Abstract
Targeted deletion of VGF, a neuronal and endocrine secreted protein and neuropeptide precursor, produces a lean, hypermetabolic mouse that is resistant to diet-, lesion-, and genetically-induced obesity and diabetes. We hypothesized that increased sympathetic nervous system activity in Vgf−/Vgf− knockout mice is responsible for increased energy expenditure and decreased fat storage, and that increased beta-adrenergic receptor stimulation induces lipolysis in white adipose tissue (WAT) of Vgf−/Vgf− mice. We found that fat mass was markedly reduced in Vgf−/Vgf− mice. Within knockout WAT, phosphorylation of protein kinase A (PKA) substrate increased in males and females, phosphorylation of hormone sensitive lipase (HSL) (Ser563) increased in females, and levels of adipose triglyceride lipase (ATGL), comparative gene identification-58 (CGI-58), and phospho-perilipin, were higher in male Vgf−/Vgf− WAT compared to wild type, consistent with increased lipolysis. The phosphorylation of AMP-activated protein kinase (AMPK) (Thr172) and levels of the AMPK kinase, transforming growth factor β-activated kinase 1 (TAK-1), were decreased. This was associated with a decrease in HSL Ser565 phosphorylation, the site phosphorylated by AMPK, in both male and female Vgf−/Vgf− WAT. No significant differences in phosphorylation of cAMP response element binding protein (CREB) or the p42/44 mitogen-activated protein kinase (MAPK) were noted. Despite this evidence supporting increased cAMP signaling and lipolysis, lipogenesis as assessed by fatty acid synthase (FAS) protein expression and phosphorylated acetyl-CoA carboxylase (pACC) was not decreased. Our data suggest that the VGF precursor or selected VGF-derived peptides dampen sympathetic outflow pathway activity to WAT to regulate fat storage and lipolysis.
Keywords: adipose tissue, beta-adrenergic receptor, diabetes, lipolysis, norepinephrine, obesity, sympathetic nervous system, VGF
INTRODUCTION
The sympathetic nervous system (SNS) is an important regulator of glucose and fat metabolism, and its dysfunction can predispose to obesity and type 2 diabetes mellitus. The melanocortin pathway projects from the hypothalamic paraventricular nucleus, innervating parasympathetic and sympathetic preganglionic cells in the brainstem and spinal cord, to form sympathetic circuits that innervate brown adipose tissue (BAT), white adipose tissue (WAT), liver, and pancreas (Foster, et al. 2010; Giordano, et al. 2005; Penn, et al. 2006; Song, et al. 2005; Voss-Andreae, et al. 2007). This pathway, through its modulation of autonomic outflow, has been proposed to regulate food intake, energy expenditure, and insulin secretion (Bray and York 1998; Fan, et al. 2000; Friedman and Halaas 1998; Li, et al. 2003). Norepineprine (NE), released from sympathetic nerve endings, stimulates lipolysis in WAT and increases thermogenesis in BAT via activation of beta-adrenergic receptors (Collins, et al. 2004), and mice that lack all three beta-adrenergic receptor subtypes (betaless) develop massive diet-induced obesity (Bachman, et al. 2002).
VGF is a secreted protein and peptide precursor, a member of the chromogranin/secretogranin family (Bartolomucci, et al. 2011), that is expressed in neurons throughout the brain and in several neuroendocrine and endocrine tissues (Levi, et al. 2004; Salton, et al. 2000). Homozygous germline VGF knockout mice are lean and hypermetabolic, and resist developing obesity and diabetes when fed a high fat diet (Hahm, et al. 1999), suggesting that VGF regulates energy balance by modulating sympathetic outflow. Consistent with this hypothesis, neonatal treatment of Vgf−/Vgf− mice with either monosodium glutamate, which damages the hypothalamus and the hypothalamic projections to the autonomic nervous system (Bergen, et al. 1998; Morris, et al. 1998; Tsukahara, et al. 1998), or guanethidine, which results in a peripheral sympathectomy (Watson, et al. 2009), blocks development of the lean phenotype (Hahm, et al. 2002; Watson, et al. 2005). Moreover, VGF knockout mice also have increased serum free fatty acid (FFA) levels, suggesting increased fat mobilization in WAT (Watson et al. 2005). Targeted deletion of Vgf also suppresses obesity, hyperinsulinemia and hyperglycemia in Ay/a agouti and melanocortin 4 receptor deficient (MC4R−/MC4R−) mice (Hahm et al. 2002; Watson et al. 2005), supporting a role for VGF in the melanocortin pathway and its projections. Here we examined WAT to determine the effect that targeted germline ablation of VGF has on the activation state and the protein expression of key lipolytic and lipogenic enzymes. We noted alterations in the level and/or phosphorylation of proteins that control fat breakdown, consistent with increased SNS activity. Our studies support the hypothesis that VGF and/or one or more VGF peptides modulate sympathetic outflow pathway activity to control fat storage and energy expenditure.
MATERIALS AND METHODS
Mouse Strains and Diets
The VGF-deficient line used here was generated by Regeneron Pharmaceuticals Inc. as previously described (Valenzuela, et al. 2003) using F1H4 ES cells (a 129B6/F1-derived cell line) and a BAC-based targeting vector with deletion of the entire Vgf coding sequence and insertion of an in frame lacZ reporter gene and neomycin-selection cassette; chimeric mice resulted from the injection of two independent Vgf−/Vgf− embryonic stem cell clones into C57BL6/J blastocysts. Male chimeras were mated with C57BL6/J females to produce F1 breeders and experiments were performed on N2F1 mice (>83% C57Bl6 background). As previously described (Watson et al. 2009), the phenotype of the Regeneron VGF-deficient line is extremely similar to an earlier line generated using R1 ES cells (Hahm et al. 1999). Mice were housed at room temperature in a 12h light, 12h dark cycle, with chow and water available ad lib unless otherwise specified. Mice fed standard chow received a 4.5% fat, 55% carbohydrate, 20% protein, 4.7% fiber diet (Purina PicoLab Rodent Diet 20-5053; 4 Kcal/gm; Purina. St. Louis, MO). All animal studies were conducted in accordance with the Guide for Care and Use of Experimental Animals, using protocols approved by Institutional Animal Care and Use Committees at Mount Sinai School of Medicine.
