Abstract
IL-4 production by leukocytes is a key regulatory event that occurs early in the type-2 immune response, which induces allergic reactions and mediates expulsion of parasites. CD4+ T-cells and basophils are thought to be the key cell types that produce IL-4 during a type-2 response. Here, we assessed the relative contribution of both CD4+ T-cell- and basophil-IL-4 production during primary and secondary responses to Nippostrongylus brasiliensis using a murine IL-4-eGFP reporter system. During infection, IL-4 producing basophils were detected systemically and tissue recruitment occurred independent of IL-4/STAT6 signaling. We observed that basophil recruitment to a tissue environment was required for their full activation. Basophil induction in response to secondary infection exhibited accelerated kinetics in comparison to primary infection. However, total basophil numbers were not enhanced, as predicted by previous models of protective immunity. Overall, the induction and migration of IL-4 producing basophils into peripheral tissues was found to be a prominent characteristic of the primary, but not memory responses to N. brasiliensis infection where CD4+ T-cells were identified as the major source of IL-4. While basophils were the major initial producers of IL-4 we determined that normal TH2 differentiation occurs independently of basophils and depletion of basophils led to an enhancement of inflammatory cell recruitment to the site of infection.
Introduction
IL-4 has been widely described as the signature cytokine of the TH2 immune response in which it plays multiple roles during adaptive immune responses. As such, IL-4 is essential for in vitro TH2 induction (1), B cell class switching to IgE production (2), and cellular migration into peripheral tissues (2, 3). IL-4 is also considered important in the secondary response, as symptoms in patients with allergic disorders are believed, at least in part, due to chronicity of IL-4 producing effector cells. However, identification of the IL-4 source is still a matter of uncertainty.
IL-4 is produced not only by differentiated TH2 CD4+ T cells, but also by basophils (4), mast cells (5), eosinophils (6) and NKT cells (7, 8). Basophils, the least common of the blood leukocytes, are increasingly being recognized as an important component of the TH2 immune response as they are recruited to sites of inflammation during type 2 responses (9) where they are believed to influence immune responses by releasing immune mediators such as histamine and leukotriene as well as rapidly producing the cytokines IL-4, IL-13 and TSLP (9–12). IL-4 producing basophils have been identified as a key feature of parasitic infections in both mice (4, 13) and humans (14, 15) and have been implicated as a significant effector cell subset in allergic inflammation (16, 17). Moreover, basophils have been demonstrated to produce early IL-4 during a memory T-dependent response using goat anti-mouse IgD Ab (18). Mechanistically a key role for basophil derived IL-4 and IL-6 was recently identified in Denzel et al (19) whereby, basophil depletion profoundly impaired B cell proliferation and immunoglobulin production, highlighting the role that basophils play in humoral memory responses. Further basophils have been suggested to play a key role in T cell activation as antigen presenting cells to CD4+ cells following papain induced activation (12, 20) and Trichuris muris infection (21). More recently the ability of basophils to both cross present Ag and activate CD8+ cells has been demonstrated (22).
The nematode Nippostrongylus brasiliensis (Nb) induces a robust TH2 immune response in mice, characterized by the production of high levels of IgE, IL-4 and IL-13 as well as an influx of eosinophils and basophils into mucosal tissues (23). Nb is a skin penetrating blood borne parasite that migrates through the lungs to the gut, before being expelled at approximately day 9 post infection, in the process eliciting immune reactions at these sites. Upon re-infection with Nb, a protective memory response is elicited, characterized by an earlier enhanced TH2 immune response, which disrupts migration and prevents worm establishment in the host (24, 25).
We have previously shown that basophils accumulate in tissues after infection with Nb and are a major source of IL-4 (4). Here, we utilized the G4 transgenic reporter system in which an eGFP construct was inserted into the first exon of the IL-4 gene, allowing identification of cellular production of IL-4 (26). Basophils detected in the G4 model system displayed low side scatter, were GFP+, FcεR1+, CD49bbright, ckitneg, Gr1neg and were furthermore identified as basophils by electron microscopy. Significant levels of these GFP+ basophils were found systemically in various peripheral tissues. Induction of basophilia was found to be STAT6 and IL-4 independent, T cell dependent and appeared to be partially dependent on IL-3 (27). Here, we sought to examine whether IL-4 producing basophils were also a feature of the memory TH2 response induced following secondary infection with Nb. Using G4 mice in which expression of GFP faithfully reports production of IL-4, we were able to directly identify GFP/IL-4 producing cells ex vivo without the need for restimulation. As Gessner et al (28) had previously determined that both activated CD4+ TH2 cells and basophils produce similar quantities of IL-4, this allowed for a direct assessment of IL-4 production by CD4+ T cells and basophils in both primary and secondary Nb responses. We determined that both GFP+ basophils and GFP+ CD4+ T cells were induced more rapidly following secondary Nb infection with a greater number of IL-4 producing CD4+ T cells detected in the lung following re-infection. However, an enhanced GFP+ basophil response was not elicited throughout the course of secondary infection. Thus, our results demonstrate that GFP+ basophils constitute the major cellular source of IL-4 during the primary response, while GFP+ CD4+ T cells were the major source of IL-4 during the secondary infection. In addition, migration of IL-4 producing basophils is a prominent feature of the primary, but not the secondary TH2 immune response, while CD4+ T cell derived IL-4 is a dominant feature of the secondary immune response in the lung.
Materials and Methods
Mice
Heterozygous GFP/IL-4 knock-in (G4) (26) and STAT6−/− (29) mice were provided by Dr William Paul at the Laboratory of Immunology (National Institute of Asthma and Infectious Disease, NIAID, NIH). The G4/IL-4 mice were bred and homozygous G4/G4 progeny were selected from the F1 generation at six weeks of age, via PCR detection of the eGFP transgene. G4/G4 mice were crossed to the STAT6−/− mice to obtain STAT6−/− G4/G4 mice. Mice were bred and maintained by the Malaghan Institute of Medical Research animal facility in Wellington, NZ. All animal procedures were approved by the Victoria University of Wellington Animal Ethics committee. 6–12 week old mice were used for all experiments and within experiments were matched for age and sex.
