Abstract
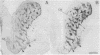
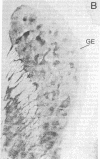
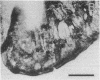
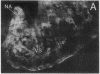
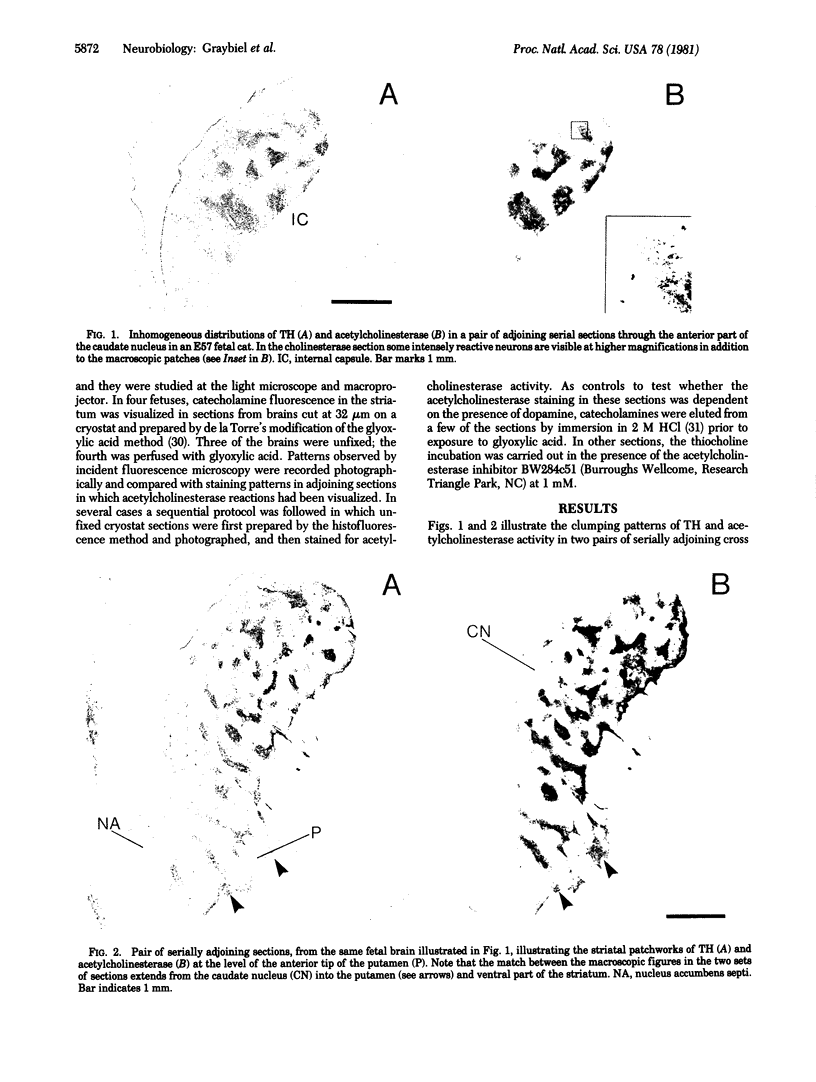
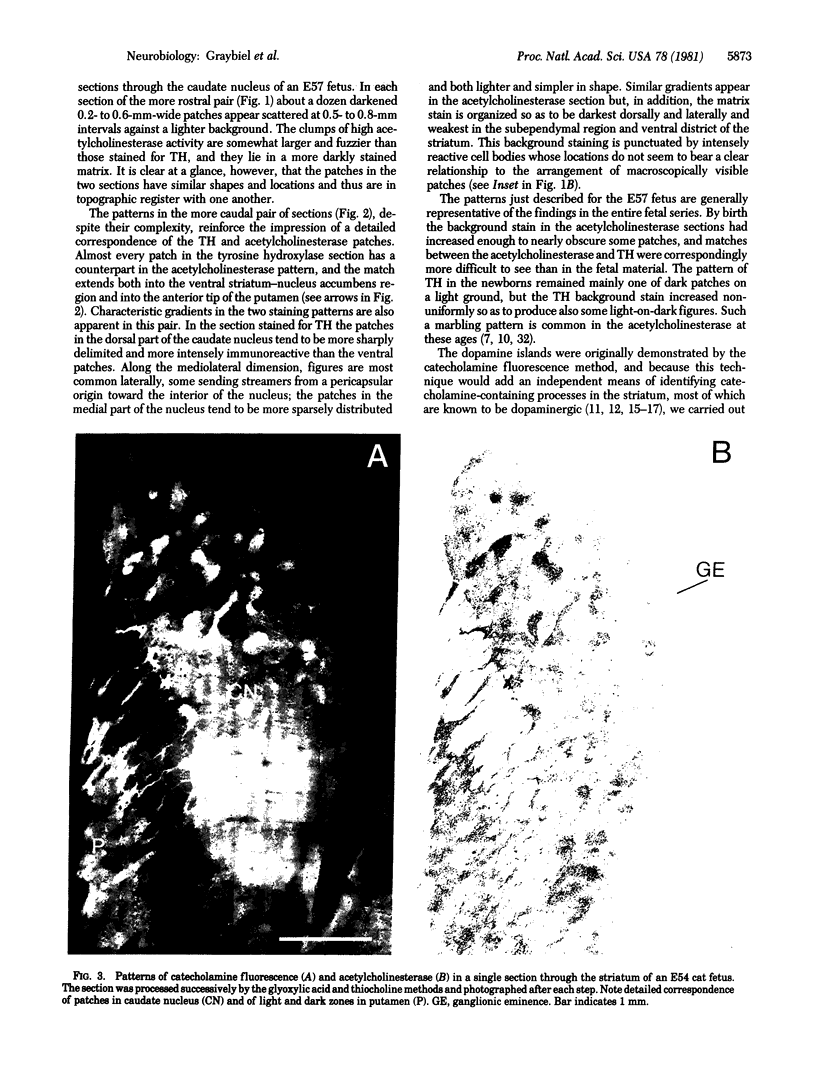
The distribution of dopamine-containing processes in the striatum of fetal and neonatal cats was studied by immunohistochemical and glyoxylic acid histofluorescence methods and compared to the distribution of acetylcholinesterase (acetylcholine acetylhydrolase, EC 3.1.1.7) observed by thiocholine histochemistry in the same or serially adjoining sections. Both methods for demonstrating the dopamine innervation revealed the characteristic patchwork of dopamine "islands" in the caudoputamen, in which catecholamine histofluorescence or tyrosine hydroxylase [tyrosine 3-monooxygenase; L-tyrosine, tetrahydropteridine:oxygen oxidoreductase (3-hydroxylating), EC 1.14.16.2]-like immunoreactivity was concentrated into 0.2- to 0.6-mm-wide patches. Both methods also demonstrated a high degree of patterning of the dopamine innervation in the ventral striatum, including the nucleus accumbens septi. A detailed and striking match was found between these configurations and the compartmental distribution of acetylcholinesterase observed in the caudoputamen and ventral striatum of the same brains. The correspondence between the dopamine and acetylcholinesterase figures was most obvious in the fetal brains, in which the background acetylcholinesterase staining was lightest, but matches between the dopamine islands and acetylcholinesterase patches could still be seen in the kittens. There was no clear alignment of striatal cell bodies stained for acetylcholinesterase with either the dopamine or the acetylcholinesterase-positive patches. Nor was there an obvious correspondence between dopamine and acetylcholinesterase in the striatal background matrix. We conclude that, at least during ontogenesis, it is the clustered arrangements of dopamine and acetylcholinesterase that are, in particular, tightly linked, and we suggest that information about the maturation of these clusters may be crucial in assessing the functions of striatal dopamine and acetylcholinesterase in the adult.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Technical considerations on the use of horseradish peroxidase as a neuronal marker. Neuroscience. 1977;2(1):141–145. doi: 10.1016/0306-4522(77)90074-4. [DOI] [PubMed] [Google Scholar]
