Summary
Background and objectives
Mineral and bone disorders (MBDs) are common in long-term dialysis patients and are risk factors for unfavorable outcomes. The associations between pretransplant levels of MBD surrogates and outcomes after kidney transplantation are not clear.
Design, setting, participants, & measurements
Data from the Scientific Registry of Transplant Recipients up to June 2007 were linked to the 5-year (July 2001–June 2006) cohort of a large dialysis organization in the United States. All dialysis patients who received a kidney transplant during this period were identified and divided into groups according to increments of pretransplant MBD markers. Unadjusted and multivariate adjusted predictors of transplant outcomes were examined.
Results
The 11,776 patients were aged 47±14 years and 39% were women. Compared with recipients with pretransplant time-averaged serum alkaline phosphatase of 80–120 U/L, recipients with pretransplant serum alkaline phosphatase of 120–160 and ≥160 U/L had 49% and 64% higher graft failure censored all-cause mortality in multivariable adjusted models. There was no significant association between time-averaged serum alkaline phosphatase categories and risk of death censored graft failure, delayed graft function (DGF), or acute rejection (AR). Compared with recipients with pretransplant time-averaged serum parathyroid hormone (PTH) levels of 150–300 pg/ml, there was no significant association with graft censored death among recipients with pretransplant serum PTH ≥800 pg/ml. In addition, the risk of graft failure, DGF, and AR did not show any association with time-averaged serum intact PTH level. There was no significant association between time-averaged serum calcium categories and risk of graft failure censored death, DGF, and AR.
Conclusions
In this cohort, hemodialysis patients with pretransplant serum alkaline phosphatase >120 U/L have unfavorable post-transplant mortality, whereas there was no association between serum PTH and serum calcium levels and post-transplant outcomes.
Introduction
A number of reports have delineated an increased risk of all-cause and cardiovascular mortality in patients with disorders of mineral metabolism (1–5). Accelerated atherosclerosis is an important cause of cardiovascular death in long-term dialysis patients (6), and shows strong association with mineral and bone disorders (MBDs) in these patients (7). High serum alkaline phosphatase was linearly associated with increased coronary calcification (5) and mortality (8) in dialysis patients. Dysregulation of parathyroid gland function is associated with serious skeletal abnormalities, ranging from high-turnover osteodystrophy bone disease to adynamic bone disease, that have been associated with nonskeletal consequences such as cardiovascular effects, vascular calcifications, calciphylaxis, and mortality in dialysis patients (9,10).
Cardiovascular disease is the leading cause of death after kidney transplantation (11). It is therefore important to evaluate the risk factors of coronary disease before transplantation. The abnormalities of MBD are well known risk factors of mortality and coronary disease in patients on maintenance hemodialysis (4,5,8). However, only few studies aimed to analyze the association between pretransplant MBDs and post-transplant outcome. We recently examined only the association of pretransplant phosphorus level and post-transplant outcome (12). However, similar investigations for other routinely measured MBD measures, such as alkaline phosphatase, intact parathyroid hormone (iPTH), and calcium, have not yet been examined in a large cohort. Both pretransplant (13) and post-transplant (14) high serum iPTH levels were associated with inferior outcome of graft function in kidney transplant recipients. Roodnat et al. examined 407 kidney transplant recipients and found a significant, linear association between a higher pretransplant PTH level and higher risk of graft failure censored death (13). Compared with a pretransplant serum PTH level of 100 pg/ml, pretransplant PTH levels of 300 pg/ml, 500 pg/ml, and 800 pg/ml were associated with approximately 20%, 40%, and 80% higher risk of graft failure censored death, respectively (13). Similarly to PTH, pretransplant (12) and post-transplant (15) serum phosphorous levels were associated with increased mortality risk in kidney transplant recipients.
Short-term delayed graft function (DGF) and acute rejection are important predictors of long-term outcome in transplant recipients. Some small studies showed that the abnormalities in markers of MBD are associated with increased risk of DGF and acute rejection (16–18).
Thus, there is very limited knowledge on the value of biochemical markers of bone and mineral metabolism (serum alkaline phosphatase, serum iPTH, and serum calcium) and short-term outcomes such as acute rejection and DGF and long-term outcomes such as mortality and graft failure after kidney transplantation. We hypothesized that higher pretransplant serum alkaline phosphatase and serum iPTH levels are associated with poor post-transplant patient and graft survival and DGF in a large prospective cohort of incident kidney transplant recipients across the United States.
Materials and Methods
Patients
We linked data on all kidney transplant recipients listed in the Scientific Registry of Transplant Recipients (SRTR) up until June 2007 to a list of individuals with CKD by using the patients’ social security numbers. These CKD patients underwent maintenance hemodialysis treatment from July 2001 to June 2006 at one of the outpatient dialysis facilities of a large, US-based dialysis organization (DaVita Inc, before its acquisition of former Gambro dialysis facilities). The study was approved by the institutional review boards of both Los Angeles Biomedical Research Institute at Harbor-UCLA and DaVita Clinical Research.
Clinical and Demographic Measures
The creation of the national DaVita dialysis patient cohort has been described previously (19–23). We collected demographic data (such as age, sex, race, type of insurance, marital status, presence of diabetes, and dialysis vintage) and details of medical history data. Dialysis vintage was defined as the duration of time between the first day of dialysis treatment and the day of kidney transplantation. DGF and nonbiopsy-confirmed acute rejection data were captured from the SRTR database. In addition, medical history data (atherosclerotic heart disease, congestive heart failure, cancer, chronic obstructive pulmonary disease, cerebrovascular disease, hypertension, peripheral vascular disease, tobacco use) were captured from US Renal Data System. To minimize measurement variability, all repeated measures for each patient during any given calendar quarter (i.e., over a 13-week interval) were averaged and the summary estimate was used in all models. Time-averaged values obtained from up to 20 calendar quarters (q1–q20) over the entire pretransplant period were used in our analyses. The first (baseline) studied quarter for each patient was the calendar quarter in which the patient’s dialysis vintage was >90 days.
Laboratory Measures
Blood samples methods were described previously (19–23). Most blood samples were collected predialysis with the exception of postdialysis serum urea nitrogen to calculate urea kinetics. Most laboratory values were measured monthly, including serum alkaline phosphatase, calcium, phosphorus, and albumin. Serum iPTH was measured at least quarterly using a first-generation immunoradiometric PTH assay (Nichols, San Juan Capistrano, CA) as described by Nussbaum et al. (24). We divided patients into five a priori–defined categories based on pretransplant time-averaged serum iPTH level (<150 pg/ml, 150–300 pg/ml, 300–500 pg/ml, 500–800 pg/ml, and ≥800 pg/ml values) to examine the dose-response association between pretransplant time-averaged serum iPTH categories and outcome risk. We also divided patients into four a priori–defined categories based on pretransplant time-averaged serum alkaline phosphatase level (<80 U/L, 80–<120 U/L, 120–<160 U/L, and ≥160 U/L values), serum time-averaged calcium level (<8.4 mg/dl, 8.4–<9.5 mg/dl, 9.5–<10.2 mg/dl, and ≥10.2 mg/dl values), and serum time-averaged calcium-phosphorous level (<45 mg2/dl2, 45–<55 mg2/dl2, 55–<65 mg2/dl2, and ≥65 mg2/dl2 values), respectively, similarly to our previous article (4).
Statistical Analyses
Data were summarized using proportions (mean ± SD). We examined P values for trends across pretransplant serum alkaline phosphatase categories. Time to event survival analyses were done to determine association of time-averaged markers of MBDs with all-cause mortality and graft failure (defined as reinitiation of dialysis treatment or retransplantation), DGF, and acute rejection. For DGF, defined as the need for any dialysis therapy in the first week after transplantation (25), time to event was not accounted for. Survival analyses to calculate hazard ratios (HRs) and 95% confidence intervals (95% CIs) of graft failure censored death or death censored graft failure used Cox proportional hazards regression. In mortality analyses, patients were followed until event (death) or censoring (graft failure or end of follow-up period), whichever happened first. In graft failure analyses, patients were followed until event (graft failure) or censoring (death or end of follow-up period), whichever occurred first. Proportional hazard assumption was tested using log(-log) against survival plots. Logistic regression models were used to estimate the odds ratio (ORs) and 95% CIs of post-transplant DGF and acute rejection.