Model Systems
For Nb infection, mice were inoculated with 600 third stage infectious larvae (L3) of Nb by subcutaneous (s.c.) injection in the nape to generate a primary immune response. To induce a secondary response, mice infected 30 days previously as above were inoculated s.c. with an additional 600 L3 larvae. Where indicated mice were depleted of basophils following intravenous (i.v.) injection of 150 μg of anti-FcεRI (MAR-1) 48 hours prior to infection with N. brasiliensis. N. brasiliensis excretory secretory product (NES) was prepared as previously described (30); 2μg L3 NES was administered via intraperitoneal (i.p.) injection in adsorbed Alum at days 0 and 14 followed by intra-nasal (i.n.) challenge at day 21 with either 100μg NES in PBS or PBS alone. OVA 2 μg was adsorbed in Alum and administered i.p. at days 0 and 14 followed by i.n. challenge at day 21 with either 100 μg OVA in PBS or PBS alone. Additionally, 4 μg KLH was adsorbed in Alum and administered i.p. at day 0 followed by i.n. challenge at day 7 with either 100 μg KLH in PBS or PBS alone. 1 HAU Influenza in PBS or PBS alone was administered via intranasal (i.n.) transfer.
Isolation and Preparation of cells
Mice were tail bled and RBC lysed with ammonium chloride treatment before counting to determine the total number of lymphocytes per ml of blood. Perfused lung was minced and incubated for 1 h in culture media (IMDM (GIBCO) supplemented with 5% FCS (GIBCO), 2mM GlutaMAX (GIBCO), 1% penicillin-streptomycin (GIBCO) and 5×105 M 2-ME (GIBCO) containing 2.4mg/ml of collagenase type I (Invitrogen) and 0.1% DNase I (Roche). A single cell suspension was layered over a 30/70% Percoll (Amersham Biosciences) gradient and centrifuged at 2,000 rpm for 20 min at room temperature. Cells at the interface were recovered and washed before counting. Livers were perfused and mechanically disrupted to single cell suspensions, followed by resuspension in 30% Percoll and centrifugation at 2,000 rpm for 10 min at room temperature. RBC were subsequently lysed and the resulting mononuclear cells were counted. Single cell suspension of the mesenteric and mediastinal lymph nodes were obtained and analyzed by flow cytometry.
Flow Cytometry
Processed cells were stained for cell surface markers to identify different cell types and their GFP expression. PE-labeled anti-FcεR1a (MAR-1) Ab was purchased from eBioscience. Anti-CD4-APC, CD45-APC and PanNK-PE (DX5) Abs were purchased from PharMingen (San Diego, CA). Data were acquired using either a FACSort or FACSCaliber (Becton Dickinson) and analyzed with a Flowjo software (Treestar).
IL-4 Quantitation
Balb/c animals were infected with 600 L3 Nb s.c. At day 9 post infection lungs were perfused and lung tissue was dissociated through cell strainers. Single cell suspensions were stimulated with either DynabeadsR Mouse T-Activator CD3/CD28 (Invitrogen NZ 1:1 ratio) + 30U/mL IL-2 or 10μg/mL anti-mouse IgE (6HD5) + IL-3 (1.2 ng/mL X63 IL-3) for 6 hours. Cells were plated at 2×106 cells in 200μl with the addition of 20μg/mL anti-mouse CD124 (IL-4Rα; BD Pharmingen, San Diego, CA) for IL-4 ELISA analysis and 1×105 in 150μL for the IL-4 ELISpot. IL-4 ELISpot was performed according to manufacturers instructions (Mabtech MacLeod Victoria Australia) and ELISA determined supernatant IL-4. Briefly, plates were coated overnight at 4°C with 100 μl of anti-mouse IL-4 Ab (11B11; 2 μg/ml) and then blocked with 200 μl of 10% Fetal Bovine Serum (FBS diluted in sterile PBS) before adding serial dilutions of supernatant samples (100 μl/well). Samples were incubated for 2 hours and biotinylated anti-mouse IL-4 Ab was added ( BVD6-24G2 BD Pharmingen 2 μg/ml, 100μl/well). After two hours of incubation, streptavidin-HRP (1:1000 dilution GE Healthcare Buckinghamshire UK) was added. Prior to the initiation of each step, plates were washed with 0.05% Tween-20 in PBS. Finally, after one-hour incubation, 100 μl of substrate (TMB Substrate Reagent Set, BD OptEIA, San Diego, CA) was added. Colorimetric reaction was stopped with 1M H2SO4 and was quantified by measuring optical density with an ELISA plate reader at 450 nm.
Results
Basophil induction and recruitment during type 2 immune responses
Although basophilia has been widely described as a key feature of the TH2 associated airway inflammatory response, little effort has previously been made to differentiate between the role that basophils play in parasitic infections and asthma/allergy type responses. Here, we investigated the comparative induction and recruitment of basophils using several common models of type 2 lung inflammation (Fig 1A). Nb infection was used as a classical model of helminthic response and was compared to the allergic type responses initiated after protein/adjuvant prime challenge with KLH or OVA in Alum. Nb Excretory Secretory product (NES) in Alum was included to determine whether a synergistic response between helminth associated factors and Alum could be elicited. Additionally, animals were infected with influenza to examine whether basophil recruitment occurs under conditions of type 1 inflammation.
Figure 1. Comparison of Basophil Induction in Murine Immune Response Models.
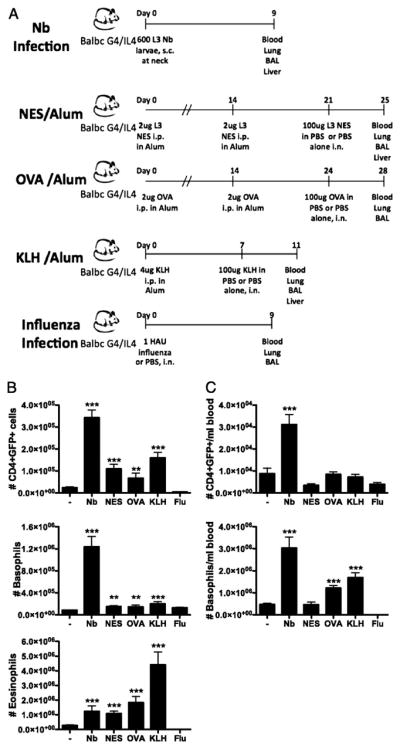
(A) Comparison of different mouse immune response models. Total numbers of CD4+GFP+, basophils and eosinophils were measured in the lungs (B) and total numbers of CD4+GFP+, basophils in BALB/c G4/IL-4 mice day 9 following Nb infection (C), day 4 post i.n. challenge with NES, OVA or KLH antigen, or day 9 following i.n. influenza (flu) infection. - shows the level of cells present in uninfected or PBS challenged control mice. NES and KLH data is representative of single 3-mice experiments, whereas OVA is an average of 6 mice from two experiments and Nb of 22 mice from seven experiments. ***, P ≤ 0.0001; **, P ≤ 0.001; *, P ≤ 0.01; no asterisk, P > 0.05, relative to naïve animal with Student’s t-test.