- Butcher L. L., Hodge G. K. Postnatal development of acetylcholinesterase in the caudate-putamen nucleus and substantia nigra of rats. Brain Res. 1976 Apr 23;106(2):223–240. doi: 10.1016/0006-8993(76)91022-2. [DOI] [PubMed] [Google Scholar]
- Butcher L. L., Marchand R. Dopamine neurons in pars compacta of the substantia nigra contain acetylcholinesterase: histochemical correlations on the same brain section. Eur J Pharmacol. 1978 Dec 1;52(3-4):415–417. doi: 10.1016/0014-2999(78)90301-1. [DOI] [PubMed] [Google Scholar]
- CARLSSON A., FALCK B., HILLARP N. A. Cellular localization of brain monoamines. Acta Physiol Scand Suppl. 1962;56(196):1–28. [PubMed] [Google Scholar]
- Chubb I. W., Hodgson A. J., White G. H. Acetylcholinesterase hydrolyzes substance P. Neuroscience. 1980;5(12):2065–2072. doi: 10.1016/0306-4522(80)90124-4. [DOI] [PubMed] [Google Scholar]
- De la Torre J. C. An improved approach to histofluorescence using the SPG method for tissue monoamines. J Neurosci Methods. 1980 Oct;3(1):1–5. doi: 10.1016/0165-0270(80)90029-1. [DOI] [PubMed] [Google Scholar]
- Geneser-Jensen F. A., Blackstad T. W. Distribution of acetyl cholinesterase in the hippocampal region of the guinea pig. I. Entorhinal area, parasubiculum, and presubiculum. Z Zellforsch Mikrosk Anat. 1971;114(4):460–481. doi: 10.1007/BF00325634. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M., Ragsdale C. W., Jr Clumping of acetylcholinesterase activity in the developing striatum of the human fetus and young infant. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1214–1218. doi: 10.1073/pnas.77.2.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A. M., Ragsdale C. W., Jr Fiber connections of the basal ganglia. Prog Brain Res. 1979;51:237–283. [PubMed] [Google Scholar]
