Abstract
Scanning electron microcopy (SEM), transmission electron microscopy (TEM), and differential scanning calorimetry (DSC) were used to evaluate structural changes in Leuconostoc mesenteroides cells as a function of high-hydrostatic-pressure treatment. This bacterium usually grows in chains of cells, which were increasingly dechained at elevated pressures. High-pressure treatments at 250 and 500 MPa also caused changes in the external surface and internal structure of cells. Dechaining and blister formation on the surface of cells increased with pressure, as observed in SEM micrographs. TEM studies showed that cytoplasmic components of the cells were affected by high-pressure treatment. DSC studies of whole cells showed increasing denaturation of ribosomes with pressure, in keeping with dense compacted regions in the cytoplasm of pressure-treated cells observed in TEM micrographs. Apparent reduction of intact ribosomes observed in DSC thermograms was related to the reduction in number of viable cells. The results indicate that inactivation of L. mesenteroides cells is mainly due to ribosomal denaturation observed as a reduction of the corresponding peak in DSC thermograms and condensed interior regions of cytoplasm in TEM micrographs.
High-hydrostatic-pressure (HHP) processing is considered for food preservation as an alternative to conventional thermal pasteurization due to its potential for microbial inactivation. HHP can be used alone or in combination with thermal or nonthermal techniques for the production of a wide variety of high-quality foods that are minimally processed, additive free, and microbiologically safe (7). Thus, HHP research has been primarily focused on the cellular targets and the mechanism of the HHP-induced inactivation of various food spoilage and food-borne pathogenic bacteria with the ultimate goal of optimizing the processing conditions. Resistance to HHP varies among bacteria and is dependent on the physiological state of the organisms at the time of pressurization (1, 8, 24, 30). Although some studies (9, 26) suggest that the cell wall and cell membrane lose their function as a result of pressure, the exact mechanism of inactivation caused by HHP is still not well understood. Dissociation of ribosomes, thermotropic phase changes in membrane lipids, and protein denaturation also are proposed as possible structural changes in the cell that cause inactivation of microorganisms subjected to high pressure (1, 9, 16, 23). Electron microscopy has been employed to characterize pressure-induced morphological changes in microorganisms in order to understand the events leading to cell inactivation (9, 16, 20, 29). Using scanning electron microscopy (SEM), Kalchayanand et al. (9) evaluated morphological changes in Leuconostoc mesenteroides cells after pressure treatment at 345 MPa. These investigators reported that while the cell size, shape, and surface structure of inactivated cells immediately after pressure treatment were not different from those of living cells, cell lysis was observed after 2 h of storage at 4°C. Transmission electron microscopy (TEM) studies by Mackey et al. (16) of the cell structure of Salmonella enterica serovar Thompson and Listeria monocytogenes after pressure treatments at 250 and 500 MPa showed structural changes specific to each organism. While electron micrographs of pressure-treated L. monocytogenes cells showed formation of vacuolar regions in the cytoplasm, vacuole formation was not reported for the pressure-treated cells of S. enterica serovar Thompson. These investigators observed fewer ribosomes in pressure-treated S. enterica serovar Thompson cells than untreated cells, suggesting apparent cell lysis. Using SEM, Tholozan et al. (33) and Ritz et al. (29) reported increasing irregularities referred to as “bud scars” on the surface of L. monocytogenes cells with increasing pressure. Although total inactivation of the population was observed at 400 MPa, bacterial cells retained their morphological characteristics with limited cell disruptions. Tholozan et al. (33) observed increasing cell invaginations with increasing pressure for Salmonella enterica serovar Typhimurium, but there was no lysis. SEM studies by Malone et al. (20) revealed low-density intracellular regions in Lactococcus lactis subsp. cremoris cells after pressure treatments at 300 and 800 MPa and cell envelope damage at 800 MPa.