To analyze the role of basophils in immune responses, lung inflammatory infiltrate was analyzed at the peak of responses (Fig 1B) and as expected following Nb, KLH, OVA and NES challenge a significant type 2 response was induced with increased numbers of CD4+GFP+ cells and eosinophils being recruited to the lung, whereas influenza infection led to a decrease in CD4+GFP+ and eosinophil numbers while lung basophil numbers were not substantially altered. Interestingly, although CD4+GFP+ cells and eosinophils were induced and recruited to the lung in large numbers by both allergic (protein/alum) models and Nb infection, significant lung basophilia was only detected following Nb infection. Nb infection also induced a significant basophilia in the lung, with a 15-fold increase in numbers above background (Fig 1B). This increase was due to both a 2.7-fold increase in total cells in the lung as well as an increase in the proportion of basophils from 0.9% of CD45+ cells in naïve mice to 4.8% at the peak of infection. Following both OVA and KLH treatment an increase in blood basophilia was observed while CD4+GFP+ levels remained similar to numbers observed in control animals. However, following both NES treatment and influenza infection a decrease in circulating CD4+GFP+ cells was observed, along with a decrease in circulating basophils following influenza treatment. During influenza infection increased levels of circulating CD4+GFP− cells were observed (Fig 1C) coincident with a pronounced infiltration of CD8+CD69+ cells to the lung (data not shown). Considering the host phase of the Nb life cycle involves migration through multiple organs and the induction of a multi-tissue immune response, it is reasonable we find a comparatively larger basophil response than that induced for protein/adjuvant challenge models. It is additionally interesting to note that Nb infection led to the recruitment of similar numbers of basophils and eosinophils to the lung (approx 1.2×106 cells/lung), while the allergic airway models resulted in significant airway eosinophilia, with only a moderate increase in basophil recruitment. Even in the case of KLH/Alum, which led to a 16-fold increase of lung eosinophils lung basophils were not markedly increased. The comparison of a primary helminth infection with allergen challenge models is not exactly parallel since mice sensitized with KLH/alum and OVA/alum both had specific IgE present (31) and loaded on their basophils at the time of intranasal allergen challenge whereas no specific IgE was present at the time of primary helminth infection. Thus we evaluated blood basophilia during KLH/alum and OVA/alum sensitization and determined that initial priming with KLH/alum and OVA/alum led to an increase in the level of circulating basophils (Fig S1A), whilst the CD4+GFP+ cell levels remained unchanged (Fig S1B). Hence these results indicate that helminths such as Nb may secrete factors that specifically induce the recruitment of basophils to peripheral tissues.
Kinetics and magnitude of basophil induction in response to a primary and secondary N. brasiliensis infection
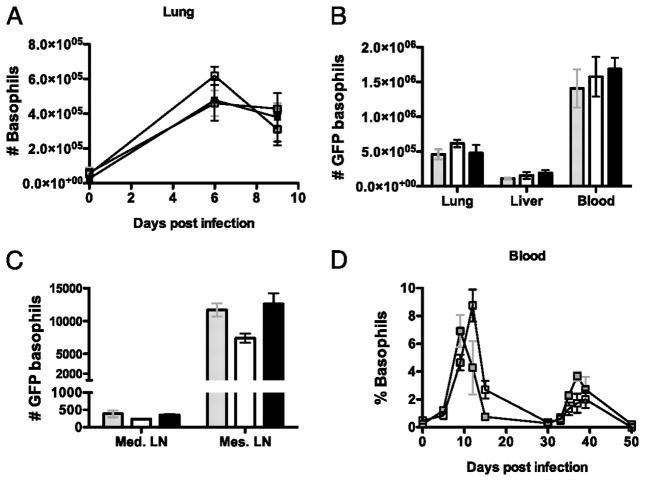
As Nb induced basophilia was the most pronounced of the models tested, we next sought to examine whether IL-4 producing basophils were also a prominent feature of the memory TH2 response induced following secondary infection with Nb. We have previously reported that both the enhanced basophil production and subsequent peripheral tissue accumulation following primary Nb infection are IL-4-independent processes (32). To compare basophil responses during primary and secondary Nb infection, groups of IL-4 deficient G4/G4 mice were infected with 600 L3 Nb and, where indicated, re-infected with 600 L3 Nb 30 days after the primary infection. Blood, liver, lung, BAL, Mediastinal lymph node (Md Ln) and Mesenteric lymph node (Ms Ln) cells (Fig. 2A) were isolated at the indicated time points and the numbers of GFP+CD4− FcεRIa+ basophils followed by flow cytometric analysis. Few GFP+ basophils were found in the tissues of naive animals; 2.5 × 105/ml blood, 9 × 104/lung and 4 × 104/liver, comprising on average 3.0%, 0.6% and 1% of total blood, lung and liver cells, respectively. Following infection, basophils were found to be significantly elevated 10- to 12- fold at the peak of the primary response (day 9), rapidly decreasing thereafter (Fig. 2A) (4). Following secondary Nb infection, mice generated a basophilic response in the blood and lung which peaked at day 6 post infection, 3 days earlier than the peak response observed following primary infection (Fig. 2A). Surprisingly, the magnitude of the secondary basophil response, was not found to be greater than that observed during the primary response (Fig. 2B). Basophil numbers in the liver during both primary and the secondary infection showed identical kinetics to that observed in the lung. Additionally, basophilic infiltration into the BAL followed a similar pattern (Fig. 2A). It was recently reported that blood basophils are recruited into the draining lymph nodes following allergen challenge (33), the reported recruitment was transient in nature and peaked at day 3 post papain challenge and rapidly disappeared from lymphoid sites thereafter (33). It was also demonstrated that the recruited basophils produce IL-4 and TSLP, both of which appear to contribute to TH2 differentiation. In support of this, depletion of basophils during papain challenge abolished the development of IL-4 producing TH2 cells (33). Thus to determine whether basophils are also recruited into the draining lymph nodes during Nb infection, basophils were measured in both the Md and the Ms lymph nodes following infection. Similar to papain challenge, basophil numbers also peaked in the Md Ln at day 3 following both primary and secondary infection (Fig. 2A). Unlike other tissues tested, basophil recruitment to the Md Ln after secondary infection was significantly higher than observed following primary infection. Basophil recruitment in the Ms Ln peaked at day 9 post primary infection and recruitment to the Ms Ln after secondary infection peaked at day 3 post infection but was reduced in comparison to primary infection (Fig 2C). Basophil recruitment was only observed in lymph nodes draining the sites of inflammation and was not observed in non-draining inguinal lymph nodes (data not shown), suggesting that the recruitment is induced by Ag drainage and possibly by the local induction of immune responses. Taken together, these results demonstrate that basophil responses following secondary Nb infection are induced more rapidly in comparison to primary infection. However no significant increase in the magnitude of the basophil response during secondary infection was observed except in the draining Md Ln.
