Abstract
Higher expression of reactive oxygen species (ROS) is implicated in neurological disorders. A major event in glaucoma, the death of retinal ganglion cells (RGCs), has been associated with elevated levels of glutamate and TNF-α in the RGCs’ local microenvironment. Herein we show that the transduction of Peroxiredoxin 6 (PRDX6) attenuates TNFα- and glutamate-induced RGC death, by limiting ROS and maintaining Ca2+ homeostasis. Immunohistochemical staining of rat retina disclosed the presence of PRDX6 in RGCs, and Western and real-time PCR analysis revealed an abundance of PRDX6 protein and mRNA. RGCs treated with glutamate and/or TNF-α displayed elevated levels of ROS and reduced expression of PRDX6, and underwent apoptosis. A supply of PRDX6 protected RGCs from glutamate and TNF-α induced cytotoxicity by reducing ROS level and NF-kB activation, and limiting increased intracellular Ca2+ influx. Results provide a rationale for use of PRDX6 for blocking ROS-mediated pathophysiology in glaucoma and other neuronal disorders.
Introduction
Reactive oxygen species (ROS) are produced intracellularly as byproducts of a variety of physiological processes in the microenvironment of cells, and can also be generated as a result of external environmental stresses. ROS-driven oxidative stress and inadequacy of antioxidant defense have important roles in initiation of diseases (Maier and Chan, 2002; Granot and Kohen, 2004). ROS-mediated insult to neuronal cells has been shown to be a cause of the development and progression of various neurodegenerative disorders, and there has been a correlation between antioxidant levels and neuronal function (Romero, 1996; Beal, 1998; Nunomura et al., 2001; Perry et al., 2003). Several lines of evidence indicate that oxidative damage and antioxidant responses lead to clinical and pathological manifestations in glaucoma (Kurysheva et al.,1996; McKinnon, 1997; Roth, 1997; Rose et al., 1998; Levin, 1999; Lieven et al., 2006).
Glaucoma, one of the leading causes of blindness in the world, is associated with selective death of retinal ganglion cells (RGCs). The disease is characterized by an elevation in intraocular pressure (IOP), which leads to increased glutamate and TNF-α levels (Tezel and Wax, 2000; Nucci et al., 2005; Okuno et al., 2006), both of which are producers of ROS (Moreno et al., 2004). The progressive loss of RGCs in glaucomatous patients has been suggested to result from long-term oxidative damage induced by ROS which were generated by higher levels of glutamate and TNF-α within the cellular microenvironment (Okuno et al., 2006). Although the underlying cause for this cell death remains unknown, the primary risk factor associated with glaucoma is an increase in IOP. Moreover, RGCs in animal studies and from patients with elevated IOP have been reported to contain high levels of metabolic products of lipid peroxidation, suggesting that oxidative stress plays a pivotal role in the pathophysiology of this disease (Muller et al., 1997; Bonne et al., 1998; Okuno et al., 2006; Tezel, 2006; Izzotti et al., 2006). Recently, ROS have gained attention because of their role in cellular signaling for various biomolecules such as glutamate (Aoun et al., 2003), cytokines such as TNF-α, and growth factors, such as PDGF and TGFβ, (Kim et al., 2006). TNF-α has been shown to cause RGC death following ischemia (Pahl and Baeuerle, 1994; Madigan et al., 1996; Fontaine et al., 2002; Tezel et al., 2004; Tezel and Yang, 2005). Moreover, recent evidence reveals that a high level of glutamate is toxic to and results in apoptosis of RGCs both in vivo if injected in vitreous, and in vitro when used as a treatment of cultured retinal neuronal cells (Aoun et al., 2003; Zhang et al., 2004; Guo et al., 2005; Guo et al., 2006). Glutamate is present in RGCs in very high concentrations and activates several types of cell receptors, including NMDA receptors, that can enhance intracellular levels of ROS and calcium concentrations (Chen and Diamond, 2002; Ullian et al., 2004) that may lead to inappropriate activation of the apoptotic program. The generation of ROS by these molecules has been associated with the activation and deactivation of several survival factors (Rhee, 1993). Studies in a variety of experimental systems have demonstrated that RelA containing NF-kB complex has an antiapoptotic effect (Barkett and Gilmore, 1999). In glaucoma, NF-kB is highly activated in RGCs and has been suggested to be proapoptotic (Kasibhatla et al., 1988; Schreck and Baeuerle, 1994; Pahl and Baeuerle, 1994; Kucharczak et al., 2003). Moreover, the levels of ROS are controlled by both their rate of production and their metabolism. To cope with deleterious factors which enhance levels of ROS, cells initiate expression and activation of various antioxidant enzymes including peroxiredoxins (Spector et al., 2001; Reddy et al., 2004; Fatma et al., 2005; Ma et al., 2006).
The peroxiredoxin (PRDX) family includes six known members (PRDX 1-6). Of particular interest is PRDX 6 cloned by our group from human lens epithelial cells cDNA library (Fatma et al., 2001). The PRDXs including PRDX6 use redox-active cysteine (Cys or C) to reduce peroxides (Kang et al., 1998a, b; Chae et al., 1994; Fatma et al., 2001 and 2005). PRDX6, a “moonlighting” protein, can protect cells from membrane, DNA, and protein damage mediated by lipid peroxidation (Manevich et al., 2002). Prdx6 from human, rat, bovine, and mouse tissues show 94% homology with conserved redox-active Cys47 residue, and mutation of this residue abolishes its protective activity (Kim et al., 1988; Leyens et al., 2003; Fatma et al., 2005). Advances in the field of gene/protein delivery with identification of several protein transduction domains (PTDs) have made it possible to deliver proteins to targeted cells or organs (Green and Loeewenstein, 1988; Frankel and Pabo, 1988). HIV-TAT domain has 11 amino acids (aa; YGRKKRRQRRR) and has been shown to hold 100% potential for intracellular delivery of proteins across the plasma membrane and the blood brain barrier, and has been found to be biologically active (Mann and Frankel, 1991; Rustani et al., 1997; Nagahara et al., 1998; Becker-Hapak et al., 2001). Taking advantage of the ability of the TAT transduction domain, we fused the PRDX6 cDNA with a gene fragment encoding the TAT protein transduction domain in a bacterial expression vector, pTAT-HA (a kind gift of Dr. Dowdy) to produce a genetic TAT-HA-PRDX6 fusion protein. Western and immunoctochemical analysis revealed that TAT-HA-PRDX6 efficiently internalized to RGCs. In the past several cell- culture based experiments have been conducted to disclose the biological protective function and mechanism of action(s) of chemical/biomolecules and have been found to show the same function in vivo (Ribeiro et al., 2003; Kwon et al., 2003; Plaisant et al., 2003; Eum et al., 2005; Kim et al., 2005). Moreover, most of the cell culture based experiments related to glutamate or TNF-α-induced toxicity have been conducted using primary cultures of different sources, which are usually heterogeneous. Therefore, we selected continuous cell lines, RGC-5 of neuronal origin (a kind gift of Dr. Agarwal) that respond to glutamate or TNF-α, these factors are implicated for RGC death, either in terms of cell death or physiological signals, to use as model system for the study of RGC death. Recently it has been demonstrated that RGC-5 cells respond to glutamate treatment with significant loss of viability (Maher and Hanneken, 2005), however, it requires higher concentration of glutamate but provides similar response as primary RGC, suggesting that this cell line may represent suitable model system to investigate some of mechanisms of glutamate or TNF-α induced-RGCs insults and its attenuation by the application of PRDX6.
Our current study has disclosed the presence of all six known PRDXs in the RGCs, with PRDX6 expressed at a higher level than the others. Hence, the present study was designed to characterize and document the TNF-α- and/or glutamate-induced ROS-driven oxidative damage to RGCs and the ability of PRDX6 in attenuation of death signaling produced by these two stressors. Also, this study describes the regulation and regulatory role of PRDX6 in RGCs facing ROS-induced oxidative stress. Collectively, finding should provide a foundation for rational use of antioxidant-based therapeutics for treating or preventing/delaying RGC death.
Results
PRDX6 was localized in cytoplasm in RGC-5 and level of PRDX6 was higher than those of other PRDX family members
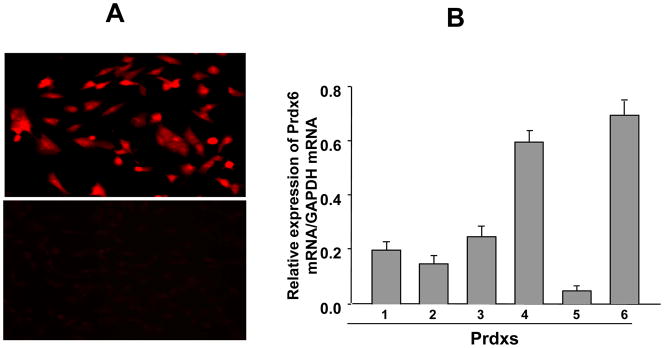
Because localization patterns and expression levels of biomolecules indicate their putative cellular function, we intended to determine the localization of PRDX6 in RGCs by using RGC-5 cells. Immunostaining with PRDX6-specific antibody resulted in cytoplasmic staining (Fig. 1, A, upper panel), while negative controls treated with neutralized PRDX6 antibody showed no staining, (Fig. 1, A, lower panel). We monitored expression levels of all six members of the Prdx family in RGC-5, because comparative expression levels would reflect their abundance, which in turn would indicate their specific importance. Real–time PCR for all six peroxiredoxins (Prdx 1-6) revealed greater abundance of PRDX6 mRNA in RGCs (Fig. 1, B), and the expression level of PRDX6 was higher than that of the other Prdxs. Thus, in the present study, we elected to explore the putative protective role of PRDX6 in RGC-5 facing TNF-α- and glutamate-induced oxidative insults. We recognize that the expression of Prdx4 level (a secretary protein) is also high and next to PRDX6, and may be involved in protecting RGCs against stressors, we will pursue that study in a separate project. However, in current study we selected to study PRDX6 since it is highly expressed in RGCs and RGC-5 and has glutathione peroxidase as well as aiPLA2 activities. Later is unique quality of PRDX6 that is not acquired by other PRDXs members.
Figure 1.
(A) Immuno- histochemical analysis of RGC-5 using PRDX6 antibody. Positive imunostaining was observed in the cytoplasm of cells (A, upper panel). While in the control cells stained with neutralized PRDX6 antibody, staining was not visible (A, lower panel). (B) Quantitative real time PCR showing differential expression of Prdx1-6 mRNA in normal RGCs. Total RNA was isolated and transcribed into cDNA. Real-time PCR was performed using specific primers (see Experimental Methods). mRNA expression of each Prdx was adjusted to the mRNA copies of GAPDH. Results indicate that mRNA expression level of PRDX6 was significantly high in comparison to other PRDXs. However, Prdx4 is also present at a significant level but could not provide protection against glutamate and/or TNF-α induced RGC death.