For each regression analysis, four levels of multivariate adjustment were examined. First, an unadjusted model included pretransplant markers of MBD categories as the predictor. Second, case-mix adjusted models included the above plus age, sex, recipient race/ethnicity (African Americans and other self-categorized blacks, non-Hispanic whites, Asians, Hispanics, and others), diabetes mellitus, dialysis vintage (<6 months, 6 months to 2 years, 2–<5 years, and ≥5 years), primary insurance (Medicare, Medicaid, private, and others), marital status (married, single, divorced, widowed, and other or unknown), standardized mortality ratio of the dialysis clinic during entry quarter, dialysis dose as indicated by Kt/V (single pool), presence or absence of a hemodialysis catheter, and residual renal function during the entry quarter and eight comorbidities. Third, markers of malnutrition or inflammation (MMI) models included all of the above covariates plus 11 surrogates of nutritional status and inflammation measured during the last calendar quarter before transplantation including body mass index and 10 laboratory variables such as normalized protein catabolic rate as an indicator of daily protein intake, also known as the normalized protein nitrogen appearance (26), and serum or blood concentrations of total iron-binding capacity, ferritin, calcium (except calcium analysis), phosphorous, iPTH (except iPTH analysis), bicarbonate, peripheral white blood cell count, lymphocyte percentage, and albumin. Fourth, models adjusted for case mix, MMI, and transplant data included all of the above plus eight transplant-related variables, including donor type (deceased or living), donor age, donor sex, panel reactive antibody titer (last value before transplant), number of HLA mismatches, cold ischemia time, DGF (except when DGF was a dependent variable in logistic regression models), and extended donor criteria using standard definition (donor history of hypertension and/or serum creatinine of donor >1.5 mg/dl and/or cause of death in donor is cerebrovascular event). We repeated our alkaline phosphatase analyses in patients without liver disease (defined if aspartate aminotransferase >40 U/L) as sensitivity analyses. All analyses were carried out with STATA software (version 11.1; STATA Corporation, College Station, TX).
Results
The original 5-year (July 2001–June 2006) national database of all DaVita patients included 164,789 adult participants. Of 65,386 DaVita patients who were identified in the SRTR database, 17,629 had undergone one or more kidney transplantations during their lifetime, but only 14,508 dialysis patients had undergone kidney transplantation for the first time. This analytic cohort was followed until death, graft failure, loss of follow-up, or survival until June 30, 2007. In the final analyses we excluded the patients who did not have serum alkaline phosphatase measurements (n=2732) or serum iPTH measurements (n=4401) or serum calcium measurements (n=2669) (Supplemental Figure 1). Accordingly, the final analyses were done in 11,776 patients for serum alkaline phosphatase, 10,107 patients for serum iPTH, and 11,839 patients for serum calcium (Supplemental Figure 1).
There were 869 deaths (7.4%) and 1320 graft failures (11.2%) irrespective of subsequent deaths in the alkaline phosphatase cohort. The median cohort time was 829 days (interquartile range, 358–1362 days). Tables 1 and 2 show the clinical, demographic, and laboratory data of the 11,776 transplanted patients across four pretransplant serum alkaline phosphatase categories. The crude all-cause mortality rate was 31.2/1000 patient-years (95% CI, 29.3–33.2).
Table 1.
Baseline characteristics of 11,776 dialysis patients who underwent renal transplantation between July 2001 and June 2006
| Pretransplant Serum Alkaline Phosphatase (U/L) | <80 | 80–<120 | 120–<160 | ≥160 | P for Trend |
|---|---|---|---|---|---|
| n (%) | 3769 (32) | 4701 (40) | 1833 (16) | 1473 (12) | N/A |
| Age (yr) | 48±14 | 48±14 | 47±14 | 42±16 | <0.001 |
| Female sex | 34 | 40 | 45 | 47 | <0.001 |
| Race (% African American) | 24 | 26 | 28 | 31 | <0.001 |
| Diabetes mellitus | 28 | 36 | 41 | 35 | <0.001 |
| Proportion of peritoneal dialysis patients | 18 | 17 | 17 | 20 | 0.07 |
| BMI (kg/m2) | 26.8±5.8 | 26.6±5.8 | 26.4±5.7 | 25.5±6.4 | <0.001 |
| Presence of ischemic heart disease | 8 | 9 | 9 | 7 | 0.24 |
| Presence of congestive heart failure | 8 | 10 | 12 | 10 | <0.001 |
| Presence of hypertension | 78 | 77 | 75 | 68 | <0.001 |
| Presence of cerebrovascular events | 2 | 3 | 2 | 2 | 0.60 |
| Presence of peripheral vascular disease | 3 | 4 | 4 | 4 | 0.02 |
| Presence of chronic obstructive pulmonary disease | 1 | 1 | 2 | 1 | 0.24 |
| Presence of cancer | 2 | 2 | 2 | 2 | 0.62 |
| Tobacco use | 4 | 4 | 3 | 3 | 0.78 |
| Dialysis vintage | |||||
| 0–6 mo | 15 | 10 | 9 | 9 | <0.001 |
| 6–24 mo | 34 | 28 | 22 | 21 | <0.001 |
| 2–5 yr | 36 | 39 | 39 | 33 | <0.001 |
| >5 yr | 15 | 23 | 30 | 37 | <0.001 |
| Kt/V | 1.57±0.36 | 1.61±0.36 | 1.61±0.34 | 1.63±0.37 | <0.001 |
| nPCR (g/kg per day) | 1.04±0.25 | 1.04±0.26 | 1.03±0.26 | 1.03±0.28 | 0.02 |
| Serum creatinine (mg/dl) | 10.9±3.5 | 10.6±3.4 | 10.3±3.3 | 10.1±3.3 | <0.001 |
| Serum albumin (mg/dl) | 4.02±0.38 | 3.99±0.39 | 3.94±0.41 | 3.87±0.48 | <0.001 |
| Serum phosphate (mg/dl) | 6.0±1.5 | 5.9±1.5 | 5.9±1.5 | 5.8±1.6 | <0.001 |
| Serum calcium (mg/dl) | 9.4±0.6 | 9.4±0.6 | 9.4±0.6 | 9.3±0.7 | <0.001 |
| Serum iPTH (pg/ml) | 305±224 | 386±299 | 499±402 | 727±668 | <0.001 |
| Blood hemoglobin (g/dl) | 12.2±1.3 | 12.3±1.3 | 12.2±1.3 | 12.1±1.4 | 0.003 |
| WBC (×103/L) | 6.9±2.0 | 6.9±2.1 | 7.0±2.2 | 7.1±2.4 | 0.004 |
| Number of HLA mismatch | 3.5±1.8 | 3.5±1.9 | 3.6±1.8 | 3.7±1.8 | <0.001 |
| Number of HLA-DR mismatch | 1.05±0.73 | 1.07±0.74 | 1.06±0.73 | 1.12±0.74 | 0.02 |
| PRA (%) | 8±21 | 11±25 | 11±25 | 14±28 | <0.001 |
| PRA >20% | 11 | 15 | 17 | 20 | <0.001 |
| Donor age (yr) | 39±15 | 39±15 | 39±15 | 38±16 | 0.02 |
| Donor sex (% women) | 49 | 47 | 49 | 46 | 0.08 |
| Donor type (% living) | 39 | 34 | 30 | 27 | <0.001 |
| EDC kidneya | 19 | 19 | 17 | 16 | 0.07 |
| Cold ischemia time (h)a | 18.4±8.4 | 18.5±8.5 | 18.0±8.6 | 18.3±8.6 | <0.001 |
Data are percentages or means ± SDs unless otherwise indicated. BMI, body mass index; nPCR, normalized protein catabolic rate; iPTH, intact parathyroid hormone; WBC, white blood cell count; PRA, panel reactive antibody (last value before transplant); EDC, extended donor criteria; N/A, not applicable.
In recipients who received kidney from deceased donors.
Table 2.
