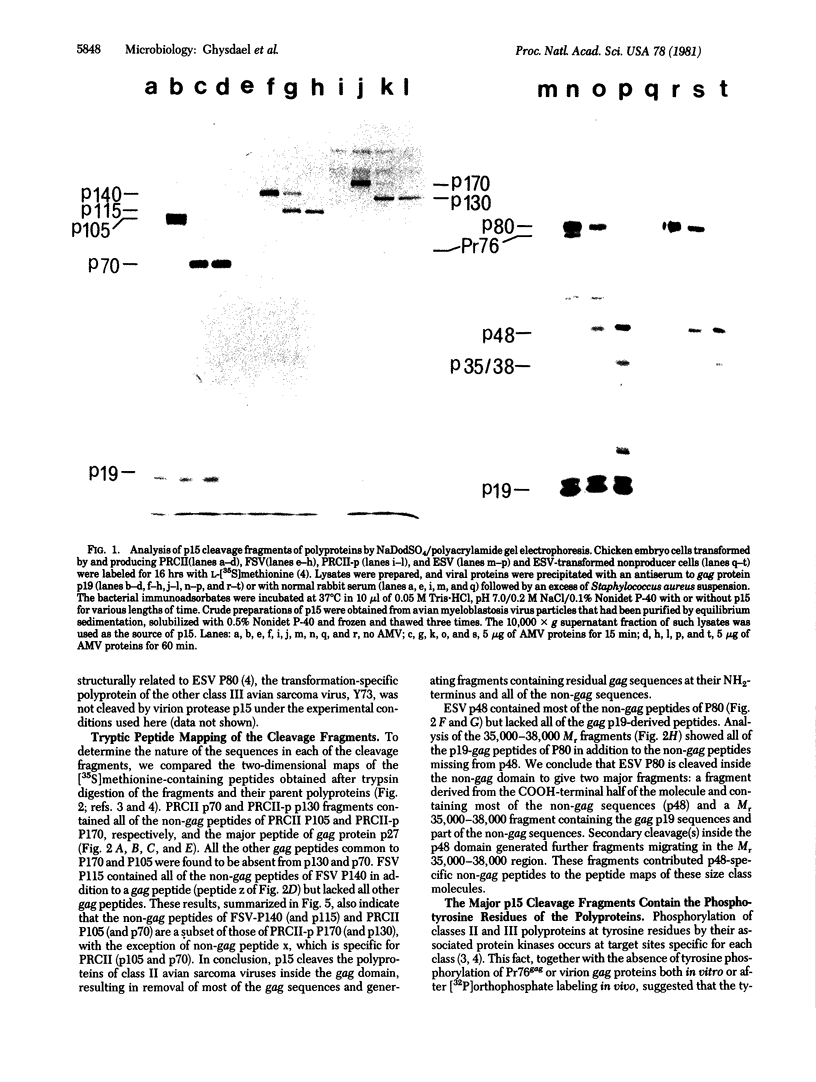
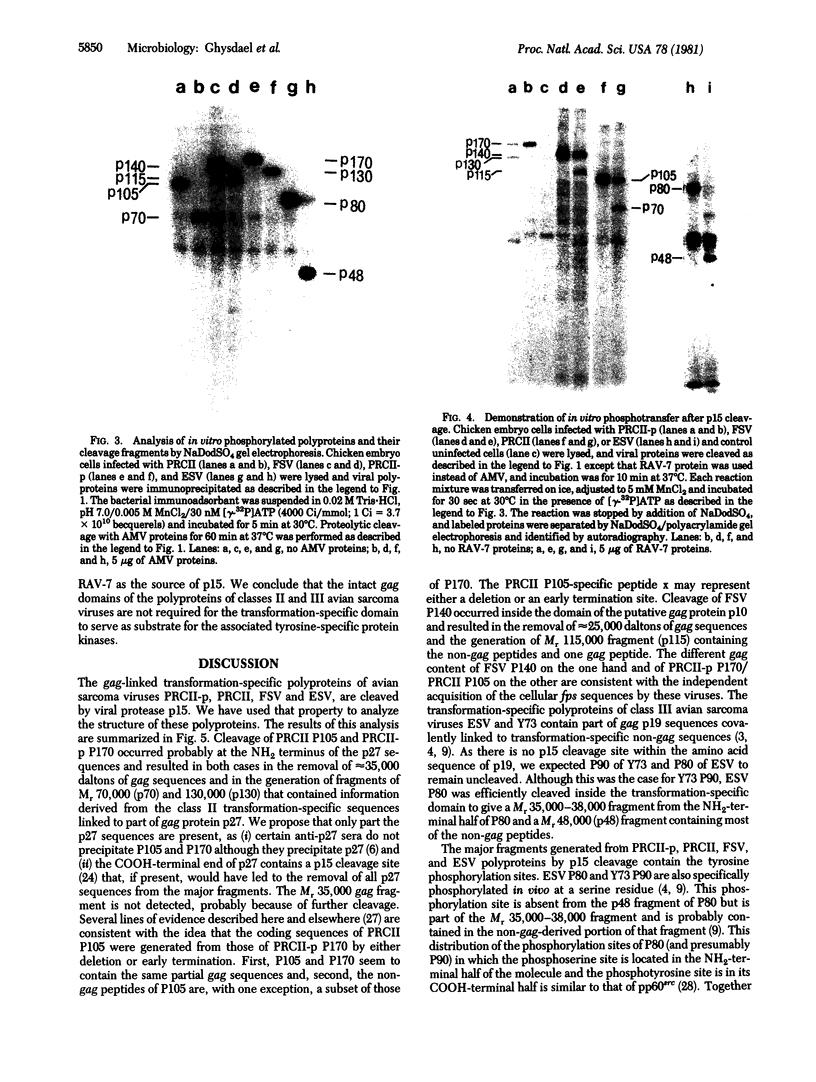
The transformation-specific polyproteins of avian sarcoma viruses PRCII, PRCII-p, Fujinami sarcoma virus (FSV), and Esh sarcoma virus (ESV) consist of two domains, one derived from a partial viral gag gene and the other representing an apparently cell-derived insert in the defective viral genome. These gag-linked proteins were cleaved with retrovirion protease p15. Cleavage of PRCII-p polyprotein P170, P105 of PRCII, and P140 of FSV occurred within the gag domain and generated fragments of Mr 130,000, 70,000, and 115,000, respectively, containing all of the transformation-specific sequences linked to a remnant of the original gag sequences. ESV P80 was cleaved inside the transformation-specific domain, yielding a Mr 35,000--38,000 fragment from the NH2-terminal half of the molecule consisting of the entire gag portion and some no-gag sequences and a Mr 48,000 fragment containing most of the transformation-specific sequences. The tyrosine phosphorylation sites of the polyproteins were found in every case in the transformation-specific fragments. The major serine phosphorylation site of ESV P80 was found to reside in the Mr 35,000--38,000 gag-containing fragment, probably within the transformation-specific sequences of that cleavage product. Removal of all of the gag domain of ESV P80 or most of the gag domain in PRCII-p P170, PRCII P105, and FSV P140 does not affect their ability to be phosphorylated by the polyprotein-associated tyrosine-specific protein kinase activities. This observation suggests that the gag sequences of the polyproteins of classes II (PRCII-p, PRCII, and FSV) and III (ESV) avian sarcoma viruses may not be required for this enzymatic function, which appears to be of importance in transformation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beemon K. Transforming proteins of some feline and avian sarcoma viruses are related structurally and functionally. Cell. 1981 Apr;24(1):145–153. doi: 10.1016/0092-8674(81)90510-9. [DOI] [PubMed] [Google Scholar]
- Breitman M. L., Neil J. C., Moscovici C., Vogt P. K. The pathogenicity and defectiveness of PRCII: a new type of avian sarcoma virus. Virology. 1981 Jan 15;108(1):1–12. doi: 10.1016/0042-6822(81)90522-5. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Erikson R. L. Structural analysis of the avian sarcoma virus transforming protein: sites of phosphorylation. J Virol. 1979 Feb;29(2):770–781. doi: 10.1128/jvi.29.2.770-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Four different classes of retroviruses induce phosphorylation of tyrosines present in similar cellular proteins. Mol Cell Biol. 1981 May;1(5):394–407. doi: 10.1128/mcb.1.5.