Summary
Background and objectives
Abnormally elevated GFR, or hyperfiltration, is a proposed mechanism for kidney injury in diabetes, prediabetes, and obesity. This study investigated whether lack of physical exercise is associated with hyperfiltration and whether exercise modifies the positive association between fasting glucose and measured GFR.
Design, setting, participants, & measurements
The Renal Iohexol Clearance Survey in Tromsø 6 measured GFR as single-sample plasma iohexol clearance in 1506 members of the general population (age 50–62 years) without diabetes, cardiovascular disease, or kidney disease. Leisure-time physical exercise was assessed by a self-administered questionnaire. Hyperfiltration was defined as GFR above the 90th percentile after adjustment for sex, age, weight, height, and use of renin-angiotensin system inhibitors.
Results
High-intensity exercise was associated with lower adjusted odds of hyperfiltration in men (odds ratio [OR], 0.47; 95% confidence interval [CI], 0.28–0.80) but not in women (OR, 1.02; 95% CI, 0.60–1.72). In both sexes, high-intensity exercise modified the association between fasting glucose and GFR. A fasting glucose level 1 mmol/L higher was associated with a GFR that was 7.3 (95% CI, 4.0–10.6) and 6.2 (95% CI, 3.4–9.0) ml/min per 1.73 m2 higher in men and women who never exercised or exercised with low intensity. There was no association between fasting glucose and GFR in men and women who exercised with high intensity (interaction, P<0.001).
Conclusions
High-intensity exercise was associated with lower odds of hyperfiltration in men and modified the association between glucose and GFR of both sexes in a population without diabetes.
Introduction
CKD is common worldwide, and the global burden of ESRD is growing (1). Obesity, hypertension, and diabetes are major risk factors for CKD, but the understanding of how to prevent CKD at the population level is limited. Epidemiologic studies strongly suggest that physical exercise reduces the risk for CKD (2–5). However, the mechanisms of a possible protective effect of exercise on CKD risk are unknown.
Abnormally elevated GFR, or renal hyperfiltration, has been proposed as a mechanism that contributes to glomerular injury and albuminuria and subsequently to CKD (6). Renal hyperfiltration is common in early diabetes and in obesity and has been associated with generalized vascular dysfunction (7–9). Recently, we found that hyperfiltration was associated with impaired fasting glucose, a condition present in some 30% of adults in the United States (10,11). Physical exercise, as opposed to hyperglycemia, confers several benefits in terms of vascular function that also could influence GFR (12,13). However, the effect of physical exercise on renal function is difficult to study when GFR is estimated using creatinine or cystatin C. Estimated GFR (eGFR) is imprecise in the normal and upper range of GFR and could be biased by metabolic factors or creatinine production, which exercise also affects (14). To overcome these limitations, we measured GFR with single-sample iohexol clearance in a representative sample of a middle-aged, general population without diabetes. Our aims were to investigate whether physical exercise is associated with a reduced risk for hyperfiltration and whether physical exercise modifies the effect of fasting glucose on measured GFR (mGFR).
Materials and Methods
Participants
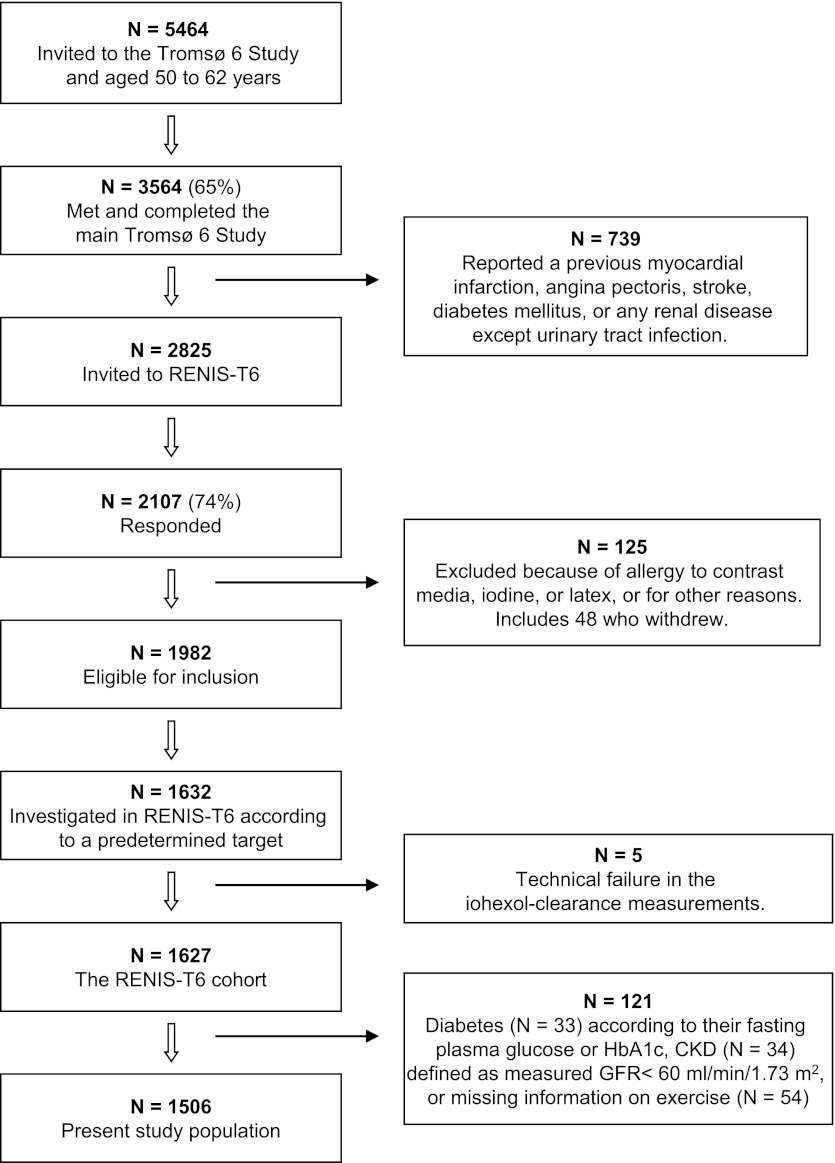
The Renal Iohexol Clearance Survey in Tromsø 6 (RENIS-T6) was conducted from November 2007 to June 2009 as a part of the population-based sixth Tromsø study (Tromsø 6) (15). Tromsø 6 included an age-stratified random sample of 12,984 inhabitants of the municipality of Tromsø in northern Norway. Among those invited to participate were 40% of all inhabitants age 50–59 years and all inhabitants age 60–62 years. In these age groups, 3564 (65%) completed the main part of Tromsø 6, which included a physical examination and a self-administered questionnaire. Of these, we excluded 739 who reported a previous myocardial infarction, angina pectoris, stroke, diabetes mellitus, or renal disease (Figure 1). The remaining 2825 persons were invited to participate in RENIS-T6, and 2107 (75%) responded positively. Seventy-seven persons were excluded because of allergies to contrast media, iodine, or latex or other reasons, and 48 persons did not appear at their appointments. Among the remaining 1982 persons, we included 1632 individuals according to a predetermined target size and stratified them by sex and age group. Five participants were excluded because the iohexol-clearance measurements were technical failures, leaving 1627 persons in the RENIS-T6 cohort.