Post-transplant outcomes of 11,776 dialysis patients who underwent renal transplantation between July 2001 and June 2006
| Pretransplant Serum Alkaline Phosphatase (U/L) | <80 | 80–<120 | 120–<160 | ≥160 | P for Trend |
|---|---|---|---|---|---|
| n (%) | 3769 (32) | 4701 (40) | 1833 (16) | 1473 (12) | N/A |
| Deaths, n (crude death rate %) | 224 (5.9) | 328 (7.0) | 169 (9.2) | 148 (10.1) | <0.001 |
| Crude all-cause mortality rate per 1000 patient-years (95% CI) | 23 (20–26) | 30 (27–33) | 42 (36–49) | 47 (40–55) | N/A |
| Cardiovascular deaths, n (crude cardiovascular death rate %) | 52 (1.4) | 80 (1.7) | 49 (2.7) | 51 (3.5) | <0.001 |
| Crude cardiovascular mortality rate per 1000 patient-years (95% CI) | 5.3 (4.0–7.0) | 7.3 (5.8–9.0) | 12.2 (9.2–16.1) | 16.3 (12.4–21.4) | N/A |
| Infectious deaths, n (crude infectious death rate %) | 40 (1.1) | 69 (1.5) | 40 (2.2) | 3.7 (2.5) | <0.001 |
| Crude infectious mortality rate per 1000 patient-years (95% CI) | 4.1 (3.0–5.6) | 6.3 (4.9–7.9) | 9.9 (7.3–13.6) | 11.8 (8.5–16.3) | N/A |
| Graft failure, n (crude graft failure rate %) | 333 (8.8) | 482 (10.3) | 261 (14.2) | 244 (16.6) | <0.001 |
| Crude graft failure rate per 1000 patient-years (95% CI) | 34 (30–38) | 44 (40–48) | 65 (57–73) | 78 (69–88) | N/A |
| DGF, n (crude DGF %) | 613 (17.0) | 888 (19.9) | 392 (22.8) | 333 (24.5) | <0.001 |
| History of acute rejection, n (%) | 71 (3.8) | 96 (3.7) | 47 (4.6) | 43 (5.5) | 0.10 |
Values in parentheses indicate the crude death rate, crude graft failure rate, and crude DGF rate in the indicated group during the 6 years of observation. 95% CI, 95% confidence interval; DGF, delayed graft function; N/A, not applicable.
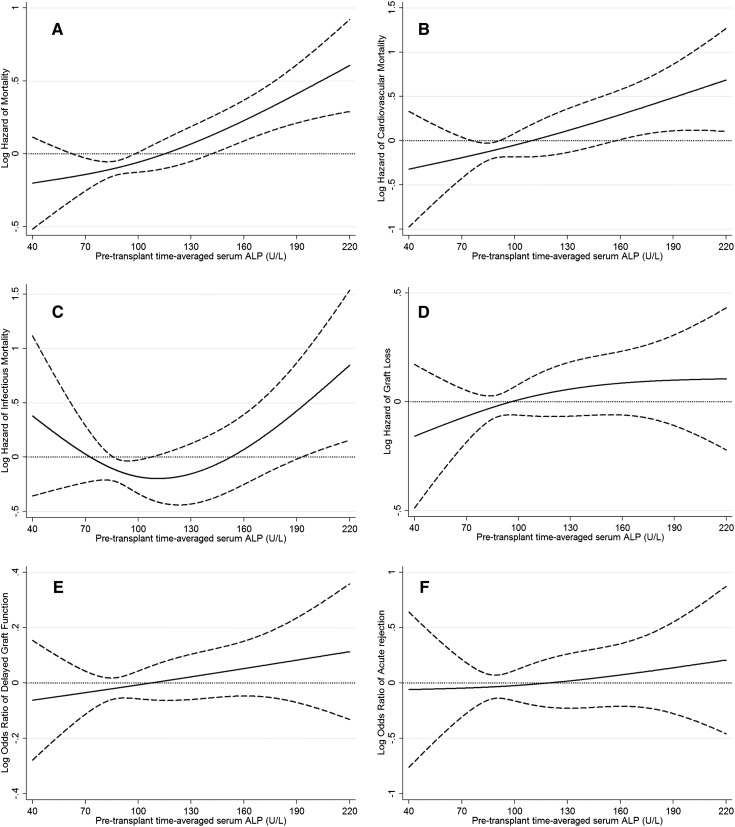
The associations of pretransplant time-averaged serum alkaline phosphatase categories with the post-transplant risk of graft failure censored death, death censored graft failure, DGF, and acute rejection are shown in Figure 1. A higher time-averaged serum alkaline phosphatase level showed a linear and significant (P=0.04 for serum alkaline phosphatase as continuous variable) association with higher risk of graft failure censored death in our cohort (Figure 1A). Similar association was found in cardiovascular mortality (Figure 1B). Similar, but nonsignificant, association was observed for graft loss, DGF, and acute rejection (Figure 1, D–F). Table 3 shows the risk of post-transplant all-cause, cardiovascular, infectious graft failure censored death or death censored graft failure or DGF or acute rejection comparing different pretransplant time-averaged serum alkaline phosphatase categories. Compared with recipients with pretransplant time-averaged serum alkaline phosphatase of 80–120 U/L, recipients with pretransplant time-averaged serum alkaline phosphatase of 120–160 and ≥160 U/L had 41% (HR, 1.41; 95% CI, 1.17–1.69) and 57% (HR, 1.57; 95% CI, 1.30–1.91) higher graft failure censored all-cause mortality, and recipients with pretransplant time-averaged serum alkaline phosphatase of <80 U/L had 23% (HR, 0.77; 95% CI, 0.65–0.92) lower unadjusted graft failure censored death risk. After additional adjustment for case-mix, MMI, and transplant-related variables, recipients with pretransplant time-averaged serum alkaline phosphatase of 120–<160 and ≥160 U/L had 49% (HR, 1.49; 95% CI, 1.14–1.93) and 64% (HR, 1.64; 95% CI, 1.21–2.23) higher graft failure censored death risk compared with recipients with pretransplant time-averaged serum alkaline phosphatase of 80–<120 U/L (Table 3). Compared with recipients with pretransplant time-averaged serum alkaline phosphatase 80–<120 U/L, recipients with pretransplant time-averaged serum alkaline phosphatase of 120–<160 and ≥160 U/L had 111% (HR, 2.11; 95% CI, 1.26–3.52) and 100% (HR, 2.00; 95% CI, 1.10–3.65) higher graft failure censored cardiovascular mortality (Table 3). There was no significant association between time-averaged serum alkaline phosphatase categories and risk of death censored graft failure, DGF, or acute rejection (Table 3). Similar results were found in patients without liver disease (Supplemental Table 1).
Figure 1.
Hazard/odds ratio (95% confidence intervals) of post-transplant outcomes across the entire range of pretransplant serum alkaline phosphatase level using Cox regression analyses in 11,776 long-term dialysis patients who underwent renal transplantation and who were observed over a 6-year study period (July 2001–June 2007). (A) All-cause mortality, (B) cardiovascular mortality, (C) infectious mortality, (D) graft loss, (E) delayed graft function, and (F) acute rejection. Model adjusted for age, sex, recipient race-ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, standardized mortality ratio of the dialysis clinic during entry quarter, dialysis dose as indicated by Kt/V (single pool), presence or absence of a hemodialysis catheter, and residual renal function during the entry quarter, eight comorbidities, body mass index, normalized protein nitrogen appearance , serum or blood concentrations of total iron-binding capacity, ferritin, calcium, phosphorous, intact parathyroid hormone, bicarbonate, peripheral white blood cell count, lymphocyte percentage, albumin, donor type, donor age, donor sex, panel reactive antibody titer (last value before transplant), number of HLA mismatches, cold ischemia time, delayed graft function, and extended donor criteria.
Table 3.
