Abstract
Mantle cell lymphoma (MCL) is an aggressive B-cell non-Hodgkin lymphoma frequently involved in the lymph nodes, bone marrow, spleen, and gastrointestinal tract. We examined the role of IL-6 in MCL. Human MCL cells expressed the membrane gp130 and soluble gp80, and some of them also secreted IL-6. Neutralizing autocrine IL-6 and/or blocking IL-6 receptors in IL-6+/gp80+ MCL cells inhibited cell growth, enhanced the rate of spontaneous apoptosis, and increased sensitivity to chemotherapy drugs. For IL-6− or gp80low MCL cells, paracrine or exogenous IL-6 or gp80 protected the cells from stress-induced death. Knockdown of gp80 in gp80high MCL cells rendered the cells more sensitive to chemotherapy drugs, even in the presence of exogenous IL-6. In contrast, overexpression of gp80 in gp80low/IL-6+ MCL cells protected the cells from chemotherapy drug-induced apoptosis in vitro and compromised the therapeutic effect of chemotherapy in vivo. IL-6 activated the Jak2/STAT3 and PI3K/Akt pathways in MCL, and the inhibition of these pathways completely or partially abrogated IL-6–mediated protection of MCL cells. Hence, our study identifies IL-6 as a key cytokine for MCL growth and survival and suggests that targeting the IL-6 pathway may be a novel way to improve the efficacy of chemotherapy in MCL patients.
Introduction
Tumor growth is regulated by accessory cells, such as stromal cells, myeloid cells, and some lymphoid immunocytes within the microenvironment. These cells secrete cytokines, chemokines, and other molecules that are associated with tumor survival, development, and metastasis.1–3 IL-6 is a pleiotropic cytokine that provokes a broad range of physiologic or pathologic actions. IL-6 binds with its receptors to affect tissue repair, neuronal differentiation, bone metabolism, hematopoiesis, inflammation, and immune regulation in addition to myocardium and liver reproduction.4–8 IL-6 also binds with its receptors on tumor cells to induce drug resistance.9 Finally, IL-6 increases the expression of acute phase proteins, such as C-reactive protein, which protects tumor cells from chemotherapy drug–induced apoptosis in an independent mechanism and stimulates tumor cells to secrete more IL-6.10
IL-6 receptors consist of 2 glycoproteins, a soluble IL-6 receptor (gp80) and a transmembrane IL-6 receptor (gp130). IL-6 signals via a heterodimeric gp80/gp130 complex, and 2 trimeric IL-6/gp80/gp130 complexes initiate the signaling and trigger activation of Janus (JAK) kinases, and the downstream effectors STAT3, SHP-2/Ras, and PI3K/Akt.11,12 Early studies implicated IL-6 and STAT3 as protumorigenic factors in most tumors and were associated with poor prognosis; IL-6 levels are increased significantly in patients with both solid tumors and hematologic malignancies.12
Mantle cell lymphoma (MCL) is a type of aggressive B-cell non-Hodgkin lymphoma characterized by constitutive activation of STAT3 and frequent resistance to conventional chemotherapy.13–15 Most patients with MCL present during the advanced stage diseases, when it frequently is involved in the bone marrow, spleen, and gastrointestinal tract.16,17 It is well known that multiple myeloma (MM) is a typical microenvironment-dependent tumor because MM cells reside mainly within the bone marrow,18 and IL-6 is a key growth and survival cytokine for MM cells.19 Thus, bone marrow stromal cells (BMSCs) may be a major source of IL-6 because they keep the MM cells growing.20 Because MCL usually involves the bone marrow, we established an in vivo mouse model of human MCL in SCID mice implanted with human fetal bone chips (SCID-hu), in which freshly isolated MCL cells from patients were engrafted directly into the human bone marrow microenvironment. Freshly isolated human MCL cells can survive and thrive after direct injection into the microenvironment of human fetal bone.21 Therefore, MCL, as well as MM, are ideal tumor models for investigating the interaction between the tumor and its microenvironment.
On the basis of the similarity between MCL and MM, we hypothesized that IL-6 also may be a key growth and survival cytokine for MCL cells. In this study, we investigated the role of IL-6 and its signaling in the survival, growth, and drug resistance of MCL. We found that MCL cells expressed IL-6 receptors, gp80 and gp130, which could be up-regulated by stress such as serum starvation or low-dose chemotherapy drug treatment. Although not all MCL cells secrete IL-6, a coculture of MCL cells with peripheral blood mononuclear cells (PBMCs) or BMSCs protected MCL cells from chemotherapy drug–induced apoptosis, which could be abrogated by IL-6–neutralizing antibodies. Our results clearly show that IL-6 and gp80, derived from MCL cells themselves or from cells in the microenvironment, play a pivotal role in MCL cell survival and drug resistance.
Methods
Reagents
Recombinant human IL-6 (rIL-6); human IL-6– and gp80-neutralizing antibodies; and ELISA kits for human IL-6 and gp80 were purchased from R&D Systems. Human gp130-blocking antibody was purchased from AbD Serotec. The Jak2 inhibitor (SD-1029), STAT3 inhibitor V, PI3K inhibitor (Ly294002), MEK inhibitor (Uo126), and mTOR inhibitor (rapamycin) were purchased from CalBiochem/EMD Biosciences. FITC-conjugated annexin V was purchased from Caltag Laboratories. Propidium iodide was purchased from Sigma-Aldrich. Fetal bovine serum was purchased from Atlanta Biologicals. Thymidine [methyl-3H] was purchased from Amersham.
Cells and fresh samples
Specimens of bone marrow aspirates and peripheral blood were obtained from patients with relapsed MCL after they provided informed consent, per the Declaration of Helsinki, and approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center. Mononuclear cells were separated by Ficoll-Hypaque density centrifugation, and MCL cells were isolated by the use of anti-CD19 magnetic microbeads (Miltenyi Biotec). PBMCs were obtained from healthy blood donors. Primary BMSCs were generated from bone marrow mononuclear cells as described previously.22 Four MCL cell lines, SP53, Mino, Granta 519, and Jeko-1, were maintained in RPMI-1640 medium (Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum; penicillin (10 000 units/mL; Sigma-Aldrich); streptomycin (10 mg/mL, Sigma-Aldrich); gentamicin (50 mg/mL; Sigma-Aldrich); and l-glutamine (29.2 mg/mL; Life Technologies).
Construction and production of lentivirus vectors
To design and clone shRNA hairpin sequences or full-length sequences of human gp80 (GenBank accession no. NM 000565) into a lentivirus vector, we used a third-generation, self-inactivating human pLKO.1 lentiviral shRNA target gene set. For gp80 knockdown, the following shRNA hairpin sequences were used (Open Biosystems): CCGGGCAGGCACTTACTACTAATAACTCGAGTTATTAGTAGTAAGTGCCTGCTTTTTG; CCGGCCACTCCTGGAACTCATCTTTCTCGAGAAAGATGAGTTCCAGGAGTGGTTTTTG; CCGGCTCTGGAAACTATTCATGCTACTC-GAGTAGCATGAATAGTTTCCAGAGTTTTTG; CCGGAGCCCTTATGACATCAGCAATCTCGAGATTGCTGATGTCATAAGGGCTTTT-TTG; CCGGCTCCTCTGCATTGCCATTGTTCTCGAGAACAATG-GCAATGCAGAGGAGTTTTTG.