Figure 1.
Inclusion of participants in the Renal Iohexol-clearance Survey in Tromsø 6 (RENIS-T6) from the main part of the sixth Tromsø Study (Tromsø 6). HbA1c, hemoglobin A1c.
For the present investigation, we excluded 33 persons with fasting plasma glucose ≥126 mg/dl or hemoglobin A1c [HbA1c] value ≥6.5%, who were considered to have diabetes (16); 34 persons with iohexol clearance <60 ml/min per 1.73 m2, who were considered to have CKD (17); and 54 whose information on the exercise questionnaire was missing (Figure 1).
The study was approved by the regional ethics committee of northern Norway. All participants provided informed written consent.
Assessment of Physical Exercise
The self-administered questionnaire included questions about physical exercise during leisure time. We used the three questions about frequency, intensity, and duration of physical exercise (Table 1). These questions were recently validated and found to perform well in a study that assessed physical activity and fitness by accelerometer and maximal oxygen consumption (18).
Table 1.
Response to questions about exercise in the sixth Tromsø Study
| Exercise | Women | Men | All |
|---|---|---|---|
| Exercise frequency: How frequently do you exercise? | |||
| Respondents (n) | 748 | 758 | 1506 |
| Never | 20 (2.7) | 36 (4.8) | 56 (3.7) |
| Less than once a week | 88 (11.8) | 151 (19.9) | 239 (15.9) |
| Once a week | 137 (18.3) | 197 (26.0) | 334 (22.2) |
| 2–3 times per week | 332 (44.4) | 282 (37.2) | 614 (40.8) |
| Almost every day | 171 (22.8) | 92 (12.1) | 263 (17.5) |
| Exercise intensity: If you exercise; how hard do you exercise? | |||
| Respondents (n) | 728 | 722 | 1450 |
| I take it easy without becoming breathless or sweaty | 334 (45.9) | 284 (39.3) | 618 (42.6) |
| I push myself so hard that I become breathless and sweaty | 374 (51.4) | 412 (57.1) | 786 (54.2) |
| I push myself to near-exhaustion | 20 (2.7) | 26 (3.6) | 46 (3.2) |
| Exercise duration: If you exercise, for how long do you exercise? | |||
| Respondents (n) | 700 | 706 | 1406a |
| <15 min | 16 (2.3) | 25 (3.5) | 41 (2.9) |
| 15–29 min | 97 (13.9) | 82 (11.6) | 179 (12.7) |
| 30–60 min | 442 (63.1) | 410 (58.1) | 852 (60.6) |
| >60 min | 145 (20.7) | 189 (26.8) | 334 (23.8) |
Unless otherwise noted, data are number (percentage) of participants.
44 missing (did not answer the question).
Measurements
All study participants met between 08:00 and 10:00 at the Clinical Research Unit at the University Hospital of Northern Norway after an overnight fast. Participants had been instructed to avoid large meals of meat and nonsteroidal anti-inflammatory drugs for 2 days before the investigation. They had also been asked to drink two to three glasses of water before their arrival. Smokers were instructed to abstain from smoking during the preceding 8 hours.
BP was measured three times at 1-minute intervals with an automatic device (A&D model UA-799); the last two readings were averaged. A Teflon catheter was placed in an antecubital vein, and fasting serum samples were drawn for glucose, insulin, creatinine, triglycerides, and cholesterol measurements. Iohexol (Omnipaque, 300 mg I/ml, Amersham Health, London, United Kingdom), 5 ml, was injected; the syringe was weighed before and after injection. The venous catheter was flushed with 30 ml of isotonic saline. The optimal time for measuring iohexol concentration after injection was calculated by the Jacobsson method based on the eGFR (19). The serum iohexol concentration was measured by HPLC as previously described by Nilsson-Ehle (20). The analytical coefficient of variation during the study period was 3.0%. GFR was calculated as described by Jacobsson (19). Further details about the iohexol clearance measurements have been published previously (21).
Creatinine levels were analyzed by the enzymatic method that was standardized against isotope dilution mass spectroscopy (CREA Plus, Roche Diagnostics, GmbH, Mannheim, Germany). Cystatin C was measured by particle-enhanced turbidimetric immunoassay using reagents from Gentian (Gentian, Moss, Norway) on a Modular E analyzer (Roche Diagnostics). GFR was estimated from creatinine (eGFRcre) or cystatin C (eGFRcys) using the recalibrated four-variable Modification of Diet in Renal Disease (eGFRMDRD) equation, the Chronic Kidney Disease Epidemiology Collaboration (eGFRCKD-EPI) equation, and the Rule cystatin C–based equation of 2006 (Supplemental Table 1) (22–24).