Figure 2. The timing and magnitude of basophil induction in response to a primary and secondary Nb infection.

The total numbers of non-CD4 FcεR1α+ GFP+ basophils were measured in the blood, lung, BAL, Md Ln, and Ms Ln of G4/G4 mice at various time points after primary (clear) and secondary (black) Nb infections (A). B and C, Comparison of GFP+ basophil numbers in uninfected animals (Grey), at the peak of primary (day 9, Clear) and secondary (day 6, Black) responses. GFP basophil numbers shown are per ml of blood. Blood data is the average of 9 mice from 3 independent experiments; lung data is from 6 mice from 2 experiments and liver, Md Ln and Ms Ln 3 mice per time point from a single experiment.
Basophils become fully activated upon migration into tissues
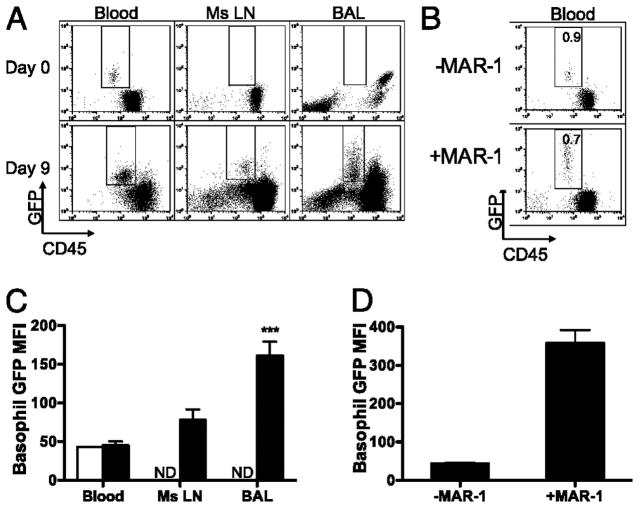
Basophil activation was assessed via measurement of GFP mean fluorescent intensity (MFI) in G4/G4 mice as a measure of potential for the production of IL-4 and was compared following primary infection in blood, Ms Ln and BAL samples (Fig 3A and Fig S2). GFP intensity was not found to be significantly increased in circulating basophils following Nb infection (Fig 3C). Upon migration into either the airways (BAL) or Ms Ln basophils were found to have an increased GFP MFI (Fig 3C), indicating that although basophils are not tissue resident, localization to tissues leads to an increase in effector function. To determine whether the circulating basophils observed following infection comprise a recently activated immature subset that are unable to respond to activation stimuli, MAR-1 was administered via intravenous injection. As MAR-1 has previously been described as a basophil-depleting(12) agent we assessed blood basophilia at 12hr after MAR-1 administration and determined that a significant proportion of basophils were still present in peripheral blood. However, the percentage of basophils observed had only decreased slightly from 0.9% (±0.1) to 0.7% (±0.2) of CD45+cells present in peripheral blood (Fig 3B). Additionally the majority of basophils present (>75%) displayed an increased GFP MFI (Fig 3D) indicating they were able to be fully activated in response to the MAR-1 induced cross linking of surface FcεRI and constitute a mature basophilic population. Thus the majority of basophils that have recently emigrated from the bone marrow are sufficiently matured such that they rapidly increase production of IL-4 in response to activation stimuli such as FcεRI crosslinking. However, during the course of a natural infection, basophils require additional activation to become fully activated as migration into a tertiary tissue environment, such as the lung, was required to induce fully enhanced IL-4 production (Fig 3A, 3C and S2) as opposed to that observed in the blood or lymph nodes. Thus it is possible that upon migration into the tissue environment basophils receive a secondary signal which allows them to fully mature or that the level of parasite Ag present in the tissue is sufficiently high compared to that encountered in the blood or lymphoid compartments, such that FcεRI crosslinking associated maturation occurs. Further investigations are required to assess these possible mechanisms.
Figure 3. Basophil GFP MFI in tissues.
(A) FACs plots of non-CD4 cells from uninfected, day 9 blood, BAL and Ms LN of primary Nb infected G4/G4 mice, showing FcεR1α+GFP+ basophils. (B) FACS plot of peripheral blood 12hr following i.v. administration of MAR-1. Indicated percentages represent the proportion of GFP+ basophils among total CD45+ cells. (C) MFI of basophils present in blood, BAL and mesenteric lymph nodes at days 0 (clear) and 9 (black) post infection. (D) MFI of basophils present in peripheral blood at day 1 ± MAR-1. Data points shown indicate mean ± SE from three individual animals from two experiments. ***, P ≤ 0.0001; **, P ≤ 0.001; *, P ≤ 0.01; no asterisk, P > 0.05, relative to day 9 blood basophils with Student’s t-test. ND, None Detected.
The number of GFP-expressing basophils induced in response to a secondary N. brasiliensis infection is not dependent on IL-4 or STAT6
We have previously demonstrated that STAT6 signaling is not required for basophil accumulation or GFP (IL-4) expression during the course of primary Nb infection (4). In order to examine the role of STAT6 during secondary infection, groups of Nb infected G4/IL-4, G4/G4, and G4/G4 STAT6 KO mice were re-infected with Nb 30 days post-primary infection as described above and basophil numbers were examined at days 6 and 9 following secondary infection. As shown in Figure 4A, the levels of basophil induction following infection in the lung were comparable between G4/IL-4, G4/G4, and G4/G4 STAT6KO groups. Total basophil numbers found in the lung, liver, and blood were also indistinguishable between all three groups, suggesting that IL-4 and STAT6 signaling has no role in the induction of basophilia during Nb infection (Fig. 4B). Furthermore, basophil recruitment into the Md Ln and Ms Ln following the secondary infection with Nb was not affected by the absence of IL-4 or STAT6 (Fig. 4C). Indeed, when blood basophils were examined in G4/IL-4 STAT6 KO and G4/G4 STAT6 KO mice throughout the 50 day period during primary and secondary Nb infection, neither the presence or absence of IL-4 in the complete absence of STAT6 altered the kinetics of circulating basophils (Fig. 4D). These results demonstrate that, as is the case with primary infection, the induction and migration of GFP producing basophils during a secondary response to Nb is not dependent on STAT6, further suggesting that other cytokines which utilize the STAT6 signaling pathway such as IL-13 are not involved in the induction of basophilic responses.