Hazard/odds ratios (95% confidence intervals) of post-transplant all-cause, cardiovascular, and infectious death or graft failure, delayed graft function, or acute rejection comparing pretransplant serum alkaline phosphatase categories (80–<120 U/L as reference) using Cox regression and logistic regression analyses in 11,776 dialysis patients who underwent renal transplantation and were observed for up to 6 years (July 2001–June 2007)
| Pretransplant Serum Alkaline Phosphatase Level (U/L) | Unadjusted | Case-Mix Adjusteda (n=) | MMI Adjustedb | Transplant Data Adjustedc | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Graft failure censored all-cause death | ||||||||
| n | 11,776 | 9246 | 7820 | 4852 | ||||
| <80 | 0.77 (0.65–0.92) | 0.003 | 0.92 (0.76–1.11) | 0.37 | 0.89 (0.72–1.10) | 0.28 | 0.97 (0.75–1.24) | 0.80 |
| 80–<120 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 120–<160 | 1.41 (1.17–1.69) | <0.001 | 1.46 (1.17–1.78) | 0.001 | 1.48 (1.19–1.85) | 0.001 | 1.49 (1.14–1.93) | 0.003 |
| ≥160 | 1.57 (1.30–1.91) | <0.001 | 1.91 (1.52–2.40) | <0.001 | 1.85 (1.44–2.39) | <0.001 | 1.64 (1.21–2.23) | 0.002 |
| Graft failure censored cardiovascular death | ||||||||
| <80 | 0.75 (0.53–1.06) | 0.10 | 1.01 (0.68–1.50) | 0.94 | 1.12 (0.73–1.71) | 0.61 | 1.12 (0.67–1.89) | 0.66 |
| 80–<120 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 120–<160 | 1.66 (1.16–2.37) | 0.005 | 1.86 (1.24–2.80) | 0.003 | 1.83 (1.19–2.83) | 0.006 | 2.11 (1.26–3.52) | 0.004 |
| ≥160 | 2.20 (1.55–3.13) | <0.001 | 2.33 (1.50–3.63) | <0.001 | 1.85 (1.12–3.08) | 0.02 | 2.00 (1.10–3.65) | 0.02 |
| Graft failure censored infectious death | ||||||||
| <80 | 0.66 (0.45–0.98) | 0.04 | 0.82 (0.52–1.27) | 0.36 | 0.78 (0.48–1.26) | 0.30 | 0.97 (0.53–1.77) | 0.93 |
| 80–<120 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 120–<160 | 1.58 (1.07–2.33) | 0.02 | 1.34 (0.83–2.15) | 0.23 | 1.23 (0.74–2.04) | 0.44 | 1.15 (0.59–2.23) | 0.68 |
| ≥160 | 1.86 (1.25–2.78) | 0.002 | 2.07 (1.27–3.37) | 0.004 | 1.93 (1.12–3.32) | 0.02 | 1.85 (0.94–3.66) | 0.08 |
| All-cause death censored graft failure | ||||||||
| <80 | 0.79 (0.69–0.91) | 0.001 | 1.08 (0.91–1.29) | 0.37 | 1.12 (0.93–1.35) | 0.22 | 1.14 (0.89–1.45) | 0.29 |
| 80–<120 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 120–<160 | 1.47 (1.26–1.71) | <0.001 | 1.30 (1.07–1.59) | 0.008 | 1.29 (1.05–1.59) | 0.01 | 1.23 (0.94–1.61) | 0.14 |
| ≥160 | 1.75 (1.50–2.04) | <0.001 | 1.20 (0.96–1.49) | 0.11 | 1.10 (0.86–1.40) | 0.45 | 1.16 (0.85–1.59) | 0.35 |
| Delayed graft function | ||||||||
| n | 11,143 | 8801 | 7457 | 4852 | ||||
| <80 | 0.83 (0.74–0.93) | 0.001 | 1.00 (0.88–1.13) | 0.97 | 0.97 (0.84–1.11) | 0.65 | 1.01 (0.86–1.19) | 0.86 |
| 80–<120 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 120–<160 | 1.19 (1.04–1.36) | 0.01 | 1.12 (0.96–1.31) | 0.14 | 1.13 (0.96–1.33) | 0.16 | 1.10 (0.91–1.33) | 0.32 |
| ≥160 | 1.31 (1.14–1.51) | <0.001 | 1.20 (1.01–1.43) | 0.04 | 1.16 (0.95–1.41) | 0.14 | 1.14 (0.91–1.43) | 0.25 |
| Acute rejection | ||||||||
| n | 6307 | 5206 | 4123 | 2793 | ||||
| <80 | 1.04 (0.76–1.42) | 0.82 | 1.16 (0.81–1.65) | 0.41 | 1.16 (0.77–1.74) | 0.49 | 1.08 (0.65–1.81) | 0.77 |
| 80 –<120 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 120–<160 | 1.29 (0.90–1.84) | 0.17 | 1.28 (0.85–1.93) | 0.23 | 1.42 (0.90–2.22) | 0.13 | 1.38 (0.81–2.35) | 0.23 |
| ≥160 | 1.52 (1.05–2.20) | 0.03 | 1.42 (0.92–2.20) | 0.12 | 1.40 (0.83–2.35) | 0.20 | 1.12 (0.59–2.12) | 0.72 |
MMI, markers of malnutrition or inflammation; HR, hazard ratio; 95% CI, 95% confidence interval; iPTH, intact parathyroid hormone.
Adjusted for age, sex, recipient race/ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, standardized mortality ratio of the dialysis clinic during entry quarter, dialysis dose as indicated by Kt/V (single pool), presence or absence of a hemodialysis catheter, and residual renal function during the entry quarter and eight comorbidities.
Adjusted for all of the covariates plus body mass index, normalized protein nitrogen appearance , serum or blood concentrations of total iron-binding capacity, ferritin, calcium, phosphorous, iPTH, bicarbonate, peripheral white blood cell count, lymphocyte percentage, and albumin; N/A, not applicable.
Adjusted for all of the above plus donor type, donor age, donor sex, panel reactive antibody titer (last value before transplant), number of HLA mismatches, cold ischemia time, delayed graft function (except when delayed graft function was a dependent variable in our logistic regression models), and extended donor criteria.
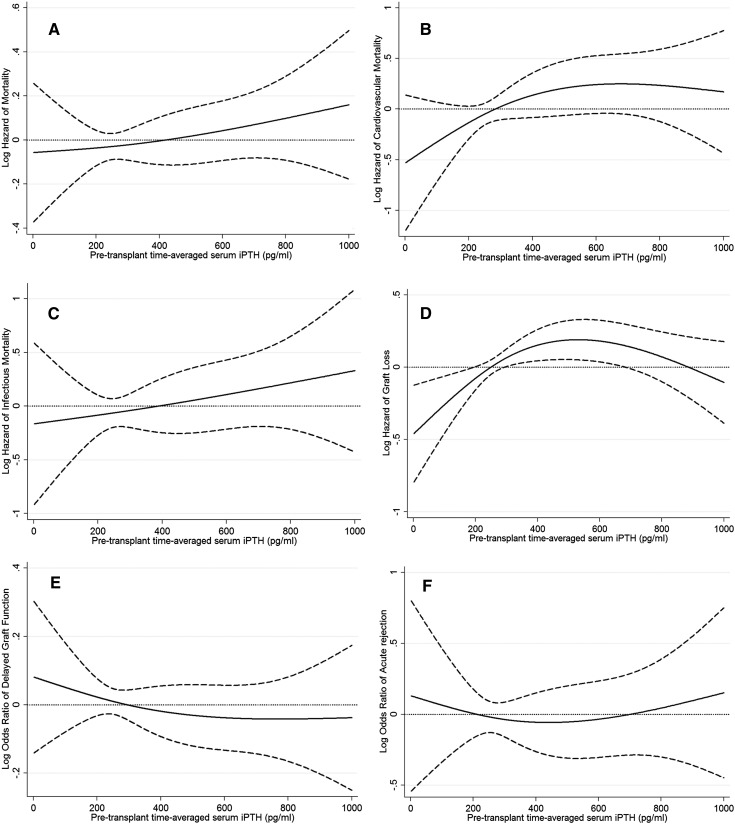
The associations of pretransplant time-averaged serum iPTH categories with the post-transplant risk of graft failure censored death, death censored graft failure, DGF, and acute rejection are shown in Figure 2. Higher time-averaged serum iPTH level did not show (P=0.15 for iPTH as continuous variable) any association with risk of graft failure censored death in our cohort (Figure 2A). Similar results were found in cardiovascular and infectious mortality (Figure 2, B and C).