Serum glucose, triglycerides, and cholesterol were measured on a Modular P800 (Roche Diagnostics). HbA1c, urinary albumin excretion (UAE), and urinary creatinine were measured in the main part of Tromsø 6. HbA1c was measured with a liquid chromatographic method (Variant II instrument, Bio-Rad Laboratories, Hercules, CA). Three samples of first-void morning spot urine were collected on separate days. UAE and urinary creatinine were measured with commercial kits as described in a previous study (3). Albumin-to-creatinine ratio (ACR) was calculated for each urine specimen and the mean ACR value was used in the analyses. The insulin concentration was measured with an ELISA kit (DRG Instruments, Marburg, Germany). The intra- and interassay coefficients of variation were 4.7% and 6.3%, respectively. Insulin resistance was expressed by the homeostasis model assessment (HOMA-IR), which was calculated by multiplying fasting glucose (mmol/L) by fasting insulin (mU/L), and dividing the result by 22.5 (25).
Ambulatory BP was measured during the period after the measurement of iohexol clearance to the next day. BP was measured using the appropriate cuff size by Spacelab 90207 (Spacelab, Inc., Redmond, WA) at 20-minute intervals from 08:00 to 22:00 and at 45-minute intervals from 22:00 to 08:00. The registration was considered valid in accordance with criteria adopted from the International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcome study (26). Persons with invalid measurements had their measurements repeated as soon as possible.
Statistical Analyses
Characteristics of the participants are presented according to three groups of physical exercise: those who never exercised, those who exercised with low intensity (“easy exercise without becoming breathless or sweaty”), and those who exercised with high intensity (“I push myself so hard that I become breathless or sweaty” or “I push myself to near-exhaustion”). Mean values, or median values in cases of skewed data, were adjusted for age and sex. Differences across groups were tested by analysis of covariance for mean values, quantile regression for median values, and multiple logistic regression for dichotomous variables.
Renal hyperfiltration was defined as measured absolute GFR (in ml/min) above the 90th percentile after adjustment for sex, age, weight, height, and angiotensin-converting enzyme inhibitor (ACEI) or angiotensin-receptor blocker (ARB) use (10). This was done by selecting all participants above the 90th percentile in the distribution of residuals from a multiple linear regression analysis in which the dependent variable was the logarithm of absolute GFR and the independent variables were sex, ACEI or ARB use, and the logarithms of age, weight, and height. The association between hyperfiltration (yes/no) and categories of exercise was assessed by multiple logistic regression; stratified by sex; and adjusted for age, weight, height, current smoking, ambulatory diastolic BP, and current ACEI or ARB use. Physical exercise was categorized according to the frequency, intensity, and duration of exercise (Table 1). Because few individuals reported the lowest category of frequency, the lowest category of duration, and the highest category of intensity, we merged the two lower-frequency and -duration categories and the two upper-intensity categories. Accordingly, physical exercise was categorized into four categories of frequency, two categories of intensity, and three categories of duration of exercise. We also calculated an “exercise volume” variable by combining the frequency and duration of exercise into minutes of exercise per week (<30 minutes, 30–89 minutes, 90–180 minutes, and >180 minutes/week). The Spearman rank correlation coefficient was used to test the correlations between frequency, duration, and intensity of exercise.
To test whether physical exercise modified the effect of fasting glucose on GFR, we performed multiple linear regression analysis with GFR expressed in ml/min per 1.73 m2 as the dependent variable and fasting glucose as the independent variable, stratified by category of exercise and sex. We adjusted for the following known or possible determinants of GFR: age, weight, height, current smoking, ambulatory diastolic BP, and current ACEI or ARB use. We also tested for a possible interaction between fasting glucose and category of exercise on GFR. Similarly, to test whether exercise modified the association between glucose and hyperfiltration, we performed a multivariable adjusted logistic regression analysis, with hyperfiltration as the dependent variable and glucose as the independent variable, stratified by category of exercise and sex. A possible interaction between category of exercise and glucose on hyperfiltration was tested. To assess the independent effects of exercise frequency, intensity, and duration, we also repeated analyses in which all three variables were included in the same model.
Stata software, version 11 (Stata Corp., College Station, TX), was used for the statistical analyses. Statistical significance was set at P<0.05.
Results
There were small differences in the characteristics of participants included versus those not included in the RENIS-T6 cohort (Supplemental Table 2).
The reported frequency, intensity, and duration of exercise are shown separately for women and men in Table 1. Frequency and intensity of exercise were correlated (r = 0.30; P<0.001), as were exercise duration and intensity (r = 0.33; P<0.001) and duration and frequency (r = 0.15; P<0.001).
Table 2 shows the age- and sex-adjusted characteristics of the participants according to exercise intensity. More intensive exercise was associated with more frequent exercise, a lower percentage of smoking; higher eGFRcys; higher HDL cholesterol; and lower ambulatory heart rate, triglycerides, insulin, HOMA-IR, ACR, and eGFRcre. Measured GFR, however, did not differ between exercise levels.
Table 2.