Figure 4. The number of GFP producing basophils in response to a secondary Nb infection is not dependent on IL-4 or STAT6.
The total number of GFP expressing basophils were detected following a secondary Nb infection in G4/IL-4 (grey), G4/G4 (clear) and G4/G4 xSTAT6 ko (black) mice. (A) Kinetics of GFP basophil induction in the lung. (B) Total number of GFP basophils in the lung, liver, blood, (C) mediastinal lymph node and mesenteric lymph node day 6 after infection. (D) Kinetics of GFP basophil induction in the BAL following primary (day 0) and secondary (Day 30) infection with Nb in G4/IL-4xSTAT6 ko (grey) and G4/G4xSTAT6 ko (black) mice. Data points shown indicate mean ± SE from three individual animals from two experiments.
The timing and magnitude of CD4+ T cell and eosinophil induction in response to a primary and secondary N. brasiliensis infection
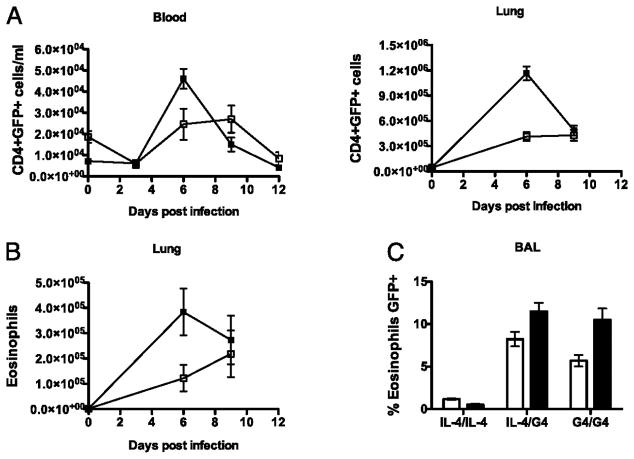
CD4+ T cells and eosinophils are also important sources of IL-4 in TH2 immune responses(13, 34, 35). Here we examined the involvement of such GFP/IL-4 producing cell types in our primary and secondary Nb models. Induction of GFP expressing CD4+ T cells after secondary Nb infection closely followed the faster kinetics of GFP+ basophil induction, with peak GFP expression by CD4+cells occurring at day 6 p.i. in both blood and lung (Fig. 5A). In contrast to the basophilic response where secondary infection did not increase the magnitude of the response, ~ 3-fold more GFP+ CD4+ T cells were detected in the lung following secondary infection.
Figure 5. The timing and magnitude of CD4 T cells and eosinophil induction in response to primary and secondary Nb infection.
The total number of GFP expressing CD4+ T cells (A) and GFPexpressing eosinophils (B) were measured in the blood and lung of G4/G4 mice at various time points after primary (clear) and secondary (black) Nb infections. (C) Proportion of GFP expressing eosinophils in BAL at the peak of primary (day 9, clear) and secondary (day 6, black) responses. Blood, Lung and BAL fluid was collected and expression of CD4 and GFP by SSCHi and SSCLo cells was examined. SSCHi cells had previously been identified as >95% eosinophils following fluorescence assisted cell sorting and identification by Difquick staining according to using standard morphological and cytochemical criteria. Data points shown indicate mean ± SE from three individual animals from two experiments.
Others have previously observed a significant number of GFP+ eosinophils in the lungs of Nb infected 4get mice (13, 34). Eosinophils were defined here as the population of lung cells that exhibited high side scatter, low forward scatter and lacked expression of the FcεR1 and CD4 This eosinophilic phenotype was confirmed using standard cytochemical and histological criteria following fluorescence assisted cell sorting (data not shown). Analysis of BAL eosinophilia revealed a significant proportion of both G4/IL-4 and G4/G4 eosinophils expressed GFP 8.6%, and 7.4%, respectively (Fig. 5C). GFP+ eosinophils were induced with faster kinetics and were detected in greater numbers following secondary infection in comparison to primary infection (Fig. 5B), comprising 1% and 0.7% of total cells at the peak of the secondary and primary response respectively. GFP+ eosinophils were not detected in the blood or lymph nodes (data not shown). Therefore both the kinetics and magnitude of the GFP+ CD4+ and GFP+ eosinophil response is enhanced upon secondary infection.
The differential contribution to IL-4 production by basophils, CD4+T cells and eosinophils in response to primary and secondary Nb infection
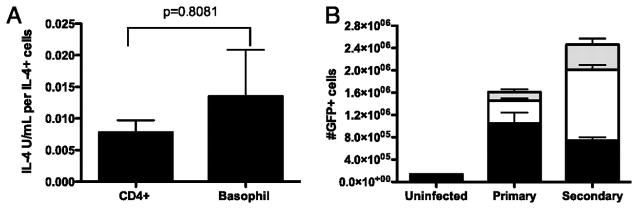
We have shown that GFP-expressing basophils, CD4+ T cells and to a lesser extent eosinophils are induced in both the primary and secondary Nb-infected lung. Previously Gessner et al(28) have reported that TH2 cells and basophils isolated from blood produced similar amounts of IL-4 on a per cell basis following activation, and that eosinophils produced approximately 0.6 -fold as much IL-4/cell. However, as we previously observed that lung associated basophils are more highly activated we felt it important to directly compare the amount of IL-4 produced by basophils and TH2 cells which had been specifically recruited to the lung. Thus at day 9 post infection with live Nb whole lungs were processed to a single cell suspension, TH2 cells were activated with T cell activator anti-mouse CD3/CD28 beads and basophils activated with anti-mouse IgE (6HD5) for six hours in the presence of αIL-4Rα to prevent protein degradation (Fig 6A). Specific numbers of IL-4 producers were measured by ELISPOT and ELISA determined total IL-4, thus a direct comparison of IL-4 production per cell was able to be determined ex vivo. Our results indicated that there was no statistically significant difference in the production of IL-4 by basophils (0.13U/cell ±0.07) and TH2 cells (0.08U/cell ±0.05). In order to determine the relative contribution of these cell types to total IL-4 production, we compared the number of GFP expressing basophils, CD4+ cells and eosinophils in the lung day 9 following a primary and day 6 following a secondary Nb infection (Fig. 6B). The majority of GFP producing cells during the primary response were basophils (65%), with CD4+ T cells (25%) and eosinophils (10%) comprising the remainder of the GFP+ population. Consistent with the notion that re-infection induces a stronger immune response, secondary infection resulted in 1.5 fold more GFP+ cells in the lung than did the primary. While the magnitude of the GFP+ CD4+ T cell and GFP+ eosinophil response tripled, indicating that CD4+ T cells were the main source of IL-4 during a recall response, there were in fact 0.7 fold fewer GFP+ basophils induced during secondary infection. Implying that while GFP+ basophils are a key feature of the secondary response to Nb, they are not subject to the enhanced response that is characteristic of memory responses associated with the adaptive immune system. Overall this data indicates that basophils are the main source of IL-4 in the primary lung response to Nb, while CD4+ T cells are the predominant IL-4 producing cells during secondary infection.