Western Blot Analysis
Gonadal WAT from wild type (Vgf+/Vgf+), heterozygous knockout (Vgf+/Vgf−), and homozygous knockout (Vgf−/Vgf−) (n=4–17 mice) was homogenized in ice cold lysis buffer. Two different buffers were used with comparable results: (1) 50 mM Tris-HCl (pH 8) 150 mM NaCl, 0.1% SDS, 0.5% deoxycholate and 1% NP40 supplemented with protease and phosphatase inhibitor cocktails (Roche Diagnostics, Mannheim, Germany; Thermo Scientific, Waltham, MA) (Watson et al. 2009), and (2) 20 mM MOPS, 2 mM EGTA, 5 mM EDTA, 30 mM sodium fluoride, 40 mM b-glycerophosphate, 10 mM sodium pyrophosphate, 2 mM sodium orthovanadate, 0.5% NP-40 and complete protease inhibitor cocktail (Roche, Nutley, NJ) (Scherer, et al. 2011). Lysates were cleared by centrifugation (14,000 rpm, 10 min at 4°C), and the protein concentration of the supernatant determined by the BCA protein method (Thermo Scientific, Waltham, MA). Protein samples (25 μg) were separated by SDS-PAGE and transferred to PVDF membranes (Millipore, Bedford, MA). Membranes were blocked with 5% (W/V) BSA in phosphate-buffered saline or with Odyssey LI-COR Blocking Buffer (LI-COR, Lincoln, NE) 1:1 in TBS.
Membranes were incubated overnight at 4°C with the following rabbit antisera (Cell Signaling, Boston, MA), unless otherwise indicated, diluted in blocking buffer: phospho-AMPKα (Thr172) [1:1000 (v/v)]; total AMPKα [1:1000 (v/v)]; TAK1 [1:1000 (v/v)]; mouse monoclonal anti-GAPDH [1:1000 (v/v)]; phospho-TAK1 (Thr187) [1:1000 (v/v)]; TORC3 [1:1000 (v/v)]; phospho-HSL Ser563 [1:1000 (v/v)]; phospho-HSL Ser565 [1:1000 (v/v)]; phospho-HSL Ser660 [1:1000 (v/v)]; HSL total [1:2000 (v/v)]; ATGL [1:1000 (v/v)]; phospho-PKA substrate [1:1000 (v/v)]; phospho-ACC [1:1000 (v/v)]; ACC [1:1000 (v/v)]; p42/44 MAPK [1:1000 (v/v)]; phospho-p42/44 MAPK [1:1000 (v/v)]; GLUT4 [1:1000 (v/v)]; and phospho-CREB [1:1000 (v/v)]. Other antisera included: FAS [1:1000 (v/v)] (BD Bioscience, San Jose, CA); insulin receptor beta [1:500 (v/v)] (Santa Cruz Biotechnology, Santa Cruz, CA); beta-Actin [1:10,000 (v/v)] (Abcam, Cambridge, MA); GADPH [1:5000 (v/v)] (Abcam, Cambridge, MA); perilipin [1:2000 (v/v)] (Souza, et al. 2002) (gift from Dr. Andrew Greenberg, Tufts University, MA); and CGI-58 [1:2000 (v/v)] (Subramanian, et al. 2004) (gift from Dawn Brasaemle, Rutgers University, NJ).
After 3 consecutive 5 min washes in PBS-T or TBS-T (0.1% Tween20 in PBS or TBS, respectively), membranes were incubated for 1 hour at room temperature with either HRP-conjugated secondary antibody [1:2,000 (v/v) in PBS-T] (GE Healthcare, Piscataway, NJ) containing 5% non-fat dry milk, or with Dylight 680-conjugated goat anti–rabbit IgG and Dylight 800-conjugated goat anti–mouse IgG (both Thermo Scientific, Waltham, MA) in blocking buffer containing 0.1% TBS-T and 0.1% SDS. After 3 washes in PBS-T or TBS-T, bound antibodies were detected using either ECL (Thermo Scientific), exposure to HyBlot CL (Denville Scientific, Metuchen, NJ), and densitometric quantification using NIH ImageJ, or were scanned with the LI-COR Odyssey (LI-COR, Lincoln, NE) and quantified with Odyssey 3.0 software based on direct fluorescence measurement.
Data and Statistical Analysis
Data are expressed as mean ± SEM. The statistical significance of differences among Vgf−/Vgf−; Vgf−/Vgf+ and Vgf +/Vgf+ groups was subjected to a one-way analysis of variance and Tukey’s multiple-range test; comparisons were performed using Prism 5.0 (GraphPad Software Inc; La Jolla, CA). P-values less than 0.05 were considered significant. For western blot assays, values (± SEM) from Vgf−/Vgf− and Vgf +/Vgf+ adipose samples were compared by two-tailed Student‘s t test.