Immunohistochemical localization of PRDX6 in rat retina
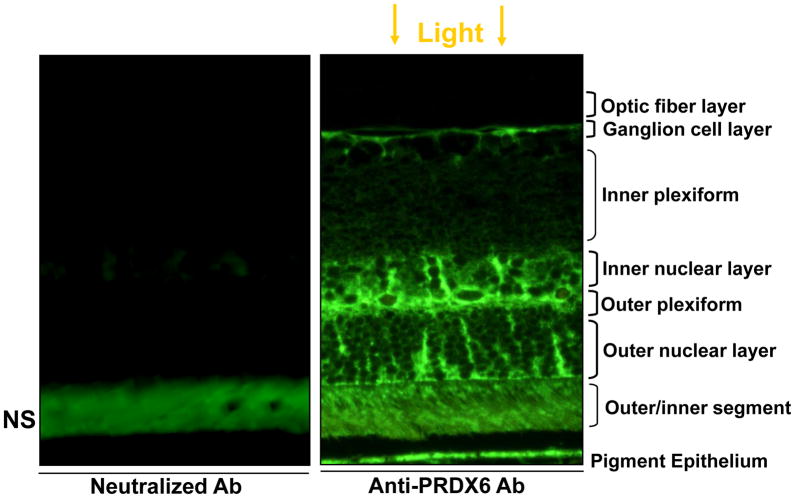
Although RGC-5 is derived from rat retina and did bear higher PRDX6 expression, we wished to know the expression pattern of PRDX6 in rat retina in situ. Results of immunohistochemical analysis are presented in Fig. 2 (paraffin section). The immunostaining pattern revealed that PRDX6 was localized in retinal pigment epithelium, outer and inner nuclear layers, outer plexiform, and ganglion cell layer. Spotted PRDX6 positive staining in the outer and inner nuclear layers suggested specific staining of certain cells. Although we did not identify the cell specificity, based on the literature (Jin et al., 2005) and localization pattern we surmised that PRDX6 may also be localized in the astrocytes/glia. However, further work is required to show the PRDX6 localization pattern in these layers to identify specific cell types. Our aim in the present study was to document whether PRDX6 linked to TAT domain could attenuate the glutamate or TNF-α induced death of RGC-5. Moreover, the RGC layer of retina was specifically stained with PRDX6 antibody, indicating that PRDX6 was indeed expressed in RGCs in vivo (Fig. 2 right panel). No staining was visible with neutralized antibody (Fig. 2 left panel), validating the specificity of localization pattern of PRDX6 and specificity of antibody. However, outer and inner segments also showed staining, but this staining pattern was also present when PRDX6 neutralized antibody was used, suggesting that staining of these regions was non-specific. A gap between RPE and the outer segment layer is artifact; during processing of the section they were separated from each other due to mechanical stretch.
Figure 2.
Immuno-histochemical localization of PRDX6 in rat retina. Rat eye was paraffin-embedded and sectioned after being fixed in 4% paraformaldehyde, and sections were immunostained using antibody specific to PRDX6. Right panel; Green color of positive immunostaining was observed. No staining was visible with neutralized Ab (left Panel) suggesting specificity of antibody.
Concentration and time-dependent toxicity of glutamate and/or TNF-α on RGC-5
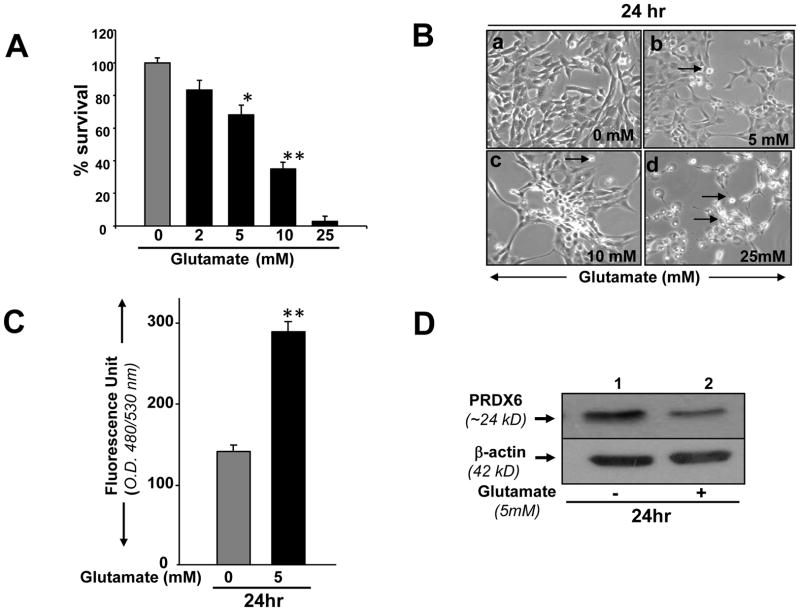
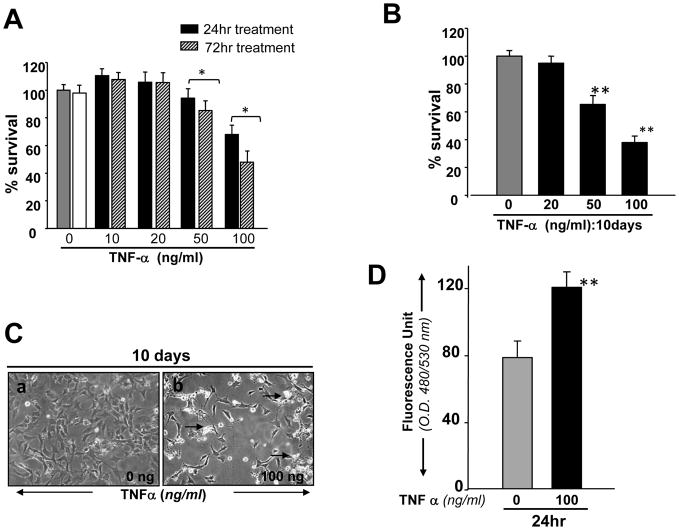
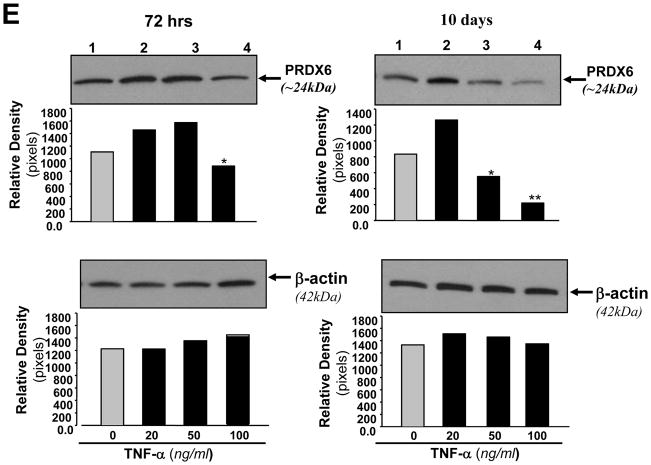
Glutamate and TNF-α have been implicated as causes of RGC death during glaucoma. We sought to examine whether the RGC-5 model system was vulnerable to glutamate and TNF-α toxicity. Using variable concentrations of glutamate and TNF-α to treat RGC-5 for variable times, we found that applying increasing concentrations of either caused a dose-dependent decrease in cell survival. Treatment with glutamate at 5 and 10 mM reduced the survival rates by ~30% and ~50%, respectively (Fig. 3 A). Treatment with 25 mM of glutamate was even more toxic to RGCs, and 95% of cells were dead when examined at 24hr (Fig. 3 A). These cells were photomicrographed and recorded (Fig. 3 B, a, b, c and d). Notably, Cellular effects produced by TNF-α seem to be largely associated with its concentration as well as the duration of its exposure to cells. Cell viability assay showed the inhibition of cell growth and cell survival with treatment involving high dose of TNF-α (100ng/ml) for 24hr and cell death increased with increasing exposure time, whereas lower doses of TNF-α (10 and 20ng/ml) enhanced the cell growth up to observation period of 72hr (Fig., 4 A) as well as 10 days (data not shown) compared to control (there was significant death of untreated RGCs, but TNF-α-induced was significantly higher). In contrast, RGC-5 exposed to TNF-α at higher concentration (50 or 100 ng/ml) for 10 days displayed significant inhibition of cell growth and increased cell death (Fig. 4, B) and these cells were photomicrographed, and Fig. 4 C is representative of the experiment. Based on these results, we decided to use 5 mM glutamate and 50 or 100 ng/ml TNF-α to induce toxicity and for the evaluation of the protective efficacy of PRDX6 against these molecules.
Figure 3.
(A) MTS assay showing effect of Glutamate on viability of RGC-5. Cells were cultured in 24-well plate in DMEM containing 10% FBS. After 24 hrs, cells were washed and treated with different concentrations (black bars 2, 5, 10 and 25 mM) of glutamate for a period of 24hr. Results are means ± SD of three individual experiments. *p< 0.05, **p< 0.001. (B) Photomicrograph of RGCs with or without treatment of glutamate (B: b, c, d) showing significant cell death compared to control (B; a). Arrow heads denotes dead cells. (C) RGC-5 exposed to glutamate displayed higher levels of ROS (3 C, black bar). Intracellular ROS levels were measured as described in Experimental Methods. (D) Western analysis showing reduced expression of PRDX6 protein in RGC-5 treated with glutamate. Cells were cultured in 6-well plates, and these cells were treated with glutamate at 5 mM. 24hr later culture was terminated, cell extracts were prepared for Western analysis. A significant diminution of PRDX6 protein level was observed (D, lane 2), while no change was detected in the expression of β-actin level, suggesting glutamate specifically reduced the expression of PRDX6.
Figure 4.
(A and B) Concentration and time–dependent effect of TNF-α on viability of RGC-5. Cells were cultured with or without TNF-α at different concentrations for variable time periods, and cell viability was assessed by MTS assay. RGCs were treated with different concentrations of TNF-α for 24hr (A, black bar), 72hr (A, stripped bar) and for 10 days (Fig, 4 B: black bar). (C) Cells were culture and exposed to TNF-α for 10 days. Cells were photomicrographed and (C, a and b) are representative of the experiment. (D) Histogram showing effect of TNF-α on the expression of intracellular ROS in RGCs, Cells were treated with TNF-α (100ng/ml) for 24 hr and ROS were measured with H2-DCFH-DA dye. OD, optical dencity. Results are means ± SD of 3 experiments. *p<0.05, **p <0.001 vs. control. (E) Western analysis showing modulation of PRDX6 protein expression in RGCs treated with different concentrations of TNF-α for variable time periods. Cells were treated with different concentrations of TNF-α for 24, 48, 72hr and 10 days. Cell extracts were prepared and Western analysis was carried out as described in Experimental Methods. Upper, left panel (72 hr): Lane 1, untreated control; Lane2, 20ng/ml; lane3, 50ng/ml; Lane 4, 100ng/ml. Upper, right panel (10 days): The same concentrations of TNF-α were used as described in upper left panel (72hr). Results are derived from 3 different cell preparations. No change was observed in β-actin expression level (lower left and lower right panels; β-actin band and black bars), suggesting that modulation in expression of PRDX6 level is specific. Histograms show relative density of protein bands. *p<0.05 and **P<0.001.