Figure 2.
Hazard/odds ratio (95% confidence intervals) of post-transplant outcomes across the entire range of pretransplant serum intact parathyroid hormone level using Cox regression analyses in 10,107 long-term dialysis patients who underwent renal transplantation and who were observed over a 6-year study period (July 2001–June 2007). (A) All-cause mortality, (B) cardiovascular mortality, (C) infectious mortality, (D) graft loss, (E) delayed graft function, and (F) acute rejection. Model adjusted for age, sex, recipient race-ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, standardized mortality ratio of the dialysis clinic during entry quarter, dialysis dose as indicated by Kt/V (single pool), presence or absence of a hemodialysis catheter, and residual renal function during the entry quarter, eight comorbidities, body mass index, normalized protein nitrogen appearance , serum or blood concentrations of total iron-binding capacity, ferritin, calcium, phosphorous, bicarbonate, peripheral white blood cell count, lymphocyte percentage, albumin, donor type, donor age, donor sex, panel reactive antibody titer (last value before transplant), number of HLA mismatches, cold ischemia time, delayed graft function , and extended donor criteria.
In addition, the risk of graft failure, DGF, and acute rejection did not show any association with time-averaged serum iPTH level (Figure 2, D–F). Table 4 shows the risk of post-transplant graft failure censored all-cause, cardiovascular, infectious death, death censored graft failure, DGF, or acute rejection comparing different pretransplant time-averaged serum iPTH categories. Compared with recipients with pretransplant time-averaged serum PTH level of 150–300 pg/ml, there was no significant association with graft censored death among recipients with pretransplant serum PTH of ≥800 pg/ml (HR, 1.28; 95% CI, 0.87–1.88) (Table 4). Compared with recipients with pretransplant time-averaged serum iPTH level 150–300 pg/ml, recipients with pretransplant time-averaged serum iPTH of 500–800 pg/ml iPTH had higher (HR, 2.21; 95% CI, 1.24–3.91) cardiovascular mortality risk in our multivariable adjusted model (Table 4). There was no association between iPTH categories and risk of graft failure, DGF, and acute rejection.
Table 4.
Hazard/odds ratio (95% confidence intervals) of post-transplant all-cause, cardiovascular, and infectious death or graft failure, delayed graft function, or acute rejection comparing pretransplant serum iPTH categories (150–<300 pg/ml the reference) using Cox regression and logistic regression analyses in 10,107 dialysis patients who underwent renal transplantation and observed for up to 6 years (July 2001–June 2007)
| Pretransplant Serum iPTH Level (pg/ml) | Unadjusted | Case-Mix Adjusteda | MMI Adjustedb | Transplant Data Adjustedc | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Graft failure censored all-cause death | ||||||||
| n | 10,107 | 8082 | 7820 | 4852 | ||||
| <150 | 1.05 (0.86–1.28) | 0.63 | 0.99 (0.79–1.26) | 0.97 | 1.04 (0.82–1.33) | 0.72 | 1.06 (0.79–1.41) | 0.70 |
| 150–<300 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 300–<500 | 0.90 (0.75–1.08) | 0.24 | 0.99 (0.81–1.22) | 0.94 | 0.98 (0.79–1.21) | 0.83 | 1.12 (0.88–1.44) | 0.36 |
| 500–<800 | 0.72 (0.56–0.92) | 0.01 | 0.98 (0.74–1.29) | 0.87 | 1.02 (0.76–1.37) | 0.88 | 1.15 (0.81–1.63) | 0.43 |
| ≥800 | 0.79 (0.61–1.03) | 0.08 | 1.22 (0.90–1.66) | 0.21 | 1.27 (0.92–1.75) | 0.14 | 1.28 (0.87–1.88) | 0.20 |
| Graft failure censored cardiovascular death | ||||||||
| <150 | 1.17 (0.77–1.78) | 0.46 | 1.09 (0.66–1.80) | 0.72 | 1.02 (0.61–1.72) | 0.94 | 1.00 (0.54–1.87) | 0.99 |
| 150–<300 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 300–<500 | 1.12 (0.78–1.63) | 0.53 | 1.08 (0.71–1.64) | 0.74 | 1.03 (0.67–1.58) | 0.88 | 1.04 (0.67–1.80) | 0.79 |
| 500–<800 | 1.52 (1.01–2.28) | 0.04 | 1.81 (1.14–2.89) | 0.01 | 1.70 (1.03–2.79) | 0.04 | 2.21 (1.24–3.91) | 0.007 |
| ≥800 | 1.19 (0.74–1.91) | 0.47 | 1.31 (0.72–2.39) | 0.37 | 1.29 (0.70–2.38) | 0.41 | 1.21 (0.58–2.54) | 0.62 |
| Graft failure censored infectious death | ||||||||
| <150 | 1.32 (0.86–2.03) | 0.20 | 0.96 (0.57–1.63) | 0.89 | 0.97 (0.56–1.67) | 0.90 | 1.16 (0.59–2.29) | 0.66 |
| 150–<300 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 300–<500 | 1.03 (0.69–1.54) | 0.89 | 0.93 (0.58–1.48) | 0.76 | 0.94 (0.58–1.52) | 0.80 | 1.18 (0.64–2.16) | 0.59 |
| 500–<800 | 0.92 (0.55–1.54) | 0.75 | 1.03 (0.55–1.91) | 0.93 | 1.05 (0.55–1.99) | 0.89 | 1.45 (0.66–3.19) | 0.36 |
| ≥800 | 1.07 (0.63–1.81) | 0.82 | 1.51 (0.78–2.91) | 0.22 | 1.52 (0.77–2.99) | 0.23 | 1.52 (0.62–3.70) | 0.36 |
| All-cause death censored graft failure | ||||||||
| <150 | 0.88 (0.72–1.08) | 0.21 | 0.88 (0.68–1.14) | 0.34 | 0.87 (0.66–1.14) | 0.31 | 0.99 (0.70–1.41) | 0.96 |
| 150–<300 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 300–<500 | 1.43 (1.23–1.67) | <0.001 | 1.24 (1.04–1.49) | 0.02 | 1.25 (1.04–1.51) | 0.02 | 1.42 (1.11–1.82) | 0.005 |
| 500–<800 | 1.34 (1.12–1.61) | 0.002 | 1.07 (0.85–1.35) | 0.58 | 1.03 (0.81–1.31) | 0.82 | 1.26 (0.93–1.72) | 0.14 |
| ≥800 | 2.10 (1.77–2.49) | <0.001 | 1.11 (0.87–1.42) | 0.40 | 1.07 (0.83–1.38) | 0.59 | 1.10 (0.79–1.55) | 0.57 |
| Delayed graft function | ||||||||
| n | 9592 | 7705 | 7457 | 4852 | ||||
| <150 | 0.90 (0.77–1.06) | 0.21 | 1.06 (0.88–1.27) | 0.52 | 1.10 (0.91–1.33) | 0.31 | 1.14 (0.91–1.41) | 0.26 |
| 150–<300 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 300–<500 | 1.12 (0.99–1.27) | 0.08 | 1.12 (0.97–1.29) | 0.13 | 1.09 (0.94–1.27) | 0.24 | 1.10 (0.93–1.30) | 0.29 |
| 500–<800 | 1.04 (0.89–1.22) | 0.60 | 1.01 (0.84–1.22) | 0.90 | 0.94 (0.77–1.14) | 0.50 | 0.97 (0.77–1.21) | 0.78 |
| ≥800 | 1.23 (1.04–1.45) | 0.01 | 1.26 (1.03–1.54) | 0.03 | 1.14 (0.92–1.41) | 0.23 | 1.03 (0.80–1.32) | 0.82 |
| Acute rejection | ||||||||
| n | 4985 | 4209 | 4123 | 2793 | ||||
| <150 | 1.51 (0.95–2.40) | 0.08 | 1.35 (0.78–2.32) | 0.28 | 1.37 (0.79–2.37) | 0.26 | 1.27 (0.65–2.51) | 0.49 |
| 150–<300 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 300–<500 | 1.51 (1.05–2.18) | 0.03 | 1.48 (0.99–2.22) | 0.06 | 1.46 (0.97–2.20) | 0.07 | 1.25 (0.76–2.05) | 0.38 |
| 500–<800 | 1.41 (0.91–2.21) | 0.13 | 1.26 (0.75–2.11) | 0.38 | 1.21 (0.72–2.04) | 0.48 | 1.01 (0.52–2.00) | 0.97 |
| ≥800 | 1.53 (0.95–2.46) | 0.08 | 1.20 (0.67–2.16) | 0.54 | 1.07 (0.58–1.99) | 0.82 | 1.41 (0.70–2.85) | 0.34 |
iPTH, intact parathyroid hormone; MMI, markers of malnutrition or inflammation; HR, hazard ratio; 95% CI, 95% confidence interval.