Figure 6. The differential contribution to IL-4 production by basophils, CD4T cells and eosinophils in a primary and secondary Nb infection.
(A) BALB/c animals were infected s.c. with 600L3 Nb, at day 9 post infection lung resident CD4+ cells were stimulated with anti-mouse CD3/CD28 beads and basophils stimulated with anti-mouse IgE. IL-4 production was quantitated by ELISA and the number of cells producing IL-4 determined by ELISPOT. IL-4 production on a per cell basis was calculated as units of IL-4 produced per IL-4 producing cell. Data points shown indicate mean ± SEM from three replicates from two individual experiments. (B) The total number of GFP+ basophils, GFP+ CD4 cells and GFP+ eosinophils were detected in lungs of G4/G4 mice 9 days following a primary and 6 days following a secondary Nb infection and compared to levels seen in uninfected mice. Data points shown indicate mean ± SE from three individual animals from two experiments.
Basophils do not influence TH2 differentiation in response to Nb infection
As previous papers (20, 21, 36) have indicated that basophils may play a significant role in the presentation of antigen to CD4+ cells and the induction of TH2 priming we sought to ascertain whether basophils were required for TH2 induction using the dead Nb model of infection (23) which allowed us to study a localized response to Nb independent from secondary features of inflammation which may be induced by the migration of Nb through host tissues. IL-4/G4 animals were depleted of basophils following i.v. administration of MAR-1 at day -2 (Fig. 7A) following this depleted and non-depleted animals were infected i.d. with dead Nb and the development of a localized TH2 response determined. At the peak of response (day 7 p.i.) there was no discernable difference in the total numbers of CD45+, CD4+ or CD4+GFP+ cells present in the draining lymph nodes of infected animals. Following the administration of MAR-1 background levels of basophils were detected in both naïve and depleted animals (~20–30 per Lymph node), where as a 50–60 fold increase in the number of basophils was detectable in PBS treated non-depleted animals following infection (Fig 7B). These data suggest that the recruitment of basophils to the lymph node does not have a significant effect on the induction of TH2 differentiation. Previous studies(12, 36) using papain as an adjuvant have indicated that basophil derived IL-4 may be important for the induction of TH2 differentiation. To determine whether IL-4 was required for TH2 differentiation in this system we transferred either +/G4 or G4/G4 Rag deficient CD4+ cells specific for PCC (5CC7) into either w.t. B10.A or IL-4 deficient G4/G4 hosts and primed them with papain and PCC. At day 4 p.i. there was no significant difference observed in the total number of cells or number of CD4+ cells present in draining lymph nodes (Fig S3). In the absence of IL-4, TH2 differentiation was not found to be impaired as measured by the induction of GFP expression by CD4+ cells present (Fig S3).
Figure 7. Basophil and the initiation of type 2 immune responses.
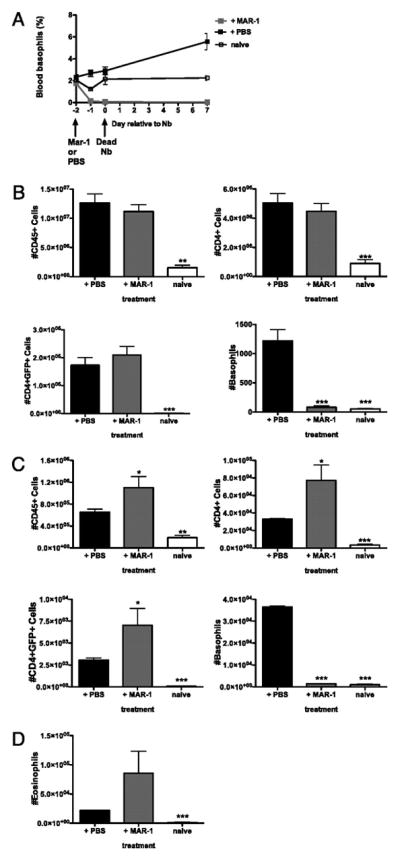
IL-4/G4 animals were administered either MAR-1 or PBS via iv injection 48 hours prior to intra-dermal infection with 600 dead N. brasiliensis. (A) Time course of basophils present in the blood as a percentage of CD45+ cells present. Total numbers of CD45+, CD4+ and CD4+GFP+ cells present were determined by flow cytometry in the draining lymph node (B) and ear (C) at 7 days post infection. (D) Total number of GFP+ eosinophils present in ear dermis. Data points shown indicate mean ± SE from three individual animals from two experiments. ***, P ≤ 0.0001; **, P ≤ 0.001; *, P ≤ 0.01; no asterisk, P > 0.05, relative to infection +PBS with Student’s t-test.
We additionally examined the effect of basophil depletion on cellular infiltrate to the inflamed dermis and determined that treatment with MAR-1 actually increased the total number of CD45+, CD4+, CD4+GFP+ cells (Fig. 7C) and eosinophils (Fig. 7D) present. Following MAR-1 treatment background levels of basophils were present at levels comparable to naïve controls (Fig. 7C), whereas significant numbers of basophils were recruited to the dermis following treatment with PBS, thus indicating that basophils may actually be playing a role in regulating the recruitment of effector cells to the dermis during type 2 inflammation.
Discussion
The role of basophil derived IL-4 has so far remained elusive, in part due to difficulties in basophil identification and the lack of appropriate knockout animal models. The utilization of G4 mice has enabled us to directly investigate the involvement of IL-4 expressing basophils in response to infection with Nb, without the need for ex vivo restimulation. Following both primary and secondary Nb infection, IL-4 expressing basophils were found systemically at various tissue sites including the lung, liver, lymph nodes and blood. Basophils were also present in mice lacking IL-4 and STAT6, indicating their induction and migration to these sites does not require IL-4 or STAT6 signaling. Alternatively, as basophils have been observed to enhance humoral immunity through the production IL-6 (19) it is possible that basophils recruited to the lymph node are required for enhanced levels of IgG2a, IgG2b and IgG3 observed in response to Nb in the absence of IL-4, may form an alternate positive feedback loop to increase basophil production in the absence of specific IgE or IgG1.