General characteristics of the study population by category of leisure-time exercise intensity
| Characteristic | Never Exercise (n=56) | Low-Intensity Exercise (n=618)a | High-Intensity Exercise (n=832)b | P Value for Trend |
|---|---|---|---|---|
| Age (yr) | 58.3 (57.3–59.3) | 58.3 (58.0–58.6) | 57.8 (57.5–58.1) | 0.38 |
| Men (%) | 64 | 46 | 53 | 0.09c |
| Current daily smoking (%) | 57 | 28 | 13 | <0.001 |
| Body mass index (kg/m2) | 26.9 (25.9–27.9) | 27.5 (27.2–27.8) | 27.0 (26.7–27.2) | 0.93 |
| Conventional SBP (mmHg) | 127.8 (123.4–132.1) | 129.6 (128.3–130.9) | 129.5 (128.3–130.6) | 0.46 |
| Conventional DBP (mmHg) | 83.3 (80.8–85.7) | 83.1 (82.4–83.9) | 83.7 (83.1–84.3) | 0.73 |
| Ambulatory SBP (mmHg) | 123.9 (120.9–127.0) | 123.5 (122.5–124.4) | 122.9 (122.1–123.7) | 0.52 |
| Ambulatory DBP (mmHg) | 76.3 (74.3–78.3) | 76.5 (75.9–77.1) | 76.6 (76.1–77.1) | 0.78 |
| Ambulatory heart rate (beats/min) | 74.4 (72.1–76.6) | 71.6 (70.9–72.2) | 68.6 (68.0–69.2) | <0.001 |
| Total cholesterol (mg/dl) | 212 (202–221) | 219 (216–222) | 218 (215–220) | 0.23 |
| LDL cholesterol (mg/dl) | 136 (127–145) | 143 (140–145) | 141 (139–143) | 0.26 |
| HDL cholesterol (mg/dl) | 54.5 (50.3–58.4) | 58.0 (56.8–59.6) | 60.3 (59.6–61.5) | 0.004 |
| Triglycerided (mg/dl) | 97.5 (66.4–135.1) | 97.5 (66.4–131.6) | 88.6 (66.4–123.0) | 0.06e |
| Fasting glucose (mg/dl) | 95.3 (93.3–97.5) | 96.6 (96.0–97.3) | 95.3 (94.8–95.8) | 0.97 |
| Fasting insulind (μU/ml) | 8.3 (5.7–12.0) | 9.0 (6.4–12.5) | 8.2 (5.9–11.5) | 0.28e |
| HOMA-IRd | 2.0 (1.4–2.7) | 2.1 (1.5–3.0) | 1.9 (1.4–2.8) | 0.31e |
| Albumin-to-creatinine ratiod (mg/g) | 4.1 (1.9–7.5) | 3.4 (1.9–6.1) | 3.1 (1.8–5.7) | 0.02 |
| Measured GFRf (ml/min per 1.73 m2) | 93.8 (90.6–97.1) | 92.7 (91.8–93.7) | 91.9 (91.1–92.7) | 0.25 |
| eGFRMDRDg (ml/min per 1.73 m2) | 99.1 (95.0–103.3) | 96.3 (95.0–93.4) | 92.3 (91.3–93.4) | 0.002 |
| eGFRCKD-EPIh (ml/min per 1.73 m2) | 97.3 (95.0–99.5) | 96.0 (95.4–96.7) | 94.1 (93.5–94.7) | 0.008 |
| eGFRcysi (ml/min per 1.73 m2) | 91.6 (87.3–95.8) | 91.6 (90.3–92.9) | 93.3 (92.2–94.4) | 0.44d |
| Exercise more than once a week (%) | 0 | 42 | 75 | <0.001 |
Values are mean (95% confidence intervals), median (interquartile range) in case of skewed data, or percentages and were adjusted by age and sex. Differences between groups were tested by analysis of covariance for mean values, quantile regression for median values and logistic regression for proportions. SBP, systolic BP; DBP, diastolic BP; HOMA-IR, homeostasis model assessment of insulin resistance.
Easy exercise; without becoming breathless or sweaty.
Hard exercise; becoming breathless and sweaty, or exhausted.
P=0.002 for quadratic trend.
Values are median (interquartile range).
P<0.05 for the difference between the low-intensity exercise group and the high-intensity exercise group.
GFR measured by single-sample iohexol clearance and adjusted for body surface area.
GFR estimated by the Modification of Diet in Renal Disease equation.
GFR estimated by the Chronic Kidney Disease-Epidemiology Collaboration equation.
GFR estimated by Rules cystatin C–based equation of 2006.
Table 3 gives the sex-specific associations between hyperfiltration and physical exercise, adjusted for age, height, weight, current smoking, ambulatory diastolic BP, and the use of ACEI or ARB. High-intensity exercise was associated with lower odds of hyperfiltration in men (odds ratio, 0.47; 95% confidence interval [CI], 0.28–0.80) but not in women (odds ratio, 1.02; 95% CI, 0.60–1.72) (interaction for sex; P=0.02). The results remained similar and significant after adjustment for exercise frequency and duration (not shown).
Table 3.
Multivariable-adjusted odds ratio for hyperfiltration
| Variable | Women | Men | ||
|---|---|---|---|---|
| Adjusted Odds Ratio (95% CI)a | P Value for Trend | Adjusted Odds Ratio (95% CI)a | P Value for Trend | |
| Exercise frequency | ||||
| Never or less than once a weekb | Reference | Reference | ||
| Once a week | 0.80 (0.37–1.69) | 0.89 (0.46–1.70) | ||
| 2–3 times per week | 0.43 (0.21–0.89) | 0.89 (0.48–1.67) | ||
| Almost every day | 0.68 (0.31–1.49) | 0.16 | 0.68 (0.27–1.69) | 0.42 |
| Exercise intensity | ||||
| Low intensityc | Reference | Reference | ||
| High intensityd | 1.02 (0.60–1.72) | 0.92e | 0.47 (0.28–0.80) | 0.005e |
| Exercise duration | ||||
| <30 minb | Reference | Reference | ||
| 30–60 min | 1.03 (0.50–2.15) | 1.21 (0.59–2.51) | ||
| >60 min | 1.14 (0.48–2.73) | 0.76 | 0.84 (0.35–1.98) | 0.88 |
| Duration of exercise per week | ||||
| <30 min (n=336) | Reference | Reference | ||
| 30–89 min (n=344) | 0.56 (0.26–1.22) | 0.76 (0.40–1.47) | ||
| 90–180 min (n=416) | 0.49 (0.24–1.01) | 0.93 (0.48–1.80) | ||
| >180 min (n=376) | 0.65 (0.32–1.34) | 0.28 | 0.58 (0.25–1.30) | 0.29 |
CI, confidence interval.
Adjusted for age, height, weight, current smoking, ambulatory diastolic BP, and angiotensin-converting enzyme inhibitor, or angiotensin-receptor blocker use.
The two lowest categories were merged into one (reference) group.
Easy exercise; without becoming breathless or sweaty.
Hard exercise; becoming breathless and sweaty or exhausted.
P=0.02 for the interaction between sex and exercise intensity on hyperfiltration when men and women were analyzed in the same model.