RGC-5 exposed to glutamate and TNF-α showed reduced expression of PRDX6 and elevated levels of ROS
Because TNF-α and glutamate induce the generation of ROS and thereby produces cell injury by altering survival signaling to deleterious signaling (Tezel and Wax, 2000; Nucci et al., 2005; Okuno et al., 2006) and PRDX6 protein provides cytoprotection against ROS-induced oxidative stress, whether generated externally or internally by such physiological stressors as growth factor/cytokines, we examined the levels of both ROS and PRDX6 in RGC-5 treated with TNF-α or glutamate using H2DCFH-DA assay and Western analysis, respectively. Results demonstrated that ROS level was significantly higher (Fig. 3, C and 4, D) and expression of PRDX6 protein (Fig. 3, D) was reduced in RGC-5 exposed to glutamate for 24hr. Surprisingly, lower doses of TNF-α (10, 20 or 50ng/ml) for 24 or 48 (data not shown) or 72hr upregulated the expression of PRDX6 in RGC-5 (Fig. 4 E, upper panel), and did not significantly alter ROS levels (data not shown). Higher doses of TNF-α significantly downregulated expression of PRDX6 in RGC-5 exposed for 72hr and longer, and these cells displayed elevated levels of ROS (Fig. 4 D). These results suggest that in acute stress induced by TNF-α (50 or 100ng/ml for 24hr, data not shown) or in presence of sublethal doses for longer time (Fig. 4 E, 10 days; 20ng/ml), PRDX6 is upregulated to counteract TNF-α induced cellular damage, but if stress is continued for a longer time (Fig. 4 E, 10days; 50 or 100ng/ml); chronic stress that may occur during the progression of glaucoma, PRDX6 expression is attenuated. The finding indicates that PRDX6 expression level plays an important role in survival of RGCs. Therefore, we decided to examine whether an extrinsic supply of PRDX6 to the cells would show protective ability against damage induced by TNF-α and glutamate.
TAT-HA-PRDX6 fusion protein was transduced into cells in a concentration-dependent manner
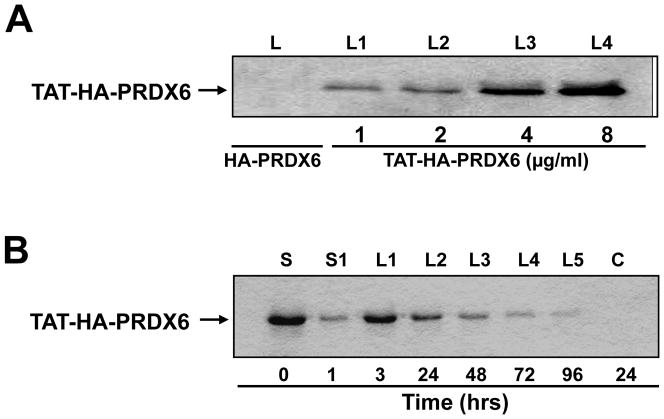
From the above experiments, we believed that a reduced level of PRDX6 might be a cause of higher ROS levels that in turn could induce RGCs death. To provide an extrinsic supply of PRDX6 to RGCs, we prepared TAT transduction linked PRDX6, and evaluated the transduction potential of recombinant TAT-HA-PRDX6 protein in RGC-5 as described in Materials and Methods (Kubo et al., 2008). To this end, we cultured RGC-5 in 6 well plates, and after 24hr supplied these cells with TAT-HA-PRDX6 protein (1μg, 2μg, 4μg and 8μg/ml) by adding it to the culture medium. After 3hr cells were thoroughly washed several times, and protein was extracted and used for Western analysis. We used anti-His HRP antibody (Invitrogen) to monitor the internalization of TAT-HA-PRDX6 in cells. Western analysis assay disclosed that TAT-HA-PRDX6 could be efficiently transduced into the cells in a concentration-dependent manner (Fig. 5 A) as reported by others (Becker-Hapak et al., 2001). Next we examined the duration of retention of TAT-HA-PRDX6 in these cells. We added 5 μg/ml protein in culture media; protein was extracted after 1, 3, 24, 48, 72 and 96hr; and Western analysis was performed. We found that recombinant protein was internalized and could be detected inside the cells after 30 minutes (data not shown), and it was retained in the cells at detectable levels up to 96hr (Fig. 5 B, Lanes L1–L5). We did not detect any band when HA-PRDX6 only (without TAT) was added to the culture media (Fig. 5 A, Lane L(s) and Fig. 5 B, C).
Figure 5.
(A) Concentration dependent transduction of TAT-HA-PRDX6. Recombinant protein was added to culture media and transduction of TAT-HA-PRDX6 was assessed after 24 hr. Cells were washed, protein was extracted and used for Western analysis using anti-His HRP antibody. Lanes, L1–L4: cell lysates from the cells cultured with 1, 2, 4, 8 μg/ml protein, and lane L denotes the lysate extracted from cells cultured with HA-PRDX6 only. (B) Western analysis showing stability of TAT-HA-PRDX6 transduced into RGCs. Lane, S: culture media just after addition of recombinant protein (0hr); lane, S1: Culture supernatant after 1 hr; Lanes, L1 to L5: Cell lysate after 1, 3, 24, 48, 72 and 96 hrs; C: Control (HA-PRDX6 without TAT). Results revealed the intracellular transduction of TAT-HA-PRDX6 where as (HA)-PRDX6 with flag tag (HA) only could not internalize into cells (lane C).
TAT-HA-PRDX6 attenuated glutamate- or TNF-α- or their synergistically-induced RGC death
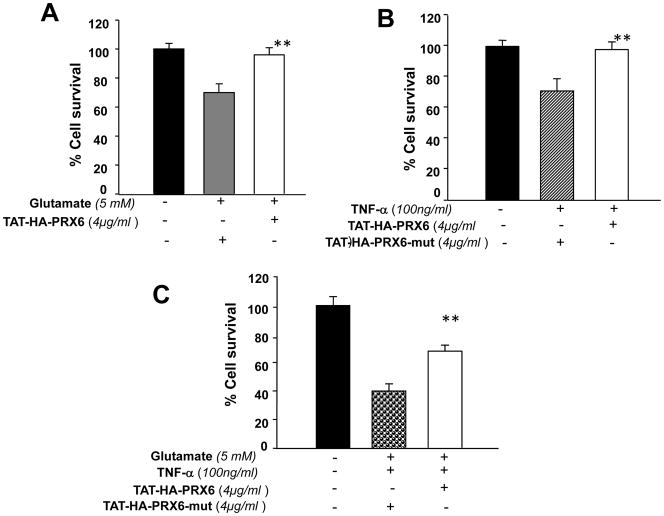
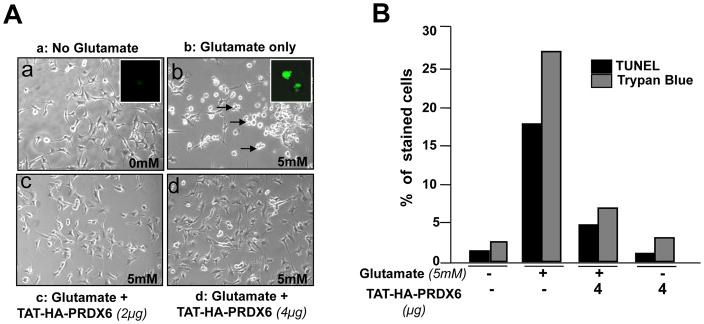
We sought to determine the efficiency and functionality of PRDX6 linked to TAT transduction domain in attenuating gluatamate- or TNF-α- or their synergistically-induced RGC toxicity. At the first step we used variable concentrations of TAT-HA-PRDX6 (2, 4 and 8 μg/ml) to assess their optimum concentration showing highest protection against these two stressors (data not shown). To this end, 4μg/ml PRDX6 was found to optimum concentration to nullify the toxic effects of glutamate and TNF-α. While PRDX6 supplied at 8μg/ml concentration was found to be toxic to cells (data not shown). Thus, we supplied TAT-HA-PRDX6 (4μg/ml) or mutant TAT-HA-PRDX6 (Cystine (C)47 to Isoleucine (I)47) to RGC-5 cultured in vitro. After 3hr, these pretreated cells were exposed to glutamate (Fig. 6 A, 5mM for 24hr) or TNF-α (Fig. 6 B, 100ng/ml for 24hr) or their combination (Fig. 6 C). Survival assay (MTS assay) was performed; cell viability was determined and expressed as the percent cell survival (Fig. 6 A, B and C). Results indicate that cell death occurred when glutamate or TNF-α or their combinations was added (Fig. 6 A, gray bar B, stripped bar and C, dotted Bar), but cells survived well in the presence of TAT-HA-PRDX6 protein (4 μg/ml) (Fig. 6 A, B and C, white bars). Fig. 7A is a representative of the experiment photographs of RGCs cultured with and without glutamate or glutamate plus TAT-HA-PRDX6. Similarly, results of TNF-α, and combination of glutamate and TNF-α without TAT-HA-PRDX6 were photomicrographed and recorded (data not shown). Next, we determined the type of cell death, whether necrosis or apoptosis, by performing TUNEL and trypan blue assays. Results (Fig. 7 B) demonstrated that the percentage of TUNEL-positive and trypan blue stained cells was significantly high following glutamate treatment, but TAT-HA-PRDX6 transduced cells showed remarkably reduced cell death (apoptosis or necrosis) after glutamate exposure. Cells exposed to TNF-α underwent apoptosis only (data not shown).
Figure 6.
MTS assay showing influence of TAT-HA-PRDX6 in protecting RGC-5 against glutamate (A)- or TNF-α (B)- or their synergistically-induced cytotoxicity (C). Cells were seeded (10K cells/well) in 24 well plates containing complete DMEM. An overnight culture of these cells were subjected to varying concentrations of TAT linked PRDX6. Three hours later cells were exposed to glutamate (A) or TNF-α (B) or glutamate plus TNF-α. After 24hr, cell viability was estimated using MTS assay. Protective ability of TAT-HA-PRDX6 is evident (white bars). Results are presented in percent survival. While TAT-HA-PRDX6 failed to attenuate glutamate, TNF-α and/or their synergistically-induced cytotoxic activity against RGC-5 (Fig. 6, A: gray bar; B: stripped bar; C: dotted bar. Results are means ± SD of three individual experiments. **; p< 0.001
Figure 7.
(A) Photomicrograph of RGCs showing protective effect of transduced TAT-HA-PRDX6 against glutamate. RGCs were cultured over-night. Next day, cells were supplied with TAT-HA-PRDX6. 3hr later these cells were exposed to 5mM glutamate. A: a, without glutamate; b, with glutamate; c and d, glutamate plus TAT-HA-PRDX6. Arrow heads indicate dead cells. Insets: glutamate induced apoptosis (b). (B) TUNEL and Trypan blue staining of glutamate-induced RGCs death. Results disclosed that a supply of PRDX6 can spare RGC-5 from glutamate-induced apoptotic and/or necrotic cell death (TUNEL: black bars, Trypan blue, necrosis: gray bars). Results are means ± SD of three individual experiments.
