Abstract
Abstract:
The prevalence of asthma is increasing in developing countries and the burden of uncontrolled asthma affects patients, families, and the health system. This is to summarize, evaluate, and discuss previous reports on the impact of a targeted and comprehensive approach to the most severe cases of asthma in a low-income setting. A Program for Control of Asthma (ProAR) was developed in Salvador, Bahia, Brazil, prioritizing the control of severe asthma. By facilitating referrals from the public health system and providing proper multidisciplinary but simple management including education and medication, for free, the Program enrolled 2385 patients in 4 reference clinics. They are offered regular follow up and discharged back to primary health care only when asthma control can be maintained without requirement of a combination of an inhaled corticosteroid and a long-acting β2 agonist. ProAR has markedly reduced health resource utilization and decreased the rate of hospital admissions because of asthma in the entire City (2.8 million inhabitants) by 74%. Moderate to severe rhinitis was associated with lack of control of asthma. The average income of the families in the ProAR was US$2955 a year, and they spent 29% of all their income attempting to control the severe asthma of one member, a unbearable expenditure for a low-income family. The ProAR was shown to be cost-effective, reducing costs to the public health system (US$387 patient/year) and the families (US$789 patient/year). In a low-income setting of Brazil, an intervention prioritizing the control of severe asthma was feasible, effective, and reduced costs.
Key Words: asthma, control, treatment, prevention, hospitalization
INTRODUCTION
Asthma is associated with emergency room visits, risk of hospitalizations, and deaths. Asthma exacerbations place a considerable burden on the health care system.1 According to World Health Organization (WHO) estimates, 300 million people have asthma and 250,000 die every year.2 Most of the deaths are preventable with treatment, which is often not affordable or available in developing countries.
Surveys using the ISAAC (International Study of Asthma and Allergy in Childhood) methodology conducted in 3 cities in Bahia–Brazil demonstrate a high prevalence of symptoms of asthma: 24.6% in Salvador, 21.6% in Feira de Santana, and 30.5% in Vitoria da Conquista, among adolescents.3 In Recife, Pernambuco, Brazil, 19.1% of the adolescents have symptoms of asthma.3 Asthma is one of the leading causes of admissions and emergency room visits in Bahia and Pernambuco, as it is in the country in general.4 Some 3000 people die of asthma every year in Brazil,5 as proper treatment is not used by the majority of those who need.6
A global evaluation of trends in prevalence of asthma from surveys repeated on average 7 years apart with the same questionnaire (ISAAC) and methods in 56 countries indicates it has reached a high plateau in many of them, including Brazil, which has figures for prevalence of asthma similar to those of the United States. However, it seems to continue to increase in various developing countries such as South Africa, Kenya, Nigeria, Algeria, Morocco, Tunisia, China, Indonesia, Iran, Pakistan, Argentina, Mexico, Romania, Russia, and Ukraine.7 In Maputo, Mozambique, a recent survey found 13% of the adolescents to have symptoms of asthma.8 In addition, in this African City asthma is one of the leading causes of emergency room visits and hospital admissions. An analysis of the current situation and a forecast of the short-medium term perspectives bring no reason for optimism. Despite remarkable advances in the understanding of the mechanisms involved in the pathogenesis of asthma and the development of effective alternatives for treatment, the interaction between genetic predisposition and multiple complex environmental factors leading to varied phenotypes,9,10 has not allowed scientists to devise clearly its causality. Therefore, no effective strategy for large-scale prevention is available at the moment, and the prevalence of asthma is likely to continue to grow in developing countries as their populations adopt a westernized urban lifestyle, which has repeatedly shown to be associated to higher risk.11 Severe asthma represents some 10% of all asthma cases but accounts for most of the health resource utilization, morbidity, and mortality related to the disease.
As the complex causality of asthma is not fully understood, neither are there clear associations between phenotypes and specific risk factors, an effective and generalizable strategy for primary prevention is yet to be identified. Second-hand tobacco smoke exposure is currently the only avoidable exposure for which there is evidence to support population intervention. A series of systematic reviews of observational studies evaluated the relation between second-hand tobacco smoke exposure and respiratory diseases. They generally reported an increased risk of asthma, wheeze, and chronic cough in children from families where a parent smoked. Second-hand smoke increases the severity and frequency of symptoms in children with asthma.12
Several randomized trials of birth cohorts evaluated the effect of multifaceted interventions on the development of allergy and/or asthma. In these trials, families of children considered to be at high risk of developing allergy and/or asthma were randomly assigned to interventions or usual care. Five studies reported results after 3 to 8 years of follow-up. Interventions were associated with lower risk of wheezing.13
Reduction of exposure to house dust mites for prevention of asthma, as a single measure, is ineffective. A systematic review assessed the efficacy of house dust mite avoidance measures in patients with asthma14 and 2 additional randomized trials published afterward confirmed these findings.15,16 However, most of the randomized trials that examined multifaceted interventions to reduce environmental allergen exposure in children with atopic asthma found fewer days with symptoms, unscheduled clinic visits, and less use of beta-agonist inhalers in the intervention group, compared with the controls.17
Evidence on benefits of changes in lifestyle including diet18 and physical activity19 on subjects with asthma are still scarce to support clear recommendations aimed at prevention of asthma. Among subjects with asthma who smoke, cessation is clearly beneficial.20