Table 4 shows the association between fasting glucose and mGFR or eGFR, stratified by intensity of exercise and sex. A glucose level 1 mmol/L (18 mg/dl) higher was associated with an mGFR that was 7.3 (95% CI, 4.0–10.6) and 6.2 (95% CI, 3.4–9.0) ml/min per 1.73 m2 higher in men and women who never exercised or exercised with low intensity and with an mGFR that was 0.5 (95% CI, −2.1 to 3.1) and 0.1 (95% CI, −2.7 to 2.9) ml/min per 1.73 m2 higher in men and women who exercised with high intensity (P<0.001 for interaction). Attenuated or nonsignificant results were found for eGFRcys and eGFRcre (shown for eGFRCKD-EPI). The estimates remained unchanged for mGFR and eGFR in both sexes after additional adjustment for HOMA-IR, HDL cholesterol, triglycerides, body mass index, ACR, and ambulatory heart rate (not shown). Exercise frequency or exercise duration did not modify the association between glucose and mGFR (not shown).
Table 4.
Multiple linear regression analyses with measured GFR or estimated GFR as dependent variable and fasting glucose as independent variable, stratified by intensity of physical exercise
| Independent Variable | Never Exercise or Low-Intensitya Exercise (n=674) | High-Intensity Exerciseb (n=832) | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted Estimate (95% CI) (ml/min per 1.73 m2) | P Value | Adjustedc Estimate (95% CI) (ml/min per 1.73m2) | P Value | Unadjusted Estimate (95% CI) (ml/min per 1.73 m2) | P Value | Adjustedc Estimate (95% CI) (ml/min per 1.73 m2) | P Value | |
| Measured GFR | ||||||||
| Women | ||||||||
| Glucose (per 18 mg/dl) | 3.0 (0.2–5.8) | 0.03 | 6.2 (34–9.0) | <0.001 | −0.4 (−3.1 to 2.3) | 0.77 | 0.1 (−2.7 to 2.9) | 0.94d |
| Men | ||||||||
| Glucose (per 18 mg/dl) | 5.2 (2.0–8.5) | 0.002 | 7.3 (4.0–10.6) | <0.001 | 0.6 (−1.9 to 3.1) | 0.62 | 0.5 (−2.1 to 3.1) | 0.70d |
| eGFRcys | ||||||||
| Women | ||||||||
| Glucose (per 18 mg/dl) | −0.1 (−4.1 to 3.9) | 0.96 | 4.0 (0.1–8.1) | 0.05 | −2.6 (−6.4 to 1.2) | 0.18 | −0.02 (−4.0 to 4.0) | 0.99e |
| Men | ||||||||
| Glucose (per 18 mg/dl) | 3.2 (−0.5 to 7.0) | 0.09 | 4.7 (1.0–8.4) | 0.01 | −0.6 (−3.8 to 2.5) | 0.69 | 1.0 (−2.2 to 4.2) | 0.55e |
| eGFRCKD-EPI | ||||||||
| Women | ||||||||
| Glucose (per 18 mg/dl) | −1.2 (−3.3 to 1.0) | 0.29 | 0.2 (−1.9 to 2.4) | 0.84 | −1.2 (−3.3 to 1.1) | 0.30 | −0.4 (−2.7 to 1.8) | 0.72 |
| Men | ||||||||
| Glucose (per 18 mg/dl) | 0.6 (−1.4 to 2.5) | 0.56 | 2.3 (0.4–4.2) | 0.02 | −0.9 (−2.8 to 0.9) | 0.32 | −0.1 (−1.9 to 1.7) | 0.91 |
CI, confidence interval; eGFRcys, GFR estimated by Rule's cystatin C–based equation of 2006; eGFRCKD-EPI, GFR estimated by the Chronic Kidney Disease-Epidemiology Collaboration equation.
Easy exercise; without becoming breathless or sweaty.
Hard exercise; becoming breathless and sweaty or exhausted.
Adjusted for age, height, weight, current smoking, ambulatory diastolic BP, and angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker use.
P<0.001 for interaction between fasting glucose and intensity of exercise on GFR when men and women were analyzed in the same model.
P=0.03 for interaction between fasting glucose and intensity of exercise on GFR when men and women were analyzed in the same model.
A similar pattern of effect modification by exercise was found on the association between glucose and hyperfiltration. For men and women, higher fasting glucose was associated with increased odds of hyperfiltration in the never-exercise or low-intensity exercise groups, but not in the high-intensity group (Table 5). However, the interaction between category of exercise and glucose on hyperfiltration did not reach statistical significance when all participants were analyzed in the same model (P=0.25).
Table 5.
Multivariable-adjusted odds ratio for hyperfiltration, stratified by intensity of physical exercise
| Independent Variable | Never Exercise or Low-Intensitya Exercise (n=674) | High-Intensityb Exercise (n=832) | ||
|---|---|---|---|---|
| Adjusted Odds Ratio (95% CI)c | P Value | Adjusted Odds Ratio (95% CI)c | P Value | |
| Women | ||||
| Fasting glucose (per 18 mg/dl) | 3.4 (1.5–7.6) | 0.003 | 1.7 (0.7–4.0) | 0.21d |
| Men | ||||
| Fasting glucose (per 18 mg/dl) | 2.1 (1.1–4.1) | 0.04 | 1.2 (0.5–0.3) | 0.68d |
CI, confidence interval.
Easy exercise; without becoming breathless or sweaty.
Hard exercise; becoming breathless and sweaty or exhausted.
Adjusted for age, height, weight, current smoking, ambulatory diastolic BP, and angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker use.
P=0.31 for interaction between fasting glucose and intensity of exercise on hyperfiltration when men and women were analyzed in the same model.
We repeated all analyses when persons with mGFR <60 ml/min per 1.73 m2 and persons with diabetes according to their fasting glucose or HbA1c were included. These analyses yielded similar results.
Discussion
In this study, we found that performing high-intensity exercise during leisure time was associated with lower odds of hyperfiltration in men but not in women. Furthermore, high-intensity exercise eliminated the association between fasting glucose and mGFR in both sexes. An elevation in fasting glucose was associated with a markedly higher mGFR, but only in individuals who reported that they never exercised or exercised with low intensity.
To our knowledge, this work is the first population-based study on the cross-sectional relationship between physical exercise and mGFR. Previous studies have reported the association between physical activity and eGFR, but the results have been divergent. In the third National Health and Nutrition Examination Survey (NHANES III), frequency of physical activity was associated with lower eGFRcre, and physical activity calculated as metabolic equivalents was associated with increased eGFRcre (27).