TAT-HA-PRDX6 protected RGC-5 by limiting glutamate- and TNF-α-induced intracellular ROS level
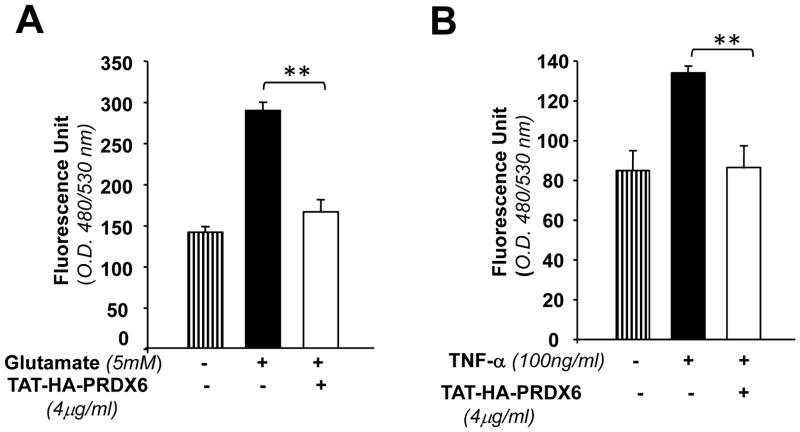
Higher levels of ROS are toxic to cells, while lower non-toxic levels are beneficial and thereby play a part in cellular survival signaling (Hancock et al., 2001; Wood et al., 2003). We envisaged that PRDX6, having GSH peroxidase activity, might exert its protective effect by limiting the levels of intracellular ROS induced by TNF-α and glutamate as shown in Figs. 3C and 4D. We delivered PRDX6 linked to TAT to RGC-5 cultured in vitro as described in Material and Methods. We measured the levels of ROS in PRDX6-treated RGCs following glutamate or TNF-α treatment. As expected, delivery of PRDX6 to RGCs was able to reduce the level of glutamate or TNF-α induced intracellular ROS (Fig. 8 A and B). Interestingly, the results also disclosed that cells exposed to glutamate displayed higher “Fluorescence Unit” demonstrating that glutamate is more potent in generating ROS driven oxidative stress that lead more RGC-5 death in comparison to TNF-α.
Figure 8.
TAT-HA-PRDX6 attenuates glutamate-and/or TNF-α-induced elevated levels of intracellular ROS, one of the causative factors of deleterious signaling. Cells were treated with TAT-HA-PRDX6 (4ug/ml). 3hr later glutamate (5 mM) (A) or TNF-α (100 ng/ml) (B) were added in culture. ROS was measured at different time periods using H2-DCFH-DA as described in materials and methods. Results showing that a supply of 4μg TAT-HA-PRDX6 to RGCs could attenuate Glutamate- or TNF-α-induced over production of ROS Results are a representative of the experiments. Results also disclosed that RGC-5 exposed to glutamate generates more ROS than TNF-α (compare Fluorescence Unit Fig. 8 A and B). Results are means ± SD of 3 individual experiments. **p <0.001 vs. control.
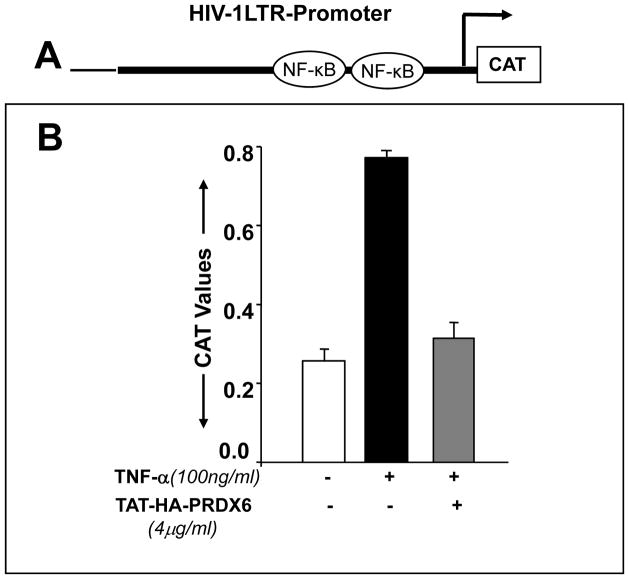
Supply of PRDX6 reduced TNF-α mediated over-activation of NF-κB
To test whether PRDX6 administration attenuates NF-kB activation in RGC-5 following TNF-α treatment, we used transactivation assay in RGC-5 using pLTR-CAT construct (EcoRI) (Kretz-Remy et al., 2001), which consists of binding sites for NF-κB (Fig. 9 A). We found that a remarkable increase in the promoter activity following treatment with TNF-α could be inhibited by a supply of TAT-HA-PRDX6 (Fig. 9 B), suggesting that PRDX6 has the ability to reverse NF-kB mediated adverse signaling in RGCs. In a parallel transactivation experiments with mutated construct pLTR-CAT (PstI) (Kretz-Remy et al., 2001) where NF-κB binding sites are disrupted (data not shown), activity was not observed, validating the earlier result. We believe that the overstimulation in promoter activity was due to TNF-α induced ROS-driven oxidative stress. These results suggests that overstimulation of NF-kB, one of causes of RGC death during progression of glaucoma, can be attenuated by supplying PRDX6.
Figure 9.
Extrinsic supply of TAT-HA-PRDX6 down-regulates NF-kB-dependent transcriptional activation of HIV-1LTR in RGC-5 exposed to TNF-α. RGC-5 were transiently transfected with HIV-1LTR-CAT construct (A) (see Experimental Methods). Cells were transduced with TAT-HA-PRDX6 and after 3 hrs and treated with TNF-α (100 ng/ml). Transactivation assay was performed after 72 hrs (B). Results indicate that NF-κB was up-regulated following TNF-α treatment (black bar) but it was down-regulated when PRDX6 was supplied to cells (gray bar).
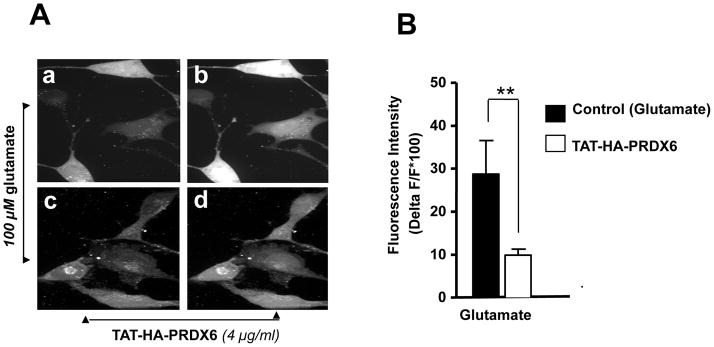
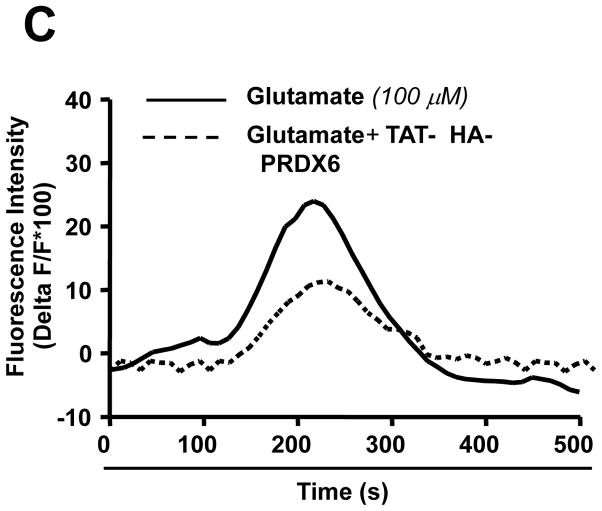
PRDX6 inhibited Ca2+ influx into RGC-5 following glutamate treatment
Glutamate-induced Ca2+ influx and oxidative stress are known to be involved in selective death of RGC in glaucoma. Based on our results showing glutamate-induced reduced expression of PRDX6 and higher ROS levels in RGCs (Figs. 3 and 4), we surmised that permeability of plasma membrane would be altered, and thereby Ca2+ influx would increase (Farber et al., 1990; Orrenious et al., 1992; Hallestrap et al., 1993). PRDX6 has acidic Ca2+ independent phopholipase activity that provides membrane protection (Manevich et al., 2005). To test this, we measured calcium influx in RGCs treated or untreated with TAT-HA-PRDX6 using the membrane-permeable fluorescent calcium indicator dye fluo-4 AM, as described in Materials and Methods. Time series images were made by collecting fluorescence images at a rate of 0.5 second. Analysis of confocal image files showed that RGCs transduced with TAT-HA PRDX6 followed by glutamate treatment showed less Ca2+ mediated fluorescence (Fig. 10 A, c and d) compared to cells pretreated with mutant TAT-HA-PRDX6 (Fig. 10 A, a and b). Results are presented in a histogram (Fig. 10 B). The relative increase in fluorescence was calculated by dividing the pixel intensities of the image during stimulation by the pixel intensities of the control image before stimulation (Fig. 10 C). These results show TAT-HA-PRDX6 mediated protection against glutamate-induced RGC death through the novel mechanism of limiting intracellular ROS levels and thereby maintaining Ca2+ homeostasis.
Figure 10.
PRDX6 inhibits Ca2+ influx into RGC-5 following glutamate treatment. Intracellular Ca2+ level was measured using the cell permeable Fluo-4 AM, fluorescent calcium indicator dye. Cells were cultured on cover slips in DMEM medium with TAT-HA-PRDX6 or mutant PRDX6 protein followed by glutamate treatment. (A) Fluorescent images were taken before and during application of 100 μM glutamate without (a, b) or with 4 μg/ml TAT-HA-PRDX6 (c, d). Pretreatment with TAT-HA-PRDX6 significantly reduced the increase in Fluo-4 fluorescence produced by application of 100 μM glutamate indicating TAT-HA-PRDX6 controls the glutamate-induced increase in Ca2+. (B) Histogram showing fluorescent intensity of the cells treated with glutamate in presence (empty bar, **p <0.001 vs. control) or absence of TAT-HA-PRDX6 (black bar). (C) Graph showing fluorescent intensity of cells at different time points (seconds).
Discussion
Oxidative stress driven by reactive oxygen species (ROS) and reduced expression of antioxidant molecules have been implicated in the development of neurodegenerative diseases, including glaucoma. When ROS exceed the antioxidant capacity, the accumulated ROS-induced oxidative modifications of proteins, lipids, and DNA cause various neurodegenerative processes. We believe that the progressive loss of RGCs in patients with glaucoma is associated with the long-term effects of oxidative damage induced by ROS that are locally generated by higher levels of glutamate and TNF-α within the cellular microenvironment. In our research, RGC-5 exposed to TNF-α and/or glutamate in vitro showed higher levels of ROS and reduced expression of PRDX6, and underwent spontaneous apoptosis (Fig. 3, 4 and 7). In vivo studies in humans and animals have demonstrated that TNF-α and glutamate released from glial cells and dead RGCs, respectively, are responsible for RGC death (Garcia-Valenzuele et al., 1995; Quigley et al., 1995; Yuan and Neufeld, 2003; Guo et al., 2006). In the present study, we observed the increased expression of PRDX6 in the presence of lower, sublethal doses of TNF-α (Fig. 4) and noted that, after withdrawal of TNF-α, these cells grew better in DMEM containing 5% FBS than did untreated controls (Data not shown). In contrast, RGCs exposed to TNF-α (100 ng/ml) for longer periods showed reduced expression of PRDX6 and increased cell death (Fig. 4). Importantly, at every concentration tested, glutamate suppressed the expression level of PRDX6 in RGCs. Thus our results demonstrate a novel mechanism of TNF-α regulation of PRDX6, in which concentration and time of exposure of RGC-5 to TNF-α play a pivotal role in determining the fate of the RGCs, which is associated with the expression of PRDX6. Also, our present work has demonstrated that TNF-α- or glutamate-induced elevated levels of ROS in RGCs is the major cause of cell death, and that the increase of ROS is eliminated by a supply of PRDX6. As other PRDXs did not counteract the changes in RGCs, we consider the role of PRDX6 to be major in those cells. Furthermore, we found that acute stress induced by TNF-α is beneficial to RGCs, and TNF-α confers this benefit by upregulating the level of PRDX6 as an adaptive control mechanism that attenuates the extent of cellular ROS (Fig 4). In light of our findings, it is likely that RGC death during glaucoma may be a cumulative effect of TNF-α and glutamate, both of which produce ROS-driven oxidative damage (Duchen, 2000; Aoun et al., 2003; Kaltschmidt et al., 2005; Henshall and Simon, 2005; Parfenova et al, 2006). Furthermore, elevated levels of ROS have been observed in rabbit retina during ischemia induced by high IOP, and there is evidence of elevated glutamate and TNF-α during ischemia and elevated IOP (Tezel and Wax, 2000; Nucci et al., 2005). We recently reported that LECs deficient in PRDX6 bear higher levels of ROS, are vulnerable to oxidative stress, and undergo spontaneous apoptosis (Fatma et al., 2005). Collectively, our results suggest that the reduced expression of PRDX6 in RGCs exposed to glutamate and/or TNF-α may be one cause of RGC death. Moreover, ROS-driven oxidative stress has been related, to varying extents, to a number of diseases and disorders. In fact, it is possible that most pathologies involve oxidative stress, at least some extent, and this may occur due to suppression of antioxidant such as PRDX6. Our present work has demonstrated that TNF-α and/or glutamate could suppress PRDX6 expression that in turn led to RGCs death. Moreover, identification of genes or their products involved in etiology of oxidant-mediated pathology has already led to important insights into the cellular response to stress and mechanisms of oxidant damage and will continue to do so as more and more involved genes are uncovered. These genes also represent potential clinical targets for oxidant related disease and disorders. In present study, we found hyperactivation NF-kB, a probable cause of RGC death. This activation of NF-kB could be reversed by a supply of PRDX6, suggesting death signaling initiated by oxidative stress could be prevented by PRDX6, a “moonlighting” protein having GSH peroxidase and acidic Ca 2+- independent phospholipase A2 (aiPLA2) activities.