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar K. J., Moelling K. Biochemical properties of p15-associated protease in an avian RNA tumor virus. J Virol. 1978 Oct;28(1):106–118. doi: 10.1128/jvi.28.1.106-118.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. A., Hanafusa T., Hanafusa H. Characterization of protein kinase activity associated with the transforming gene product of Fujinami sarcoma virus. Cell. 1980 Dec;22(3):757–765. doi: 10.1016/0092-8674(80)90552-8. [DOI] [PubMed] [Google Scholar]
- Ghysdael J., Neil J. C., Vogt P. K. A third class of avian sarcoma viruses, defined by related transformation-specific proteins of Yamaguchi 73 and Esh sarcoma viruses. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2611–2615. doi: 10.1073/pnas.78.4.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysdael J., Neil J. C., Wallbank A. M., Vogt P. K. Esh avian sarcoma virus codes for a gag-linked transformation-specific protein with an associated protein kinase activity. Virology. 1981 Jun;111(2):386–400. doi: 10.1016/0042-6822(81)90342-1. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Mathey-Prevot B., Feldman R. A., Hanafusa H. Mutants of Fujinami sarcoma virus which are temperature sensitive for cellular transformation and protein kinase activity. J Virol. 1981 Apr;38(1):347–355. doi: 10.1128/jvi.38.1.347-355.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Wang L. H., Anderson S. M., Karess R. E., Hayward W. S., Hanafusa H. Characterization of the transforming gene of Fujinami sarcoma virus. Proc Natl Acad Sci U S A. 1980 May;77(5):3009–3013. doi: 10.1073/pnas.77.5.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Yoshida M., Segawa K., Sugiyama H., Ishizaki R., Toyoshima K. Characterization of Y73, an avian sarcoma virus: a unique transforming gene and its product, a phosphopolyprotein with protein kinase activity. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6199–6203. doi: 10.1073/pnas.77.10.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. H., Bister K., Pawson A., Robins T., Moscovici C., Duesberg P. H. Fujinami sarcoma virus: an avian RNA tumor virus with a unique transforming gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2018–2022. doi: 10.1073/pnas.77.4.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- MOMMAERTS E. B., ECKERT E. A., BEARD D., SHARP D. G., BEARD J. W. Dephosphorylation of adenosine triphosphate by concentrates of the virus of avian erythromyeloblastic leucosis. Proc Soc Exp Biol Med. 1952 Mar;79(3):450–455. doi: 10.3181/00379727-79-19409. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Breitman M. L., Vogt P. K. Characterization of a 105,000 molecular weight gag-related phosphoprotein from cells transformed by the defective avian sarcoma virus PRCII. Virology. 