RESULTS
Vgf−/Vgf− knockout mice showed significantly reduced body and gonadal fat pad weights compared to Vgf+/Vgf− and Vgf+/Vgf+ mice
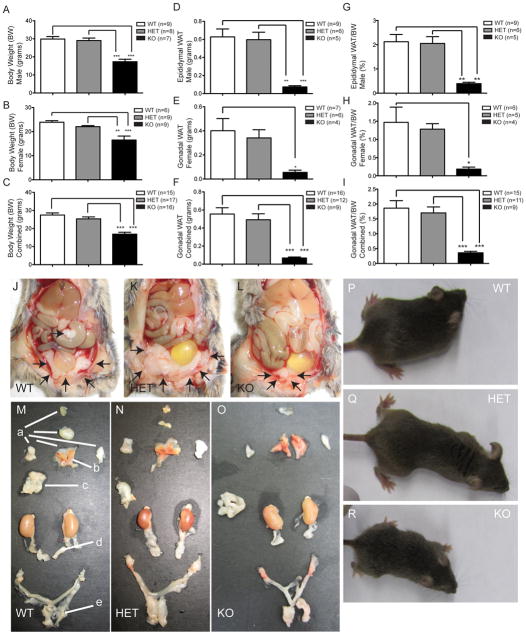
Quantification of Vgf−/Vgf−, Vgf+/Vgf−, and Vgf+/Vgf+ body and adipose tissue weights was initially performed. Because fat mass is often dramatically reduced in homozygous VGF knockout mice, only gonadal WAT could be reliably isolated and quantified from all mice. Vgf−/Vgf− male & female mice are lean with 42% & 31% less body weight, respectively, than Vgf+/Vgf+ mice (Fig. 1A and B), which was accompanied by a significant 8 fold decrease in gonadal fat pad mass (Fig. 1F, ***P<0.0001, ANOVA), that was also significantly decreased when fat pad mass was examined as a percentage of body mass (Fig. 1I, ***P < 0.0001 ANOVA). In addition, similar differences between Vgf−/Vgf+ heterozygous and Vgf−/Vgf− mice were noted (Fig. 1C, ***P<0.0001, ANOVA). Neither body weight nor fat pad weight differed significantly between Vgf−/Vgf+ and Vgf+/Vgf+ mice. Photographs of representative 8 week-old female mice of each genotype, and adipose depots dissected from each, are shown in Fig. 1J–R.
Figure 1. Body weight and gonadal WAT are reduced in VGF knockout mice.
Body weights (grams) of male and female Vgf−/Vgf−, Vgf+/Vgf−, and Vgf+/Vgf+ mice were measured at 9 weeks of age (panels A–C); male and female Vgf−/Vgf− mice had significantly lower body weights compared to Vgf+/Vgf−, and Vgf+/Vgf+ mice. In panel D, gonadal fat pad weights (grams) were significantly reduced in Vgf−/Vgf− (n=5 male, 4 female), compared to Vgf+/Vgf− (n=6 male, 7 female) and Vgf+/Vgf+ (n=9 male, 7 female). No significant differences in body weight, fat pad weight, or the ratio of fat pad weight to body weight, between Vgf+/Vgf− and Vgf+/Vgf+ mice, were noted (panels A–I). Body weights and epididymal and ovarian fat pad weights were measured for each genotype at 9–10 weeks of age; values are mean ± SEM, *p = 0.0430, **p = 0.0031, ***p < 0.0001 compared to wild type (ANOVA and Tukey’s multiples comparison). Eight week old female mice [Vgf+/Vgf+ WT (24.6 grams body weight), Vgf+/Vgf− HET (20.5 grams), and Vgf−/Vgf− KO (13.6 grams)] (panels P–R, respectively) were transcardially perfused with 4% paraformaldehyde in PBS, intraperitoneal fat depots were exposed (panels J–L), and the subcutaneous and visceral depots were dissected and placed on a mouse template to show their location in the body (panels M–O). Fat depots are labeled with lower case letters: a (subcutaneous anterior: deep & superficial cervical, interscapular, subscapular, axillo-thoracic); b (interscapular brown adipose tissue); c (mesenteric); d (perirenal, periovarian, parametrial, perivesical); e (subcutaneous posterior: dorsolumbar, inguinal, gluteal) (Murano, et al. 2009).
Altered protein expression in Vgf−/Vgf− WAT is consistent with increased lipolysis
Western analysis of adipose tissues from Vgf−/Vgf−, Vgf+/Vgf−, and Vgf+/Vgf+ mice was performed to investigate potential mechanisms underlying decreased adiposity in VGF knockout mice. Expression of proteins involved in glucose uptake or lipogenesis was unchanged: GLUT4, the main transporter involved in glucose uptake into WAT, Fatty Acid Synthase (FAS), and additional enzymes important in adipose tissue triglyceride accumulation, including total and phosphorylated acetyl-CoA carboxylase (ACC), were unchanged in VGF knockout WAT compared with wild type (Table 1).
Table 1. Expression of proteins that regulate lipolysis or lipogenesis: comparison of VGF knockout and wild type gonadal WAT.
Protein levels were determined in gonadal fat pads from 9–10 week old male and female mice using western blot analysis as described in Materials and Methods.
| Protein Quantified in Male WAT | Protein Expression (Arbitrary Units) Mean ± SEM |
P- Value | Protein Quantified in Female WAT | Protein Expression (Arbitrary Units) Mean ± SEM |
P- Value |
|---|---|---|---|---|---|
| FAS | Vgf+/+ = .19±.21 Vgf−/− = .17±.06 |
.93 | FAS | Vgf+/+ = .27±.04 Vgf−/− = .37±.11 |
.45 |
| Perilipin | Vgf+/+ = .46±.12 Vgf−/− = .55±.05 |
.62 | Perilipin | Vgf+/+ = 1.14±.22 Vgf−/− = 1.01±.32 |
.79 |
| TORC3 | Vgf+/+ = .015±.003 Vgf−/− = .017±.002 |
.55 | TORC3 | Vgf+/+ = .03±.01 Vgf−/− = .04±.01 |
.58 |
| pCREB | Vgf+/+ = .006±.002 Vgf−/− = .002±.001 |
.25 | pCREB | Vgf+/+ = .01±0.00 Vgf−/− = .01±0.00 |
.30 |
| IRbeta | Vgf+/+ = .06±.003 Vgf−/− = .08±.02 |
.55 | IRbeta | Vgf+/+ = .05±0.01 Vgf−/− = .07±0.02 |
.57 |
| ACC | Vgf+/+ = .008±.002 Vgf−/− = .074±.046 |
.23 | ACC | Vgf+/+ = .114±.06 Vgf−/− = .077±.02 |
.63 |
| pACC | Vgf+/+ = .15±.061 Vgf−/− = .42±.146 |
.16 | pACC | Vgf+/+ = .39±.104 Vgf−/− = .22±.045 |
.19 |
| p42/44 MAPK | Vgf+/+ = .41±.053 Vgf−/− = .56±.044 |
.11 | p42/44 MAPK | Vgf+/+ = .42±.04 Vgf−/− = .41±.015 |
.99 |
| phospho- p42/44 MAPK | Vgf+/+ = .04±.002 Vgf−/− = .25±.083 |
.06 | phospho- p42/44 MAPK | Vgf+/+ = .06±.008 Vgf−/− = .17±.065 |
.18 |
| Glut4 | Vgf+/+ = .03±.006 Vgf−/− = .04±.021 |
.58 | Glut4 | Vgf+/+ = .038±.009 Vgf−/− = .057±.008 |
.20 |
| HSL total | Vgf+/+ = .16±.017 Vgf−/− = .14±.060 |
.80 | HSL total | Vgf+/+ = .15±.061 Vgf−/− = .076±.024 |
.32 |
All data are normalized to the loading control β-actin, and are expressed in arbitrary units (mean ± SEM); statistical significance was determined using the two-tailed Student’s t test with *p < 0.05 considered significant [male, n=3–6 (Vgf+/Vgf+), n=3–5 (Vgf−/Vgf−); female, n=3–4 (Vgf+/Vgf+), n=3–5 (Vgf−/Vgf−)].