Differential scanning calorimetry (DSC) has been employed to monitor the conformational transitions specific to various cellular components of intact cells as a function of temperature in order to understand the sequence of events leading to inactivation of microorganisms (1, 2, 4, 12-14, 17-19, 22, 23, 34). The thermal stability of ribosomes has been shown to correlate with growth temperature of the cells, and the denaturation of ribosomes has been proposed as a mechanism for cell injury or death (1, 4, 12-14, 17-19, 22, 31). In addition to thermal treatment-induced changes, DSC has been used to evaluate the effect of various physical and chemical factors on bacterial inactivation by comparing the thermograms before and after treatment (1, 23). DSC analysis of pressure-treated bacteria indicated a correlation between cell viability and a reduction in the apparent enthalpy associated with ribosome denaturation, suggesting that cell inactivation and ribosomal denaturation are closely related (1, 23). While some interesting observations have been reported, pressure-induced structural changes at the cellular and molecular levels and their implications on cell inactivation have not been characterized thoroughly.
The goal of this study was to investigate HHP-induced morphological changes and their relation to cell inactivation in L. mesenteroides. SEM and TEM were used to characterize chain arrangement and the surface and internal morphology of cells as a function of HHP treatment. DSC was employed to detect and monitor changes in the thermal stabilities of DNA and ribosomes as well as the apparent enthalpy of whole cells.
MATERIALS AND METHODS
Source and preparation of organisms.
L. mesenteroides OSU553 (isolated from a local source) was obtained from the Culture Collection, Department of Microbiology, The Ohio State University, Columbus. The culture was stored frozen (−80°C) in 30% (vol/vol) sterile glycerol. A loopful of L. mesenteroides was revived in 10 ml of MRS broth (Difco, Detroit, Mich.) and incubated at 30°C for 16 h.
L. mesenteroides culture was inoculated (1% [vol/vol]) into MRS broth and incubated at 30°C. Duplicate samples were taken every 1 h and pour plated with MRS agar (MRS broth plus Bacto-Agar; Difco). The plates were incubated at 30°C for 36 h, and a growth curve for L. mesenteroides was constructed. Once the late-exponential phase was reached, cells were harvested for HHP treatment, DSC analysis, viable count, and electron microscopy studies.
HHP treatment.
Cells grown to a final concentration of 1.3 × 108 ± 0.1 × 108 CFU ml−1 in MRS broth (200 ml) were placed in 2-mil-thick sterile polyethylene bags (3.8 by 15 cm) (Fisher Scientific, Inc., Pittsburgh, Pa.) for HHP treatment. Air was removed from the bags prior to heat sealing. The bags were placed inside a second polyethylene bag and heat-sealed under vacuum to prevent contamination of the high-pressure unit if the primary package were to fail. A hydrostatic pressurization unit (Quintus QFP-6; ABB Autoclave Systems, Inc., Columbus, Ohio) that was capable of operating up to 900 MPa was used to apply pressure to the L. mesenteroides cell suspensions. A water-propylene glycol (Houghton-Safe 620-TY; Houghton Intl., Inc., Valley Forge, Pa.) mixture (1:1 [vol/vol]) was used as the pressure-transmitting fluid. Prior to pressurization, the fluid was heated to the desired temperature by an electrical heating system surrounding the unit. The rate of pressure increase was approximately 400 MPa/min, and the pressure release time was less than 20 s. Pressurization times reported in this study exclude the pressure increase and release times. The pressure level, time, and temperature of pressurization were set manually and were recorded as a function of time during the treatment.
The polyethylene bags containing cell suspensions were pressurized for 5 min at pressures of 250 and 500 MPa at 35°C. Duplicate samples were prepared for each treatment. Both pressure-treated and untreated cell suspensions were centrifuged (J2-21; Beckman, Palo Alto, Calif.) at 10,000 × g for 10 min at 4°C to form pellets prior to SEM, TEM, and DSC analysis.
Enumeration of cells.
Pressure-treated and untreated cell suspensions were serially diluted in 0.1% sterile peptone (Becton Dickinson, Cockeysville, Md.) solution. From the selected dilutions, 1-ml portions were pour plated in duplicate plates by using MRS agar media. The plates were incubated at 30°C for 36 h, and plates containing 25 to 250 CFU ml−1 were selected for counting.