Moreover, PRDX6 protects cells by removing ROS (Lim et al., 1993; Kim et al., 2000; Pak et al., 2002; Fatma et al., 2005). Our results show that supplying PRDX6 to RGCs engenders resistance against TNF-α- and glutamate-induced cell damage, by limiting ROS-driven oxidative damage. Several lines of evidence indicate that PRDX6 confers this resistance by attenuating ROS-mediated cellular damage (Fatma et al., 2005; Kubo et al., 2004 and 2008). Because lower doses of TNF-α enhance the expression of PRDX6, we believe that TNF-α mediated elevation of PRDX6 is physiologically important in maintaining normal physiological function of cells. Earlier reports by our group and others have demonstrated that cells exposed to lower doses of TNF-α display elevated PRDX6 expression (Kubo et al, 2006, Gallagher and Phelan, 2007). However, sensitive cells can be made resistant to TNF-α challenge by prior exposure to sublethal concentrations of TNF-α (Planck et al., 1994; Dicktion et al., 1995; Karsan et al., 1996; Mehlen et al., 1995). Also, cells overexpressing several protective genes show significant resistance against TNF-α toxicity (Planck et al., 1994; Dicktion et al, 1995; Mehlen et al., 1995; Karsan et al., 1996). These findings imply that, under certain conditions, TNF-α leads to the induction of genes that provide cellular protection. We observed higher expression of PRDX6 in these cells, and found that they grew even better than control cells (untreated with TNF-α). Nevertheless, it is unlikely that PRDX6 alone causes the overall increase in cell survival, because survival may also be associated with other proteins, HSP27, or αB-crystallin, a negative regulator of death signaling (Mehlen. et al., 1995).
Furthermore, glutamate is the main excitatory neurotransmitter and is present in RGCs in very high concentrations. High glutamate concentration activates several types of cell receptors, including NMDA receptors that can enhance intracellular levels of ROS as well as Ca2+ influx that may lead to inappropriate activation of the apoptotic program (Aoun et al., 2003; Zhang et al., 2004; Guo et al., 2005; Guo et al., 2006). Our results showed that RGC-5 exposed to glutamate revealed higher levels of ROS as well as Ca2+, and these cells underwent spontaneous apoptosis. Accumulating evidence shows that oxidative stress induced by glutamate causes an increase of cytosolic calcium concentration. This may occur because elevated expression of ROS can change the permeability of cell membrane, and disturbance of Ca2+ has been associated with cell death (Farber et al., 1990; Orrenious et al., 1992; Halestrap et al., 1993). PRDX6 is a bifunctional protein with glutathione peroxidase and aiPLA2 (acidic Ca2+-independent phospholipase A2) activities, and thus protects membrane from oxidative damage (Manevich et al., 2005). We found that RGC-5 pretreated with PRDX6 could maintain Ca2+ homeostasis, a novel biological function of PRDX6. However, further work is required to more fully explore this role of PRDX6. Our study found that RGC-5 treated with glutamate underwent apoptosis as well as necrosis. (Fig. 7). Necrosis typically follows severe disruptions in Ca2+ homeostasis, characterized by large increases in intracellular Ca2+ concentration, while apoptosis may be caused by smaller increases. We used higher than normal physiological concentrations of glutamate to induce toxicity in RGC-5 because RGC-5 appears to require higher concentrations before displaying features similar to those of primary RGCs. This resistance of RGCs to oxidants is associated with higher expression of antioxidants in these cells (Maher and Hanneken, 2005).
Furthermore, the generation of ROS has been associated with the activation and deactivation of the transcriptional protein NF-kB (Pahl and Baeuerle, 1994; Kaltschmidt et al., 2005; Mattson and Meffert, 2006). The modulation in the activity of NF-kB in neuronal cells is strongly associated with cellular fate (Mattson, 2006), and NF-kB can have an antiapoptotic (Cheng et al., 1994; Mattson, 1997; Tamatani et al., 1999; Marchetti et al., 2004) as well as a proapoptotic function, depending upon cell type or cellular microenvironment (Grilli et al., 1996; Clemens et al., 1997; Schneider et al., 1999; Shou et al., 2000; Nurmi et al., 2004; Charles et al., 2005). Importantly, in RGCs, activation of NF-kB has been found to induce apoptotic signaling, and suppression of its activation significantly enhances the viability of RGCs (Charles et al., 2005). Our results also vividly demonstrate that addition of PRDX6 in RGCs attenuates NF-kB activation induced by TNF-α or glutamate (Fig. 9), suggesting that PRDX6 can block the NF-kB-induced death pathway in RGCs. Within the retina, the TNF-α gene may be subjected to autoregulation by activated NF-kB (Collart, 1990), and TNF-α may generate increased expression of ROS. On the other hand, we found that lower doses of TNF-α enhanced the expression of PRDX6 (Fig. 4), and these cells survived well. We believe the survival of RGCs is associated with repression of NF-kB activation due to TNF-α upregulation of PRDX6 in these cells during acute stress. However, hyperactivation or inadequate activation of NF-kB in RGCs may be disastrous (Bhakar et al., 2002). Thus modulation of NF-kB activation should be an important strategy for reducing cellular injury. Overall, it appears that RGC death induced by glutamate or TNF-α should be associated with hyperactivation of NF-kB as well as failure of calcium homeostasis due to higher levels of ROS during glaucoma. Interestingly, we found that TAT-HA-PRDX6 was transduced in RGCs, and protected the RGCs from TNF-α or glutamate induced oxidative stress (Fig. 5, 6, 7 and 8).
In conclusion, we have described a novel mechanism by which TNF-α and/or glutamate induce ROS-driven RGC death that occurs due to a feed-forward-process within the local microenvironment of RGCs during glaucoma. The process is driven by excessive release of glial TNF-α and glutamate from dead RGCs, which further enhances the ROS-driven oxidative stress. A supply of the antioxidant PRDX6 may attenuate RGC death by limiting intracellular ROS and inhibiting Ca2+ influx and NF-kB activation. The present characterization of the novel protective role of PRDX6 in attenuating toxicity to RGCs is an initial step toward understanding the molecular mechanism involved in RGC death during glaucoma. Further detailed studies will be needed to elucidate the mechanisms involved in the PRDX6-mediated protection of RGCs.
Experimental Procedures
Culture of the Retinal Ganglion Cell
RGC5 (a kind gift from Neeraj Agarwal, University of North Texas Health Science, Fort Worth, TX) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μl/ml streptomycin at 37°C with 5 % CO2. The cells reaching confluency, were trypsinized and subcultured using 1:20 split. RGCs of 5 to 7 passages were used to carry out the experiments.
Immunocytochemical localization of PRDX6 in RGC-5
RGC-5 cells were grown on chamber slides (Nalge Nunc International, Naperville, IL). Immunostaining was performed using PRDX6 specific antibody. Cells were fixed for 10 minutes in 4% paraformaldehyde, washed three times with PBS, and permeabilized with o.1% Triton-X-100 in PBS for 5 minutes. After blocking, cells were incubated with PRDX6 monoclonal antibody (Lab Frontier, Seoul) overnight at 4° followed by addition of anti-mouse Ig-TR (Texas Red conjugated) secondary antibody (SantaCruz Biotechnology). Cells were viewed by fluorescent microscope (Nikon, Eclipse TE 2000-U) and microphotographs were taken by MAGNAFIRE (a computer program). In control cells, neutralized antibody was added to validate the results (Fatma et al 2001, Kubo et al., 2006).
Immunohistochemical analysis of PRDX6 expression in rat retina
Immunohistochemistry was performed using the Tyramide Signal Amplification (TSA™) Kit (Molecular Probes Inc., Eugene, OR), following the manufacturer’s protocol. Briefly, retina were fixed in 4% paraformaldehyde in PBS, embedded in paraffin and sectioned at 4 μm. The specimens were incubated with blocking reagent and then exposed to the anti-PRDX6 rabbit polyclonal antibody (Lab Frontier, Seoul) overnight, followed by incubation in horseradish peroxidase-conjugated goat anti-rabbit IgG diluted to 1:100. Tyramide working solution was applied to the specimens for 10 min. Negative controls were incubated with PRDX6 protein-neutralized preparation.
Real-time PCR
To monitor the levels of Prdxs mRNA in mouse lens, total RNA was isolated using the single-step guanidine thiocynate/phenol/chloroform extraction method (Trizol Reagent;Invitrogen) and converted to cDNA using Supercsript II Rnase H− Reverse Transcriptase. Quantitative real-time PCR was performed with TaqMan Universal Master Mix (Applied Biosystems, Foster City, CA) in an ABI ® 7000 Sequence detector system (Applied Biosystems). We used primers specific for Prdx 1 43 (forward, 5′-ACACCCAAGAAACAAGGAGGATT-3′ and reverse, 5′-CAACGGGAAGATCGTTTATTGTTA-3′), Prdx2 (forward, 5′-AACGCGCAAATCGGAAAGT-3′ and reverse, 5′-AGTCCTCAGCA TGGTCGCTAA-3′), Prdx 3 (Forward, 5′-GGCCACATGAACATCACACTGT-3′ and reverse, 5′-CAAACTGGAACGCCTTTACCA-3′), Prdx 4 (Forward, 5′-TCCTGTTGCGGACCGAAT-3′ and reverse, 5′-GAAAGAAGCAGGTTGGGAGTGT-3′), Prdx 5 (Forward, 5′-GAAAGAAGCAGGTTGGGAGTGT-3′ and reverse, 5′-CCCAGGGACTCCAAACAAAA-3′), and Prdx 6 (Forward, 5′-TTGATGATAA GGG CAGGGAC-3′ and reverse, 5′-CTACCATCACGCTCTCTCC C-3′). PRDX6 and GAPDH primers were purchased from Custom TaqMan® Gene Expression Assays (Applied Biosystems). The comparative Ct method was used to calculate relative fold expression levels using the 7000 SDS ver1.1 RQ software (Applied Biosystems). The Cts of target genes were normalized to the levels of GAPDH as an endogenous control in each group (Fatma et al., 2005; Kubo et al., 2006).