No significant differences in the levels of these proteins were found between Vgf−/Vgf− and Vgf+/Vgf+ WAT.
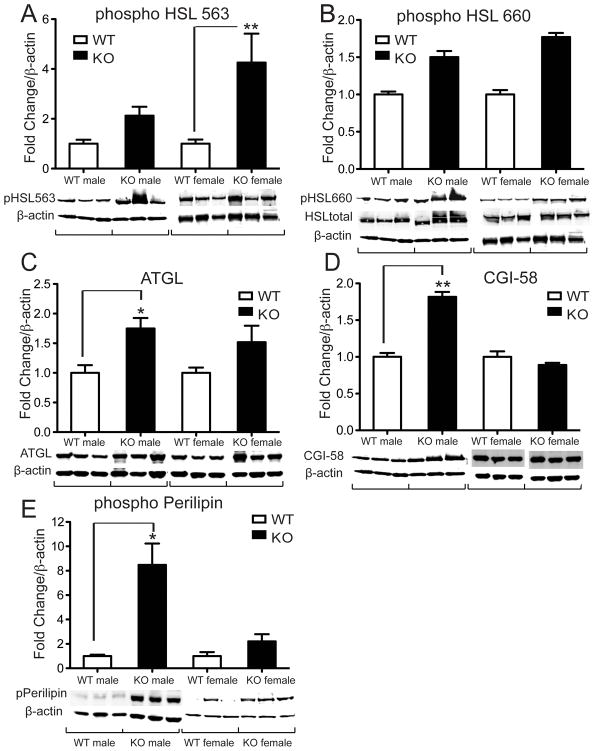
Next we analyzed whether reduced gonadal fat pad weight might be due to increased lipolysis. Phosphorylation of hormone sensitive lipase (HSL), an enzyme responsible for the mobilization of free fatty acids (FFA) from adipose tissue, on serine-563 was significantly increased in female Vgf−/Vgf− WAT (Fig. 2A), although phosphorylation of HSL at serine 660 was unchanged (Fig. 2B). We found that levels of adipose triglyceride lipase (ATGL), the main lipase responsible for the first step in intracellular triglyceride hydrolysis, were significantly up regulated in male Vgf−/Vgf− WAT (Fig. 2C). This was consistent with increased expression of Comparative Gene Identification-58 (CGI-58, also known as a/b hydrolase domain containing protein 5; Fig. 2D) and increased levels of phospho-perilipin (Fig. 2E), both important activators of ATGL-mediated lipolysis, in male VGF knockout WAT.
Figure 2. Altered expression of proteins involved in lipolysis in VGF knockout compared to wild type WAT.
WAT tissues were collected from Vgf−/Vgf− knockout mice (n=11–13) and Vgf+/Vgf+ wild type littermate controls (n=9–11), and protein expression was determined using western blot analysis and Image J as described in Materials and Methods. Values are expressed as fold change in knockout relative to wild type WAT, normalized to β-actin as a loading control, and statistical significance was determined using the two-tailed Student’s t test (*p < 0.05). Levels of phospho-HSL (ser563) were significantly increased in VGF KO WAT (panel A, *p = 0.0012), while phospho-HSL (ser660) levels were unchanged (panel B). Total HSL levels were also unchanged (see Table 1). Expression of ATGL (panel C, *p = 0.0312), CGI-58 (panel D, *p = 0.0074), and phospho-perilipin (panel E, *p = 0.0317) were significantly increased in VGF knockout WAT.