Adjusted for age, sex, recipient race/ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, standardized mortality ratio of the dialysis clinic during entry quarter, dialysis dose as indicated by Kt/V (single pool), presence or absence of a hemodialysis catheter, and residual renal function during the entry quarter and eight comorbidities.
Adjusted for all of the covariates plus body mass index, normalized protein nitrogen appearance , serum or blood concentrations of total iron-binding capacity, ferritin, phosphorous, calcium, bicarbonate, peripheral white blood cell count, lymphocyte percentage, and albumin.
Adjusted for all of the above plus donor type, donor age, donor sex, panel reactive antibody titer (last value before transplant), number of HLA mismatches, cold ischemia time, delayed graft function (except when delayed graft function was a dependent variable in our logistic regression models), and extended donor criteria.
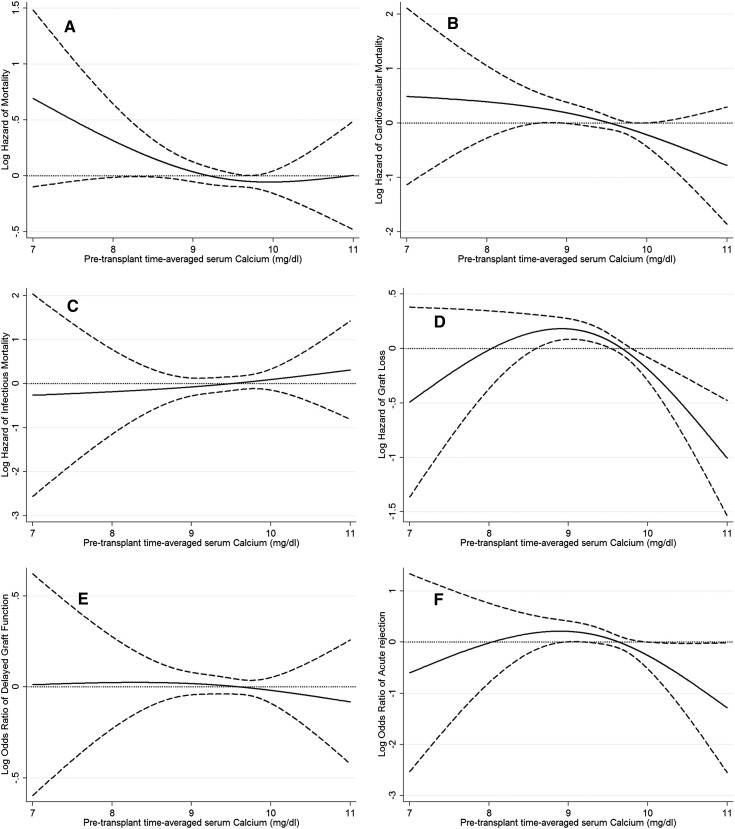
The associations of pretransplant time-averaged serum calcium categories with the post-transplant risk of graft failure censored death, death censored graft failure, DGF, and acute rejection is shown in Figure 3. Lower time-averaged serum calcium level showed a nonsignificant trend toward higher all-cause mortality risk (P=0.10 for serum calcium as continuous variable), but there was no association between serum calcium level and the risk of DGF or acute rejection. Table 5 shows the risk of post-transplant graft failure censored all-cause, cardiovascular, and infectious death or death censored graft failure, DGF, or acute rejection comparing different pretransplant time-averaged serum calcium categories. Compared with recipients with pretransplant time-averaged serum calcium of 8.4–9.5 mg/dl, recipients with pretransplant time-averaged serum calcium of 9.5–<10.2 and ≥10.2 mg/dl calcium had 26% (HR, 0.74; 95% CI, 0.60–0.92) and 40% (HR, 0.60; 95% CI, 0.41–0.88) lower risk of graft failure in our models adjusted for case mix, MMI, and transplant variables (Table 5). There was no significant association between time-averaged serum calcium categories and risk of graft failure censored death, DGF, and acute rejection (Table 5).
Figure 3.
Hazard/odds ratio (95% confidence intervals) of post-transplant outcomes across the entire range of pretransplant serum calcium level using Cox regression analyses in 11,839 long-term dialysis patients who underwent renal transplantation and who were observed over a 6-year study period (July 2001–June 2007). (A) All-cause mortality, (B) cardiovascular mortality, (C) infectious mortality, (D) graft loss, (E) delayed graft function, and (F) acute rejection. Model adjusted for age, sex, recipient race-ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, standardized mortality ratio of the dialysis clinic during entry quarter, dialysis dose as indicated by Kt/V (single pool), presence or absence of a hemodialysis catheter, and residual renal function during the entry quarter, eight comorbidities, body mass index, normalized protein nitrogen appearance , serum or blood concentrations of total iron-binding capacity, ferritin, bicarbonate, phosphorous, intact parathyroid hormone, peripheral white blood cell count, lymphocyte percentage, albumin, donor type, donor age, donor sex, panel reactive antibody titer (last value before transplant), number of HLA mismatches, cold ischemia time, delayed graft function , and extended donor criteria.
Table 5.