Assay for intracellular redox state
Intracellular redox state levels were measured using the fluorescent dye, H2-DCFH-DA as described earlier (Fatma et al., 2005; Kubo et al., 2008). Briefly, cells were washed once with HBSS and incubated in the same buffer containing 5–10 μg of DCFH-DA for 30 min at 37° C. Intracellular fluorescence was detected with excitation at 485 nm and emission at 530 nm using Spectra Max Gemini EM (Molecular Devices, CA).
Cell survival assay (MTS and apoptotic cell assays)
A colorimetric MTS assay (Promega) was performed as described earlier (Fatma et al., 2005). This assay of cellular proliferation uses 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2 to 4-sulfophenyl)-2H-tetrazolium salt (MTS; Promega, Madison, MI, USA). Upon being added to medium containing viable cells, MTS is reduced to a water-soluble formazan salt. The OD490 nm value was measured after 4 hr with an ELISA reader.
A TUNEL assay was employed to assess and validate apoptotic cell death. TUNEL staining was performed using an in situ cell death detection kit, Fluorescein (Roche Diagnostics GmbH, Germany), following the company’s protocol. Briefly, cells were washed with PBS and fixed in freshly prepared 4% paraformaldehyde in PBS (pH 7.4), followed by incubation in permeabilization solution (0.1% Triton X-100, 0.1% sodium citrate) for 2 min on ice. Cells were rinsed twice with PBS, and incubated in a TUNEL reaction mixture for 60 min at 37°C in the dark. Cells were rinsed three times with PBS. After mounting, samples were microphotographed using a microscope (Nikon, ECLIPSE TE 300), and analyzed. To determine the total dead cells, other than only the apoptotic cells, RGCs were stained with trypan blue solution (0.4%), where non viable cells are stained with trypan blue which is normally excluded by the live cells.
Expression and purification of TAT-HA-PRDX6 fusion protein
A full length cDNA of PRDX6 was isolated from human lens epithelial cell cDNA library and cloned into TAT-HA prokaryotic expression vector and recombinant protein was purified as described earlier (Kubo et al., 2008). Briefly, the host E. coli BL21 (DE3) was transformed with pTAT-HA-Prdx6, and the selected colonies were cultured in a LB medium containing ampicillin at 37°C. After the cells had grown until OD600 = 0.5–1.0, IPTG was added to a concentration of 0.5 mM, and the incubation was continued for 4 hr. The cells were harvested in binding buffer (50 mM NaH2PO4, 0.5 M NaCl, 10 mM imidazole, pH 8.0) and sonicated. After centrifugation, supernatant containing TAT-HA-PRDX6 was immediately loaded onto a 2.5 ml Ni2+-nitrilotriacetic acid Sepharose column. After the column was washed with 10 volumes of a binding buffer and six volumes of a washing buffer (50 mM NaH2PO4, 0.5 M NaCl, 10 mM imidazole, pH 8.0), the fusion protein was eluted with an elution buffer (50 mM NaH2PO4, 0.5 M NaCl, 250 mM imidazole, pH 8.0) and dialyzed to remove immidazole. A batch of recombinant protein, TAT-HA-PRDX6 was passed through Detoxi-Gel™ Endotoxin Removing Gel column (Pierce, Product No. 20344) to remove endotoxin contamination, if any. The purified protein can be either used directly for protein transduction or aliquoted and stored frozen in 10% glycerol at −80° C for further use. Similarily, a mutant constructs of PRDX6 Cystine (C)47 to isoleucine (I) were prepared using Site Directed Mutagenesis (SDM) with defined complementary primers (5′-CTTTACCCCAGTGATAACCACAGAGCTTGGCAGAGC-3′).
Transduction of TAT-HA-PRDX6 fusion protein into RGCs
To test transduction efficiency of TAT-HA-PRDX6, RGC-5 were grown overnight on a six well plate and then different concentrations of fusion protein (1, 2, 4. and 8μg/ml) were added to the culture media. After incubation period of 30 min, 1, 3, 6, 24, 48, 72 and 96 hr. cells were washed and harvested for the preparation of cell extract. Western analysis was performed using anti-HisG HRP antibody (Invitrogen).
Western analysis
Cell lysates were prepared in ice-cold Radioimmune precipitation buffer (RIPA buffer), as described previously (Fatma et al., 2005). Equal amounts of protein samples were loaded onto a 10% SDS gel, blotted onto PVDF membrane, and immunostained with primary antibody; PRDX6 monoclonal antibody (1:1000) or β-actin antibody (Sigma) (1:2000) or anti-His antibody (Invitrogen) (1:5000). The membranes were further incubated with horseradish peroxidase-conjugated secondary antibodies (1:1500 dilution) following washing. Specific protein bands were visualized by incubating the membrane with luminol reagent (Santa Cruz Biotechnology) and exposing to film (X-OMAT; Eastman Kodak).
Effect of L-Glutamate and TNF-α or their combination on RGC
The RGC-5 cells were seeded at 5,000 or 10,000 cells/well in 24 well plates in DMEM containing 10% FBS. After 24 hr, cells were exposed to DMEM containing 0.2% BSA and L-glutamate at varying concentrations (2, 5, 10 and 25 mM) for different time. Cell viability was determined at 24 hr or onwards. Similarly, to examine the effect of TNF-α on RGC-5 cells, cells were plated (1 to 5×104/well) in twenty four-well plates (Falcon) or onto 35mm dishes for 24hr, washed twice, and replaced with DMEM with 0.2% BSA or 5% FBS and TNF-α (Pepro Tech) at different concentrations (10, 20, 50 and 100 ng/ml) for variable time (upto10 days). In parallel experiments, variable concentrations of TNF-α along with a fixed concentration of glutamate or vice-versa were used to assay the combinatorial effect of these molecules on cell viability. To determine the protective effect of TAT- HA-PRDX6, cells were pretreated with TAT- HA-PRDX6 (4μg/ml). Cells were photomicrographed and cell viability was determined using MTS assay at predefined time.
Determination of NF-κB activation using HIV-1LTR-CAT
HIV-1LTR-CAT constructs (a kind gift from Dr Carole Kretz-Remy, France) were used to transfect RGC-5. HIV-1 promoter contains binding sites for many transcriptional factors including NF-κB and can be up-regulated 12 to 150–fold following various stresses including oxidative stress (Kretz-Remy et al., 2001). We used pLTR-CATWT, pLTR-CAT EcoR1 (where two κB consensus sequences are mutated to perfect palendromic κB sites) and pLTR-CAT Pst1 (where NF-κB sites are disrupted). We transfected cells with these constructs and 24 hr later cells were treated with TNF-α at different concentrations. In another set of experiment, cells were transduced with TAT-HA-PRDX6 before treatment with TNF-α.
Measurement of intracellular Ca2+ concentration
Calcium was measured using the membrane-permeant fluorescent calcium indicator dye, fluo-4 AM. RGCs (5.0 × 103 cells) were plated on cover slips overnight, transduced with TAT- HA -PRDX6 (4ug) for 3 hr and treated with L-glutamate. For dye loading, cells were incubated in Ringer solution (Nacl 140mM, Kcl 5.0 mM, Cacl2 2.0 mM, Mgcl2 0.5 mM, HEPES 10 mM, Glucose 10 mM, pH 7.4) containing 10 uM Fluo-4 AM for 1h at 37°C. A laser scanning confocal system (UltraVIEW LCI; PerkinElmer life sciences, UK) attached to a micsoscope and equipped with a ion laser, was used to visualize Ca2+ mediated fluorescence in the RGCs. The excitation illumination was 488nm and emitted fluorescence was collected through a 515 nm long pass filter. Images were collected in standard confocal modes using a transmitted light detector. Time series images were made by collecting fluorescence images at a rate of 0.5 second. Confocal image files were analyzed by computer (software). The relative increase in fluorescence was calculated by dividing the pixel intensities of the image during stimulation by the pixel intensities of the control image before stimulation. The cells were allowed 5 minutes to return to the resting level.
Statistical Method
Data are presented as means ± S.D. of the indicated number of experiments. Data were analyzed by Student’s t-test when appropriate. A p value of <0.05 was defined as indicating a statistically significant difference.