There is limited information on the impact of population-based interventions for asthma control, but some studies from developed and developing countries indicate it is cost-effective. Most asthma exacerbations can be quickly relieved with a combination of inhaled salbutamol and prednisolone. These medications or similar products are commonly the only options for treatment of asthma available free of charge in developing countries. However, they are not appropriate to control symptoms of persistent asthma. A glucocorticosteroid for inhalation, such as beclomethasone, listed as an essential medicine by WHO,21 controls the airway inflammation and persistent symptoms of asthma, avoiding exacerbations, hospital admissions, and deaths.22 Inhaled corticosteroids, within the recommended doses, are safe enough to be used long term.23 For cases of moderate to severe asthma, a combination of a long acting bronchodilator and an inhaled corticosteroid is more effective than the corticosteroid alone,24 and therefore has been recommended by current guidelines.25,26
The first large scale national program based on prescriptions of inhaled corticosteroids for control of persistent asthma implemented and evaluated was the Finnish National Asthma Program, undertaken from 1994 to 2004 aiming to reduce the burden of asthma to individuals and society. The action focused on implementation of new knowledge, especially for primary care. The number of hospital days fell by 54% from 1993 to 2003. In 1993, 7212 patients received a disability pension and it was reduced to 1741 in 2003. The total increasing costs with asthma begun to decline, being reduced from 218 million euro to 213.5 million euro per year. Costs per patient per year have decreased 36%.27 According to the authors, the key for the success was an effective network of focal points for asthma and an evaluation strategy. A multicentric controlled study among inner-city children with asthma in the US had favorable outcomes.28 Observations after interventions on pediatric asthma in 2 cities in Brazil also reported reduction in morbidity.29 The results obtained in Finland after the implementation of the asthma program could potentially be reproduced in affluent societies, and it is likely to work in low- and middle-income countries as well. However, there is no evidence of the feasibility of the implementation and evaluation of the impact of such country wide approach elsewhere.
In Brazil, as in most Latin American countries, despite several attempts, there is no countrywide strategy for asthma prevention or outpatient management in public health facilities in place. Isolated nonstandardized initiatives restricted to a few cities and focused on distinct ages and asthma severity groups have been implemented. The combination of high prevalence and limited access to secondary prevention may cause high morbidity and unacceptable mortality. Morbidity resulting from asthma is not easy to measure, but hospital admission rates disclose the most severe episodes and consequently represent a relevant indicator of the burden of uncontrolled asthma in a population that has access to hospitals.
This is a review paper, aiming to summarize, evaluate, and discuss the impact of a public health intervention on hospitalizations and costs related to asthma in Salvador, Bahia, Brazil. It might be taken as a case study of feasibility and comparative effectiveness of knowledge translation practices for asthma control in low-income settings.
METHODS
Salvador City is the capital of the state of Bahia, located in northeastern region of Brazil with a population of 2.8 million. The proportional National Gross Product per capita estimate for Salvador is $2700 US dollars/year. A major part of the population has no supplementary health insurance and is covered only by the universal public health policies. ProAR encompass a public health intervention for prevention and control of asthma followed by various observational studies to investigate the outcomes of the intervention at individual and population levels. As there were no human neither financial resources to tackle the problem in general, during the initial years, the project aimed at the control of severe asthma (2385 patients registered until April 2007) by providing free medication and multidisciplinary care in 4 governmental reference centers in the City to low-income patients. In addition, ProAR has offered training to primary health care teams for prevention and control of mild to moderate asthma and educational sessions for the patients and families.
ProAR was launched in 2003 and has developed 3 categories of core activities: health care, capacity building, and research. It is lead by a team of academicians which interact with all levels of public health management to provide appropriate multidisciplinary care including education for health and medication, for free. The central reference clinic and administration of ProAR are located within facilities of the Department of Health of the City of Salvador, Bahia, which has a memorandum of understanding for cooperation with UFBA, and also provides part of ProARs health care work force.
Current ProAR Approach for Asthma Control
Facing the lack of resources to initiate a full scale intervention for prevention and control of asthma, the initiators of ProAR decided to start a program focusing on the most severe patients by opening up reference centers to offer specialized care, patients education, and pharmaceutical assistance. A concommitant series of 14 workshops (6 hours each) were carried out aiming to raise recognition of the burden of asthma, to increase awareness on the availability of effective interventions, build capacity for management of mild and moderate cases and to stimulate referrals of severe cases, having in the audience primary health care physicians, registered nurses, pharmacists, managers, and policy makers. More than 500 attendants were registered from 2005 to 2007.
ProAR takes advantage of policies of the Ministry of Health for reimbursement of expenditures with medications in cases of severe asthma. The reference clinics are accessible to all population and free at the point of use. In accordance to current guidelines,26 patients with severe asthma are treated with regular use of combined inhaled corticosteroid and a long-acting β2 agonist for maintenance, and short acting inhaled β2 agonist for rescue, as needed. Patients with persistent rhinitis receive topical nasal beclomethasone concomitantly. Educational sessions for patients and family members emphasize prevention and early control of exacerbations. A particular effort is placed on improving compliance to treatment and proper use of inhalers.