As is characteristic of a memory response, IL-4 expressing basophils were induced more rapidly upon secondary infection, peaking at day 6 following infection, 3 days prior to the primary peak at day 9. Surprisingly, the magnitude of the secondary GFP+ basophil response was not enhanced and was even reduced in some tissues. Other IL-4 producing subsets were also examined, both GFP+ CD4+ T cells and GFP+ eosinophils exhibited faster secondary kinetics, peaking on day 6 post infection. In contrast, the numbers of basophils found in the lung were increased 3-fold at the peak of the primary response. Interestingly, when the various contributors to overall IL-4 production are compared, it is evident that basophils are the main cellular source of IL-4 during the primary response while CD4+ T cells are the major source of IL-4 during memory responses. Additionally we extend the findings of Sokol et al (33) concerning the recruitment of basophils to lymph nodes in an allergen based TH2 model, by demonstrating that the kinetics of basophilic accumulation following parasitic infection in primary and secondary infection generally precedes total lymphoid expansion and TH2 induction via a transient recruitment to draining lymphoid organs. Basophil-dependent antigen presentation and production of both IL-4 and TSLP have been identified as potential enhancers of TH2 differentiation(20, 21, 36). However, we have previously observed that in vivo TH2 differentiation occurs independently of IL-4/STAT6(23) and we have additionally noted here that maximal basophil IL-4 production as measured by GFP does not occur in the lymphoid compartment, but was only observed upon recruitment of basophils to tissue sites such as the liver, lung and BAL. This would indicate that basophils most likely exert the majority of their IL-4 dependent effects in peripheral tissues. Our finding that basophil depletion did not alter TH2 differentiation in the lymphoid compartment, but instead led to an increase in the recruitment of type 2 effector cells to peripheral tissues further supports this view (Fig 7D). As our data indicates that basophils are activated in response to MAR-1 injection (Fig 3B) prior to their depletion (Fig 7A), it is possible that administration of MAR-1 may be responsible for the increase in skin inflammation observed. However, we noted that basophil depletion was almost complete at day -1 prior to Nb infection with the percentage of blood basophils decreasing from 1.9% in naive animals to 0.1% with a further reduction in blood basophilia to 0.05% by the time of infection, thus indicating the virtual absence of basophils, activated or not at the time of infection (Fig 7A). In addition, it has previously been noted that the in vivo half life of cytokines is very short (37–39) with IL-4 having a reported half life of 5–30 minutes (40). Thus, any cytokines released through the activation of basophils via MAR-1 would likely be cleared prior to infection with Nb and the initiation of the TH2 response.
Previous studies(12, 21, 36) noting that basophils may play a role in TH2 development have relied on in vitro models of TH2 differentiation where the requirement for IL-4 is well described (1, 31, 32). Further, a recent study by Massacand et al (41) has indicated that the absence of TSLP signaling had no impact on TH2 memory responses or the development of a protective immune response to Nb thus calling into question the previously perceived role for basophil produced TSLP in TH2 differentiation.
Overall this data indicates that basophils represent a significant source of IL-4 in both primary and secondary responses to parasites and allergens, while the production of CD4+ T cell derived IL-4 is greatly increased in the secondary response as compared to basophilic IL-4. Basophil derived IL-4 though not required for TH2 differentiation is highly influential in amplifying both peripheral and humoral aspects of the broader type 2 responses.
Supplementary Material
Acknowledgments
We would like to thank Dr Ronald Germain and Dr Cedric Dewas for helpful discussions, the NIH Fellows Editorial Board for help with manuscript preparation and Catherine Plunkett for technical assistance.
This work was supported by Research Program funding from the Health Research Council of New Zealand, the Marjorie Barclay Trust, the Marsden Fund, AMI Insurance Ltd, and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases/ National Institutes of Health through Project Z01 AI000493.22. The study was in part supported by the NIH grant AI080908 (B.M.). NVP is funded by the New Zealand Foundation for Research, Science and Technology.
Abbreviations used in this paper
- BAL
Bronchial Alveolar Lavage
- i.d.
Intra-dermal
- L3
Larval stage 3
- Md Ln
Mediastinal Lymph node
- Ms Ln
Mesenteric Lymph node
- Nb
Nippostrongylus brasiliensis
- NES
Nb Excretory Secretory product
- p.i.