In the present study, physical exercise was associated with lower eGFRcre and higher eGFRcys but was not associated with mGFR, after adjustment for age and sex (Table 2). In multiple regression, the association between glucose and mGFR, and the effect modification by exercise, was attenuated or was not significant with eGFRcys and eGFRcre. These findings demonstrate the pitfalls of studying the association between physical exercise or hyperglycemia and eGFR. Physical exercise is likely to increase muscle mass, which increases serum creatinine and thus lowers eGFRcre. Cystatin C is also influenced by non-GFR determinants, particularly obesity, smoking, and triglycerides (28). However, cystatin C may be less influenced by physical exercise and may have a better ability to detect changes in GFR provoked by hyperglycemia (29).
In longitudinal studies, physical activity has been shown to protect against elevated UAE or renal function decline in diabetes (30,31). Similar findings emerged from the following three studies of a general population: In a 12- to 16-year follow-up of 9082 adults in the United States (NHANES II), inactive individuals had an increased risk for ESRD or CKD-related death compared with very active individuals, also after adjustment for BP, obesity, and diabetes (2). In a previous Tromsø study, initiation of hard physical activity for ≥1 hour per week reduced the risk for increased UAE in men but not in women (3). In the Cardiovascular Health Study, high-intensity exercise, but not moderate- or low-intensity exercise, reduced the hazard ratio for rapid kidney function decline (eGFRcys) during 7 years of follow-up of older adults (4). In the latter study, no significant interaction with sex was found. However, in the AusDiab study of 6318 adults followed for 5 years, physical activity was not associated with incident CKD, defined as de novo albuminuria, eGFRcre decline >10%, or final eGFRcre <60 ml/min per 1.73 m2 (32). This study did not report sex-specific analyses and did not differentiate between low- and high-intensity exercises.
In the present study, high-intensity exercise reduced the odds of hyperfiltration in men, independent of possible confounders, such as body mass index and BP. No association was found between exercise and hyperfiltration in women. Because hyperfiltration has been associated with the development of microalbuminuria (7,33), the current finding may suggest an explanation for why hard physical activity in a previous study predicted reduced UAE progression in men but not in women (3). Possible mechanisms for this sex disparity are unknown. Subjective interpretation of exercise questions, which causes misclassification, could differ between men and women. However, it is unlikely that such misclassification could be large because intensive exercise tends to be better reported than less intensive exercise (34,35). Sex-specific risk factors for early kidney dysfunction have been reported previously. In a study of the general Dutch population, increasing age, BP, and plasma glucose were stronger predictors of increased UAE in men than in women (36).
The second main finding of the present study was that high-intensity exercise eliminated the positive association between fasting glucose and GFR and possibly between glucose and hyperfiltration in men and women. Animal experiments support that hyperfiltration combined with hyperglycemia may induce podocyte stress, podocyte injury, and cell apoptosis (37). Whether hyperfiltration or elevated GFR caused by borderline hyperglycemia is associated with renal injury in humans has not been investigated in longitudinal studies. However, increasing levels of fasting glucose, within the nondiabetic range, have been associated with the progression of albuminuria and decline in renal function in both sexes in the general population (38,39). Also, in the general population, an inverse U-shaped association was found between plasma glucose and eGFR, possibly indicating a phase of hyperfiltration that precedes GFR decline (40).
The present study suggests that elevated fasting glucose could convey different renal risk in individuals who never exercise or exercise with low intensity compared with those who exercise with high intensity. By analogy, a previous study suggested differential risk of obesity relative to CKD between physically inactive persons versus moderately or very active persons (5). Obesity or ambulatory BP did not mediate the modifying effect of exercise on the relationship between borderline hyperglycemia and GFR in the present study because we adjusted for both. We are not aware of any longitudinal reports on the effect of exercise on renal injury caused by hyperglycemia.
In the current work, the modifying effect of exercise on the association between glucose and mGFR and the reduced odds of hyperfiltration in men clearly depended on exercise intensity. Physical exercise improves endothelial function, decreases inflammation, and reduces activation of the renin-angiotensin-aldosterone system and renal sympathetic activity (12,13,41), all factors that could potentially influence vascular tone and thus affect GFR. Moreover, emerging evidence indicates that the intensity of exercise is important for obtaining benefits for the vasculature and cardiovascular disease (12,41–43). Randomized, controlled trials, one involving participants with impaired glucose tolerance and one involving patients with type 2 diabetes, have shown that high-intensity exercise but not low-intensity exercise decreased levels of inflammatory biomarkers (30,44) and albuminuria (30).
In accordance with previous studies, we found lower heart rate, lower ACR, lower insulin resistance, and higher HDL cholesterol in the high-intensity exercise group. However, these metabolic factors had no influence on the association between exercise, fasting glucose, and mGFR. We did not measure markers of inflammation or oxidative stress. Kidney disease in general, and particularly in diabetes, is clearly related to increased inflammation and oxidative stress (45). Furthermore, increasing evidence support that inflammation and oxidative stress, which are increased in hyperglycemia and reduced by exercise, may initiate hyperfiltration (46–50).
The most important limitation of this study was the cross-sectional design, which precludes conclusions about causality. There is no consensus on how to define renal hyperfiltration, which may not represent hyperfiltration at a glomerular level. Thus, the meaning of renal hyperfiltration should be interpreted with caution in cross-sectional studies. Also, the study population consisted of middle-aged white persons only, and generalizations to other age groups or ethnicities should be made with caution. The data on physical exercise were based on a questionnaire and not on objective measures of physical activity or fitness, and the questionnaire did not differentiate between different exercise activities (i.e., walking, skiing, and sports activities). However, in contrast with many previous observational studies and in accordance with the updated recommendation of the American Heart Association, this study included details on frequency, intensity, and duration of exercise (42). The main strength of this study is that GFR was measured with an exact method in a large population-based study.
Physical exercise was associated with lower odds of hyperfiltration in men and modified the association between glucose and mGFR in both sexes of a middle-aged general population without diabetes. These associations were dependent on exercise intensity and not mediated through body mass index, BP, ambulatory resting heart rate, HDL cholesterol, triglycerides, or insulin resistance. Longitudinal studies are needed to investigate the effect of exercise on the risk of CKD.