Evaluation of the Impact of ProAR at the Individual Level, An Observational Cohort
On the first day of medical evaluation in ProAR the patients are asked about the number of hospital admissions, emergency room visits, cycles of systemic corticosteroids, and school/work days missed because of an asthma exacerbation within the 12 months before their enrollment in the program, according to pre-established standard forms filled out by the attending physician or nurses. In the first year of the follow-up period, visits are scheduled 3 months apart, at most. During each medical visit, the patients are asked about the occurrence of the events above mentioned during the follow-up, and another specific standardized form is filled out to register this information, which is subsequently entered into a database. The variables are expressed as total number of events and in number of events per patient per year.
The diagnosis of severe asthma follows the guidance of Global Initiative for Asthma (GINA).30 A Spirometry is mandatory, performed with a KoKo spirometer (Software PDS Instrumentation, Inc., Louisville, CO), according to the norms of the American Thoracic Society,31 using the parameters of normality devised by Pereira et al.32
To estimate rates of adherence to treatment in patients with severe asthma, to identify predictors of adherence to treatment and evaluate the relationship between adherence to treatment, clinical and functional parameters, a random subset of patients enrolled was followed during 180 days to objectively evaluate adherence.
Evaluation of the Impact of ProAR in the Entire City (2.8 Million Inhabitants)
Data on hospitalization in the public health system has been collected from all events registered in the city of Salvador. Events do not represent the number of hospitalized patients, but the number of admissions occurred in Salvador hospitals. For comparison, we collected the same information relative to the city of Recife, northeast of Brazil.
Data on hospital admissions events from every month can be obtained from the national statistical database (SIH/DATASUS, IBGE www.ibge.gov.br). Relevant studies using this same database have already been published.33 The number of events of hospitalizations because of asthma is collected according to place of residence (City), ICD-10, age group, and sex. The number of hospitalization events are converted in hospitalization rates and analyzed separately according to sex and 2 age groups: ≤10 years old and >10 years old. The number of units of inhaled corticosteroids and fixed combination of corticosteroid and long-acting β2 agonist bronchodilator are obtained from the pharmacies supplying medication to patients from ProAR, for comparison with health outcomes. Hospitalization rates are obtained dividing absolute number of events per local and year-by-year population of each city, and multiplying by 10,000 (inhabitants). This procedure was chosen to avoid trends influenced by migration seasonality, and variations in population numbers caused by birth and deaths.
Evaluation of Costs of Severe Asthma to the Families
A sample of 198 consecutive patients with severe asthma for at least 1 year, referred to ProAR and aged 12 to 75 years old, was selected for detailed evaluation of costs. Patients typically have continuous asthma symptoms, and daily limitation to exercise, frequent exacerbations, and night symptoms, requiring daily use of a bronchodilator. The majority reported frequent emergency visits, hospitalizations, and had a low-forced expiratory volume in one second (FEV1). Some reported admission to intensive care units. The study design is that of an observational clinical cohort of patients with severe asthma. We aimed to compare the direct and indirect costs of treatment for the families before and after the intervention by ProAR. Patients were recruited when coming for the first time. At baseline they answer 3 questionnaires: AFCQ (asthma family costs questionnaire), AQLQ (Asthma Quality of Life Questionnaire),34 and ACQ (Asthma Control Questionnaire), which has just been evaluated within ProAR for accuracy, responsiveness, and reproducibility.35 Family's costs of severe asthma were measured and compared for the 2 different treatment strategies (regular public health system care and the intervention by ProAR), using accounting procedures. Costs are brought up to current values and the necessary depreciation estimated.
The AFCQ, AQLQ, and ACQ
The AFCQ is used to estimate family's costs. The questionnaire has 33 questions about direct and indirect costs for the treatment of a family member with asthma. It is divided in 6 sets of items (family income, financial help, transportation, loss of job or income because of asthma, medicines, and other expenses). Patients are asked to bring evidence for their information, such as pay slips or bank statements, transportation or meals tickets, medicine's boxes, or receipts of any purchase or expenditure. The AFCQ reliability and reproducibility were evaluated and confirmed in asthma among 30 patients before wide utilization. The group of researchers interested in health economics in ISC UFBA made it available in Portuguese and English.36
Evaluation of Costs to the Health System and Cost-Effectiveness of ProAR Strategy
A subsample of 81 patients with severe asthma, from 12 to 75 years old, living in Salvador and its metropolitan area, were consecutively selected from those attending regularly the central reference outpatient clinic of ProAR for an in depth analysis of cost-effectiveness. To be included in this study, patients were required to have more than 1 year of severe asthma. It is a before and after study (pre-post) of patients managed in ProAR central reference clinic. The objective is to compare the estimated cost-effectiveness, for families and the public health system, of 2 strategies for management of asthma experienced by the same patients: the regular asthma care available in the Salvador public health system and that offered by ProARs intervention. During the initial visit researchers collected economic and clinical retrospective information regarding health resource utilization because of asthma in the past 12 months. It included patient's and family's income, doctor's visits, medications, therapeutic devices and diagnostic tests, emergency room visits, hospitalizations, and intensive care admissions because of asthma. The same information was then collected prospectively on monthly visits during ProAR follow up and intervention for 1 year, for comparison with the previous year. To compare the cost-effectiveness of treatment of severe asthma for both strategies (regular public health system care before, and the ProAR after), the costs for the public health system, for ProAR and for the families are estimated using accounting procedures. Cost-effectiveness incremental analysis is performed by comparing the costs and health results, dividing the difference in costs per the difference of health results obtained on each strategy.37 A sensitivity analysis was carried out to ascertain the reliability of the cost-effectiveness estimates under varied assumptions regarding costs of health care.38
RESULTS
The evaluations of the impact of ProAR demonstrate control of asthma in the individuals enrolled, resulting in major reduction in health care utilization. An analysis of the first 269 subjects with severe asthma joining ProAR central reference clinic to complete 1 year of follow up found a reduction of 85% in emergency department visits, 90% in hospitalizations, and 67% in use of oral corticosteroids.39
The analysis of a subsample of 197 patients demonstrated a median family income of US$2955/year. In 47% of the cases, a family member had lost a job because of asthma. Total cost of asthma management took 29% of family income. After proper treatment asthma control scores improved by 50% and quality of life by 74%. The income of the families increased by US$711/year, as their members went back to work. The total cost of asthma to the families was reduced by a median US$789/family/year. Consequently, an annual surplus of US$1500/family became available (Fig. 1).40
FIGURE 1.