Hazard/odds ratio (95% confidence intervals) of post-transplant all-cause, cardiovascular, and infectious death or graft failure, delayed graft function, or acute rejection comparing pretransplant serum calcium categories (8.4–<9.5 mg/dl the reference) using Cox regression and logistic regression analyses in 11,839 dialysis patients who underwent renal transplantation and observed for up to 6 years (July 2001–June 2007)
| Pretransplant Serum Calcium Level (mg/dl) | Unadjusted | Case-Mix Adjusteda | MMI Adjustedb | Transplant Data Adjustedc | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Graft failure censored all-cause death | ||||||||
| n | 11,839 | 9255 | 7820 | 4852 | ||||
| <8.4 | 1.27 (0.99–1.62) | 0.05 | 1.21 (0.88–1.67) | 0.23 | 1.12 (0.79–1.60) | 0.52 | 1.00 (0.63–1.59) | 0.99 |
| 8.4–<9.5 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 9.5–<10.2 | 0.77 (0.66–0.90) | 0.001 | 0.76 (0.64–0.90) | 0.002 | 0.80 (0.66–0.97) | 0.02 | 0.81 (0.65–1.01) | 0.06 |
| ≥10.2 | 0.94 (0.74–1.19) | 0.61 | 0.86 (0.64–1.15) | 0.31 | 0.95 (0.69–1.31) | 0.75 | 1.00 (0.69–1.44) | 0.97 |
| Graft failure censored cardiovascular death | ||||||||
| <8.4 | 0.96 (0.58–1.58) | 0.87 | 0.92 (0.48–1.78) | 0.81 | 0.89 (0.44–1.82) | 0.76 | 1.01 (0.42–2.44) | 0.98 |
| 8.4–<9.5 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 9.5–<10.2 | 0.58 (0.43–0.79) | 0.001 | 0.57 (0.40–0.82) | 0.002 | 0.57 (0.40–0.86) | 0.007 | 0.62 (0.40–0.98) | 0.04 |
| ≥10.2 | 0.82 (0.51–1.30) | 0.39 | 0.56 (0.30–1.06) | 0.07 | 0.59 (0.30–1.16) | 0.13 | 0.58 (0.25–1.32) | 0.20 |
| Graft failure censored infectious death | ||||||||
| <8.4 | 1.49 (0.49–2.50) | 0.13 | 1.46 (0.72–2.95) | 0.29 | 1.44 (0.67–3.11) | 0.35 | 0.60 (0.14–2.60) | 0.50 |
| 8.4–<9.5 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 9.5–<10.2 | 0.97 (0.70–1.34) | 0.85 | 0.91 (0.62–1.33) | 0.61 | 1.09 (0.71–1.65) | 0.70 | 1.05 (0.63–1.76) | 0.84 |
| ≥10.2 | 1.13 (0.68–1.88) | 0.64 | 1.07 (0.57–2.01) | 0.84 | 1.37 (0.70–2.67) | 0.36 | 1.40 (0.61–3.22) | 0.43 |
| All-cause death censored graft failure | ||||||||
| <8.4 | 1.27 (1.04–1.55) | 0.02 | 0.93 (0.63–1.26) | 0.62 | 0.87 (0.62–1.22) | 0.43 | 0.71 (0.44–1.17) | 0.18 |
| 8.4–<9.5 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 9.5-<10.2 | 0.87 (0.77–0.98) | 0.02 | 0.78 (0.67–0.91) | 0.002 | 0.84 (0.71–0.99) | 0.04 | 0.74 (0.60–0.92) | 0.006 |
| ≥10.2 | 0.71 (0.57–0.89) | 0.03 | 0.59 (0.44–0.78) | <0.001 | 0.65 (0.48–0.88) | 0.005 | 0.60 (0.41–0.88) | 0.008 |
| Delayed graft function | ||||||||
| n | 11,204 | 8810 | 7457 | 4852 | ||||
| <8.4 | 1.14 (0.94–1.38) | 0.20 | 1.24 (0.97–1.59) | 0.08 | 1.20 (0.91–1.59) | 0.19 | 1.15 (0.82–1.61) | 0.43 |
| 8.4–<9.5 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 9.5–<10.2 | 1.13 (1.02–1.25) | 0.02 | 1.01 (0.90–1.13) | 0.90 | 1.02 (0.90–1.16) | 0.71 | 1.00 (0.87–1.16) | 0.97 |
| ≥10.2 | 0.95 (0.79–1.14) | 0.57 | 0.84 (0.68–1.05) | 0.12 | 0.85 (0.67–1.08) | 0.18 | 0.89 (0.68–1.16) | 0.37 |
| Acute rejection | ||||||||
| n | 6335 | 5210 | 4123 | 2793 | ||||
| <8.4 | 1.27 (0.76–2.13) | 0.37 | 1.29 (0.71–2.36) | 0.40 | 1.39 (0.70–2.75) | 0.35 | 1.53 (0.65–3.61) | 0.33 |
| 8.4–<9.5 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 9.5–<10.2 | 1.15 (0.88–1.50) | 0.31 | 1.06 (0.78–1.75) | 0.69 | 0.96 (0.67–1.38) | 0.83 | 0.73 (0.48–1.13) | 0.16 |
| ≥10.2 | 0.88 (0.49–1.58) | 0.68 | 0.57 (0.26–1.25) | 0.16 | 0.53 (0.21–1.34) | 0.18 | 0.43 (0.15–1.23) | 0.12 |
MMI, markers of malnutrition or inflammation; HR, hazard ratio; 95% CI, 95% confidence interval; iPTH, intact parathyroid hormone.
Adjusted for age, sex, recipient race/ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, standardized mortality ratio of the dialysis clinic during entry quarter, dialysis dose as indicated by Kt/V (single pool), presence or absence of a hemodialysis catheter, and residual renal function during the entry quarter and eight comorbidities.
Adjusted for all of the covariates plus body mass index, normalized protein nitrogen appearance , serum or blood concentrations of total iron-binding capacity, ferritin, bicarbonate, phosphorous, iPTH, peripheral white blood cell count, lymphocyte percentage, and albumin.
Adjusted for all of the above plus donor type, donor age, donor sex, panel reactive antibody titer (last value before transplant), number of HLA mismatches, cold ischemia time, delayed graft function (except when delayed graft function was a dependent variable in our logistic regression models), and extended donor criteria.
In addition, there was no significant association between time-averaged serum calcium-phosphorous product levels and risk of graft failure censored all-cause, cardiovascular, infectious death, DGF, and acute rejection (Supplemental Table 2 and Supplemental Figure 2).
Discussion
In this retrospective analysis of >10,000 primary kidney transplant recipients, we describe an association between pretransplant serum alkaline phosphatase levels >120 U/L with an increased risk of graft failure censored all-cause and cardiovascular death; however, there was no association between serum iPTH and mortality. Neither pretransplant serum iPTH nor pretransplant serum alkaline phosphatase level was associated with graft loss, DGF, or acute rejection. Interestingly, pretransplant serum calcium >9.5 mg/dl was associated with decreased risk of graft loss.
The first important finding was the association of pretransplant serum alkaline phosphatase levels >120 U/L with increased risk of post-transplant graft failure censored all-cause and cardiovascular death. The association between pretransplant serum alkaline phosphatase and survival was monotonic. Supporting this observation, the same association was detected in the cohort without liver disease. Similar associations were found in our previous studies in maintenance hemodialysis patients (4,8). This linear association is indicative of the relation between the increasing severity of high-turnover bone disease during the dialysis period and increased mortality risk in the post-transplant period (27–29).
We did not find any association between pretransplant serum iPTH level and post-transplant outcome. In contrast, Roodnat et al. showed that serum pretransplant PTH levels were independently associated with the risk for graft failure censored for death in kidney transplant recipients (13). However, they did not find any association between serum iPTH level and post-transplant mortality (13). This cohort was different from our cohort in several variables: the prevalence of diabetic nephropathy was only 9.6% and the cohort was smaller (n=407).
Interestingly, pretransplant serum calcium levels >9.5 mg/dl were associated with decreased risk of graft loss. High serum calcium level during the dialysis period may be associated with usage of vitamin D and/or calcium containing phosphate binders. Vitamin D receptor is found in significant concentrations in the T lymphocyte and macrophage populations (30). The significant role of vitamin D compounds as selective immunosuppressant is illustrated by their ability to either prevent or markedly suppress animal models of autoimmune disease (30). The vitamin D hormone stimulates TGF-β1 and IL-4 production, which in turn may suppress inflammatory T cell activity (30). The decreased inflammatory T cell activity may be associated with lower risk of graft loss. Unfortunately, we do not have data about vitamin D utilization; therefore, we are not able to test this speculative hypothesis. Further studies are needed to confirm this result and develop additional hypotheses to explain this observation.
Our study should be qualified for several potential limitations. Like all observational studies, ours too cannot prove causality. Post-transplant laboratory measures, type of immunosuppressive regimen, and other relevant medications (such as vitamin D and phosphate binders) were not available in the SRTR database; however, in the full model, we did adjust for a number of transplant-related variables. We did not have access to data pertaining to death after graft loss, which is another important outcome. Patients who did not have measured serum iPTH, alkaline phosphatase, and calcium levels were excluded from the analyses. The excluded patients might have been different from those included in our study, which may have biased our results. It is important to note that missing data for covariates led to exclusion of over half of the cohort for the final multivariable models. Another potential limitation is that we did not have bone-specific alkaline phosphatase data. Moreover, we cannot exclude patients with liver disease from our analysis; however we found qualitative similar results when we reanalyzed our data with patients aspartate aminotransferase ≤40 U/L. In addition, we did not have data about 25(OH) vitamin D, 1,25(OH)2 vitamin D, fibroblast growth factor-23, and osteocalcin levels. Moreover, we do not have data about treatment of MBD in these patients.
Strengths of this study are multilevel adjustment, which includes several important pretransplant measures, the high number of patients, and the relatively long follow-up time.
Individuals with pretransplant serum alkaline phosphatase >120 U/L have increased risk of graft failure censored all-cause and cardiovascular mortality. There was no significant association between time-averaged serum alkaline phosphatase categories and risk of death censored graft failure, DGF, or acute rejection. There was no clinically meaningful association between pretransplant serum iPTH and calcium level and post-transplant outcomes. Further clinical trials are needed to better define optimal target levels of MBD markers in waitlisted dialysis patients.