We constructed 5 shRNA-gp80 lentivirus vectors, M-B1, M4-B1, M2-B2, M4-B2, B3, and A12. To summarize, oligonucleotides were annealed, digested, and inserted between Ndel and EcoRI restriction sites of the pLKO.1 vector. Some mutations were introduced in the sense sequence of the hairpin structure to facilitate sequencing and avoid destruction by bacteria during amplification in the bacterial host. Correct insertions of shRNA cassettes were confirmed by restriction mapping and direct DNA sequencing. Similarly, we also constructed a conditional gp80 overexpression vector and a constitutive gp80 overexpression vector (Open Biosystems) after subcloning of gp80 into the pcDNA3.1/nV5-DEST Vector (Invitrogen). Recombinant lentivirus vectors and empty lentivirus vectors were packaged by 293T cells through calcium phosphate transfection by use of the lentivirus expression plasmid, psPAX2 packaging plasmid, and pMD2.G envelop plasmid. Infectious lentivirus vectors were harvested after 48 hours of incubation, spun down to remove cell debris, and filtered through 0.45-mm cellulose acetate filters.
Infection of MCL cells with lentiviral supernatants
MCL cells were infected with packaged lentivirus vectors in 6-well culture plates at 30% confluence and incubated overnight in the presence of 8 μg/mL polybrene (Sigma-Aldrich). The old medium was removed the next day and replaced with medium containing 1 μg/mL of puromycin. To detect the knockdown or overexpression of gp80 in MCL cells, ELISA was used to measure secreted gp80 in the medium after 10 days of culture.
ELISA
ELISAs for IL-6 and gp80 were used to measure the secreted cytokines by MCL cells and BMSCs. Cell culture supernatants were collected on the indicated times, and the amounts of secreted IL-6 or gp80 in the supernatants were quantified with the use of a commercially available ELISA kit (R&D Systems).
Cell growth assay
Growth inhibition of MCL cell lines was assessed by a 3H-thymidine incorporation assay. To summarize, cells were plated in 96-well plates at a concentration of 5 × 104 cells/well and treated with different concentrations of agents for indicated times. One μCi 3H-thymidine was added to each well and incubated for the last 16 hours. All experiments were performed in triplicate. Radioactivity was measured by adding scintillation cocktail and counting on a scintillation β-counter (PerkinElmer Life and Analytical Sciences).
Cell viability
Cell viability of primary MCL cells from patients was assessed by a nonradioactive cell proliferation MTS assay. To summarize in brief, freshly isolated primary MCL cells from patients and PBMCs from blood donors were coincubated overnight in noncontacting transwell 96-well plates with or without IL-6–neutralizing and/or gp130-blocking antibodies. Cells were treated with bortezomib (BTZ) for an additional 24 hours, and then PBMCs in the upper Transwell inserts were removed. Twenty milliliters of CellTiter 96 Aqueous One Solution Reagent was added to primary MCL cells in the culture wells and incubated for 3 hours at 37°C in 5% CO2. Light absorbance of formazan was measured at 495 nm on a universal microplate reader equipped with KC4 software (Biotek Instruments).
Apoptosis assay
Annexin V–binding assay was used to detect cell apoptosis. Cells were seeded in 48-well plates incubated under serum starvation or with chemotherapy drugs for 48 hours. After culture, cells were washed twice with cold PBS and resuspended in binding buffer at a concentration of 1 × 106 cells/mL. This was followed by transferring 100 μL of the solution to a 5-mL tube, to which 5 μL of annexin V–FITC and 5 μL of propidium iodide were added. The tube was gently vortexed and incubated for 15 minutes at room temperature in the dark. At the end of incubation, 300 μL of binding buffer was added. Flow cytometric analysis was performed immediately with a FACScan flow cytometer (Becton Dickinson).
Western blot analysis
Cells were harvested, washed twice with cold PBS, and lysed with lysis buffer (Cell Signaling). Cell lysates were kept on ice for 30 minutes and centrifuged at 13 000g for 10 minutes at 4°C. Samples were boiled in loading buffer and separated by 10% SDS-PAGE. After electrophoresis, proteins were transferred onto a nitrocellulose membrane (Bio-Rad), which was incubated with blocking solution (5% nonfat dry milk in PBS containing 0.05% Tween-20) for 2 hours and immunoblotted with anti-STAT3 (Cell Signaling) and β-actin (Santa Cruz Biotechnology) antibodies. The membrane was finally visualized by incubation with a chemiluminescence Western blot kit (Pierce Biotechnology).
In vivo effects of IL-6 in MCL-bearing SCID mice
Six- to 8-week-old male CB-17 SCID mice (Harlan) were housed and monitored in our animal research facility. All experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee at the University of Texas MD Anderson Cancer Center. SCID mice (5 or 10 per group) were subcutaneously inoculated on the right flank with 5 × 106 MCL cells suspended in 50 μL of PBS. After palpable tumors (≥ 3 mm in diameters) developed, the mice received intraperitoneal injections of BTZ at a dosage of 1 mg/kg on days 1, 4, 7, and 10. Tumor burdens were evaluated by measuring the tumor size. Mice were humanely euthanized when they became moribund or when the subcutaneous tumors reached 15 mm in diameter. Once the mice were killed, subcutaneous tumor masses were taken out and subjected to IHC staining.
Statistical analysis
All assays were performed in triplicates and expressed as mean values ± SE. In animal experiments, overall survival was measured using the Kaplan-Meier method. Statistical significance of differences observed between experimental groups was determined by use of the Student t test; P < .05 was considered significant.