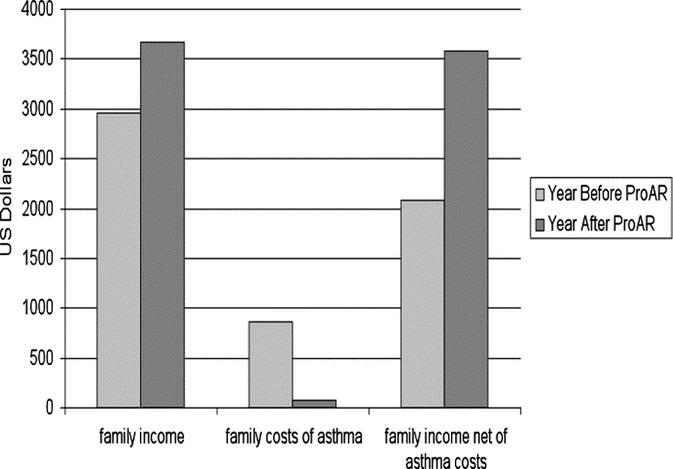
Family income, family direct and indirect cost per patient/year due to severe asthma and family annual net income, before and after ProAR intervention (median ± quartiles). Reprinted with permission from Allergy.40.
In Salvador, hospital admissions because of asthma declined by 82.3% (1998–2006). A greater proportion of this reduction (74%) happened after 2003. While there were also trends for reduction in admissions in Recife, the magnitude was smaller (22%). The rates of hospitalization because of asthma in 2006 were: 2.25/10,000 inhabitants in Salvador and 17.06 in Recife. Furthermore, in Salvador, an inverse correlation between dispensation of inhaled medication for asthma and hospitalization rates were found (−0.801; P < 0.001) (Fig. 2).41
FIGURE 2.
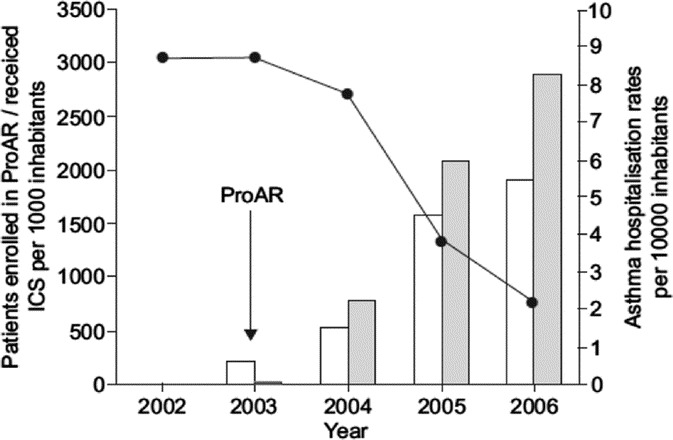
Asthma hospitalization rates, number of patients enrolled in the Program for Control of Asthma in Bahia (ProAR) and number of dispensed units of medication containing inhaled corticosteroids (ICS)/1000 in Salvador-Bahia, Brazil, from 2002 to 2006. Reprinted with permission from European Respiratory Society Journals.41.
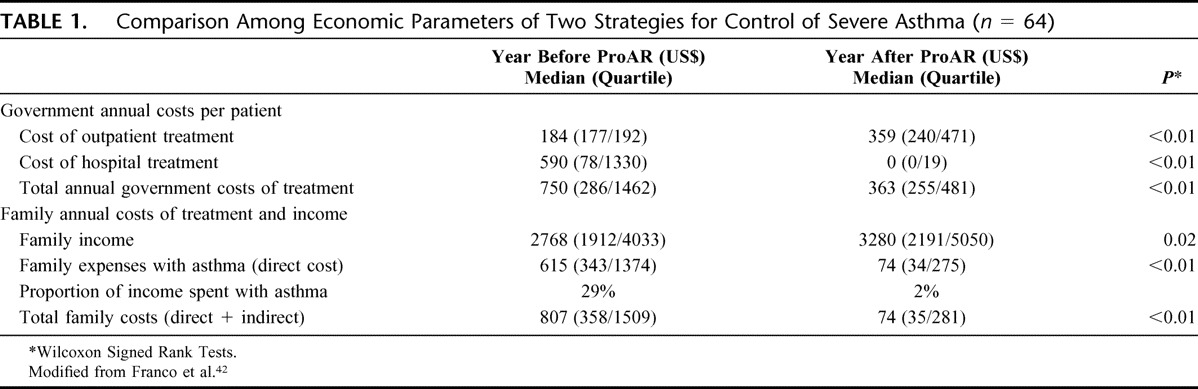
During the initial 12-months of follow-up within the program, a subsample of consecutive severe cases of asthma chosen for health economic analysis had 5 fewer days of hospitalization and 68 fewer visits to emergency/nonscheduled medical visits per year, on average. Asthma control scores improved by 50% and quality of life by 74%. The annual saving in public resources was US$387/patient (Table 1).42
TABLE 1.