Electron microscopy.
The L. mesenteroides cell pellet was prepared from untreated and pressure-treated cell suspensions by centrifugation at 10,000 × g for 10 min at 4°C and washed once with 150 ml of sterile distilled water. Cell pellets (1 mm3) were transferred to sterile vials and resuspended in 1 ml of 0.1 M phosphate buffer at pH 7.4. Suspended bacteria were filtered (0.45-μm pore size) and fixed on the membrane with 10 ml of 3% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). Fixative was left in contact with the cells overnight at 4°C.
For SEM analysis, the fixed cells were washed with buffer and postfixed for 1 h in 1% osmium tetroxide in phosphate buffer. Filters were rinsed with buffer and dehydrated through a series of ethanol solutions with increasing concentrations (50, 70, 95, and 100% ethanol). Ethanol was replaced with liquid CO2, and the samples were dried in a critical point dryer. Cells were sputter coated with gold-palladium and examined in a Philips XL-30 scanning electron microscope at 30 kV (FEI, Inc., Hillsboro, Oreg.).
For TEM analysis, fixed cells were rinsed with buffer and centrifuged, and the pellet was embedded in 2% agar. Agar was cut into 1-mm3 pieces and postfixed for 1 h in 1% osmium tetroxide in phosphate buffer. Samples were rinsed in distilled water and stained en bloc for 1 h in 1% aqueous uranyl acetate. After dehydration through an ascending series of ethanol solutions (50, 70, 95, and 100% ethanol), cells in agar were transferred to propylene oxide and infiltrated and embedded in Spurr's resin (Ted Pella, Redding, Calif.). Sections (70 nm) were obtained with an ultramicrotome and stained with Reynolds' lead citrate (27) prior to examination in a Philips CM-12 TEM at 60 kV (FEI, Inc.).
DSC analysis.
A portion (∼100 mg) of the L. mesenteroides pellet was transferred into a tared (1.5 ml) polyethylene tube, weighed, freeze-dried (Freezone 4.5; Labconco Freeze Dry System, Kansas City, Mo.), and reweighed to determine the percentage of dry matter in the pellet. The amount of moisture in the L. mesenteroides pellet used in the DSC experiments was 83% ± 0.3% (wt/wt).
A differential scanning calorimeter (DSC 111; Setaram, Lyon, France) was used to collect thermograms of untreated control and pressure-treated L. mesenteroides. A DSC thermogram with an empty stainless steel sample and reference crucibles was collected to measure the empty crucible baseline. Temperature calibration was confirmed with an indium sample in a stainless steel crucible. All thermograms were collected at a constant heating rate of 4°C min−1. Pellets of cells were transferred into the sample crucible and weighed (70 ± 0.3 mg wet weight). When the reference crucible was left empty, an artifact due to an imbalance of heat capacity between crucibles was observed at the initiation of temperature scanning. A known quantity of water, similar in mass to the moisture in the sample, was placed in the reference crucible to eliminate the artifact. The reference crucible was filled with 58 ± 0.2 mg (83% of sample weight) of distilled water. Both crucibles were sealed with aluminum O-rings. The sealed crucibles were refrigerated (4°C) until used for DSC. The sample and reference crucibles were placed in the DSC and equilibrated at 1°C with liquid nitrogen and scanned to 140°C at 4°C min−1. Samples were reweighed after DSC measurements to check for loss of mass during heating. Thermograms of samples showing signs of leakage were not used.
Analysis of DSC data.
An empty crucible thermogram was subtracted from a sample thermogram to correct for differences in the empty crucibles. Total heat levels corresponding to the area between the endothermic peaks and the baseline (apparent enthalpy in joules per gram) were determined by integrating the temperature versus heat flow curve by using software provided by the instrument's manufacturer. A curved baseline using three-temperature points was utilized to calculate the apparent enthalpy of whole cells (12). The curved baseline was constructed between the segment of the thermogram prior to the first thermally induced transition (∼36°C) and the segment of the thermogram after the last peak (∼115°C). The total peak area was determined for both the control and pressure-treated samples. Peak temperatures for the thermally induced transitions were also determined.