Results
MCL cells secrete IL-6 and express IL-6 receptors gp80 and gp130
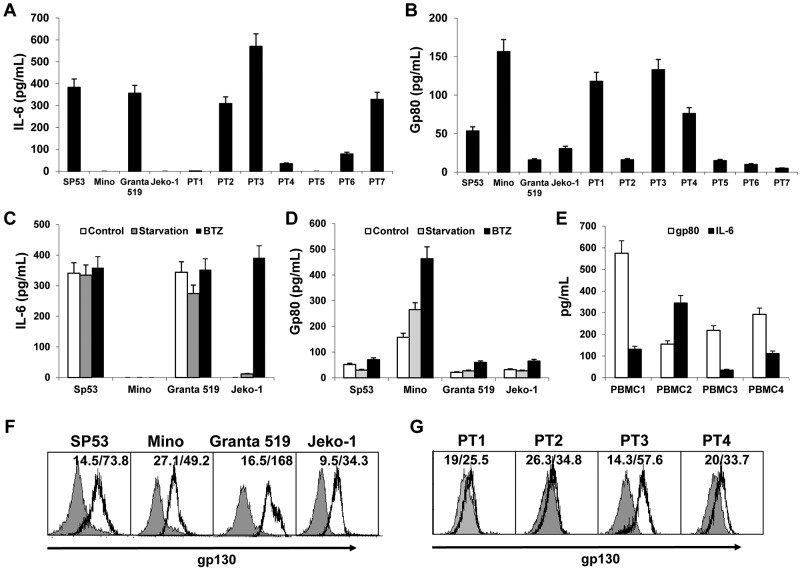
To examine whether MCL cells express IL-6 and its receptors, we first measured IL-6 and its soluble receptor gp80 in MCL culture media by ELISA. The results showed that the established MCL cell lines SP53 and Granta 519 secreted high amounts of IL-6, but Mino and Jeko-1 did not (Figure 1A). Similar patterns of IL-6 secretion also were observed in primary MCL cells from patients (n = 7; Figure 1A), indicating that most MCL cells secrete IL-6. We then examined the secretion of gp80 by the cells. Interestingly, all 4 MCL lines secreted gp80, with Mino cells secreting the greatest level of gp80 among them (Figure 1B). Similarly, primary MCL cells from all 7 patients secreted different amounts of gp80 (Figure 1B).
Figure 1.
Expression of IL-6 and its receptors by MCL. ELISA detected the levels of (A) IL-6 or (B) gp80 in culture media of MCL cell lines and primary MCL cells from 7 patients (PT1-PT7), (C) IL-6, and (D) gp80 in culture media of MCL cells after serum starvation or treatment with a low dose (2nM) of BTZ for 12 hours, and (E) IL-6 and gp80 in culture media of PBMCs from 4 blood donors (PBMC1-PBMC4). Also shown is flow cytometry analysis for the expression of gp130 on (F) MCL cell lines and (G) primary MCL cells from 4 patients (PT1-PT4).
Because IL-6 was undetectable in Mino and Jeko-1, we wondered whether IL-6 secretion could be induced in these cells. We stressed the cells by serum starvation, or a low dose of BTZ (2nM) that does not induce apoptosis in the cells,23 for 12 hours and found that stressed Jeko-1, but not Mino cells, could secrete IL-6 (Figure 1C). Stressed MCL cells generally increased the secretion of gp80 (Figure 1D).
Because not all MCL cells secrete IL-6 or sufficient amounts of gp80, MCL cells may depend on paracrine IL-6 or gp80. To test this possibility, we examined MCL microenvironment cells, such as PBMCs or BMSCs. We detected moderate levels of secreted IL-6 and gp80 in PBMC cultures from 4 blood donors (Figure 1E). Significantly, human BMSCs secreted a dramatically greater amount of IL-6 (around 6000 pg/mL) but no gp80, detected by ELISA. Thus, these cells could be the external sources of IL-6 and/or gp80 for MCL cells in vivo.
Finally, flow cytometry analysis revealed that all 4 MCL cell lines expressed gp130 on their surface (Figure 1F). Similarly, gp130 was also detected on primary MCL cells from 4 patients examined (Figure 1G). Taken together, our results demonstrated that MCL cells, including established cell lines and primary tumor cells from patients, secrete IL-6 and gp80 and express gp130 on their surfaces.
Autocrine and exogenous IL-6 protects MCL cells from apoptosis
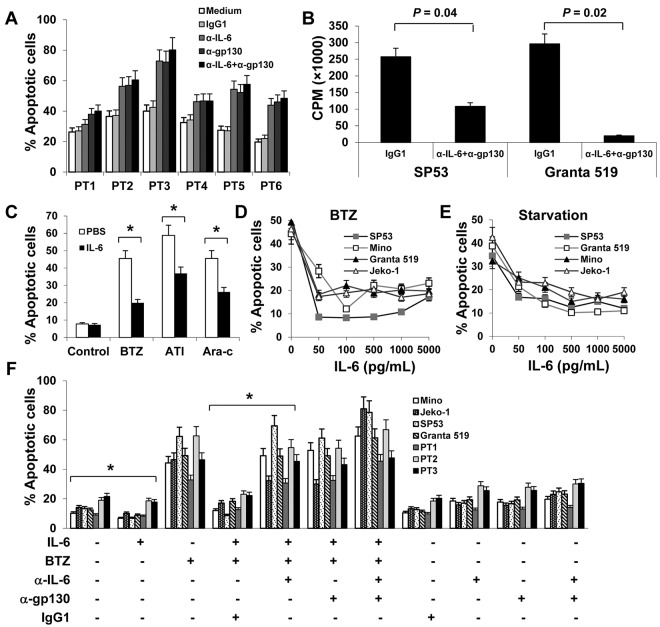
On the basis of the aforementioned results, we hypothesized that autocrine and/or paracrine IL-6 may be involved in MCL growth or survival. To examine our hypothesis, we first used IL-6–neutralizing or gp130-blocking antibodies to block the autocrine IL-6 stimulation of MCL cells. As shown in Figure 2A, primary MCL cells underwent spontaneous apoptosis ex vivo, and the addition of IL-6–neutralizing and/or gp130-blocking antibodies resulted in more cell deaths in a 48-hour culture (P ≤ .01, compared with IgG1 controls), suggesting that autocrine IL-6 signaling played a role in sustaining the survival of the cells ex vivo. Because patient-derived primary MCL cells cannot be cultured ex vivo for long times, we used MCL cell lines to further investigate the effect of autocrine IL-6 on the growth of MCL cells. Our results showed that addition of IL-6–neutralizing and gp130-blocking antibodies, but not control IgG, to the culture of SP53 and Granta 519, which secreted high amounts of IL-6, significantly inhibited the growth of the cells in a 7-day culture (Figure 2B; P < .05, compared with medium or IgG1 controls).
Figure 2.
Autocrine and exogenous IL-6 protects MCL cells from apoptosis. (A) Percentages of apoptotic cells in 48-hour cultures of primary MCL cells of 6 patients (PT1-PT6) in medium with or without addition of IL-6–neutralizing antibodies (α-IL-6; 50 μg/mL) and/or gp130-blocking antibodies (α-gp130; 5 μg/mL) or control IgG1. (B) Proliferation (CPM) of IL-6+ MCL SP53 and Granta 519 cells in a 7-day culture in medium in the presence or absence of α-IL-6 (50 μg/mL) and α-gp130 (5 μg/mL) antibodies or control IgG1. (C) Percentages of apoptotic Mino cells in 48-hour cultures in the presence of BTZ (10nM), atiprimod (ATI; 2μM), or cytarabine (Ara-c; 2μM). Mino cells were precultured with rIL-6 (0.5 ng/mL) for 2 hours before the drugs were added. Percentages of apoptotic cells induced by (D) BTZ (10nM) or (E) serum starvation. MCL cell cultures were first added with rIL-6 (50-5000 pg/mL) and 2 hours later were treated with BTZ or serum starvation. (F) Pooled data from 3 patients (PT1-PT3) showing that rIL-6 protected both MCL cell lines and primary MCL cells against BTZ-induced apoptosis. The presence of 50 μg/mL of α-IL-6 and/or 5 μg/mL of α-gp130 antibodies abrogated the protection of rIL-6 against BTZ-induced apoptosis. Results of 3 independent experiments are shown. The P values in the graphs show comparison as indicated. *P < .01.