Comparison Among Economic Parameters of Two Strategies for Control of Severe Asthma (n = 64)
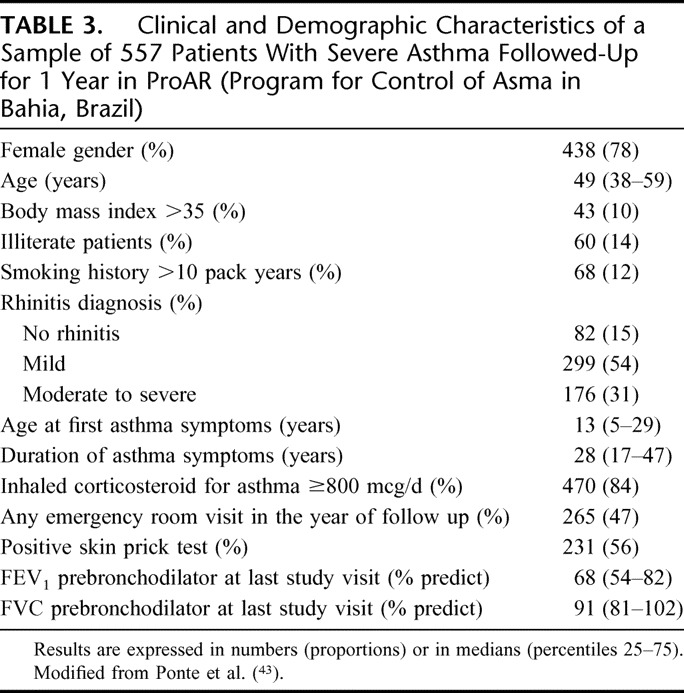
A subsample of 557 patients was investigated for the inter-relation between rhinitis and asthma. During 1 year of follow-up each patient was evaluated every 3 months with a record of emergency room visits and supply of topical corticosteroids for treatment of asthma and rhinitis. In the 1-year of follow-up visit, patients were checked for rhinitis diagnosis and severity and answered questionnaires for asthma symptoms and quality of life. Eighty-two (15%) patients had no rhinitis, 299 (54%) had mild rhinitis, and 176 (31%) moderate/severe rhinitis. In logistic regression models, moderate/severe rhinitis was a strong predictor for any emergency room visit in the follow up period [odds ratio (OR) 3.83 (2.00–7.35)], for the presence of uncontrolled asthma after 1 year of follow up [OR 12.68 (1.73–92.85)], for less than 10% improvement of the airway obstruction [OR 2.94 (1.48–5.85)], and less than 50% reduction in the number of emergency room visits [2.90 (1.02–8.26)] in the year of follow up. It was also associated with a smaller chance of more than 90% reduction in the number of emergency room visits [OR 0.27 (0.12–0.60)]. In a multivariate linear regression model, the severity of rhinitis was positively correlated with a score of asthma severity and inversely correlated to an index of quality of life (Table 2).43 Table 3 presents a summary of the clinical and demographic characteristics of a sample of 557 consecutive patients with severe asthma followed up for 1 year in ProAR.43
TABLE 2.
Asthma Severity and Response to Treatment According to Co-Existence and Severity of Rhinitis
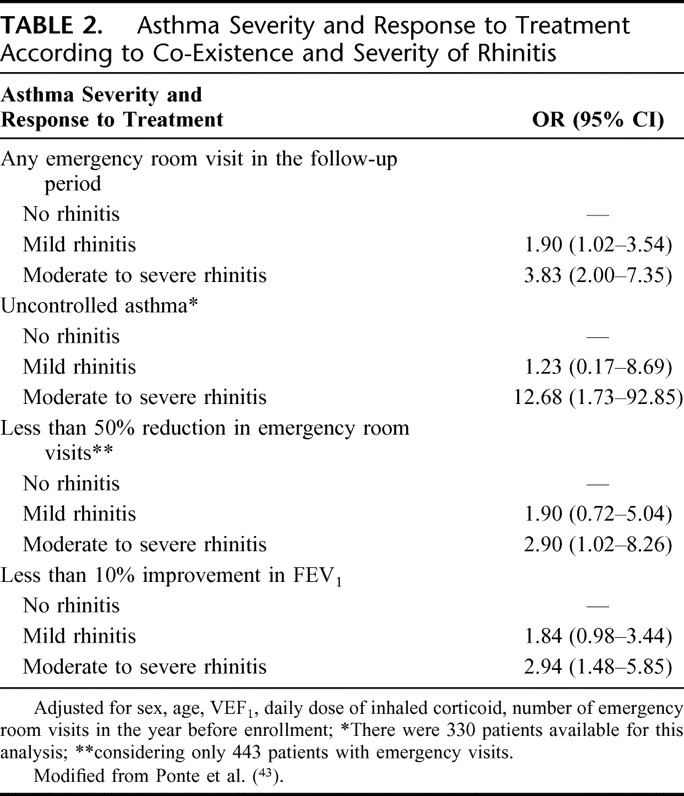
TABLE 3.