RESULTS
Viability.
The effect of HHP treatment on the viability of L. mesenteroides cells was determined by plate counting. The initial number of cells prior to pressure treatment was found to be 1.3 × 108 CFU ml−1. Pressure treatment at 250 MPa and 35°C for 5 min reduced the cell count to 2.1 × 106 CFU ml−1. Viable cell counts were not detected following pressure treatment at 500 MPa and 35°C for 5 min. MRS medium is somewhat selective, and therefore severely injured cells may not have been recovered.
SEM.
Pressure treatment at 250 and 500 MPa produced morphological changes on the surface and in the internal structure of the cells, as observed by SEM and TEM. When grown in rich media, the bacteria formed characteristic chains of up to five coccidal lenticular cells with constrictions at the junctions between cells (Fig. 1A). Electron micrographs of typical arrangements of untreated and pressure-treated cells are shown in Fig. 1. The number of long chains (three cells or more) decreased as the pressure increased relative to untreated samples. More than 50% of untreated cells, counted in SEM micrographs, were in chains of three or more (Fig. 2). The combined percentage of single cells and cells in chains of two increased progressively as the pressure increased.
FIG. 1.
SEM micrographs of L. mesenteroides cells. (A) Untreated cells. (B) Cells treated with 250 MPa of pressure at 35°C for 5 min. (C) Cells treated with 500 MPa of pressure at 35°C for 5 min. Original magnification, ×3,500.
FIG. 2.
Effect of pressure on dechaining of L. mesenteroides cells. The total number of cells counted in each case was approximately 1,000. A pressure of 0.1 MPa was used for untreated cells. Open bars, single cells and cells in chains of two; solid bars, cells in chains of three or more.
Untreated control cells exhibited a smooth surface structure (Fig. 3A). The surface appearance became rough and cracked when the cells were exposed to 500 MPa (Fig. 3B). Some cells showed even rougher surface structure and blister-like protrusions after pressure treatment at 500 MPa (Fig. 3C). Pressure treatment at 250 MPa and 35°C for 5 min produced cells with a surface morphology characteristic of both untreated cells and cells treated at 500 MPa (results not shown). Blister-like formations appeared to be in rows parallel to the division sites of lenticular cells.
FIG. 3.
SEM micrographs of L. mesenteroides cells. (A) Untreated. Original magnification, ×25,000. (B) Cells treated with 500 MPa of pressure at 35°C for 5 min. Original magnification, ×25,000. (C) Higher magnification (×50,000) of some cells treated with 500 MPa of pressure at 35°C for 5 min.
TEM.
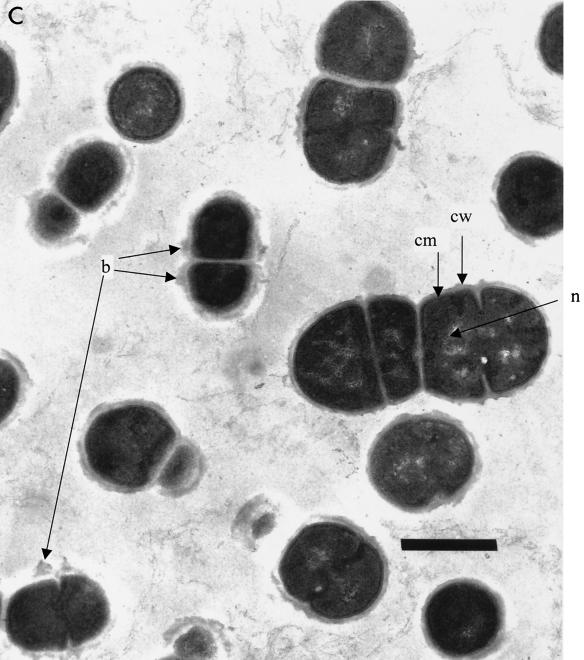
Thin sections of untreated L. mesenteroides cells displayed intact cell membrane, uniform cell cytoplasm, and electron-transparent regions of nucleoid in electron micrographs (Fig. 4A). Application of pressure resulted in morphological changes in the internal structures. The most notable changes with application of pressure were a double-track bilayer structure of the membrane instead of the single-thick-layer appearance and enlargement of electron-transparent areas in the cytoplasm. Application of pressure at 250 MPa and 35°C for 5 min resulted in expanded nucleoid regions and compacted interior regions (Fig. 4B). Most cells maintained a distinct membrane. In some cells, breakdown of the peptidoglycan layer was evident, because parts of the outer layer appeared to have sloughed off after pressure treatment at 500 MPa (35°C for 5 min) (Fig. 4C). Aggregation of cytoplasmic material and enlarged electron-transparent regions of nucleoid with a fibrous appearance were also observed (Fig. 4C). Blister formations were notable on cell walls of some cells after pressure treatment at 500 MPa (Fig. 4C).