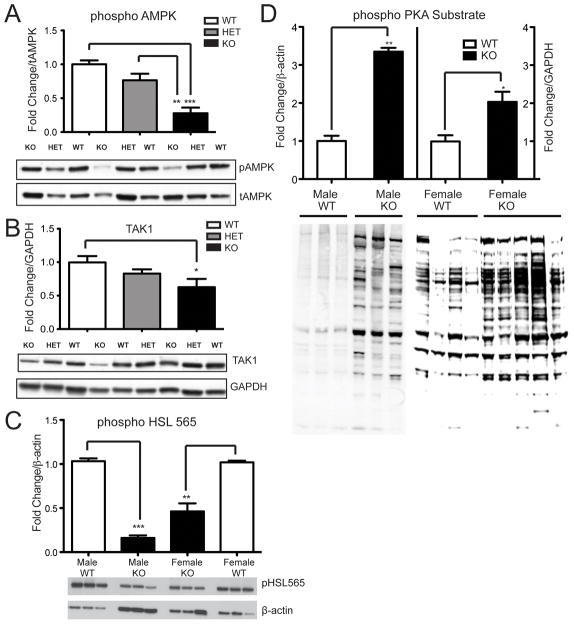
AMP-activated protein kinase (AMPK), an enzyme that is regulated by phosphorylation, has been implicated in the regulation of HSL. Phosphorylation of AMPKα, the catalytic subunit of AMPK, was significantly reduced in Vgf−/Vgf− relative to Vgf+/Vgf+ WAT (Fig. 3A; ***P = 0.0001). In support of these findings, previous studies noted that activation of AMPK decreased HSL phosphorylation of ser-563, while dominant negative inhibitors or targeted ablation of AMPK increased phospho-HSL (563) levels in adipocytes (Daval, et al. 2005). Levels of transforming growth factor β-activated kinase 1 (TAK-1), a mitogen-activated protein (MAP) kinase kinase kinase and AMPK kinase (Momcilovic, et al. 2006; Xie, et al. 2006), decreased in Vgf−/Vgf− WAT (Fig. 3B; *P = 0.0207). Although we were unable to detect TAK-1 (Thr187) phosphorylation in our WAT samples, phosphorylation of HSL at serine 565, a downstream target of AMPK (Anthonsen, et al. 1998), was found to be significantly decreased in Vgf−/Vgf− WAT (Fig. 3C; **p = 0.0039), suggesting reduced activation of AMPK in male and female VGF knockout WAT. In addition, the overall pattern of phosphorylation of PKA substrate (Fig. 3D) was significantly increased in male and female Vgf−/Vgf− WAT, consistent with increased PKA activity.
Figure 3. Phospho-AMPK, TAK1, and phospho-HSL (ser565) levels are decreased and PKA substrate phosphorylation is increased in VGF knockout WAT.
Phospho− AMPK (panel A), TAK1 (panel B), and phospho− HSL (ser565) (panel C) protein levels in gonadal WAT from Vgf +/Vgf+, Vgf+/Vgf−, and Vgf−/Vgf− mice were determined by western blotting, densitometry, and quantification using NIH image. Results were normalized to total AMPK (panel A), GAPDH (panel B), and β-actin (panels C and D). VGF ablation significantly decreased AMPK phosphorylation, TAK1 levels, and HSL (ser565) phosphorylation, in Vgf−/Vgf− compared to Vgf+/Vgf+ WAT [n=5−6 mice of each genotype per group, mean ± SEM; ***p = 0.0001; **p = 0.0053 (panel A), **p = 0.0039 (panel C); *p = 0.0207, ANOVA and Tukey’s multiples comparison]. In panel D, phosphorylation of PKA substrate in epididymal/gonadal WAT tissue from male and female Vgf−/Vgf− (n=3 males and 5 females per group) and Vgf+/Vgf+ (n=3 males and 4 females per group) mice was measured by western analysis. Values are mean ± SEM relative to the Vgf+/Vgf+ control, normalized to β-actin. A significant increase in PKA substrate phosphorylation was noted (**p = 0.0072, *p = 0.0167) in male and female Vgf−/Vgf− WAT.
No changes in the expression of p42/44 mitogen-activated protein kinase (MAPK) were detected (Table 1), which is consistent with our finding that phosphorylation of HSL at serine 660 was not altered; phosphorylation at serine 660 by p42/44 MAPK enhances the enzymatic activity of HSL (Greenberg, et al. 2001). Levels of Perilipin, a protein associated with the lipid droplet, were not changed (Table 1). Lastly, no significant differences in the levels of phosphorylated cAMP response element binding protein (pCREB), its coactivator transducer of regulated CREB activity 3 (TORC3), or the insulin receptor protein IRbeta were noted between Vgf−/Vgf− and Vgf+/Vgf+ WAT (Table 1).
DISCUSSION
We examined the effect that targeted germline Vgf gene ablation has on fat pad weight and WAT lipolysis and lipogenesis. Characterization of VGF knockout mice previously revealed increased circulating free fatty acid levels, consistent with increased lipolysis (Watson et al. 2005). Here we found that VGF knockout mice have reduced body weight, decreased gonadal fat pad weight, and alterations in a number of key lipolytic proteins by western blot analysis, compared to wild type mice. Phosphorylation of HSL on serine 563, which activates the enzyme, was increased in VGF knockout mice in comparison to wild type mice, significantly in females. This site is generally phosphorylated by protein kinase A (PKA) (Anthonsen et al. 1998), and consistent with increased PKA activity, significant changes were noted in PKA substrate phosphorylation (Fig. 3D) in both male and female knockout WAT. In addition we showed that the lipase ATGL, and its activators CGI-58 and phospho-perilipin, were significantly increased in WAT from male Vgf−/Vgf− mice. Recent studies have demonstrated that interaction of CGI-58 with ATGL enhances its activity up to 20 fold (Gruber, et al. 2010; Lass, et al. 2006), so taken together, our western results are consistent with increased lipolysis in VGF knockout adipose tissue, although there may be some sex-specific differences that need further exploration.
The role that AMPK plays in regulating lipid storage in the adipocyte is incompletely understood. Phosphorylation of HSL at serine 565 by AMPK reduces HSL phosphorylation at serine 563 by PKA, inhibiting HSL activity (Anthonsen et al. 1998), while a number of other studies have shown an inhibitory effect of AMPK on adipocyte lipolysis (Anthony, et al. 2009; Daval et al. 2005; Gaidhu, et al. 2009). Consistent with the former, we noted decreased pAMPK, decreased pHSL (ser565), and increased pHSL (ser563), in VGF knockout WAT. The anti-lipolytic role of AMPK was demonstrated using adipocytes from AMPKα1-knockout mice, treatment of adipocytes with AMPK inhibitors, and transfection of dominant negative and constitutively active AMPK constructs into adipocytes (Daval et al. 2005). On the other hand, treatment of rodents with AMPK agonists is associated with leanness (Narkar, et al. 2008), most likely through the inhibition of ACC2 activity, which leads to reduced CPT1 activity, increased long-chain FA entry into mitochondria, and increased fatty acid oxidation, although the mechanism remains uncertain and likely differs with chronic and acute treatment (Hoehn, et al. 2010). Targeted ablation of ACC2 in mice has resulted in marked reduction in whole body adiposity (Abu-Elheiga, et al. 2001), increased fatty acid oxidation coupled with elevated energy expenditure (Choi, et al. 2007), or no net effect on energy balance or adiposity (Hoehn et al. 2010), depending on the line analyzed. The latter study (Hoehn et al. 2010) suggests that chronically increased fatty acid oxidation, increased AMPK activity, and decreased ACC activity per se do not drive increased energy expenditure and reduced adiposity.