Clinical and Demographic Characteristics of a Sample of 557 Patients With Severe Asthma Followed-Up for 1 Year in ProAR (Program for Control of Asma in Bahia, Brazil)
DISCUSSION
Asthma, a global health problem, causes relevant morbidity, numerous preventable deaths and high costs to the families and to the health systems.1,2 The costs of asthma are related to severity of disease,1 and hospitalizations represent 50% of all the expenditures with asthma.25,26
Our main findings are: 1) ProAR has reduced health resource utilization and remarkably reduced the rate of hospital admissions because of asthma in the entire City; 2) severe asthma leads to a unberable expenditure for low-income families, ProAR was shown to be cost-effective, reduced costs to the public health system and to the families (by US$789/patient/year); 3) moderate to severe rhinitis was associated with lack of control of asthma.
A limitation of our study is that information from the year before intervention was collected retrospectively from patient reports. It would not be ethical to have a parallel control group of severe asthmatics followed up without access to ProAR, once free preventive inhaled medication was made available. Therefore, the only way we could study patients inside and outside the program was comparing their own profile before and after the intervention. In this study, patients apparently had no difficulty to recall hospitalizations, emergency room visits, income, financial help, medicine prices, and transportation expenses. They were able to recall and to present evidence of their recent expenses with medicine, bringing priced medicine boxes or drugstore receipts, and medical reports and prescriptions from hospitalizations and emergency visits as well. In favor of findings of this report, it shall be considered any possible loss of information related with the retrospective period, acts toward decreasing the estimated economic costs during this period. Therefore the impact of the asthma on the family income can be even greater than presented here. The presence of an interviewer might have influenced the patient's answers. However, this was needed as the majority of patients had low educational level and some were illiterate. The interviewer was trained to avoid influencing the answers, and was the same for all patients throughout all the visits.
Although asthma hospitalizations have decreased in recent years in Brazil, in large urban centers such as Recife and Salvador, they seem to decrease slowly since 1998. In Salvador, the implementation of ProAR in 2003 has likely accelerated these declining trends.
The most important novel observation of this study is the rapid reduction in asthma admissions in a City of 2.8 million inhabitants, after a very focused intervention targeting patients at greater risk. This observation needs to be reproduced in other locations, as it would be of remarkable importance to public health in countries with high morbidity because of asthma and limited resources. We speculate that the most important factors that determined our favorable results were: 1) considerable proportion of patients with severe asthma with no assess to good quality care including affordable medication at baseline; 2) establishment of treatment with the most effective and safe medications; 3) education program to increase compliance to proper medication use; 4) referral system made easy to patients and health services. Given these 4 conditions, it seems unlikely that a similar intervention may fail.
The future of ProAR depends on renewed support from research agencies and public health authorities in Brazil. It is fully nested in the public health system and has all prerequisites of sustainability: qualified human resources, infrastructure, demand, effectiveness, and efficiency. If adequate support is obtained the severe asthma program should be expanded to all other cities in the state of Bahia, and its experts should help the State Department of Health in building capacity of the primary health care professionals to handle the milder forms of the disease.
In this study, moderate/severe rhinitis was strongly associated with parameters that indicate greater asthma severity. This was demonstrated by the increased risk of any emergency room visit for acute asthma in the year of follow up, poor asthma control, less reduction in the number of emergency room visit in the follow up period, and limited improvement in airway obstruction after 1 year of follow up. Patients with mild rhinitis also had an increased risk of any emergency room visit for acute asthma and a smaller reduction in the number of emergency room visits in the year of follow up. These results are in accordance with data from previous retrospective studies, which observed more severe symptoms of asthma and increased risk for emergency room visits and for hospital admissions for acute asthma among patient with concomitant rhinitis.44–46
The consequence of increased asthma severity is a compromised quality of life and increased cost of asthma. In this study, it was also possible to demonstrate that rhinitis severity contribute to a decreased asthma related quality of life in a multivariate model. Rhinitis severity was positively correlated with the annual cost of asthma. Although the present study reinforces the association between rhinitis, asthma severity, and cost of asthma, it is not possible to conclude that rhinitis is the cause of asthma severity and increased cost. The possibility that they are both the manifestation of one only disease may be the most important factor underlying these strong associations. The proposed paradigm of unicity of disease in asthma and rhinitis47 would imply the existence of an intrinsic link between the severity of the symptoms in the upper and in the lower airways.
It is not possible to conclude that the treatment of rhinitis will reduce asthma cost either. This is an important issue, because the concept that rhinitis impacts on asthma control is largely accepted. Although the interrelationship between asthma and rhinitis is largely demonstrated, the impact of rhinitis treatment on asthma is still a matter of investigation. Our results suggest that future research should focus on evaluating the cost-effectiveness of adding nasal corticosteroids for the treatment of severe asthma in patients with moderate/severe rhinitis.
Some messages to policy makers should arise from the experience of ProAR, in line with the vision of the Global Alliance against Chronic Respiratory Diseases of “a world where all people breathe freely”2: 1) recommendations for the treatment of severe and/or uncontrolled asthma from best evidence-based current asthma guidelines are applicable and effective in low-resource settings; 2) free management of the most severe cases of asthma according to the guidelines, in reference centers, not only restores the patient's capability to breathe, but also alleviate the economical suffocation of poor families further impoverished by the cost of the ailment; 3) the strategy of ProAR, in Salvador, Bahia, Brazil, reduced health resource utilization, decreased the costs with asthma to the public health system, and could be adapted to similar settings elsewhere.48 This provides a remarkable opportunity to use current knowledge and available technology to attenuate social inequalities.