FIG. 4.
TEM micrographs of L. mesenteroides cells. (A) Untreated. (B) Cells treated with 250 MPa of pressure at 35°C for 5 min. (C) Cells treated with 500 MPa at 35°C for 5 min. cw, cell wall; cm, cell membrane; n, nucleoid; b, blisters. Scale bar, 0.5 μm.
DSC.
DSC thermograms of control and pressure-treated cells are shown in Fig. 5. An increase in pressure resulted in a decreased transition peak area (apparent enthalpy, ΔH in joules per gram). A large reduction was observed in the first major transition over a temperature region of the thermogram of 50 to ∼85°C (peak a). In addition to the area of the peak, both the onset and peak temperatures of the transition decreased as the treatment pressure increased. While the enthalpy of the DNA transition (peak b) remains unchanged as a result of pressure treatment at 250 MPa, a 25% decrease in the enthalpy of the transition was observed after a 500-MPa pressure treatment. The thermal stability of DNA decreased progressively due to pressure treatment. The peak temperatures for the DNA melting transition are 100°C for untreated control cells, 95°C for cells treated at 250 MPa, and 91°C for cells treated at 500 MPa.
FIG. 5.
DSC thermograms of L. mesenteroides cells. (A) Untreated. (B) Cells treated with 250 MPa of pressure at 35°C for 5 min. (C) Cells treated with 500 MPa of pressure at 35°C for 5 min. Arrows mark the peak temperatures of endotherms corresponding to ribosome denaturation (peak a) and DNA melting (peak b).
The DSC thermograms exhibited differences in the apparent specific heat capacity change associated with heating of the live and inactivated cells. These changes in heat capacity were observed as deviations between the pre- and posttransition baselines. The change in heat capacity became progressively smaller as the pressure level increased due to the increase in specific heat of cells inactivated by pressure treatment (Fig. 5).
DISCUSSION
Leuconostoc spp. are gram-positive chain-forming cocci that occur singly or in pairs (diplococci) and short chains (streptococci) (25). Dechaining as a result of pressure treatment may be due to a lower volume achieved by single cells and doublets, because a smaller volume is favored thermodynamically at increased pressure (3). Dechaining has been proposed to be due to enzymatic activity and is associated with autolysins in Streptococcus spp. (6, 15, 28, 32). McCarty (21) noted that streptococcal chains are difficult to disrupt without killing the organisms, but the relationship between cell death and dechaining has not been established. The present work shows that both viability and chain length decreased as the level of pressure treatment increased. Chains containing more than three cells were present after a 500-MPa treatment, although the cells were not viable.
Blister-like formations increased with pressure, and the highest numbers of blisters appeared after a pressure treatment at 500 MPa. Similar surface formations have been reported for heat- and pressure-treated bacteria. Surface blisters have been observed on the cell envelope of Escherichia coli upon heating to 55°C for 15 s (11). Longer heating times resulted in a decrease of cells with surface blisters. Deformations on the surface of L. monocytogenes cells, described as bud scars, pimples, and swellings, have been reported as a result of pressure treatment at 400 MPa and 20°C for 10 min (29, 33). Both studies reveal an increase in the number of deformations at pressures between 275 and 400 MPa. The blisters observed in our work intensified at treatment at 500 MPa and 35°C, and thus their presence on L. monocytogenes cells after pressure treatment at 400 MPa and 20°C shows that blisters were induced by pressure treatment.