Acknowledgments
Grants provided by the National Eye Institute, NIH (EY-13394) (to DPS) and Research for Preventing Blindness (R.P.B.) are gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoun P, Simpkins JW, Agarwal N. Role of PPAR-gamma ligands in neuroprotection against glutamate-induced cytotoxicity in retinal ganglion cells. Invest Ophthalmol Vis Sci. 2003;44(7):2999–3004. doi: 10.1167/iovs.02-1060. [DOI] [PubMed] [Google Scholar]
- Barkett M, Gilmore TD. Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene. 1999;18(49):6910–69124. doi: 10.1038/sj.onc.1203238. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondrial dysfunction in neurodegenerative diseases. Biochim Biophys Acta. 1998;13366:211–223. doi: 10.1016/s0005-2728(98)00114-5. [DOI] [PubMed] [Google Scholar]
- Becker-Hapak M, McAllister SS, Dowdy SF. TAT-mediated protein transduction into mammalian cells. Methods. 2001;24(3):247–256. doi: 10.1006/meth.2001.1186. [DOI] [PubMed] [Google Scholar]
- Bhakar AL, Tannis LL, Zeindler C, Russo MP, Jobin C, Park DS, et al. Constitutive nuclear factor-kappa B activity is required for central neuron survival. J Neurosci. 2002;22(19):8466–8475. doi: 10.1523/JNEUROSCI.22-19-08466.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonne C, Muller A, Villain M. Free radicals in retinal ischemia. Gen Pharmacol. 1998;30(3):275–280. doi: 10.1016/s0306-3623(97)00357-1. [DOI] [PubMed] [Google Scholar]
- Chae HZ, Robison K, Poole B, Church G, Storz G. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: Alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc Natl Acad Sci. 1994;9:7017–7021. doi: 10.1073/pnas.91.15.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles I, Khalyfa A, Kumar DM, Krishnamoorthy RR, Roque RS, Cooper N, Agarwal N. Serum deprivation induces apoptotic cell death of transformed rat retinal ganglion cells via mitochondrial signaling pathways. Invest Ophthalmol Vis Sci. 2005;46(4):1330–1338. doi: 10.1167/iovs.04-0363. [DOI] [PubMed] [Google Scholar]
- Chen S, Diamond JS. Synaptically released glutamate activates extrasynaptic NMDA receptors on cells in the ganglion cell layer of rat retina. J Neurosci. 2002;22(6):2165–2173. doi: 10.1523/JNEUROSCI.22-06-02165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B, Christakos S, Mattson MP. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron. 1994;12(1):139–153. doi: 10.1016/0896-6273(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Clemens JA, Stephenson DT, Smalstig EB, Dixon EP, Little SP. Global ischemia activates nuclear factor-kappa B in forebrain neurons of rats. Stroke. 1997;28(5):1073–1080. doi: 10.1161/01.str.28.5.1073. [DOI] [PubMed] [Google Scholar]
- Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol Cell Biol. 1990;10(4):1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson JL, Bates EJ, Ferrant A, Antalis TM. Plasminogen activator inhibitor type 2 inhibits tumor necrosis factor alpha-induced apoptosis. Evidence for an alternate biological function. J Biol Chem. 1995;270(46):27894–27904. doi: 10.1074/jbc.270.46.27894. [DOI] [PubMed] [Google Scholar]
- Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J Physiol. 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eum WS, Jang SH, Kim DW, Choi HS, Choi SH, Kim SY. Enhanced transduction of Cu, Zn-superoxide dismutase with HIV-1 Tat protein transduction domains at both termini. Mol Cell. 2005;19(2):191–197. [PubMed] [Google Scholar]
- Farber JL, Kyle ME, Coleman JB. Mechanisms of cell injury by activated oxygen species. Lab Invest. 1990;62(6):670–679. [PubMed] [Google Scholar]
- Fatma N, Kubo E, Sharma P, Beier DR, Singh DP. Impaired homeostasis and phenotypic abnormalities in Prdx6−/− mice lens epithelial cells by reactive oxygen species: increased expression and activation of TGF beta. Cell Death Differ. 2005;12(7):734–50. doi: 10.1038/sj.cdd.4401597. [DOI] [PubMed] [Google Scholar]
- Fatma N, Singh DP, Shinohara T, Chylack LT., Jr Transcriptional regulation of the antioxidant protein 2gene, a thiol-specific antioxidant, by lens epithelium-derived growth factor to protect cells from oxidative stress. J Biol Chem. 2001;276(52):48899–48907. doi: 10.1074/jbc.M100733200. [DOI] [PubMed] [Google Scholar]
- Fontaine V, Mohand-Said S, Hanoteau N, Fuchs C, Pfizenmaier K, Eisel U. Neurodegenerative and neuroprotective effects of tumor Necrosis factor (TNF) in retinal ischemia: opposite roles of TNF receptor 1 and TNF receptor 2. J Neurosci. 2002;22(7):RC216. doi: 10.1523/JNEUROSCI.22-07-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodifficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Gallagher BM, Phelan SA. Investigating transcriptional regulation of Prdx6 in mouse liver cells. Free Radic Biol Med. 2007;42(8):1270–1277. doi: 10.1016/j.freeradbiomed.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Garcia-Valenzuela E, Shareef S, Walsh J, Sharma SC. Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp Eye Res. 1995;61(1):33–44. doi: 10.1016/s0014-4835(95)80056-5. [DOI] [PubMed] [Google Scholar]
- Granot E, Kohen R. Oxidative stress in Childhood-in health and disease states. Clin Nutr. 2004;23:3–11. doi: 10.1016/s0261-5614(03)00097-9. [DOI] [PubMed] [Google Scholar]
- Green M, Loewenstein PM. Autinomous functional domains of chemically synthesized human immuno-difficiency virus tat trans-activator protein. Cell. 1988;55:1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- Grilli M, Pizzi M, Memo M, Spano P. Neuroprotection by aspirin and sodium salicylate through blockade of NF-kappaB activation. Science. 1996;274(5291):1383–1385. doi: 10.1126/science.274.5291.1383. [DOI] [PubMed] [Google Scholar]
- Guo L, Moss SE, Alexander RA, Ali RR, Fitzke FW, Cordeiro MF. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure (IOP) and IOP-induced effects on extracellular matrix. Invest Ophthalmol Vis Sci. 2005;46:175–182. doi: 10.1167/iovs.04-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Salt TE, Maass A, Luong V, Moss SE, Fitzke FW, Cordeiro MF. Assessment of neuroprotective effects of glutamate modulation on glaucoma-related retinal ganglion cell apoptosis in vivo. Invest Ophthalmol Vis Sci. 2006;47(2):626–633. doi: 10.1167/iovs.05-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Griffiths EJ, Connern CP. Mitochondrial calcium handling and oxidative stress. Biochem Soc Trans. 1993;21(2):353–358. doi: 10.1042/bst0210353. [DOI] [PubMed] [Google Scholar]
- Hancock JT, Desikan R, Neill SJ. Role of reactive oxygen species in cell signalling pathways. Biochem Soc Trans. 2001;29:345–350. doi: 10.1042/0300-5127:0290345. [DOI] [PubMed] [Google Scholar]
- Henshall DC, Simon RP. Epilepsy and apoptosis pathways. J Cereb Blood Flow Metab. 2005;25(12):1557–1572. doi: 10.1038/sj.jcbfm.9600149. [DOI] [PubMed] [Google Scholar]
- Izzotti A, Bagnis A, Sacca SC. The role of oxidative stress in glaucoma. Mutat Res. 2006;612(2):105–114. doi: 10.1016/j.mrrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Jin MH, Lee YH, Kim JM, Sun HN, Moon EY, Shong MH. Characterization of neural cell types expressing peroxiredoxins in mouse brain. Neurosci Lett. 2005;381(3):252–257. doi: 10.1016/j.neulet.2005.02.048. [DOI] [PubMed] [Google Scholar]
- Kang SW, Baines IL, Rhee SG. Characterization of a mammalian peroxiredoxin that contains one conserved cysteine. J Biol Chem. 1998;273:6303–6311. doi: 10.1074/jbc.273.11.6303. [DOI] [PubMed] [Google Scholar]
- Kang SW, Chae HS, Seo MS, Kim KH, Baines IC, Rhee SG. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-alpha. J Biol Chem. 1998;273:6297–6302. doi: 10.1074/jbc.273.11.6297. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt B, Widera D, Kaltschmid C. Kaltschmidt B 2005 Signaling via NF-kappaB in the nervous system. Biochim Biophys Acta. 2005;1745(3):287–299. doi: 10.1016/j.bbamcr.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Karsan A, Yee E, Harlan JM. Endothelial cell death induced by tumor necrosis factor-alpha is inhibited by the Bcl-2 family member, A1. J Biol Chem. 1996;271(44):27201–27214. doi: 10.1074/jbc.271.44.27201. [DOI] [PubMed] [Google Scholar]
- Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green DR. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappa B and AP-1. Mol Cell. 1998;1(4):543–551. doi: 10.1016/s1097-2765(00)80054-4. [DOI] [PubMed] [Google Scholar]
- Kim DW, Eum WS, Jang SH, Kim SY, Choi HS, Choi SH. Transduced Tat-SOD fusion protein protects against ischemic brain injury. Mol Cell. 2005;19(1):88–96. [PubMed] [Google Scholar]
- Kim H, Lee TH, Park ES, Suh JM, Park SJ, Chung HK, et al. Role of peroxiredoxins in regulating intracellular hydrogen peroxide and hydrogen peroxide-induced apoptosis in thyroid cells. J Biol Chem. 2000;275(24):18266–18270. doi: 10.1074/jbc.275.24.18266. [DOI] [PubMed] [Google Scholar]
- Kim K, Kim IH, Lee KY, Rhee SG, Stadtman ER. The isolation and purification of a specific ‘Protector’ protein which inhibits enzyme inactivation by a thiol/Fe (III)/O2 mixed -function oxidation system. J Biol Chem. 1988;263:4704– 4711. [PubMed] [Google Scholar]
- Kim YK, Bae GU, Kang JK, Park JW, Lee EK, Lee HY. Cooperation of H2O2-mediated ERK activation with Smad pathway in TGF-beta1 induction of p21WAF1/Cip1. Cell Signal. 2006;18(2):236–243. doi: 10.1016/j.cellsig.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Kretz-Remy C, Munsch B, Arrigo AP. NFkappa B-dependent transcriptional activation during heat shock recovery. Thermolability of the NF-kappaB Ikappa B complex. J Biol Chem. 2001;276(47):43723–33. doi: 10.1074/jbc.M010821200. [DOI] [PubMed] [Google Scholar]
- Kubo E, Fatma N, Akagi Y, Beier DR, Singh SP, Singh DP. TAT-mediated PRDX6 protein transduction protects against eye lens epithelial cell death and delays lens opacity. AJP: Cell Physiology. 2008;294(3):842–855. doi: 10.1152/ajpcell.00540.2007. [DOI] [PubMed] [Google Scholar]
- Kubo E, Miyazawa T, Fatma N, Akagi Y, Singh DP. Development- and age-associated expression pattern of peroxiredoxin 6, and its regulation in murine ocular lens. Mech Ageing Dev. 2006;127(3):249–256. doi: 10.1016/j.mad.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Kubo E, Urakami T, Fatma N, Akagi Y, Singh DP. Polyol pathway-dependent osmotic and oxidative stresses in aldose reductase-mediated apoptosis in human lens epithelial cells: role of AOP2. Biochem Biophys Res Commun. 2004;314(4):1050–1056. doi: 10.1016/j.bbrc.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Kucharczak J, Simmons MJ, Fan Y, Gelinas C. To be, or not to be: NF-kappaB is the answer--role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene. 2003;22(56):8961–8982. doi: 10.1038/sj.onc.1207230. [DOI] [PubMed] [Google Scholar]
- Kurysheva NI, Vinetskaia MI, Erichev VP, Artamonov VP, Uspenskaia AP. Contribution of free-radical reactions of chamber humor to development of primary open angle glaucoma. Vestn Oftalmol. 1996;112:3–5. [PubMed] [Google Scholar]
- Kwon HY, Eum WS, Jang HW, Kang JH, Ryu J, Ryong Lee B, Jin LH, Park J, Choi SY. Transduction of Cu, Zn-superoxide dismutase mediated by an HIV-1 Tat protein basic domain into mammalian cells. FEBS Lett. 2000;485(2–3):163–167. doi: 10.1016/s0014-5793(00)02215-8. [DOI] [PubMed] [Google Scholar]