REFERENCES
- 1.Bousquet J, Bousquet PJ, Godard P, Daures JP. The public health implications of asthma. Bull World Heath Organ. 2005;83:548–554 [PMC free article] [PubMed] [Google Scholar]
- 2.Bousquet J, Khaltaev N. Global surveillance, prevention and control of chronic respiratory diseases. A comprehensive approach. World Health Organization, Geneva, 2007;146p [Google Scholar]
- 3.Solé D, Wandalsen GF, Camelo-Nunes IC, Naspitz CK, ISAAC-Brazilian Group Prevalence of symptoms of asthma, rhinitis, and atopic eczema among Brazilian children and adolescents identified by the International Study of Asthma and Allergies in Childhood (ISAAC)-Phase 3. J Pediatr (Rio J). 2006;82:341–346 [DOI] [PubMed] [Google Scholar]
- 4.Brasil Ministério da Saúde. Informações de Saúde. Available at: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sih/cnv/mruf.def];. Accessed October 20, 2008. [Google Scholar]
- 5.Brasil Ministério da Saúde. Informações de Saúde. Available at: http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sim/cnv/obtuf.def]. Accessed October 23, 2008. [Google Scholar]
- 6.Neffen H, Fritscher C, Schacht FC, Levy G, Chiarella P, et al. Asthma control in Latin America: the Asthma Insights and Reality in Latin America (AIRLA) survey. Rev Panam Salud Publica. 2005;17:191–197 [DOI] [PubMed] [Google Scholar]
- 7.Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multi-country cross-sectional surveys. Lancet. 2006;368:733–743 [DOI] [PubMed] [Google Scholar]
- 8.Mavale-Manuel S, Joaquim O, Macome C, Almeida L, Nunes E, et al. Asthma and allergies in schoolchildren of Maputo. Allergy. 2007;62:265–271 [DOI] [PubMed] [Google Scholar]
- 9.Barnes KC, Grant AV, Hansel NN, Gao P, Dunston GM. African Americans with asthma: genetic insights. Proc Am Thorac Soc. 2007;4:58–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez FD. Genes, environments, development and asthma: a reappraisal. Eur Respir J. 2007;29:179–184 [DOI] [PubMed] [Google Scholar]
- 11.Nicolaou N, Siddique N, Custovic A. Allergic disease in urban and rural populations: increasing prevalence with increasing urbanization. Allergy. 2005;60:1357–1360 [DOI] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Chapter 6. Respiratory Effects in Children from Exposure to Second-hand Smoke. Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. [Google Scholar]
- 13.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update. Allergy. 2008;63:(Suppl1):868–160 [DOI] [PubMed] [Google Scholar]
- 14.Gotzsche PC, Johansen HK, Schmidt LM, Burr ML. House dust mite control measures for asthma. Cochrane database of systematic reviews (Online). 2004;CD001187. [DOI] [PubMed] [Google Scholar]
- 15.de Vries MP, van den Bemt L, Aretz K, Thoonen BP, Muris JW, et al. House dust mite allergen avoidance and self-management in allergic patients with asthma: randomised controlled trial. Br J Gen Pract. 2007;57:184–190 [PMC free article] [PubMed] [Google Scholar]
- 16.Luczynska C, Tredwell E, Smeeton N, Burney P. A randomized controlled trial of mite allergen-impermeable bed covers in adult mite-sensitized asthmatics. Clin Exp Allergy. 2003;33:1648–1653 [DOI] [PubMed] [Google Scholar]
- 17.Morgan WJ, Crain EF, Gruchalla RS, O'Connor GT, Kattan M, et al. Inner-City Asthma Study Group Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068–1080 [DOI] [PubMed] [Google Scholar]
- 18.Wood LG, Garg ML, Powell H, Gibson PG. Lycopene-rich treatments modify noneosinophilic airway inflammation in asthma: proof of concept. Free Radic Res. 2008;42:94–102 [DOI] [PubMed] [Google Scholar]
- 19.Basaran S, Guler-Uysal F, Ergen N, Seydaoglu G, Bingol-Karakoç G, Ufuk Altintas D. Effects of physical exercise on quality of life, exercise capacity and pulmonary function in children with asthma. J Rehabil Med. 2006;38:130–135 [DOI] [PubMed] [Google Scholar]
- 20.Chaudhuri R, Livingston E, McMahon AD, Lafferty J, Fraser I, et al. Effects of smoking cessation on lung function and airway inflammation in smokers with asthma. Am J Respir Crit Care Med. 2006;174:127–133 [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization (WHO) Model List of Essential Medicines. Geneva, World Health Organization; 2007 [Google Scholar]
- 22.Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332–336 [DOI] [PubMed] [Google Scholar]
- 23.Bacharier LB, Raissy HH, Wilson L, McWilliams B, Strunk RC, Kelly HW. Long-term effect of budesonide on hypothalamic-pituitary-adrenal axis function in children with mild to moderate asthma. Pediatrics. 2004;113:1693–1699 [DOI] [PubMed] [Google Scholar]
- 24.Pauwels RA, Löfdahl CG, Postma DS, Tattersfield AE, O'Byrne P, Barnes PJ, Ullman A. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med. 1997;337:1405–1411 [DOI] [PubMed] [Google Scholar]