1981 Jan 15;108(1):98–110. doi: 10.1016/0042-6822(81)90530-4. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Delamarter J. F., Vogt P. K. Evidence for three classes of avian sarcoma viruses: comparison of the transformation-specific proteins of PRCII, Y73, and Fujinami viruses. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1906–1910. doi: 10.1073/pnas.78.3.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil J. C., Ghysdael J., Vogt P. K., Smart J. E. Homologous tyrosine phosphorylation sites in transformation-specific gene products of distinct avian sarcoma viruses. Nature. 1981 Jun 25;291(5817):675–677. doi: 10.1038/291675a0. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Ghysdael J., Vogt P. K. Tyrosine-specific protein kinase activity associated with p105 of avian sarcoma virus PRCII. Virology. 1981 Feb;109(1):223–228. doi: 10.1016/0042-6822(81)90493-1. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Varmus H. E. The structure and protein kinase activity of proteins encoded by nonconditional mutants and back mutants in the sec gene of avian sarcoma virus. Virology. 1981 Jan 15;108(1):47–70. doi: 10.1016/0042-6822(81)90526-2. [DOI] [PubMed] [Google Scholar]
- Pawson T., Guyden J., Kung T. H., Radke K., Gilmore T., Martin G. S. A strain of Fujinami sarcoma virus which is temperature-sensitive in protein phosphorylation and cellular transformation. Cell. 1980 Dec;22(3):767–775. doi: 10.1016/0092-8674(80)90553-x. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Shealy D. J., Mosser A. G., Rueckert R. R. Novel p19-related protein in Rous-associated virus type 61: implications for avian gag gene order. J Virol. 1980 May;34(2):431–437. doi: 10.1128/jvi.34.2.431-437.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa T., Hanafusa H., Stephenson J. R. Homology exists among the transforming sequences of avian and feline sarcoma viruses. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6536–6540. doi: 10.1073/pnas.77.11.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Wight A., Eisenman R. In vitro cleavage of avian retrovirus gag proteins by viral protease p15. Virology. 1979 Oct 15;98(1):154–167. doi: 10.1016/0042-6822(79)90534-8. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Kawai S., Toyoshima K. Unifected avian cells contain structurally unrelated progenitors of viral sarcoma genes. Nature. 1980 Oct 16;287(5783):653–654. doi: 10.1038/287653a0. [DOI] [PubMed] [Google Scholar]
- von der Helm K. Cleavage of Rous sarcoma viral polypeptide precursor into internal structural proteins in vitro involves viral protein p15. Proc Natl Acad Sci U S A. 1977 Mar;74(3):911–915. doi: 10.1073/pnas.74.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]