To examine the effects of exogenous IL-6 on MCL cells, we first tested the effect of IL-6 on cell proliferation of IL-6– MCL cells. We found that exogenous IL-6 slightly increased the proliferation of Mino and Jeko-1 cells (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). We then used IL-6−/gp80high MCL cell line Mino to test the effect of IL-6 on cell apoptosis. As shown in Figure 2C, exogenous IL-6 partially protected Mino cells from chemotherapy drugs BTZ-, atiprimod-, and cytarabine-induced apoptosis (P < .01; compared with PBS control). We then used BTZ as the representative chemotherapy drug to test the effect of IL-6 on MCL cells. As shown in Figure 2D, the addition of IL-6 significantly protected 2 MCL (Jeko-1 and Mino, both were IL-6−) cell lines from BTZ-induced apoptosis (P < .01; compared with medium controls). Interestingly, exogenous IL-6 also protected other MCL cell lines, such as SP53 and Granta 519 that secreted autocrine IL-6, from BTZ-induced apoptosis (Figure 2D; P < .01; compared with medium controls). In addition, exogenous IL-6 also protected MCL cells from serum starvation-induced cell apoptosis (Figure 2E; P < .01; compared with medium controls). Importantly, addition of IL-6 partially protected primary MCL cells from ex vivo spontaneous apoptosis (supplemental Figure 1C).
To further confirm the effects of IL-6 on MCL cells, we used IL-6–neutralizing and gp130-blocking antibodies to block IL-6–mediated signaling. Results showed that exogenous IL-6 not only was able to decrease the spontaneous apoptosis but also protect IL-6− Mino and Jeko-1 and IL-6+ SP53 and Granta 519 cells and primary MCL cells from BTZ-induced apoptosis (Figure 2F; P < .01; compared with medium or IgG1 controls). Neutralizing IL-6 by IL-6–neutralizing and/or gp130-blocking antibodies abrogated the protective effect of exogenous IL-6 on the cells. The results demonstrated that both autocrine and paracrine IL-6 play a role in MCL growth and survival and in the development of MCL drug resistance.
Role of microenvironment-derived IL-6 in MCL drug resistance
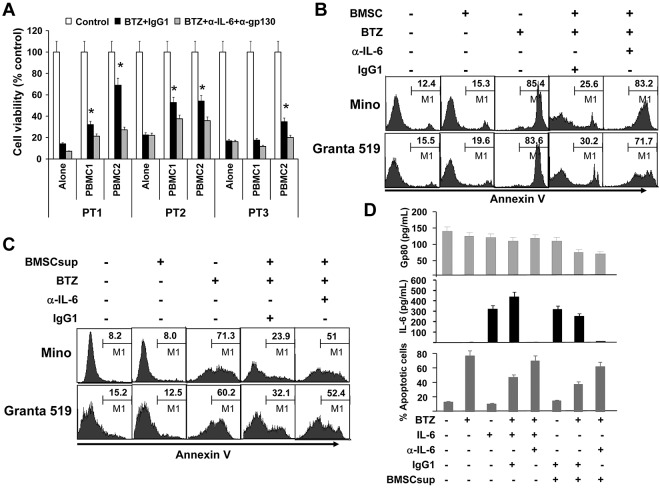
To examine the importance of microenvironment-derived IL-6, we cocultured freshly isolated primary MCL cells from patients with PBMCs from blood donors, separating the cells by Transwell inserts, in the presence or absence of IL-6–neutralizing and gp130-blocking antibodies overnight. BTZ was then added and the cells were cultured for additional 24 hours. After that, PBMCs in the transwell inserts were removed and MCL cells in the culture wells were tested for viability by MTS assay. As shown in Figure 3A, PBMCs protected primary MCL cells against BTZ-induced death (P < .05; PBMC/MCL Transwell coculture compared with MCL alone), and IL-6–neutralizing and gp130-blocking antibodies reversed the protection of PBMCs on MCL cells. Furthermore, PBMCs provided partially protection to MCL ex vivo survival, and IL-6–neutralizing and gp130-blocking antibodies, by neutralizing IL-6 secreted by both MCL cells and PBMCs, not only reduced the protective effects of PBMCs but only induced more cell death in primary MCL cells (supplemental Figure 2; P < .01).
Figure 3.
Paracrine or exogenous IL-6 protects MCL cells against chemotherapy drug–induced apoptosis. (A) Viability of freshly isolated primary MCL cells from 3 patients (PT1-PT3) cocultured for 24 hours with PBMCs from 2 blood donors (PBMC1 and PBMC2) in Transwell inserts in the presence of BTZ (10nM) with the addition of IL-6–neutralizing (α-IL-6; 50 μg/mL) and gp130-blocking (α-gp130; 5 μg/mL) antibodies or control IgG1. After the culture, PBMCs in Transwell inserts were removed and MCL cells in the culture wells were subjected to MTS assay (*P < .05; compared with culture alone). Flow cytometry analysis showing the percentages of apoptotic Mino and Granta 519 cells in (B) culture alone or coculture with BMSCs (as a source of exogenous IL-6; cultured BMSCs secreted around 6000 pg/mL of IL-6) at a ratio of 1:300 of BMSCs:MCL cells in the presence or absence of BTZ (10nM) and IL-6–neutralizing antibodies (α-IL-6; 50 μg/mL) or control IgG1; or (C) cultures with or without the addition of BMSCsup (1000-fold diluted, as a source of IL-6) in the presence or absence of BTZ (10 nM) and IL-6-neutralizing antibodies (α-IL-6; 50 μg/mL) or control IgG1. Cell apoptosis was examined by Annexin V–binding assay. (D) Levels of gp80 and IL-6, and percentages of apoptotic MCL cells in cultures of Mino cells, with or without the addition of BMSCsup (1000-fold diluted, as a source of IL-6) in the presence or absence of BTZ (10nM), exogenous IL-6 (0.5 ng/mL), IL-6–neutralizing antibodies (α-IL-6; 50 μg/mL), or control IgG1. Cells were cultured as indicated previously for 48 hours and analyzed for apoptosis by annexin V–binding assay. Culture supernatants were collected and assayed by ELISA for gp80 and IL-8 secreted by MCL cells or provided as BMSCsup or added IL-6. Results of 3 independent experiments are shown.