- 25.National Asthma Education and Prevention Program (NHLBI, NIH)-Expert Panel Report 3 (Publication No. 07–4051). Guidelines for the Diagnosis and Management of Asthma. Bethesda: US Department of Health and Human Services; 2007;416p [Google Scholar]
- 26.Global Strategy for Asthma Prevention and Management, full report. Global Initiative for Asthma (GINA); 2006;109pp [Google Scholar]
- 27.Haahtela T, Tuomisto LE, Pietinalho A, Klaukka T, Erhola M, et al. A 10 year asthma programme in Finland: major change for the better. Thorax. 2006;61:663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans R, 3rd, Gergen PJ, Mitchell H, Kattan M, Kercsmar C, et al. A randomized clinical trial to reduce asthma morbidity among inner-city children: results of the National Cooperative Inner-City Asthma Study. J Pediatr. 1999;135:332–338 [DOI] [PubMed] [Google Scholar]
- 29.Fischer GB, Camargos PA, Mocelin HT. The burden of asthma in children: a Latin American perspective. Paediatr Respir Rev. 2005;6:8–13 [DOI] [PubMed] [Google Scholar]
- 30.Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA); 2002 [Google Scholar]
- 31.American Thoracic Society (ATS) Standardization of Spirometry. 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–1136 [DOI] [PubMed] [Google Scholar]
- 32.Pereira CAC, Barreto SP, Simões JG, Pereira FWL, Gerstler JG, Nakatani J. Valores de Referência para Espirometria em uma amostra da população brasileira adulta. J Bras Pneumol. 1992;18:10–12 [Google Scholar]
- 33.Okie S. Fighting HIV: lessons from Brazil. N Engl J Med. 2006;54:191–197 [DOI] [PubMed] [Google Scholar]
- 34.Juniper EF, Guyatt GH, Epstein RS. Evaluation of impairment of health-related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax. 1992;47:76–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leite M, Ponte EV, Petroni J, D'Oliveira A, Jr, Pizzichini E, Cruz AA. Evaluation of the Asthma Control Questionnaire validated for use in Brazil. J Bras Pneumol. 2008;34:756–763 [DOI] [PubMed] [Google Scholar]
- 36.Universidade Federal da Bahia, Instituto de Saúde Coletiva Programa de Economia da Saúde. Available at: http://www.pecs.ufba.br/scripts/arquivos/default.asp]. Accessed November 9, 2008. [Google Scholar]
- 37.Eddy D. Cost-effectiveness analysis: a conversation with my father. JAMA. 1992;267:1669–1675 [DOI] [PubMed] [Google Scholar]
- 38.Drummond M, O'Brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation. In: Methods for the Economic Evaluation. London: Oxford University; 1997 [Google Scholar]
- 39.Ponte E, Franco RA, Souza-Machado A, Souza-Machado C, Cruz AA. Impact that a program to control severe asthma has on the use of Unified Health System resources in Brazil. J Bras Pneumol. 2007;33:15–19 [DOI] [PubMed] [Google Scholar]
- 40.Franco R, Nascimento HF, Cruz AA, Santos AC, Souza-Machado C, et al. The economic impact of severe asthma to low-income families. Allergy. 2009;64:478–483 [DOI] [PubMed] [Google Scholar]
- 41.Souza-Machado C, Souza-Machado A, Franco R, Ponte EV, Barreto ML, et al. Rapid reduction in hospitalizations after an intervention to manage severe asthma. Eur Respir J. 2010;35:1–7 [DOI] [PubMed] [Google Scholar]
- 42.Franco R, Santos AC, do Nascimento HF, Souza-Machado C, Ponte E, et al. Cost-effectiveness analysis of a state funded programme for control of severe asthma. BMC Public Health. 2007;7:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ponte EV, Franco R, Nascimento HF, Souza-Machado A, Cunha S, et al. Lack of control of severe asthma is associated with co-existence of moderate-to-severe rhinitis. Allergy. 2008;63:564–569 [DOI] [PubMed] [Google Scholar]
- 44.Kocevar VS, Thomas J, Jonsson L, Yin DD, Bisgaard H. Association between allergic rhinitis and hospital resource use among asthmatic children in Norway. Allergy. 2005;60:338–342 [DOI] [PubMed] [Google Scholar]
- 45.Thomas M, Kocevar VS, Zhang Q, Yin DD, Price D. Asthma related heath resource use among asthmatic children with and without concomitant allergic rhinitis. Pediatrics. 2005;115:129–134 [DOI] [PubMed] [Google Scholar]
- 46.Price D, Zhang Q, Kocevar VS, Yin DD, Thomas M. Effect of a concomitant diagnosis of allergic rhinitis on astma-related heath care use by adults. Clin Exp Allergy. 2005;35:282–287 [DOI] [PubMed] [Google Scholar]
- 47.Cruz AA. The united airways require an holistic approach to management. Allergy. 2005;60:871–874 [DOI] [PubMed] [Google Scholar]
- 48.Cruz AA, Bousquet PJ. The unbearable cost of severe asthma in underprivileged populations. Allergy. 2009;64:319–321 [DOI] [PubMed] [Google Scholar]