Tholozan et al. (33) reported cell membrane invaginations in S. enterica serovar Typhimurium without any blister formation. In our laboratory, blister formation has not been observed in high-pressure-treated (300 to 700 MPa) E. coli cells even at the highest-pressure treatment (unpublished data). Heat-induced blisters reported by Katsui et al. (11) consisted of outer membrane and had a multilayered structure and short life. The blisters on L. mesenteroides cells observed here formed outside the cell wall and were composed of extracellular materials of gram-positive cells; no internal structures were evident in these blisters.
DSC thermograms of microorganisms document endothermic transitions indicating the denaturation of cellular components (2, 10, 14, 18, 22, 34). The main peaks observed in thermograms of untreated L. mesenteroides cells are identified as ribosomal subunits and DNA by comparison to the transition temperatures of isolated cell components of E. coli (13, 18). DSC data in the literature (1, 23) indicate that inactivation of bacteria by pressure correlates with the denaturation of the main ribosomal subunit. The thermograms in Fig. 5 are in agreement with the proposed denaturation of ribosomes by high pressure within this temperature envelope. Reduction in the area of the ribosomal peak as a function of pressure indicates irreversible changes with pressure and may be due to denaturation, with possible aggregation (an exothermic event) of ribosomes. The denatured ribosomes may manifest themselves as the compacted interior regions of the cytoplasm observed in TEM micrographs.
The transition attributed to melting of cellular DNA exhibited progressive changes by pressure treatment. The decrease in thermal stability of the DNA peak may be due to partial dissociation of a DNA duplex during pressure treatment, followed by refolding to a thermally less stable state upon return to atmospheric pressure. Given the small volume change (and concomitant small pressure sensitivity) associated with DNA duplex disruption (5), the reduction in transition temperature of the DNA peak may be due to pressure-induced changes in DNA packaging in the cell. The expansion of electron-transparent nucleiod regions correlates with the changes in the DNA peak in DSC thermograms with pressure.
This study demonstrates structural changes that occur during high-pressure treatment in the arrangement of chain-forming bacteria, blister formations on the external surface, and condensation of nucleoid and cytoplasmic material in the cell interior. The corresponding thermodynamic changes in cellular components—specifically in ribosomes and DNA—are also shown in this paper. Calorimetric data showed increasing denaturation of ribosomes with pressure, in keeping with the dense compacted regions in the cytoplasm of pressure-treated cells observed in TEM micrographs. The data further our understanding of complex events induced by pressure treatment leading to cell injury and death. The results provide additional characterization of HHP inactivation of cells. At lower pressures, the inactivation may be due to ribosomal denaturation, based on the DSC results. However, membrane damage cannot be eliminated as a potential source of lethality solely on the basis of the electron micrographs. Once the events causing death have been identified and characterized, a rational selection of optimal pressure and temperature treatment to prevent spoilage or disease will be forthcoming.
Acknowledgments
This study was supported by National Science Foundation grant INT-0096915 and The Scientific and Technical Research Council of Turkey (TUBITAK; project no. TOGTAG-NSF-2001-1).
We thank Brian Kemmenoe and Kathy Wolken of the Microscopy and Imaging Facility at the Ohio State University for assistance with the SEM and TEM studies.