Disclosures
K.K.Z. is the medical director of DaVita Harbor-UCLA/MFI in Long Beach, California, and received a research grant from Shire Inc. I.B.S. is the medical director of Pediatric Dialysis at DaVita Century City, Los Angeles, California.
Supplementary Material
Acknowledgments
We thank DaVita Clinical Research for providing the clinical data and review for this research project.
This study was supported by a research grant from the American Heart Association (0655776Y to K.K.Z). K.K.Z. has also received funding from the National Institute of Diabetes, Digestive and Kidney Diseases of the National Institutes of Health (R01 DK078106), a research grant from DaVita Clinical Research, and a philanthropic grant from Mr. Harold Simmons. M.Z.M. received grants from the National Developmental Agency (KTIA-OTKA-EU 7KP-HUMAN-MB08-A-81231) from the Research and Technological Innovation Fund, and is a recipient of the Hungarian Eötvös Scholarship (MÖB/77-2/2012).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01910212/-/DCSupplemental.
References
- 1.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Young EW, Albert JM, Satayathum S, Goodkin DA, Pisoni RL, Akiba T, Akizawa T, Kurokawa K, Bommer J, Piera L, Port FK: Predictors and consequences of altered mineral metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int 67: 1179–1187, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Slinin Y, Foley RN, Collins AJ: Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: The USRDS waves 1, 3, and 4 study. J Am Soc Nephrol 16: 1788–1793, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Lukowsky LR, Molnar MZ, Zaritsky JJ, Sim JJ, Mucsi I, Kovesdy CP, Kalantar-Zadeh K: Mineral and bone disorders and survival in hemodialysis patients with and without polycystic kidney disease. Nephrol Dial Transplant 27: 2899–2907, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shantouf R, Kovesdy CP, Kim Y, Ahmadi N, Luna A, Luna C, Rambod M, Nissenson AR, Budoff MJ, Kalantar-Zadeh K: Association of serum alkaline phosphatase with coronary artery calcification in maintenance hemodialysis patients. Clin J Am Soc Nephrol 4: 1106–1114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shoji T, Maekawa K, Emoto M, Okuno S, Yamakawa T, Ishimura E, Inaba M, Nishizawa Y: Arterial stiffness predicts cardiovascular death independent of arterial thickness in a cohort of hemodialysis patients. Atherosclerosis 210: 145–149, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Raggi P, Giachelli C, Bellasi A: Interaction of vascular and bone disease in patients with normal renal function and patients undergoing dialysis. Nat Clin Pract Cardiovasc Med 4: 26–33, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Regidor DL, Kovesdy CP, Mehrotra R, Rambod M, Jing J, McAllister CJ, Van Wyck D, Kopple JD, Kalantar-Zadeh K: Serum alkaline phosphatase predicts mortality among maintenance hemodialysis patients. J Am Soc Nephrol 19: 2193–2203, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rostand SG, Drüeke TB: Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int 56: 383–392, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Hörl WH: The clinical consequences of secondary hyperparathyroidism: Focus on clinical outcomes. Nephrol Dial Transplant 19[Suppl 5]: V2–V8, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Howard RJ, Patton PR, Reed AI, Hemming AW, Van der Werf WJ, Pfaff WW, Srinivas TR, Scornik JC: The changing causes of graft loss and death after kidney transplantation. Transplantation 73: 1923–1928, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Sampaio MS, Molnar MZ, Kovesdy CP, Mehrotra R, Mucsi I, Sim JJ, Krishnan M, Nissenson AR, Kalantar-Zadeh K: Association of pretransplant serum phosphorus with posttransplant outcomes. Clin J Am Soc Nephrol 6: 2712–2721, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roodnat JI, van Gurp EA, Mulder PG, van Gelder T, de Rijke YB, de Herder WW, Kal-van Gestel JA, Pols HA, Ijzermans JN, Weimar W: High pretransplant parathyroid hormone levels increase the risk for graft failure after renal transplantation. Transplantation 82: 362–367, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Gwinner W, Suppa S, Mengel M, Hoy L, Kreipe HH, Haller H, Schwarz A: Early calcification of renal allografts detected by protocol biopsies: Causes and clinical implications. Am J Transplant 5: 1934–1941, 2005 [DOI] [PubMed]
- 15.Connolly GM, Cunningham R, McNamee PT, Young IS, Maxwell AP: Elevated serum phosphate predicts mortality in renal transplant recipients. Transplantation 87: 1040–1044, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Ahmadi F, Ali-Madadi A, Lessan-Pezeshki M, Khatami M, Mahdavi-Mazdeh M, Razeghi E, Maziar S, Seifi S, Abbasi M: Pre-transplant calcium-phosphate-parathormone homeostasis as a risk factor for early graft dysfunction. Saudi J Kidney Dis Transpl 19: 54–58, 2008 [PubMed] [Google Scholar]
- 17.Boom H, Mallat MJ, de Fijter JW, Paul LC, Bruijn JA, van Es LA: Calcium levels as a risk factor for delayed graft function. Transplantation 77: 868–873, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Krüger B, Schnitzbauer AA, Böger CA, Hoffmann U, Banas B, Farkas S, Schlitt HJ, Obed A, Krämer BK: Pretransplant calcium levels have no predictive value for delayed graft function, long-term graft function, cardiovascular events, or graft and patient survival in renal transplantation. Transplant Proc 38: 697–700, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Molnar MZ, Kovesdy CP, Mucsi I, Bunnapradist S, Streja E, Krishnan M, Kalantar-Zadeh K: Higher recipient body mass index is associated with post-transplant delayed kidney graft function. Kidney Int 80: 218–224, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molnar MZ, Kovesdy CP, Bunnapradist S, Streja E, Mehrotra R, Krishnan M, Nissenson AR, Kalantar-Zadeh K: Associations of pretransplant serum albumin with post-transplant outcomes in kidney transplant recipients. Am J Transplant 11: 1006–1015, 2011 [DOI] [PMC free article] [PubMed]
- 21.Streja E, Molnar MZ, Kovesdy CP, Bunnapradist S, Jing J, Nissenson AR, Mucsi I, Danovitch GM, Kalantar-Zadeh K: Associations of pretransplant weight and muscle mass with mortality in renal transplant recipients. Clin J Am Soc Nephrol 6: 1463–1473, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molnar MZ, Huang E, Hoshino J, Krishnan M, Nissenson AR, Kovesdy CP, Kalantar-Zadeh K: Association of pretransplant glycemic control with posttransplant outcomes in diabetic kidney transplant recipients. Diabetes Care 34: 2536–2541, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molnar MZ, Mehrotra R, Duong U, Bunnapradist S, Lukowsky LR, Krishnan M, Kovesdy CP, Kalantar-Zadeh K: Dialysis modality and outcomes in kidney transplant recipients. Clin J Am Soc Nephrol 7: 332–341, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nussbaum SR, Zahradnik RJ, Lavigne JR, Brennan GL, Nozawa-Ung K, Kim LY, Keutmann HT, Wang CA, Potts JT, Jr, Segre GV: Highly sensitive two-site immunoradiometric assay of parathyrin, and its clinical utility in evaluating patients with hypercalcemia. Clin Chem 33: 1364–1367, 1987 [PubMed] [Google Scholar]
- 25.Yarlagadda SG, Coca SG, Garg AX, Doshi M, Poggio E, Marcus RJ, Parikh CR: Marked variation in the definition and diagnosis of delayed graft function: A systematic review. Nephrol Dial Transplant 23: 2995–3003, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shinaberger CS, Greenland S, Kopple JD, Van Wyck D, Mehrotra R, Kovesdy CP, Kalantar-Zadeh K: Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr 88: 1511–1518, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovesdy CP, Kalantar-Zadeh K: Vitamin D receptor activation and survival in chronic kidney disease. Kidney Int 73: 1355–1363, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Hill AB: The environment and disease: Association or causation? Proc R Soc Med 58: 295–300, 1965 [PMC free article] [PubMed] [Google Scholar]
- 29.Kalantar-Zadeh K: What is so bad about reverse epidemiology anyway? Semin Dial 20: 593–601, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Deluca HF, Cantorna MT: Vitamin D: Its role and uses in immunology. FASEB J 15: 2579–2585, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.