- Graybiel A. M., Ragsdale C. W., Jr Histochemically distinct compartments in the striatum of human, monkeys, and cat demonstrated by acetylthiocholinesterase staining. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5723–5726. doi: 10.1073/pnas.75.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A. M., Ragsdale C. W., Jr, Moon Edley S. Compartments in the striatum of the cat observed by retrograde cell labeling. Exp Brain Res. 1979 Jan 2;34(1):189–195. doi: 10.1007/BF00238352. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M., Ragsdale C. W., Jr, Yoneoka E. S., Elde R. P. An immunohistochemical study of enkephalins and other neuropeptides in the striatum of the cat with evidence that the opiate peptides are arranged to form mosaic patterns in register with the striosomal compartments visible by acetylcholinesterase staining. Neuroscience. 1981;6(3):377–397. doi: 10.1016/0306-4522(81)90131-7. [DOI] [PubMed] [Google Scholar]
- Greenfield S., Cheramy A., Leviel V., Glowinski J. In vivo release of acetylcholinesterase in cat substantia nigra and caudate nucleus. Nature. 1980 Mar 27;284(5754):355–357. doi: 10.1038/284355a0. [DOI] [PubMed] [Google Scholar]
- Joh T. H., Geghman C., Reis D. Immunochemical demonstration of increased accumulation of tyrosine hydroxylase protein in sympathetic ganglia and adrenal medulla elicited by reserpine. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2767–2771. doi: 10.1073/pnas.70.10.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil K. Patch-like termination of thalamic fibers in the putamen of the rhesus monkey: an autoradiographic study. Brain Res. 1978 Jan 27;140(2):333–339. doi: 10.1016/0006-8993(78)90464-x. [DOI] [PubMed] [Google Scholar]
- Künzle H. Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res. 1975 May 2;88(2):195–209. doi: 10.1016/0006-8993(75)90384-4. [DOI] [PubMed] [Google Scholar]
- Loizou L. A. The postnatal ontogeny of monoamine-containing neurones in the central nervous system of the albino rat. Brain Res. 1972 May 26;40(2):395–418. doi: 10.1016/0006-8993(72)90142-4. [DOI] [PubMed] [Google Scholar]
- Mesulam M. M., Van Hoesen G. W. Acetylcholinesterase-rich projections from the basal forebrain of the rhesus monkey to neocortex. Brain Res. 1976 Jun 4;109(1):152–157. doi: 10.1016/0006-8993(76)90385-1. [DOI] [PubMed] [Google Scholar]
- Nobin A., Björklund A. Topography of the monoamine neuron systems in the human brain as revealed in fetuses. Acta Physiol Scand Suppl. 1973;388:1–40. [PubMed] [Google Scholar]