Our analysis of germline homozygous VGF knockout mice here and previously (Watson et al. 2009), demonstrating increased whole body energy expenditure and increased uncoupling protein expression in BAT, together with decreased pAMPK, decreased pHSL (ser565), increased pHSL (ser563), and no change in ACC or pACC protein in WAT, are most consistent with an anti-lipolytic role for AMPK, and chronically stimulated lipolysis in VGF knockout WAT that is likely driven by increased sympathetic nervous system activity and beta-adrenergic signaling. Decreased pAMPK levels in VGF knockout WAT could be due to decreased TAK1 levels, an AMPK kinase, but may also reflect increased lipolysis and lowering of the AMP/ATP ratio in the adipocyte. Previous findings of increased serum free fatty acid levels in VGF knockout mice are consistent with increased sympathetic outflow pathway activity and WAT lipolysis (Watson et al. 2005). In our analysis of protein levels in VGF knockout WAT presented here, we noted several differences from previous studies of lipolytic enzyme mRNA levels in WAT (Watson et al. 2009). Increased HSL and FAS mRNA levels and decreased ACC mRNA levels in knockout WAT (Watson et al. 2009) were not predictive of similar changes in protein levels (see Table 1). We previously found that increased mitochondrial number and UCP1 protein levels in VGF knockout BAT were associated with decreased rather than increased UCP1 mRNA levels compared to wild type (Watson et al. 2009). So although RNA levels often correlate with protein levels, greater understanding of the roles that specific gene products play in the regulation of metabolic flux ultimately relies on the determination of protein levels and activation state.
The VGF-derived peptide TLQP21 (VGF556–576) robustly potentiates beta-adrenergic receptor-induced lipolysis, increases sympathetic tone, increases energy expenditure, and prevents diet-induced obesity in mice (Bartolomucci, et al. 2006; Possenti, et al. 2012). This is somewhat paradoxical given the lean, hypermetabolic phenotype of germline VGF knockout mice, and our current and previous findings (Watson et al. 2009; Watson et al. 2005), which suggest increased sympathetic tone and lipolysis in these mice. Morphological alterations in WAT and BAT from VGF deficient mice, including decreased lipid accumulation in WAT, smaller interscapular WAT depots that are associated with BAT, and regions of fat accretion in BAT, and increased fatty acid oxidation, increased UCP1 and UCP2 protein levels, and increased mitochondrial number and cristae density in BAT, are all consistent with increased sympathetic nervous system activity (Watson et al. 2009; Watson et al. 2005). Individual VGF-derived peptides may therefore have opposing activities, much as has been shown previously for the proopiomelanocortin (POMC)-derived peptides, alpha-melanocyte stimulating hormone (α-MSH) and beta-endorphin (β-endorphin), which reduce and increase feeding, respectively (Raffin-Sanson, et al. 2003), while germline ablation of the pomc gene in mice and humans leads to profound obesity (Yaswen, et al. 1999). Moreover, increased feeding was found following intracranial administration of beta-endorphin (Grandison and Guidotti 1977), and also following selective ablation of the beta-endorphin coding sequence from the mouse Pomc gene (Appleyard, et al. 2003), a similarly paradoxical situation to the increased lipolysis noted in TLQP21-treated and VGF knockout mice. Ablation of VGF-derived peptides other than TLQP21, including the neuroendocrine regulatory peptide-2 (NERP-2) that regulates feeding and energy expenditure via an orexin-dependent mechanism, could therefore be responsible for the observed germline VGF knockout phenotype (Toshinai, et al. 2010). Alternatively, germline ablation of VGF could result in developmental abnormalities in hypothalamic/sympathetic outflow pathways, or lack of this chromogranin- and secretogranin-like protein could impact dense core secretory vesicles (DCV) structure and function, possibly altering catecholamine release from sympathetic terminals that innervate WAT, leading to chronic changes in beta-adrenergic signaling. Additional experimentation utilizing conditional knockout mice should allow these different hypotheses to be further tested.
Acknowledgments
FUNDING
Supported in part by: NIH Endocrine Training Grant 5T32DK07645 (SF); DK071308 and MH086499 (SRS); DK074873, DK083568 and DK082724 (CB); Diabetes Action Research and Education Foundation (SRS); Hope for Depression Research Foundation (SRS); ADA Basic Research Award (CB); European Foundation for the Study of Diabetes Grant (TS). CB is the recipient of a Hirschl-Weill-Caulier Career Scientist Award.
Footnotes
DECLARATION OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
AUTHOR CONTRIBUTION STATEMENT
SS and CB designed the study; MS generated and genotyped the mice, and assisted SF with sample preparation; SF, TS, and AS carried out the protein analysis; SF, TS, CB and SS wrote the manuscript; all authors approved the final version of the manuscript.
Contributor Information
Samira Fargali, Email: samira.fargali@mssm.edu.
Thomas Scherer, Email: thomas.scherer@meduniwien.ac.at.
Andrew C. Shin, Email: andrew.shin@mssm.edu.
Masato Sadahiro, Email: masato.sadahiro@mssm.edu.
Christoph Buettner, Email: christoph.buettner@mssm.edu.
Stephen R. Salton, Email: stephen.salton@mssm.edu.