Disclosures
U.D.M. received reimbursement for travel expenses from Gentian AS (Moss, Norway). None of the other authors have any conflicts of interest to report.
Supplementary Material
Acknowledgments
We thank Bjørg Skog Høgset, Saskia van Heusden, and the rest of the staff at the Clinical Research Unit (University Hospital of North Norway) for performing the study; Harald Strand and the staff at the Department of Medical Biochemistry (University Hospital of North Norway) for HPLC analyses of iohexol; Åse Lund and Gro Bolstad for insulin analyses; Inger Sperstad and Ingrid Dorthea Sandstad (Clinical Research Centre, University Hospital of North Norway) for database support; and Tom Wilsgaard, Sriharan Sivasingarajah, and Kurt Jøran Nyland (Institute of Community Medicine, University of Tromsø) for identifying eligible participants from the Tromsø 6 cohort.
This study was funded by the North Norway Regional Health Authority. The sponsor had no role in study design; collection, analysis, or interpretation of data; writing the report; or the decision to submit the report for publication.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02980312/-/DCSupplemental.
References
- 1.Tsukamoto Y, Wang H, Becker G, Chen HC, Han DS, Harris D, Imai E, Jha V, Li PK, Lee EJ, Matsuo S, Tomino Y, Tungsanga K, Yamagata K, Hishida A: Report of the Asian Forum of Chronic Kidney Disease Initiative (AFCKDI) 2007. “Current status and perspective of CKD in Asia”: Diversity and specificity among Asian countries. Clin Exp Nephrol 13: 249–256, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Stengel B, Tarver-Carr ME, Powe NR, Eberhardt MS, Brancati FL: Lifestyle factors, obesity and the risk of chronic kidney disease. Epidemiology 14: 479–487, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Solbu MD, Kronborg J, Eriksen BO, Jenssen TG, Toft I: Cardiovascular risk-factors predict progression of urinary albumin-excretion in a general, non-diabetic population: A gender-specific follow-up study. Atherosclerosis 201: 398–406, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Robinson-Cohen C, Katz R, Mozaffarian D, Dalrymple LS, de Boer I, Sarnak M, Shlipak M, Siscovick D, Kestenbaum B: Physical activity and rapid decline in kidney function among older adults. Arch Intern Med 169: 2116–2123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hallan S, de Mutsert R, Carlsen S, Dekker FW, Aasarød K, Holmen J: Obesity, smoking, and physical inactivity as risk factors for CKD: Are men more vulnerable? Am J Kidney Dis 47: 396–405, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Brenner BM, Lawler EV, Mackenzie HS: The hyperfiltration theory: A paradigm shift in nephrology. Kidney Int 49: 1774–1777, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG: Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 52: 691–697, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Wuerzner G, Pruijm M, Maillard M, Bovet P, Renaud C, Burnier M, Bochud M: Marked association between obesity and glomerular hyperfiltration: A cross-sectional study in an African population. Am J Kidney Dis 56: 303–312, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Cherney DZ, Miller JA, Scholey JW, Nasrallah R, Hébert RL, Dekker MG, Slorach C, Sochett EB, Bradley TJ: Renal hyperfiltration is a determinant of endothelial function responses to cyclooxygenase 2 inhibition in type 1 diabetes. Diabetes Care 33: 1344–1346, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melsom T, Mathisen UD, Ingebretsen OC, Jenssen TG, Njølstad I, Solbu MD, Toft I, Eriksen BO: Impaired fasting glucose is associated with renal hyperfiltration in the general population. Diabetes Care 34: 1546–1551, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Williams DE, Gregg EW, Bainbridge KE, Saydah SH, Geiss LS: Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care 32: 287–294, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribeiro F, Alves AJ, Duarte JA, Oliveira J: Is exercise training an effective therapy targeting endothelial dysfunction and vascular wall inflammation? Int J Cardiol 141: 214–221, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Meredith IT, Friberg P, Jennings GL, Dewar EM, Fazio VA, Lambert GW, Esler MD: Exercise training lowers resting renal but not cardiac sympathetic activity in humans. Hypertension 18: 575–582, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Stevens LA, Levey AS: Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol 20: 2305–2313, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen BK, Eggen AE, Mathiesen EB, Wilsgaard T, Njolstad I: Cohort profile: The Tromso Study [published online ahead of print April 21, 2011]. Int J Epidemioldoi: 10.1093/ije/dyr049 [DOI] [PMC free article] [PubMed]
- 16.International Expert Committee : International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 32: 1327–1334, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G, National Kidney Foundation : National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med 139: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Kurtze N, Rangul V, Hustvedt BE, Flanders WD: Reliability and validity of self-reported physical activity in the Nord-Trøndelag Health Study: HUNT 1. Scand J Public Health 36: 52–61, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Jacobsson L: A method for the calculation of renal clearance based on a single plasma sample. Clin Physiol 3: 297–305, 1983 [DOI] [PubMed] [Google Scholar]
- 20.Nilsson-Ehle P: Iohexol clearance for the determination of glomerular filtration rate: 15 years´ experience in clinical practice. E J Int Fed Clin Chem Lab Med 13, 2006 [PMC free article] [PubMed] [Google Scholar]