Next, we cocultured BMSCs and MCL cells in the presence of BTZ, in a cell ratio of 1:300 between BMSCs and MCL cells, which was based on our preliminary studies (data not shown). As shown in Figure 3B, BMSCs significantly protected Mino and Granta 519 cells from BTZ-induced apoptosis (P < .01, compared with controls). However, the addition of IL-6–neutralizing antibodies almost completely abrogated the protective effect of BMSCs (P < .01, compared with IgG1 control). The same results were obtained when the supernatant from the BMSC culture (BMSCsup) was used as a source of paracrine IL-6 to the cultures of Mino and Granta 519 in the presence of BTZ and/or IL-6–neutralizing antibodies (Figure 3C). We measured the levels of IL-6 and gp80 in the media of Mino cell cultures with different treatment conditions. As shown in Figure 3D, gp80 was detected in the media of all treatment conditions, whereas IL-6 was undetected in the media of any culture conditions, except those with addition of exogenous IL-6 or BMSCsup. However, in cultures with added exogenous IL-6 or BMSCsup and IL-6–neutralizing antibodies, no soluble IL-6 was detectable, confirming that IL-6–neutralizing antibodies were able to completely neutralize IL-6 in the media and thus abrogate IL-6–mediated protection of MCL cell apoptosis induced by BTZ.
Autocrine or paracrine gp80 protects MCL cells against apoptosis
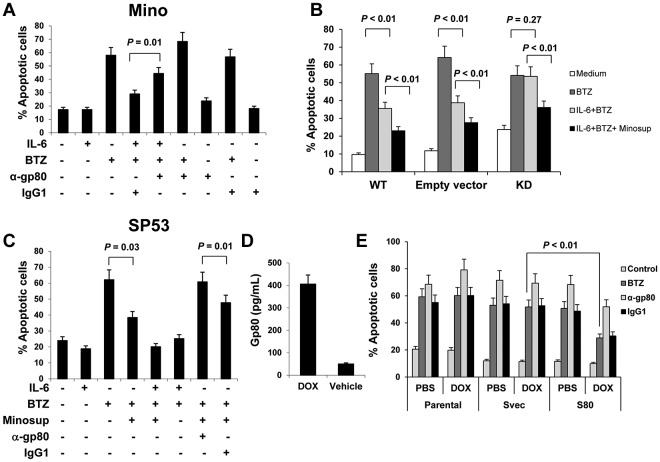
Similar to IL-6, gp80 also plays an important role in IL-6 complex–mediated cell survival signaling.24 Because Mino cells secreted a greater amount of gp80 (gp80high) than the other 3 MCL cell lines (Figure 1B), we first used gp80-neutralizing antibodies to block gp80 binding with IL-6 in cultures of Mino cells in the presence or absence of exogenous IL-6 and/or BTZ. Figure 4A shows that exogenous IL-6 protected Mino cells from BTZ-induced apoptosis, and gp80-neutralizing antibodies reversed the protective effects of exogenous IL-6 (P = .01).
Figure 4.
Autocrine or paracrine gp80 protects MCL cells against apoptosis. Percentages of apoptotic: (A) Mino cells in cultures with or without addition of IL-6 (0.5 ng/mL), gp80-neutralizing antibodies (α-gp80; 10 μg/mL), or control IgG1 for 2 hours before BTZ (10nM) was added to the culture for additional 48 hours; (B) gp80-knockdown (KD), wild-type (WT), or empty vector-transfected Mino cells (Empty vector) in cultures with or without addition of IL-6 (0.5 ng/mL) or Minosup for 2 hours before BTZ (10nM) was added to the culture for additional 48 hours; or (C) SP53 cells in cultures with or without addition of IL-6 (0.5 ng/mL), Minosup, gp80-neutralizing antibodies (α-gp80; 10 μg/mL) or control IgG1 for 2 hours before BTZ (10nM) was added to the culture for additional 48 hours. (D) ELISA showing the levels of gp80 secreted by conditional, gp80-expressing stable SP53 cells induced by doxycycline (DOX) or vehicle. (E) Percentage of apoptotic gp80-transfected (S80), empty vector-transfected (Svec), or parental SP53 cells, pretreated with PBS or DOX overnight, in cultures with addition of gp80-neutralizing antibodies (α-gp80; 10 μg/mL) or control IgG1, and/or BTZ (10nM) for 48 hours. Results of 3 independent experiments are shown. The P values in the graphs show comparison as indicated.
Next, we used pLKO.1 lentiviral transfection system to knockdown gp80 in Mino cells. We encapsulated lentivirus in 293T cells and infected Mino with 293T supernatant. We detected GFP expression as a control under fluorescence microscope (supplemental Figure 3A). We selectively cultured gp80-siRNA–transfected Mino cells after puromycin treatment. ELISA data showed that we successfully knocked down gp80 in Mino cells (supplemental Figure 3B). Figure 4B shows that gp80-knockdown Mino cells had more spontaneous apoptosis than wild-type cells (P < .05). Exogenous IL-6 protected the wild-type and vector-control, but not gp80-knockdown, Mino cells from BTZ-induced apoptosis (P < .01). The addition of Mino cell-culture supernatant (Minosup), as a source of gp80, to the cultures provided further protection against BTZ-induced apoptosis in all the cells (P < .01). These results indicated that gp80 was necessary for IL-6–mediated signaling. In other experiments, we added Minosup to SP53 cell culture, as SP53 cells secreted a high level of IL-6 but a low level of gp80. The addition of the Minosup significantly reduced the percentages of apoptotic SP53 cells induced by BTZ in the absence of exogenous IL-6 (Figure 4C; P = .03; compared with medium control). Maximum protection was seen in the cell cultures with the addition of both Minosup and exogenous IL-6. To confirm that gp80 was the effective factor in the Minosup, we used gp80-neutralizing antibodies to block gp80 binding with IL-6. As expected, gp80-neutralizing antibodies completely abrogated Minosup-mediated protection of the cells against BTZ-induced apoptosis (Figure 4C; P = .01, compared with IgG1 control).
Finally, we used the pLKO.1 lentiviral transfection system to transfect gp80 into SP53 cells. We established a conditional gp80-expressing stable SP53 cell line that could be induced to express gp80 in response to doxycycline (Figure 4D). Compared with parental or empty vector-transfected SP53 cells, gp80-transfected SP53 cells were more resistant to BTZ-mediated cytotoxicity in the presentence of doxycycline (P < .01; compared with control cells), and addition of gp80-neutralizing antibodies sensitized the cells to BTZ-mediated killing (Figure 4E).