References
- Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem. 1998;273:215–221. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- Anthony NM, Gaidhu MP, Ceddia RB. Regulation of visceral and subcutaneous adipocyte lipolysis by acute AICAR-induced AMPK activation. Obesity (Silver Spring) 2009;17:1312–1317. doi: 10.1038/oby.2008.645. [DOI] [PubMed] [Google Scholar]
- Appleyard SM, Hayward M, Young JI, Butler AA, Cone RD, Rubinstein M, Low MJ. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology. 2003;144:1753–1760. doi: 10.1210/en.2002-221096. [DOI] [PubMed] [Google Scholar]
- Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, La Corte G, Possenti R, Locatelli V, Rigamonti AE, Torsello A, Bresciani E, Bulgarelli I, Rizzi R, Pavone F, et al. TLQP-21, a VGF-derived peptide, increases energy expenditure and prevents the early phase of diet-induced obesity. Proc Natl Acad Sci U S A. 2006;103:14584–14589. doi: 10.1073/pnas.0606102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomucci A, Possenti R, Mahata SK, Fischer-Colbrie R, Loh YP, Salton SR. The extended granin family: structure, function, and biomedical implications. Endocr Rev. 2011;32:755–797. doi: 10.1210/er.2010-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen HT, Mizuno TM, Taylor J, Mobbs CV. Hyperphagia and weight gain after gold-thioglucose: relation to hypothalamic neuropeptide Y and proopiomelanocortin. Endocrinology. 1998;139:4483–4488. doi: 10.1210/endo.139.11.6324. [DOI] [PubMed] [Google Scholar]
- Bray GA, York DA. The MONA LISA hypothesis in the time of leptin. Recent Prog Horm Res. 1998;53:95–117. discussion 117–118. [PubMed] [Google Scholar]
- Choi CS, Savage DB, Abu-Elheiga L, Liu ZX, Kim S, Kulkarni A, Distefano A, Hwang YJ, Reznick RM, Codella R, et al. Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci U S A. 2007;104:16480–16485. doi: 10.1073/pnas.0706794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S, Cao W, Robidoux J. Learning new tricks from old dogs: beta-adrenergic receptors teach new lessons on firing up adipose tissue metabolism. Mol Endocrinol. 2004;18:2123–2131. doi: 10.1210/me.2004-0193. [DOI] [PubMed] [Google Scholar]
- Daval M, Diot-Dupuy F, Bazin R, Hainault I, Viollet B, Vaulont S, Hajduch E, Ferre P, Foufelle F. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem. 2005;280:25250–25257. doi: 10.1074/jbc.M414222200. [DOI] [PubMed] [Google Scholar]
- Fan W, Dinulescu DM, Butler AA, Zhou J, Marks DL, Cone RD. The central melanocortin system can directly regulate serum insulin levels. Endocrinology. 2000;141:3072–3079. doi: 10.1210/endo.141.9.7665. [DOI] [PubMed] [Google Scholar]
- Foster MT, Song CK, Bartness TJ. Hypothalamic paraventricular nucleus lesion involvement in the sympathetic control of lipid mobilization. Obesity (Silver Spring) 2010;18:682–689. doi: 10.1038/oby.2009.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Gaidhu MP, Fediuc S, Anthony NM, So M, Mirpourian M, Perry RL, Ceddia RB. Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: novel mechanisms integrating HSL and ATGL. J Lipid Res. 2009;50:704–715. doi: 10.1194/jlr.M800480-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A, Frontini A, Murano I, Tonello C, Marino MA, Carruba MO, Nisoli E, Cinti S. Regional-dependent increase of sympathetic innervation in rat white adipose tissue during prolonged fasting. J Histochem Cytochem. 2005;53:679–687. doi: 10.1369/jhc.4A6566.2005. [DOI] [PubMed] [Google Scholar]
- Grandison L, Guidotti A. Stimulation of food intake by muscimol and beta endorphin. Neuropharmacology. 1977;16:533–536. doi: 10.1016/0028-3908(77)90019-3. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Shen WJ, Muliro K, Patel S, Souza SC, Roth RA, Kraemer FB. Stimulation of lipolysis and hormone-sensitive lipase via the extracellular signal-regulated kinase pathway. J Biol Chem. 2001;276:45456–45461. doi: 10.1074/jbc.M104436200. [DOI] [PubMed] [Google Scholar]
- Gruber A, Cornaciu I, Lass A, Schweiger M, Poeschl M, Eder C, Kumari M, Schoiswohl G, Wolinski H, Kohlwein SD, et al. The N-terminal region of comparative gene identification-58 (CGI-58) is important for lipid droplet binding and activation of adipose triglyceride lipase. J Biol Chem. 2010;285:12289–12298. doi: 10.1074/jbc.M109.064469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm S, Fekete C, Mizuno TM, Windsor J, Yan H, Boozer CN, Lee C, Elmquist JK, Lechan RM, Mobbs CV, et al. VGF is required for obesity induced by diet, gold thioglucose treatment and agouti, and is differentially regulated in POMC- and NPY-containing arcuate neurons in response to fasting. J Neurosci. 2002;22:6929–6938. doi: 10.1523/JNEUROSCI.22-16-06929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm S, Mizuno TM, Wu TJ, Wisor JP, Priest CA, Kozak CA, Boozer CN, Peng B, McEvoy RC, Good P, et al. Targeted deletion of the Vgf gene indicates that the encoded secretory peptide precursor plays a novel role in the regulation of energy balance. Neuron. 1999;23:537–548. doi: 10.1016/s0896-6273(00)80806-5. [DOI] [PubMed] [Google Scholar]