- 21.Eriksen BO, Mathisen UD, Melsom T, Ingebretsen OC, Jenssen TG, Njølstad I, Solbu MD, Toft I: Cystatin C is not a better estimator of GFR than plasma creatinine in the general population. Kidney Int 78: 1305–1311, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration : Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS: Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int 69: 399–405, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 26.Kikuya M, Hansen TW, Thijs L, Björklund-Bodegård K, Kuznetsova T, Ohkubo T, Richart T, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Staessen JA, International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes Investigators : Diagnostic thresholds for ambulatory blood pressure monitoring based on 10-year cardiovascular risk. Circulation 115: 2145–2152, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Finkelstein J, Joshi A, Hise MK: Association of physical activity and renal function in subjects with and without metabolic syndrome: A review of the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 48: 372–382, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Mathisen UD, Melsom T, Ingebretsen OC, Jenssen T, Njølstad I, Solbu MD, Toft I, Eriksen BO: Estimated GFR associates with cardiovascular risk factors independently of measured GFR. J Am Soc Nephrol 22: 927–937, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cherney DZ, Sochett EB, Dekker MG, Perkins BA: Ability of cystatin C to detect acute changes in glomerular filtration rate provoked by hyperglycaemia in uncomplicated Type 1 diabetes. Diabet Med 27: 1358–1365, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Balducci S, Zanuso S, Nicolucci A, Fernando F, Cavallo S, Cardelli P, Fallucca S, Alessi E, Letizia C, Jimenez A, Fallucca F, Pugliese G: Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr Metab Cardiovasc Dis 20: 608–617, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Krop JS, Coresh J, Chambless LE, Shahar E, Watson RL, Szklo M, Brancati FL: A community-based study of explanatory factors for the excess risk for early renal function decline in blacks vs whites with diabetes: the Atherosclerosis Risk in Communities study. Arch Intern Med 159: 1777–1783, 1999 [DOI] [PubMed] [Google Scholar]
- 32.White SL, Dunstan DW, Polkinghorne KR, Atkins RC, Cass A, Chadban SJ: Physical inactivity and chronic kidney disease in Australian adults: The AusDiab study. Nutr Metab Cardiovasc Dis 21: 104–112, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Palatini P, Mormino P, Dorigatti F, Santonastaso M, Mos L, De Toni R, Winnicki M, Dal Follo M, Biasion T, Garavelli G, Pessina AC, HARVEST Study Group : Glomerular hyperfiltration predicts the development of microalbuminuria in stage 1 hypertension: the HARVEST. Kidney Int 70: 578–584, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Lee I, Blair SN, Manson J, Paffenbarger RSJ: Epidemiologic Methods in Physical Activity Studies, New York, NY, Oxford University Press, 2009 [Google Scholar]
- 35.Emaus A, Degerstrøm J, Wilsgaard T, Hansen BH, Dieli-Conwright CM, Furberg AS, Pettersen SA, Andersen LB, Eggen AE, Bernstein L, Thune I: Does a variation in self-reported physical activity reflect variation in objectively measured physical activity, resting heart rate, and physical fitness? Results from the Tromso study. Scand J Public Health 38[Suppl]: 105–118, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Verhave JC, Hillege HL, Burgerhof JG, Navis G, de Zeeuw D, de Jong PE, PREVEND Study Group : Cardiovascular risk factors are differently associated with urinary albumin excretion in men and women. J Am Soc Nephrol 14: 1330–1335, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Lewko B, Stepinski J: Hyperglycemia and mechanical stress: Targeting the renal podocyte. J Cell Physiol 221: 288–295, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Brantsma AH, Atthobari J, Bakker SJ, de Zeeuw D, de Jong PE, Gansevoort RT: What predicts progression and regression of urinary albumin excretion in the nondiabetic population? J Am Soc Nephrol 18: 637–645, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Halbesma N, Brantsma AH, Bakker SJ, Jansen DF, Stolk RP, De Zeeuw D, De Jong PE, Gansevoort RT, PREVEND study group : Gender differences in predictors of the decline of renal function in the general population. Kidney Int 74: 505–512, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Verhave JC, Hillege HL, Burgerhof JG, Gansevoort RT, de Zeeuw D, de Jong PE, PREVEND Study Group : The association between atherosclerotic risk factors and renal function in the general population. Kidney Int 67: 1967–1973, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Hespel P, Lijnen P, Van Hoof R, Fagard R, Goossens W, Lissens W, Moerman E, Amery A: Effects of physical endurance training on the plasma renin-angiotensin-aldosterone system in normal man. J Endocrinol 116: 443–449, 1988 [DOI] [PubMed] [Google Scholar]
- 42.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A, American College of Sports Medicine. American Heart Association : Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 116: 1081–1093, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Tyldum GA, Schjerve IE, Tjønna AE, Kirkeby-Garstad I, Stølen TO, Richardson RS, Wisløff U: Endothelial dysfunction induced by post-prandial lipemia: Complete protection afforded by high-intensity aerobic interval exercise. J Am Coll Cardiol 53: 200–206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herder C, Peltonen M, Koenig W, Sütfels K, Lindström J, Martin S, Ilanne-Parikka P, Eriksson JG, Aunola S, Keinänen-Kiukaanniemi S, Valle TT, Uusitupa M, Kolb H, Tuomilehto J, Finnish Diabetes Prevention Study Group : Anti-inflammatory effect of lifestyle changes in the Finnish Diabetes Prevention Study. Diabetologia 52: 433–442, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Forbes JM, Coughlan MT, Cooper ME: Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57: 1446–1454, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Pistrosch F, Herbrig K, Kindel B, Passauer J, Fischer S, Gross P: Rosiglitazone improves glomerular hyperfiltration, renal endothelial dysfunction, and microalbuminuria of incipient diabetic nephropathy in patients. Diabetes 54: 2206–2211, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Lal MA, Körner A, Matsuo Y, Zelenin S, Cheng SX, Jaremko G, DiBona GF, Eklöf AC, Aperia A: Combined antioxidant and COMT inhibitor treatment reverses renal abnormalities in diabetic rats. Diabetes 49: 1381–1389, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Hernández-Marco R, Codoñer-Franch P, Pons Morales S, Del Castillo Villaescusa C, Boix García L, Valls Bellés V: Oxidant/antioxidant status and hyperfiltration in young patients with type 1 diabetes mellitus. Pediatr Nephrol 24: 121–127, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Kodera R, Shikata K, Kataoka HU, Takatsuka T, Miyamoto S, Sasaki M, Kajitani N, Nishishita S, Sarai K, Hirota D, Sato C, Ogawa D, Makino H: Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 54: 965–978, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Persson MF, Franzen S, Catrina S.B, Dallner G, Hansell P, Brismar K, Palm F: Coenzyme Q10 prevents GDP-sensitive mitochondrial uncouplilng, glomerular hyperfiltration and proteinuria in kidneys from db/db mice as a model of type 2 diabetes. Diabetologia 55: 1535–1543, 2012. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.