Post-innoculation
- TSLP
Thymic Stromal Lymphopoeitic Protein
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkelman FD, Holmes J, Katona IM, Urban JF, Jr, Beckmann MP, Park LS, Schooley KA, Coffman RL, Mosmann TR, Paul WE. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 3.Ohnmacht C, Voehringer D. Basophil effector function and homeostasis during helminth infection. Blood. 2009;113:2816–2825. doi: 10.1182/blood-2008-05-154773. [DOI] [PubMed] [Google Scholar]
- 4.Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, Urban JF, Jr, Dvorak AM, Finkelman FD, LeGros G, Paul WE. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregory GD, Raju SS, Winandy S, Brown MA. Mast cell IL-4 expression is regulated by Ikaros and influences encephalitogenic Th1 responses in EAE. J Clin Invest. 2006;116:1327–1336. doi: 10.1172/JCI27227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435–1446. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshimoto T, Paul WE. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wedemeyer J, Tsai M, Galli SJ. Roles of mast cells and basophils in innate and acquired immunity. Curr Opin Immunol. 2000;12:624–631. doi: 10.1016/s0952-7915(00)00154-0. [DOI] [PubMed] [Google Scholar]
- 10.Miyajima I, Dombrowicz D, Martin TR, Ravetch JV, Kinet JP, Galli SJ. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gammaRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis. J Clin Invest. 1997;99:901–914. doi: 10.1172/JCI119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat Rev Immunol. 2002;2:773–786. doi: 10.1038/nri914. [DOI] [PubMed] [Google Scholar]
- 12.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–277. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 14.Mitre E, Taylor RT, Kubofcik J, Nutman TB. Parasite antigen-driven basophils are a major source of IL-4 in human filarial infections. J Immunol. 2004;172:2439–2445. doi: 10.4049/jimmunol.172.4.2439. [DOI] [PubMed] [Google Scholar]
- 15.Falcone FH, Dahinden CA, Gibbs BF, Noll T, Amon U, Hebestreit H, Abrahamsen O, Klaucke J, Schlaak M, Haas H. Human basophils release interleukin-4 after stimulation with Schistosoma mansoni egg antigen. Eur J Immunol. 1996;26:1147–1155. doi: 10.1002/eji.1830260528. [DOI] [PubMed] [Google Scholar]
- 16.Mukai K, Matsuoka K, Taya C, Suzuki H, Yokozeki H, Nishioka K, Hirokawa K, Etori M, Yamashita M, Kubota T, Minegishi Y, Yonekawa H, Karasuyama H. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity. 2005;23:191–202. doi: 10.1016/j.immuni.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Luccioli S, Brody DT, Hasan S, Keane-Myers A, Prussin C, Metcalfe DD. IgE(+), Kit(−), I-A/I-E(−) myeloid cells are the initial source of Il-4 after antigen challenge in a mouse model of allergic pulmonary inflammation. J Allergy Clin Immunol. 2002;110:117–124. doi: 10.1067/mai.2002.125828. [DOI] [PubMed] [Google Scholar]
- 18.Khodoun MV, Orekhova T, Potter C, Morris S, Finkelman FD. Basophils initiate IL-4 production during a memory T-dependent response. J Exp Med. 2004;200:857–870. doi: 10.1084/jem.20040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denzel A, Maus UA, Rodriguez Gomez M, Moll C, Niedermeier M, Winter C, Maus R, Hollingshead S, Briles DE, Kunz-Schughart LA, Talke Y, Mack M. Basophils enhance immunological memory responses. Nat Immunol. 2008;9:733–742. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 20.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N, Comeau MR, Pearce EJ, Laufer TM, Artis D. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S, Shen T, Min B. Basophils can directly present or cross-present antigen to CD8 lymphocytes and alter CD8 T cell differentiation into IL-10-producing phenotypes. J Immunol. 2009;183:3033–3039. doi: 10.4049/jimmunol.0900332. [DOI] [PubMed] [Google Scholar]
- 23.van Panhuys N, Tang SC, Prout M, Camberis M, Scarlett D, Roberts J, Hu-Li J, Paul WE, Le Gros G. In vivo studies fail to reveal a role for IL-4 or STAT6 signaling in Th2 lymphocyte differentiation. Proc Natl Acad Sci U S A. 2008;105:12423–12428. doi: 10.1073/pnas.0806372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson RH, Reed ND, Manning DD. Expulsion of Nippostrongylus brasiliensis from mice lacking antibody production potential. Immunology. 1977;32:867–874. [PMC free article] [PubMed] [Google Scholar]
- 25.Taliaferro W. The Cellular Reactions in the Skin, Lungs and Intestines of Normal and Immune Rats After Infection with Nippostrongylus Muris. Journal of Infectious Diseases. 1939;64:157–192. [Google Scholar]
- 26.Hu-Li J, Pannetier C, Guo L, Lohning M, Gu H, Watson C, Assenmacher M, Radbruch A, Paul WE. Regulation of expression of IL-4 alleles: analysis using a chimeric GFP/IL-4 gene. Immunity. 2001;14:1–11. doi: 10.1016/s1074-7613(01)00084-x. [DOI] [PubMed] [Google Scholar]
- 27.Shen T, Kim S, Do JS, Wang L, Lantz C, Urban JF, Le Gros G, Min B. T cell-derived IL-3 plays key role in parasite infection-induced basophil production but is dispensable for in vivo basophil survival. Int Immunol. 2008;20:1201–1209. doi: 10.1093/intimm/dxn077. [DOI] [PubMed] [Google Scholar]
- 28.Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174:1063–1072. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 30.Camberis M, Gros GLe, Urban J., Jr Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr Protoc Immunol. 2003;Chapter 19(Unit 19.12) doi: 10.1002/0471142735.im1912s55. [DOI] [PubMed] [Google Scholar]
- 31.Le Gros G, Schultze N, Walti S, Einsle K, Finkelman F, Kosco-Vilbois MH, Heusser C. The development of IgE+ memory B cells following primary IgE immune responses. Eur J Immunol. 1996;26:3042–3047. doi: 10.1002/eji.1830261233. [DOI] [PubMed] [Google Scholar]
- 32.Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, Zhu J, Paul WE. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci U S A. 2004;101:3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2007 doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinkai K, Mohrs M, Locksley RM. Helper T cells regulate type-2 innate immunity in vivo. Nature. 2002;420:825–829. doi: 10.1038/nature01202. [DOI] [PubMed] [Google Scholar]
- 35.Chen L, Grabowski KA, Xin JP, Coleman J, Huang Z, Espiritu B, Alkan S, Xie HB, Zhu Y, White FA, Clancy J, Jr, Huang H. IL-4 induces differentiation and expansion of Th2 cytokine-producing eosinophils. J Immunol. 2004;172:2059–2066. doi: 10.4049/jimmunol.172.4.2059. [DOI] [PubMed] [Google Scholar]
- 36.Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, Nakanishi K. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 37.Finkelman FD, Madden KB, Morris SC, Holmes JM, Boiani N, Katona IM, Maliszewski CR. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J Immunol. 1993;151:1235–1244. [PubMed] [Google Scholar]
- 38.Lotze MT, Frana LW, Sharrow SO, Robb RJ, Rosenberg SA. In vivo administration of purified human interleukin 2. I. Half-life and immunologic effects of the Jurkat cell line-derived interleukin 2. J Immunol. 1985;134:157–166. [PubMed] [Google Scholar]
- 39.Sherman ML, Spriggs DR, Arthur KA, Imamura K, Frei E, 3rd, Kufe DW. Recombinant human tumor necrosis factor administered as a five-day continuous infusion in cancer patients: phase I toxicity and effects on lipid metabolism. J Clin Oncol. 1988;6:344–350. doi: 10.1200/JCO.1988.6.2.344. [DOI] [PubMed] [Google Scholar]
- 40.Ma Y, Hurst HE, Fernandez-Botran R. Soluble cytokine receptors as carrier proteins: effects of soluble interleukin-4 receptors on the pharmacokinetics of murine interleukin-4. J Pharmacol Exp Ther. 1996;279:340–350. [PubMed] [Google Scholar]
- 41.Massacand JC, Stettler RC, Meier R, Humphreys NE, Grencis RK, Marsland BJ, Harris NL. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc Natl Acad Sci U S A. 2009;106:13968–13973. doi: 10.1073/pnas.0906367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.