STAT3 phosphorylation in IL-6–mediated protection of MCL cells
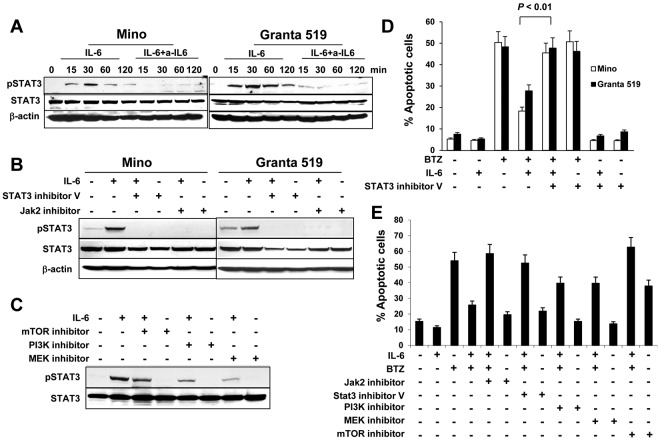
To investigate IL-6–mediated intracellular signaling, we first detected STAT3 phosphorylation in MCL cells. As shown in Figure 5A, IL-6 activated STAT3 and up-regulated its phosphorylation, and the addition of IL-6–neutralizing antibodies abrogated IL-6–mediated STAT3 phosphorylation. Next, we used both STAT3 inhibitor V and JAK2 inhibitor to pretreat Mino or Granta 519 cells. We found that STAT3 inhibitor V and JAK2 inhibitor completely blocked IL-6–mediated STAT3 phosphorylation (Figure 5B). The activation of the PI3K/mTOR and MEK pathways are associated with IL-6 complex signaling.25–28 Blockage of PI3K, mTOR, or MEK with their inhibitors partially abolished the phosphorylation of STAT3 (Figure 5C). Similarly, STAT3 inhibitor V completely blocked IL-6–mediated protection of Mino cells and Granta 519 cells against BTZ-induced apoptosis (Figure 5D; P < .01). Furthermore, we found that both Jak2 inhibitor and STAT3 inhibitor V completely abrogated IL-6–mediated protection of Mino cells against BTZ-induced apoptosis, whereas MEK and PI3K inhibitors partially abolished IL-6–mediated protection of the cells against BTZ-induced apoptosis (Figure 5E). Treatment with an mTOR inhibitor alone induced more than 35% of the apoptosis occurring in Mino cells. Taken together, these results demonstrate that STAT3 is a key transcription factor in IL-6–mediated signaling in MCL cells.
Figure 5.
Role of STAT3 activation in IL-6–mediated protection of MCL cells. Western blot showing STAT3 phosphorylation (A) in Mino and Granta 519 cells examined at 15, 30, 60, and 120 minutes in cultures without or with addition of IL-6 (0.5 ng/mL) and IL-6–neutralizing antibodies (a-IL6; 50 μg/mL), (B) in Mino and Granta 519 cells that were pretreated with 25μM of STAT3 inhibitor V or JAK2 inhibitor, respectively, for 2 hours, before culturing with IL-6 (0.5 ng/mL) for 30 minutes, or (C) in Mino cells that were pretreated with 25μM of PI3K, mTOR, or MEK inhibitors, respectively, for 2 hours before culturing with IL-6 (0.5 ng/mL) for 30 minutes. Also shown are percentages of apoptotic (D) Mino and Granta 519 cells in cultures without or with addition of BTZ (10nM), IL-6 (0.5 ng/mL), or STAT3 inhibitor V (25μM); or (E) Mino cells in cultures without or with addition of IL-6 (0.5 ng/mL), BTZ (10nM), or inhibitors (25μM) to Jak2, STAT3, PI3K, MEK, or mTOR, respectively, for 48 hours. The P value in the graph shows comparison as indicated
In vivo protective effect of IL-6/gp80 in MCL-bearing SCID mice
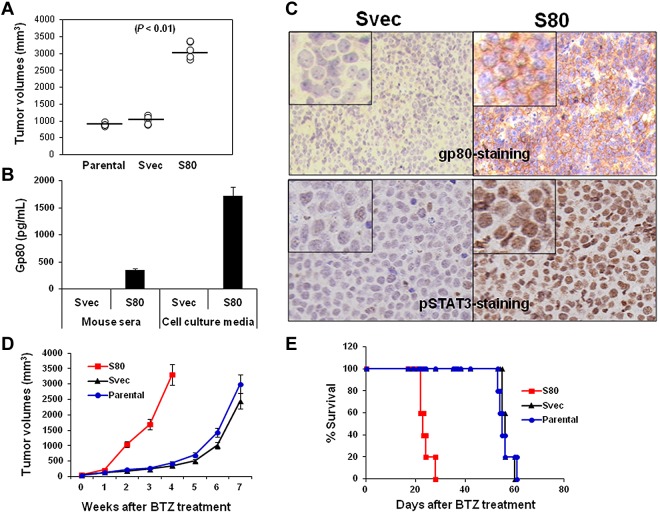
Our results showed that SP53 cells secreted high levels of IL-6 but low levels of gp80. To examine the protective effects of the IL-6 complex on MCL cells in vivo, we used a pLKO.1 lentiviral transfection system to transfect gp80 into SP53 cells and established a stable gp80-overexpressing SP53 cell line (S80). We then subcutaneously inoculated SCID mice with S80. The tumor burden data showed that S80 grew faster than parental and empty vector-transfected SP53 cells (Svec; Figure 6A, P < .01, compared with controls), indicating that overproduction of gp80 led to accelerated MCL growth in vivo. An ELISA assay revealed that greater levels of human gp80 were detected in the serum of mice bearing S80 tumors and in the culture medium of S80 tumors recovered from the mice (Figure 6B; P < .01, compared with Svec mice or cells). The IHC staining of subcutaneous tumors from the mice showed that high levels of gp80 were detected on cell surfaces of S80 tumors but not Svec tumors (Figure 6C top). Similarly, phosphorylated STAT3 also was detected in the nucleus of S80 tumors but only in a small percentage of Svec tumor cells (Figure 6C bottom). The S80 tumors were more resistant to BTZ treatment than Svec tumors, as demonstrated by the larger tumor burdens (Figure 6D, P < .05, compared with control tumors) or shorter survival time (Figure 6E, P < .05, compared with mice-bearing control tumors) of S80-bearing SCID mice. These results clearly showed that enhanced IL-6 signaling in MCL cells led to aggressive tumors and resistance to chemotherapy drug in vivo.
Figure 6.
In vivo effect of IL-6 on MCL growth and survival. (A) Tumor burdens in SCID mice (5 per group) injected with parental-, gp80-overexpressing SP53 cells (S80)–, and empty vector-transfected SP53 (Svec) cells on week 4 after tumor inoculation. (B) ELISA showing the levels of secreted human gp80 in the sera of Svec or S80 SP53-inoculated SCID mice and in culture media of dissected cells from the subcutaneous tumor mass. Mouse serum was collected when tumor mass reached 15 mm in diameter. (C) IHC staining showing the expression of gp80 (top) or phosphorylated (p) STAT3 (bottom) by S80 and Svec tumors in mice. Phosphorylated STAT3 was intensively stained in the nuclei of the tumor cells. (D) Tumor burdens and (E) survival of parental-, Svec-, and S80-bearing SCID mice (10 per group) after BTZ treatment.