- Olson L., Boréus L. O., Seiger A. Histochemical demonstration and mapping of 5-hydroxytryptamine- and catecholamine-containing neuron systems in the human fetal brain. Z Anat Entwicklungsgesch. 1973 Apr 16;139(3):259–282. doi: 10.1007/BF00519968. [DOI] [PubMed] [Google Scholar]
- Olson L., Seiger A., Fuxe K. Heterogeneity of striatal and limbic dopamine innervation: highly fluorescent islands in developing and adult rats. Brain Res. 1972 Sep 15;44(1):283–288. doi: 10.1016/0006-8993(72)90385-x. [DOI] [PubMed] [Google Scholar]
- Palkovits M., Jacobowitz D. M. Topographic atlas of catecholamine and acetylcholinesterase-containing neurons in the rat brain. II. Hindbrain (mesencephalon, rhombencephalon). J Comp Neurol. 1974 Sep 1;157(1):29–42. doi: 10.1002/cne.901570104. [DOI] [PubMed] [Google Scholar]
- Ragsdale C. W., Jr, Graybiel A. M. The fronto-striatal projection in the cat and monkey and its relationship to inhomogeneities established by acetylcholinesterase histochemistry. Brain Res. 1981 Mar 16;208(2):259–266. doi: 10.1016/0006-8993(81)90556-4. [DOI] [PubMed] [Google Scholar]
- Royce G. J. Autoradiographic evidence for a discontinuous projection to the caudate nucleus from the centromedian nucleus in the cat. Brain Res. 1978 May 5;146(1):145–150. doi: 10.1016/0006-8993(78)90224-x. [DOI] [PubMed] [Google Scholar]
- Schwarcz R., Hökfelt T., Fuxe K., Jonsson G., Goldstein M., Terenius L. Ibotenic acid-induced neuronal degeneration: a morphological and neurochemical study. Exp Brain Res. 1979 Oct;37(2):199–216. doi: 10.1007/BF00237708. [DOI] [PubMed] [Google Scholar]
- Tennyson V. M., Barrett R. E., Cohen G., Côté L., Heikkila R., Mytilineou C. The developing neostriatum of the rabbit: correlation of fluorescence histochemistry, electron microscopy, endogenous dopamine levels, and ( 3 H)dopamine uptake. Brain Res. 1972 Nov 13;46:251–285. doi: 10.1016/0006-8993(72)90019-4. [DOI] [PubMed] [Google Scholar]
- Weiner N., Rabadjija M. The effect of nerve stimulation on the synthesis and metabolism of norepinephrine in the isolated guinea-pig hypogastric nerve-vas deferens preparation. J Pharmacol Exp Ther. 1968 Mar;160(1):61–71. [PubMed] [Google Scholar]