- Levin LA. Direct and indirect approaches to neuroprotective therapy of glaucomatous optic neuropathy. Surv Ophthalmol. 1999;43:S98–101. doi: 10.1016/s0039-6257(99)00027-2. [DOI] [PubMed] [Google Scholar]
- Leyens G, Donnay I, Knoops B. Cloning of bovine peroxiredoxins-gene expression in bovine tissues and amino acid sequence comparison with rat, mouse and primate peroxiredoxins. Comp Biochem Physiol B Biochem Mol Biol. 2003;136(4):943–955. doi: 10.1016/s1096-4959(03)00290-2. [DOI] [PubMed] [Google Scholar]
- Lieven CJ, Hoegger M, Schliever CR, Levin LA. Retinal ganglion cell axotomy induces an increase in intracellular superoxide anion. Invst Ophthalmol Vis Sci. 2006;47:1477–1485. doi: 10.1167/iovs.05-0921. [DOI] [PubMed] [Google Scholar]
- Lim YS, Cha MK, Kim HK, Uhm TB, Park JW, Kim K, Kim IH. Removals of hydrogen peroxide and hydroxyl radical by thiol-specific antioxidant protein as a possible role in vivo. Biochem Biophys Res Commun. 1993;192(1):273–280. doi: 10.1006/bbrc.1993.1410. [DOI] [PubMed] [Google Scholar]
- Ma W, Nunes I, Young CS, Spector A. Catalase enrichment using recombinant adenovirus protects alphaTN4-1 cells from H(2)O(2) Free Radic Biol Med. 2006;40(2):335–340. doi: 10.1016/j.freeradbiomed.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Madigan MC, Sadun AA, Rao NS, Dugel PU, Tenhula WN, Gill PS. Tumor necrosis factor-alpha (TNF-α)-induced optic neuropathy in rabbits. Neurol Res. 1996;18:176–184. doi: 10.1080/01616412.1996.11740399. [DOI] [PubMed] [Google Scholar]
- Maher P, Hanneken A. The molecular basis of oxidative stress-induced cell death in an immortalized retinal ganglion cell line. Invest Ophthalmol Vis Sci. 2005;46(2):749–757. doi: 10.1167/iovs.04-0883. [DOI] [PubMed] [Google Scholar]
- Maier CM, Chan PH. Role of superoxide dismutases in oxidative damage and neurodegenerative disorders. Neuroscientist. 2002;8 (4):323–334. doi: 10.1177/107385840200800408. [DOI] [PubMed] [Google Scholar]
- Manevich Y, Sweitzer T, Pak JH, Feinstein SI, Muzykantov V, Fisher AB. 1-Cysperoxiredoxin over expression protects cells against phospholipid peroxidation-mediated membrane damage. Proc, Natl, Acad Sci. 2002;99(18):11599–11604. doi: 10.1073/pnas.182384499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic Biol Med. 2005;38(11):1422–1432. doi: 10.1016/j.freeradbiomed.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Mann DA, Frankel AD. Endocytosis and targeting of exogenous HIV-1 Tat protein. EMBO J. 1991;10:1733–1739. doi: 10.1002/j.1460-2075.1991.tb07697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J Biol Chem. 2004;279(31):32869–32881. doi: 10.1074/jbc.M311766200. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Neuroprotective signal transduction: relevance to stroke. Neurosci Biobehav Rev. 1997;21(2):193–206. doi: 10.1016/s0149-7634(96)00010-3. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Neuronal life-and-death signaling, apoptosis, and neurodegenerative disorders. Antioxid Redox Signal. 2006;8(11–12):1997–2006. doi: 10.1089/ars.2006.8.1997. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13(5):852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Mehlen A, Guillet D, Preville X, Arrigo AP. Tumor necrosis factor-alpha induces changes in the phosphorylation, cellular localization, and oligomerization of human hsp27, a stress protein that confers cellular resistance to this cytokine. J Cell Biochem. 1995;58(2):248–259. doi: 10.1002/jcb.240580213. [DOI] [PubMed] [Google Scholar]
- McKinnon SJ. Glaucoma, apoptosis, and neuroprotection. Current opin Ophthalmol. 1997;8:28–37. doi: 10.1097/00055735-199704000-00006. [DOI] [PubMed] [Google Scholar]
- Moreno MC, Campanelli J, Sande P, Sanez DA, Keller Sarmiento MI, Rosenstein RE. Retinal oxidative stress induced by high intraocular pressure. Free Radic Biol Med. 2004;37(6):803–812. doi: 10.1016/j.freeradbiomed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Muller A, Pietri S, Villain M, Frejaville C, Bonne C, Culcas M. Free radicals in rabbit retina under ocular hyperpressure and functional consequences. Exp Eye Res. 1997;64(4):637–643. doi: 10.1006/exer.1996.0277. [DOI] [PubMed] [Google Scholar]
- Nagahara H, Vocero-Akbani A, Snyder EL, Ho A, et al. Transduction of full-length TAT fusion proteins into mammalian cells:TAT-p27 kip1 induces cell migration. Nat Med. 1998;4:1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- Nucci C, Tartaglione R, Rombola L, Morrone LA, Fazzi E, Bagetta G. Neurochemical evidence to implicate elevated glutamate in the mechanisms of high intraocular pressure (IOP)-induced retinal ganglion cell death in rat. Neurotoxicology. 2005;26(5):935–941. doi: 10.1016/j.neuro.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK. Oxidative damage is the earliest event in Alzhiemer disease. J Neuropatholol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- Nurmi A, Lindsberg PJ, Koistinaho M, Zhang W, Juettler E, Karjalainen-Lindsberg ML, et al. Nuclear factor-kappaB contributes to infarction after permanent focal ischemia. Stroke. 2004;35(4):987–991. doi: 10.1161/01.STR.0000120732.45951.26. [DOI] [PubMed] [Google Scholar]
- Okuno T, Oku H, Sugiyama T, Ikeda T. Glutamate level in optic nerve head is increased by artificial elevation of intraocular pressure in rabbits. Exp Eye Res. 2006;82(3):465–470. doi: 10.1016/j.exer.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Burkitt MJ, Kass GE, Dypbukt JM, Nicotera P. Calcium ions and oxidative cell injury. Ann Neurol. 1992;32:S33–S42. doi: 10.1002/ana.410320708. [DOI] [PubMed] [Google Scholar]
- Pahl HL, Baeuerle PA. Oxygen and the control of gene expression. Bioessays. 1994;16(7):497–502. doi: 10.1002/bies.950160709. [DOI] [PubMed] [Google Scholar]
- Pak JH, Manevich Y, Kim HS, Feinstein SI, Fisher AB. An antisense oligonucleotide to 1-cys peroxiredoxin causes lipid peroxidation and apoptosis in lung epithelial cells. J Biol Chem. 2002;277(51):49927–49934. doi: 10.1074/jbc.M204222200. [DOI] [PubMed] [Google Scholar]
- Parfenova H, Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, Leffler CW. Glutamate induces oxidative stress and apoptosis in cerebral vascular endothelial cells: contributions of HO-1 and HO-2 to cytoprotection. Am J Physiol Cell Physiol. 2006;290(5):C1399–C1410. doi: 10.1152/ajpcell.00386.2005. [DOI] [PubMed] [Google Scholar]
- Perry G, Castellani RJ, Smith MA, Harri PLR, Kubat Z, Ghanbari K. Oxidative damage in the olfactory system in Alzheimer’s disease. Acta Neuropathol. 2003;106:552–556. doi: 10.1007/s00401-003-0761-7. [DOI] [PubMed] [Google Scholar]
- Plaisant F, Clippe A, Vander Stricht D, Knoops B, Gressens P. Recombinant peroxiredoxin 5 protects against excitotoxic brain lesions in newborn mice. Free Radic Biol Med. 2003;34(7):862–872. doi: 10.1016/s0891-5849(02)01440-5. [DOI] [PubMed] [Google Scholar]
- Planck SR, Huang XN, Robertson JE, Rosenbaum JT. Cytokine mRNA levels in rat ocular tissues after systemic endotoxin treatment. Invest Ophthalmol Vis Sci. 1994;35(3):924–930. [PubMed] [Google Scholar]
- Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36(5):774–786. [PubMed] [Google Scholar]
- Reddy VN, Kasahara E, Hiraoka M, Lin LR, Ho YS. Effects of variation in superoxide dismutases (SOD) on oxidative stress and apoptosis in lens epithelium. Exp Eye Res. 2004;79(6):859–868. doi: 10.1016/j.exer.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Rhee SG. Redox signaling: hydrogen peroxide as intracellular messenger. Exp Mol Med. 1993;31(2):53–59. doi: 10.1038/emm.1999.9. [DOI] [PubMed] [Google Scholar]
- Ribeiro MM, Klein D, Pileggi A, Molano RD, Fraker C, Ricordi C, Inverardi L, Pastori RL. Heme oxygenase-1 fused to a TAT peptide transduces and protects pancreatic beta-cells. Biochem Biophys Res Commun. 2003;305(4):876–881. doi: 10.1016/s0006-291x(03)00856-8. [DOI] [PubMed] [Google Scholar]
- Romero FJ. Antioxidant in peripheral nerve. Free Rad Biol Med. 1996;20:925–932. doi: 10.1016/0891-5849(95)02183-3. [DOI] [PubMed] [Google Scholar]
- Rose RC, Richer SP, Bode AM. Ocular oxidants and and antioxidant protection. Proc Soc Exp Biol Med. 1998;217:397–407. doi: 10.3181/00379727-217-44250. [DOI] [PubMed] [Google Scholar]
- Roth E. Oxygen free radicals and their clinical implications. Acta Chi Hung. 1997;36:302–305. [PubMed] [Google Scholar]
- Rustani M, Coltrini D, Oreste P, Zoppetti G, et al. Interaction of HIV-1 Tat protein with heparin. Role of backbone structure, sulfation, and size. J Biol Chem. 1997;272:11313–11320. doi: 10.1074/jbc.272.17.11313. [DOI] [PubMed] [Google Scholar]
- Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5(5):554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- Schreck R, Baeuerle PA. Assessing oxygen radicals as mediators in activation of inducible eukaryotic transcription factor NF-kappa B. Methods Enzymol. 1994;234:151–63. doi: 10.1016/0076-6879(94)34085-4. [DOI] [PubMed] [Google Scholar]
- Shou Y, Gunasekar PG, Borowitz JL, Isom GE. Cyanide-induced apoptosis involves oxidative-stress-activated NF-kappaB in cortical neurons. Toxicol Appl Pharmacol. 2000;164(2):196–205. doi: 10.1006/taap.2000.8900. [DOI] [PubMed] [Google Scholar]
- Spector A, Kuszak JR, Ma W, Wang RR. The effect of aging on glutathione peroxidase-i knockout mice-resistance of the lens to oxidative stress. Exp Eye Res. 2001;72(5):533–545. doi: 10.1006/exer.2001.0980. [DOI] [PubMed] [Google Scholar]
- Tamatani M, Che YH, Matsuzaki H, Ogawa S, Okado H, Miyake S, Mizuno T, Tohyama M. Tumor necrosis factor induces Bcl-2 and Bcl-x expression through NFkappaB activation in primary hippocampal neurons. J Biol Chem. 1999;274(13):8531–8538. doi: 10.1074/jbc.274.13.8531. [DOI] [PubMed] [Google Scholar]
- Tezel G. Oxidative stress in glaucomatous neurodegeneration: Mechanisms and consequences. Prog Retin Eye Res. 2006;25(5):490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Wax MB. Increased production of tumor necrosis factor-alpha by glial cells exposed to simulated ischemia or elevated hydrostatic pressure induces apoptosis in cocultured retinal ganglion cells. J Neurosci. 2000;20:8693–8700. doi: 10.1523/JNEUROSCI.20-23-08693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Yang X. Comparative gene array analysis of TNF-α induced MAPK and NFB signaling pathways between retinal ganglion cells and glial cells. Exp Eye Res. 2005;81(2):207–217. doi: 10.1016/j.exer.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Tezel G, Yang X, Yang J, Wax MB. Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice. Brain Res. 2004;996:202–212. doi: 10.1016/j.brainres.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Barkis WB, Chen S, Diamond JS, Barres BA. Invulnerability of retinal ganglion cells to NMDA excitotoxicity. Mol Cell Neurosci. 2004;26(4):544–557. doi: 10.1016/j.mcn.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- Yuan L, Neufeld AH. Tumor necrosis factor-alpha: a potentially neurodestructive cytokine produced by glia in the human glaucomatous optic nerve head. Glia. 2003;32(1):42–50. [PubMed] [Google Scholar]
- Zhang X, Cheng M, Chintala SK. Kainic acid-mediated upregulation of matrix metalloproteinase-9 promotes retinal degeneration. Invest Ophthalmol Vis Sci. 2004;45:2374–2383. doi: 10.1167/iovs.03-1239. [DOI] [PubMed] [Google Scholar]