REFERENCES
- 1.Alpas, H., J. Lee, F. Bozoglu, and G. Kaletunç. 2003. Evaluation of differential scanning calorimetry of high hydrostatic pressure sensitivity of Staphylococcus aureus and Escherichia coli O157:H7 strains. Int. J. Food Microbiol. 87:229-237. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, W. A., N. D. Hedges, M. V. Jones, and M. B. Cole. 1991. Thermal inactivation of Listeria monocytogenes studied in differential scanning calorimetry. J. Gen. Microbiol. 137:1419-1424. [DOI] [PubMed] [Google Scholar]
- 3.Balny, C., and P. Masson. 1993. Effects of high pressure on proteins. Food Rev. Int. 9:611-628. [Google Scholar]
- 4.Belliveau, B. H., T. C. Beaman, H. S. Pankratz, and P. Gerhardt. 1992. Heat killing of bacterial spores analyzed by differential scanning calorimeter. J. Bacteriol. 174:4463-4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubins, D. N., A. Lee, R. B. Macgregor, Jr., and T. V. Chalikian. 2001. On the stability of double stranded nucleic acids. J. Am. Chem. Soc. 123:9254-9259. [DOI] [PubMed] [Google Scholar]
- 6.Fan, D. P. 1970. Autolysin(s) of Bacillus subtilis as dechaining enzyme. J. Bacteriol. 103:494-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farkas, D. F., and D. G. Hoover. 2000. High pressure processing. J. Food Sci. 65(Suppl.):47-64. [Google Scholar]
- 8.Hauben, K. J. A., D. H. Bartlett, C. C. F. Soontjens, K. Cornelis, E. Y. Wuytack, and C. W. Michiels. 1997. Escherichia coli mutants resistant to inactivation by high hydrostatic pressure. Appl. Environ. Microbiol. 63:945-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalchayanand, N., C. Frethem, P. Dunne, A. Sikes, and B. Ray. 2002. Hydrostatic pressure and bacteriocin-triggered cell wall lysis of Leuconostoc mesenteroides. Innov. Food Sci. Emerg. Technol. 3:33-40. [Google Scholar]
- 10.Kaletunç, G. 2001. Thermal analysis of bacteria using differential scanning calorimetry, p. 227-235. In F. Bozoglu, T. Deak, and B. Ray (ed.), Novel process and control technologies in the food industry. IOS Press, Amsterdam, The Netherlands.
- 11.Katsui, N., T. Tsuchido, R. Hiramatsu, S. Fujikawa, M. Takano, and I. Shibasaki. 1982. Heat-induced blebbing and vesiculation of the outer membrane of Escherichia coli. J. Bacteriol. 151:1523-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee, J., and G. Kaletunç. 2002. Calorimetric determination of inactivation parameters of microorganisms. J. Appl. Microbiol. 93:178-189. [DOI] [PubMed] [Google Scholar]
- 13.Lee, J., and G. Kaletunç. 2002. Evaluation of the heat inactivation of Escherichia coli and Lactobacillus plantarum by differential scanning calorimetry. Appl. Environ. Microbiol. 68:5379-5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lepock, J. R., H. E. Frey, and W. E. Inniss. 1990. Thermal analysis of bacteria by differential scanning calorimetry: relationship to protein denaturation in situ to maximum growth temperature. Biochim. Biophys. Acta 1055:19-26. [DOI] [PubMed] [Google Scholar]
- 15.Lominski, I., J. Cameron, and G. Wyllie. 1958. Chaining and unchaining Streptococcus faecalis—a hypothesis of the mechanism of bacterial cell separation. Nature 181:1477. [DOI] [PubMed] [Google Scholar]
- 16.Mackey, B. M., K. Forestiére, N. S. Isaacs, R. Stennig, and B. Brooker. 1994. The effect of high hydrostatic pressure on Salmonella thompson and Listeria monocytogenes examined by electron microscopy. Lett. Appl. Microbiol. 19:429-432. [Google Scholar]
- 17.Mackey, B. M., S. E. Parsons, C. A. Miles, and R. J. Owen. 1988. The relationship between base composition of bacterial DNA and its intracellular melting temperature as determined by differential scanning calorimetry. J. Gen. Microbiol. 134:1185-1195. [DOI] [PubMed] [Google Scholar]
- 18.Mackey, B. M., C. A. Miles, S. E. Parsons, and D. A. Seymour. 1991. Thermal denaturation of whole cells and cell components of Escherichia coli examined by differential scanning calorimetry. J. Gen. Microbiol. 137:2361-2374. [DOI] [PubMed] [Google Scholar]
- 19.Mackey, B. M., C. A. Miles, D. A. Seymour, and S. E. Parsons. 1993. Thermal denaturation and loss of viability in Escherichia coli and Bacillus stearothermophilus. Lett. Appl. Microbiol. 16:56-58. [Google Scholar]
- 20.Malone, A. S., T. H. Shellhammer, and P. D. Courtney. 2002. Effects of high pressure on the viability, morphology, lysis, and cell wall hydrolase activity of Lactococcus lactis subsp. cremoris. Appl. Environ. Microbiol. 68:4357-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarty, M. 1990. Streptococci, p. 525-538. In B. D. Davis, R. Dulbecco, H. N. Eisen, and H. S. Ginsberg (ed.), Microbiology. J. B. Lippincott Co., Philadelphia, Pa.