Discussion
In this study, we clearly showed that IL-6 is an important cytokine involved in MCL growth, survival, and drug resistance. MCL usually originates in lymph nodes and is frequently involved in the bone marrow, spleen, and gastrointestinal tract. An earlier study showed that IL-6 and its receptors gp80 and gp130 were detected in MCL cells from patients and primary MCL cells expressed functional gp80 and gp130. We found that many MCL cells secrete different levels of IL-6 and that all MCL cells secrete different levels of gp80 and express gp130.29 For IL-6− MCL cells, our data showed that some of these cells were able to express IL-6 under stress conditions, such as serum starvation or low doses of chemotherapy drug treatment. Other studies also showed that rituximab, the anti-CD20 antibody, induced IL-6 secretion and transiently increased the concentrations of IL-6 in the peripheral blood of patients.30 We found that neutralizing autocrine IL-6 and/or gp80 inhibited cell growth, enhanced the rate of spontaneous apoptosis, and increased the sensitivity of MCL cells to chemotherapy drugs. Knockdown of gp80 in gp80high MCL cells rendered the cells more sensitive to chemotherapy, even in the presence of exogenous IL-6. In contrast, overexpression of gp80 in gp80low/IL-6+ MCL cells protected the cells from chemotherapy drug-induced apoptosis in vitro and compromised the therapeutic effect of chemotherapy in vivo. Thus, these observations indicate that autocrine IL-6 may play a role in MCL pathogenesis.
Tumor cells are protected by their microenvironment.16,17,31 Our results showed that BMSCs secreted very high levels of IL-6 and PBMCs secreted both IL-6 and gp80. These cells may be components of the MCL microenvironment and provide paracrine IL-6 and gp80 to MCL cells. We found that exogenous IL-6 or gp80 were efficacious at protecting IL-6−/gp80low MCL cells from chemotherapy drug-induced apoptosis. Furthermore, exogenous IL-6 or gp80 were also efficacious at providing additional protection to IL-6+/gp80+ MCL cells from chemotherapy drug-induced apoptosis. We cocultured freshly isolated primary MCL cells with PBMCs in Transwell inserts and found that IL-6–neutralizing antibodies abrogated the protection of PBMCs against BTZ-induced growth inhibition. We also cocultured MCL cells with BMSCs and found that coculture of MCL cells with BMSCs protected MCL cells from chemotherapy drug-induced apoptosis, which could be completely abrogated by IL-6–neutralizing antibodies. These results indicate that tumor microenvironment-derived paracrine IL-6 and gp80 may play an important role in MCL survival, growth, and drug resistance.
STAT3 is constitutively activated in MCL cells.14,32 Generally, IL-6 signaling is crucial for persistent STAT3 activation in tumor cells, which might keep tumor thriving in its microenvironment. Another mechanism of persistent STAT3 activation is associated with a G protein-coupled receptor for the lysophospholipid sphingosine-1-phosphate (S1P). STAT3 induces the expression of S1P receptor-1. The S1P–S1P receptor-1 complex induces reciprocal regulation of STAT3 activity as a major positive feedback loop for persistent STAT3 activation in tumor cells in the microenvironment and for malignant progression.33 Furthermore, studies in animals revealed that STAT3-deficient mice were resistant to skin tumor development.34 The administration of antisense oligonucleotides targeting STAT3 to animals substantially reduced the growth of the hematologic malignancies.35
In addition, STAT3 also is constitutively activated in diverse tumor-infiltrating immune cells, and ablating STAT3 in hematopoietic cells triggers an intrinsic immune-surveillance system that inhibits tumor growth and metastasis. Inhibiting STAT3 signaling in the hematopoietic system elicits multicomponent antitumor immunity.35 Our results indicated that autocrine or paracrine IL-6 was a key factor for constitutive STAT3 activation in MCL cells. We showed that phosphorylation of STAT3 was quenched after IL-6 was removed from the culture medium. Once IL-6 had been added back into the medium, MCL cells recovered STAT3 phosphorylation. Our data also showed that STAT3 phosphorylation was completely blocked by IL-6–neutralizing antibodies. Therefore, constitutive activation of STAT3 in MCL cells is dependent on the consistent presence of IL-6–mediated signaling. Baran-Marszak et al reported that the phosphorylation of STAT3 in MCL cells was dependent on IL-6 or IL-10 autocrine secretion, and/or on BCR-induced signaling, and blocking IL-6 but not IL-10 signaling pathway played the pivotal role in the inhibition of STAT3 phosphorylation in MCL cells.15 In addition, constitutive activation of STAT3 signaling confers resistance to apoptosis in hematologic malignant cells.36 In line with these results, we found that inhibition of intracellular Jak2/STAT3 signaling completely abrogated IL-6–mediated protection of MCL cells from apoptosis, whereas PI3K or MEK inhibitors partially abrogated IL-6–mediated protection.
In conclusion, our study demonstrates that IL-6 is an important growth and survival factor for human MCL cells. Autocrine and paracrine IL-6 and/or gp80 induced the resistance of MCL cells to chemotherapy. IL-6–mediated STAT3 activation is a vital signaling pathway in the microenvironment for MCL cell growth and drug resistance, and blocking IL-6–mediated STAT3 activation in MCL cells increases cells' sensitivity to chemotherapy drug-induced cytotoxicity. Therefore, targeting IL-6, gp80, and intracellular Jak2/STAT3 signaling pathway may overcome chemotherapy resistance in patients with relapsed or refractory MCL.
Supplementary Material
Acknowledgments
The authors thank Dr J. Liang for his technical assistance, Mrs Frances E. Dressman for editing the manuscript, and their departmental Lymphoma Tissue Bank for patient samples.
Supported by National Cancer Institute R01 CA138402, R01 CA138398, R01 CA163881, and P50 CA142509; Leukemia & Lymphoma Society translational research grants; the Multiple Myeloma Research Foundation, the Commonwealth Foundation for Cancer Research; and the Center for Targeted Therapy of The University of Texas MD Anderson Cancer Center.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: L.Z. and Q.Y. initiated the work, designed the experiments, and wrote the manuscript; L.Z., J.Y., J.Q., and H.L. performed the experiments and statistical analyses; J.E.R. and M.W. provided samples; and L.W.K. provided critical suggestions.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Qing Yi, MD, PhD, Department of Lymphoma/Myeloma, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 0903, Houston, TX 77030; e-mail: qyi@mdanderson.org.