- Hoehn KL, Turner N, Swarbrick MM, Wilks D, Preston E, Phua Y, Joshi H, Furler SM, Larance M, Hegarty BD, et al. Acute or chronic upregulation of mitochondrial fatty acid oxidation has no net effect on whole-body energy expenditure or adiposity. Cell Metab. 2010;11:70–76. doi: 10.1016/j.cmet.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Levi A, Ferri GL, Watson E, Possenti R, Salton SR. Processing, distribution and function of VGF, a neuronal and endocrine peptide precursor. Cell Mol Neurobiol. 2004;24:517–533. doi: 10.1023/B:CEMN.0000023627.79947.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Mobbs CV, Scarpace PJ. Central pro-opiomelanocortin gene delivery results in hypophagia, reduced visceral adiposity, and improved insulin sensitivity in genetically obese Zucker rats. Diabetes. 2003;52:1951–1957. doi: 10.2337/diabetes.52.8.1951. [DOI] [PubMed] [Google Scholar]
- Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem. 2006;281:25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- Morris MJ, Tortelli CF, Filippis A, Proietto J. Reduced BAT function as a mechanism for obesity in the hypophagic, neuropeptide Y deficient monosodium glutamate-treated rat. Regul Pept. 1998;75–76:441–447. doi: 10.1016/s0167-0115(98)00100-1. [DOI] [PubMed] [Google Scholar]
- Murano I, Barbatelli G, Giordano A, Cinti S. Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J Anat. 2009;214:171–178. doi: 10.1111/j.1469-7580.2008.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn DM, Jordan LC, Kelso EW, Davenport JE, Harris RB. Effects of central or peripheral leptin administration on norepinephrine turnover in defined fat depots. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1613–1621. doi: 10.1152/ajpregu.00368.2006. [DOI] [PubMed] [Google Scholar]
- Possenti R, Muccioli G, Petrocchi P, Cero C, Cabassi A, Vulchanova L, Riedl MS, Manieri M, Frontini A, Giordano A, et al. Characterization of a novel peripheral pro-lipolytic mechanism in mice: role of VGF-derived peptide TLQP-21. Biochem J. 2012;441:511–522. doi: 10.1042/BJ20111165. [DOI] [PubMed] [Google Scholar]
- Raffin-Sanson ML, de Keyzer Y, Bertagna X. Proopiomelanocortin, a polypeptide precursor with multiple functions: from physiology to pathological conditions. Eur J Endocrinol. 2003;149:79–90. doi: 10.1530/eje.0.1490079. [DOI] [PubMed] [Google Scholar]
- Salton SR, Ferri GL, Hahm S, Snyder SE, Wilson AJ, Possenti R, Levi A. VGF: a novel role for this neuronal and neuroendocrine polypeptide in the regulation of energy balance. Front Neuroendocrinol. 2000;21:199–219. doi: 10.1006/frne.2000.0199. [DOI] [PubMed] [Google Scholar]
- Scherer T, O’Hare J, Diggs-Andrews K, Schweiger M, Cheng B, Lindtner C, Zielinski E, Vempati P, Su K, Dighe S, et al. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 2011;13:183–194. doi: 10.1016/j.cmet.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CK, Jackson RM, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1467–1476. doi: 10.1152/ajpregu.00348.2005. [DOI] [PubMed] [Google Scholar]
- Souza SC, Muliro KV, Liscum L, Lien P, Yamamoto MT, Schaffer JE, Dallal GE, Wang X, Kraemer FB, Obin M, et al. Modulation of hormone-sensitive lipase and protein kinase A-mediated lipolysis by perilipin A in an adenoviral reconstituted system. J Biol Chem. 2002;277:8267–8272. doi: 10.1074/jbc.M108329200. [DOI] [PubMed] [Google Scholar]
- Subramanian V, Rothenberg A, Gomez C, Cohen AW, Garcia A, Bhattacharyya S, Shapiro L, Dolios G, Wang R, Lisanti MP, et al. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3-L1 adipocytes. J Biol Chem. 2004;279:42062–42071. doi: 10.1074/jbc.M407462200. [DOI] [PubMed] [Google Scholar]
- Toshinai K, Yamaguchi H, Kageyama H, Matsuo T, Koshinaka K, Sasaki K, Shioda S, Minamino N, Nakazato M. Neuroendocrine regulatory peptide-2 regulates feeding behavior via the orexin system in the hypothalamus. Am J Physiol Endocrinol Metab. 2010;299:E394–E401. doi: 10.1152/ajpendo.00768.2009. [DOI] [PubMed] [Google Scholar]
- Tsukahara F, Uchida Y, Ohba K, Ogawa A, Yoshioka T, Muraki T. The effect of acute cold exposure and norepinephrine on uncoupling protein gene expression in brown adipose tissue of monosodium glutamate- obese mice. Jpn J Pharmacol. 1998;77:247–249. doi: 10.1254/jjp.77.247. [DOI] [PubMed] [Google Scholar]
- Valenzuela DM, Murphy AJ, Frendewey D, Gale NW, Economides AN, Auerbach W, Poueymirou WT, Adams NC, Rojas J, Yasenchak J, et al. High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat Biotechnol. 2003;21:652–659. doi: 10.1038/nbt822. [DOI] [PubMed] [Google Scholar]
- Voss-Andreae A, Murphy JG, Ellacott KL, Stuart RC, Nillni EA, Cone RD, Fan W. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology. 2007;148:1550–1560. doi: 10.1210/en.2006-1389. [DOI] [PubMed] [Google Scholar]
- Watson E, Fargali S, Okamoto H, Sadahiro M, Gordon RE, Chakraborty T, Sleeman MW, Salton SR. Analysis of knockout mice suggests a role for VGF in the control of fat storage and energy expenditure. BMC Physiol. 2009;9:19. doi: 10.1186/1472-6793-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson E, Hahm S, Mizuno TM, Windsor J, Montgomery C, Scherer PE, Mobbs CV, Salton SR. VGF ablation blocks the development of hyperinsulinemia and hyperglycemia in several mouse models of obesity. Endocrinology. 2005;146:5151–5163. doi: 10.1210/en.2005-0588. [DOI] [PubMed] [Google Scholar]
- Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M, Mann DL, Taffet GE, Baldini A, Khoury DS, et al. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci U S A. 2006;103:17378–17383. doi: 10.1073/pnas.0604708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]