- 22.Miles, C. A., B. M. Mackey, and S. E. Parsons. 1986. Differential scanning calorimetry of bacteria. J. Gen. Microbiol. 132:939-952. [DOI] [PubMed] [Google Scholar]
- 23.Niven, G. W., C. A. Miles, and B. M. Mackey. 1999. The effect of hydrostatic pressure on ribosome conformation in Escherichia coli: an in vivo study using differential scanning calorimetry. Microbiology 145:419-425. [DOI] [PubMed] [Google Scholar]
- 24.Patterson, M. F., M. Quinn, R. Simpson, and A. Gilmore. 1995. Sensitivity of vegetative pathogens to high hydrostatic pressure treatment in phosphate-buffered saline and foods. J. Food Prot. 58:524-529. [DOI] [PubMed] [Google Scholar]
- 25.Pelczar, M. J., E. C. S. Chan, and N. R. Krieg. 1986. Microbiology, 5th ed., McGraw Hill, Singapore.
- 26.Ray, B., N. Kalchayanand, P. Dunne, and A. Sikes. 2001. Microbial destruction during hydrostatic pressure processing of food, p. 95-122. In F. Bozoglu, T. Deak, and B. Ray (ed.), Novel process and control technologies in the food industry. IOS Press, Amsterdam, The Netherlands.
- 27.Reynolds, E. S. 1963. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17:208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhee, S. K., and M. Y. Pack. 1980. Effect of environmental pH on chain length of Lactobacillus bulgaricus. J. Bacteriol. 144:865-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritz, M., J. L. Tholozan, M. Federighi, and M. F. Pilet. 2001. Morphological and physiological characterization of Listeria monocytogenes subjected to high hydrostatic pressure. Appl. Environ. Microbiol. 67:2240-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robey, M., A. Benito, R. H. Hutson, C. Pascual, S. F. Park, and B. M. Mackey. 2001. Variation in resistance to high hydrostatic pressure and rpoS heterogeneity in natural isolates of Escherichia coli O157:H7. Appl. Environ. Microbiol. 67:4901-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teixeira, P., H. Castro, C. Mohacsi-Farkas, and R. Kirby. 1997. Identification of sites of injury in Lactobacillus bulgaricus during heat stress. J. Appl. Microbiol. 83:219-226. [DOI] [PubMed] [Google Scholar]
- 32.Thibodeau, E., and C. Ford. 1991. Chain formation and de-chaining in Streptococus sobrinus SL-1. Oral Microbiol. Immunol. 6:313-315. [DOI] [PubMed] [Google Scholar]
- 33.Tholozan, J. L., M. Ritz, F. Juiau, M. Federighi, and J. P. Tissier. 2000. Physiological effects of high hydrostatic pressure treatments on Listeria monocytogenes and Salmonella typhimurium. J. Appl. Microbiol. 88:202-212. [DOI] [PubMed] [Google Scholar]
- 34.Verrips, C. T., and R. H. Kwast. 1977. Heat resistance of Citrobacter freundii in media with various water activities. Eur. J. Appl. Microbiol. 4:225-231. [Google Scholar]