References
- 1.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107(5):1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 2.Ayala F, Dewar R, Kieran M, et al. Contribution of bone microenvironment to leukemogenesis and leukemia progression. Leukemia. 2009;23(12):2233–2241. doi: 10.1038/leu.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res. 2008;14(9):2519–2526. doi: 10.1158/1078-0432.CCR-07-2223. [DOI] [PubMed] [Google Scholar]
- 4.Lam SP, Luk JM, Man K, et al. Activation of interleukin-6-induced glycoprotein 130/signal transducer and activator of transcription 3 pathway in mesenchymal stem cells enhances hepatic differentiation, proliferation, and liver regeneration. Liver Transpl. 2010;16(10):1195–1206. doi: 10.1002/lt.22136. [DOI] [PubMed] [Google Scholar]
- 5.Qiu Z, Sweeney DD, Netzeband JG, et al. Chronic interleukin-6 alters NMDA receptor-mediated membrane responses and enhances neurotoxicity in developing CNS neurons. J Neurosci. 1998;18(24):10445–10456. doi: 10.1523/JNEUROSCI.18-24-10445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramsay AJ, Husband AJ, Ramshaw IA, et al. The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science. 1994;264(5158):561–563. doi: 10.1126/science.8160012. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez Mdel C, Bernad A, Aracil M. Interleukin-6 deficiency affects bone marrow stromal precursors, resulting in defective hematopoietic support. Blood. 2004;103(9):3349–3354. doi: 10.1182/blood-2003-10-3438. [DOI] [PubMed] [Google Scholar]
- 8.Möllmann H, Nef HM, Troidl C. ‘Turning the right screw’: targeting the interleukin-6 receptor to reduce unfavourable tissue remodelling after myocardial infarction. Cardiovasc Res. 2010;87(3):395–396. doi: 10.1093/cvr/cvq186. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert LA, Hemann MT. Chemotherapeutic resistance: surviving stressful situations. Cancer Res. 2011;71(15):5062–5066. doi: 10.1158/0008-5472.CAN-11-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Wezeman M, Zhang X, et al. Human C-reactive protein binds activating Fcgamma receptors and protects myeloma tumor cells from apoptosis. Cancer Cell. 2007;12(3):252–265. doi: 10.1016/j.ccr.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Thiel S, Sommer U, Kortylewski M, et al. Termination of IL-6–induced STAT activation is independent of receptor internalization but requires de novo protein synthesis. FEBS Lett. 2000;470(1):15–19. doi: 10.1016/s0014-5793(00)01276-x. [DOI] [PubMed] [Google Scholar]
- 12.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41(16):2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Argatoff LH, Connors JM, Klasa RJ, et al. Mantle cell lymphoma: a clinicopathologic study of 80 cases. Blood. 1997;89(6):2067–2078. [PubMed] [Google Scholar]
- 14.Yared MA, Khoury JD, Medeiros LJ, et al. Activation status of the JAK/STAT3 pathway in mantle cell lymphoma. Arch Pathol Lab Med. 2005;129(8):990–996. doi: 10.5858/2005-129-990-ASOTSP. [DOI] [PubMed] [Google Scholar]
- 15.Baran-Marszak F, Boukhiar M, Harel S, et al. Constitutive and B-cell receptor-induced activation of STAT3 are important signaling pathways targeted by bortezomib in leukemic mantle cell lymphoma. Haematologica. 2010;95(11):1865–1872. doi: 10.3324/haematol.2009.019745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura Y, Sato K, Imamura Y, et al. Small cell variant of mantle cell lymphoma is an indolent lymphoma characterized by bone marrow involvement, splenomegaly, and a low Ki-67 index. Cancer Sci. 2011;102(9):1734–1741. doi: 10.1111/j.1349-7006.2011.01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salar A, Juanpere N, Bellosillo B, et al. Gastrointestinal involvement in mantle cell lymphoma: a prospective clinic, endoscopic, and pathologic study. Am J Surg Pathol. 2006;30(10):1274–1280. doi: 10.1097/01.pas.0000208899.15859.cb. [DOI] [PubMed] [Google Scholar]
- 18.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 19.Zhang XG, Klein B, Bataille R. Interleukin-6 is a potent myeloma-cell growth factor in patients with aggressive multiple myeloma. Blood. 1989;74(1):11–13. [PubMed] [Google Scholar]
- 20.Fulciniti M, Hideshima T, Vermot-Desroches C, et al. A high-affinity fully human anti-IL-6 mAb, 1339, for the treatment of multiple myeloma. Clin Cancer Res. 2009;15(23):7144–7152. doi: 10.1158/1078-0432.CCR-09-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang M, Zhang L, Han X, et al. A severe combined immunodeficient-hu in vivo mouse model of human primary mantle cell lymphoma. Clin Cancer Res. 2008;14(7):2154–2160. doi: 10.1158/1078-0432.CCR-07-4409. [DOI] [PubMed] [Google Scholar]
- 22.Huang N, Kawano MM, Mahmoud MS, et al. Expression of CD21 antigen on myeloma cells and its involvement in their adhesion to bone marrow stromal cells. Blood. 1995;85(12):3704–3712. [PubMed] [Google Scholar]
- 23.Wang M, Han XH, Zhang L, et al. Bortezomib is synergistic with rituximab and cyclophosphamide in inducing apoptosis of mantle cell lymphoma cells in vitro and in vivo. Leukemia. 2008;22(1):179–185. doi: 10.1038/sj.leu.2404959. [DOI] [PubMed] [Google Scholar]
- 24.Su JL, Lai KP, Chen CA, et al. A novel peptide specifically binding to interleukin-6 receptor (gp80) inhibits angiogenesis and tumor growth. Cancer Res. 2005;65(11):4827–4835. doi: 10.1158/0008-5472.CAN-05-0188. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda H, Hideshima T, Fulciniti M, et al. PI3K/p110{delta} is a novel therapeutic target in multiple myeloma. Blood. 2010;116(9):1460–1468. doi: 10.1182/blood-2009-06-222943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4(4):257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 27.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 28.Steelman LS, Pohnert SC, Shelton JG, et al. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18(2):189–218. doi: 10.1038/sj.leu.2403241. [DOI] [PubMed] [Google Scholar]
- 29.Tupitsyn N, Kadagidze Z, Gaillard JP, et al. Functional interaction of the gp80 and gp130 IL-6 receptors in human B cell malignancies. Clin Lab Haematol. 1998;20(6):345–352. doi: 10.1046/j.1365-2257.1998.00162.x. [DOI] [PubMed] [Google Scholar]
- 30.Winkler U, Jensen M, Manzke O, et al. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8). Blood. 1999;94(7):2217–2224. [PubMed] [Google Scholar]
- 31.Medina D, Goodell L, Glod J, et al. Mesenchymal stromal cells protect mantle cell lymphoma cells from spontaneous and drug-induced apoptosis through secretion of B-cell activating factor and activation of the canonical and non-canonical nuclear factor kappaB pathways. Haematologica. 2012;97(8):1255–1263. doi: 10.3324/haematol.2011.040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang M, Zhang L, Han X, et al. Atiprimod inhibits the growth of mantle cell lymphoma in vitro and in vivo and induces apoptosis via activating the mitochondrial pathways. Blood. 2007;109(12):5455–5462. doi: 10.1182/blood-2006-12-063958. [DOI] [PubMed] [Google Scholar]
- 33.Lee H, Deng J, Kujawski M, et al. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat Med. 2010;16(12):1421–1428. doi: 10.1038/nm.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan KS, Sano S, Kiguchi K, et al. Disruption of Stat3 reveals a critical role in both the initiation and the promotion stages of epithelial carcinogenesis. J Clin Invest. 2004;114(5):720–728. doi: 10.1172/JCI21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kortylewski M, Kujawski M, Wang T, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11(12):1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 36.Catlett-Falcone R, Landowski TH, Oshiro MM, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10(1):105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.