Summary
Background and objectives
Acidosis is prevalent among renal transplant recipients (RTRs) and adversely affects cardiometabolic processes. Factors contributing to acidosis are graft dysfunction and immunosuppressive drugs. Little is known about the potential influence of diet on acidosis in RTRs. This study examined the association of metabolic acid load with acidosis and with cardiovascular risk factors in RTRs and aimed to identify dietary factors associated with acidosis.
Design, participants, setting, & measurements
707 RTRs were included. Metabolic acid load was assessed by measuring 24-hour urinary net acid excretion (NAE; i.e., titratable acid + ammonium − bicarbonate). Acidosis was defined as serum [HCO3−] < 24 mmol/L. BP and insulin resistance, reflected by hemoglobin A1c, were among cardiovascular risk factors. Diet was assessed with food-frequency questionnaires. Linear regression analysis was applied to investigate association between NAE and acidosis and between dietary factors and acidosis.
Results
Mean age ± SD was 53±13 years; 57% of patients were male. Acidosis was present in 31% of RTRs. NAE was associated with acidosis (serum HCO3−: β=−0.61; serum pH: β=−0.010; both P<0.001). Patients with high intake of animal protein (i.e., from meat, cheese, and fish) and low intake of fruits and vegetables had significantly lower serum HCO3− and serum pH. No associations were observed between NAE and cardiovascular risk factors, such as hypertension and insulin resistance.
Conclusions
In addition to conventional factors contributing to acidosis, diet might influence acid-base homeostasis in RTRs. Higher intake of fruits and vegetables and lower animal protein intake is associated with less acidosis in RTRs.
Introduction
Worldwide, the prevalence of ESRD is increasing rapidly (1). Although kidney transplantation is the preferred treatment for patients with ESRD, successful transplantation is still associated with substantially elevated morbidity and mortality (2,3). Many renal transplant recipients (RTRs) have metabolic acidosis (4,5), which may adversely affect cardiometabolic processes, including BP and insulin resistance, as well as proper functioning of multiple tissues (4,6,7). Therefore, it is important to identify modifiable determinants of metabolic acidosis that might help improve acid-base homeostasis in RTRs.
Diet can influence acid-base balance in humans (8–10). Potassium salts of anions that can be metabolized, such as citrate, have an alkalinizing effect, whereas acid load originates from such precursors as cationic amino acids and organic acids. Accordingly, fruits and vegetables contribute to base load, whereas animal protein adds to acid load. The contemporary western diet, including large amounts of animal products, generates an acid load that is not compensated for by the shortage of fruit and vegetable and can subsequently lead to increasing but unnoticed metabolic acidosis (11).
The kidney plays an important role in acid-base homeostasis by excreting the excess of acids ingested. In general, persons consuming diets high in acid load have higher urinary acid excretion than persons eating diets rich in alkalinizing foods (12). In RTRs, however, the capacity to excrete acid is decreased because of impaired renal function. Therefore, it is hypothesized that dietary modifications could improve acid-base homeostasis in RTRs. Although post-transplant acidosis has been studied before (4,5), to our knowledge no studies have assessed the role of nutrition in acidosis in RTRs.
We therefore examined whether net acid excretion (NAE), reflecting metabolic acid load, was related to acidosis in a large RTR cohort. Second, we studied the association of NAE with cardiovascular risk factors, such as insulin resistance and high BP. We also aimed to identify dietary factors contributing to NAE in RTRs in order to provide tools for lowering acidosis in this population.
Materials and Methods
Design and Study Population
We conducted cross-sectional analyses in a large, single-center RTR cohort. We invited all adult RTRs (age ≥ 18 years) with a functioning graft for at least 1 year who visited our outpatient clinic between 2008 and 2011. All RTRs underwent transplantation in our center, had sufficient knowledge of the Dutch language, and had no history of drug or alcohol addiction, as reported in their patient files. Patients with overt congestive heart failure and patients diagnosed with cancer other than cured skin cancer were not considered eligible for the study. In patients with fever or other signs of infection (e.g., urinary or upper respiratory tract infection), visits were postponed until symptoms had resolved. Of 817 initially invited RTRs, 707 (87%) provided written informed consent to participate. All but 3 were white. For analyses of dietary intake, we excluded all patients with missing dietary data, resulting in 625 RTRs for analyses. RTRs with complete data and those with missing data were similar regarding age, sex, body composition, estimated GFR (eGFR), and medication use. Of RTRs who did not consent, we recorded only age, sex, body composition, and eGFR. Compared with participating RTRs, those who did not consent were slightly older (mean age ± SD, 58±13 versus 53±13 years in both other groups) and had lower eGFR (47±19 ml/min per 1.73 m2 versus 51±21 and 53±20 ml/min per 1.73 m2 for the RTRs with missing and complete data, respectively). The institutional review board approved the study protocol (METc 2008/186), which adhered to principles of the Declaration of Helsinki.
Assessment of Metabolic Acid Load
RTRs were instructed to collect a 24-hour urine sample according to strict protocol on the day before their visit to the outpatient clinic. Urine was collected under oil, and chlorhexidine was added as an antiseptic agent. After completion, electrolytes (chloride, potassium, sodium, calcium, and phosphate) were directly analyzed according to standard laboratory procedures. Urine pH and titratable acid (TA) were measured with an automated titrator (855 Robotic Titrosampler; Metrohm, Herisau, Switzerland). Directly after collection, additional urine samples were stored at −80°C and kept deeply refrigerated for a maximum of 2 years. Ammonium (NH4+) and bicarbonate (HCO3−) were measured via chromatography in freshly thawed 24-hour urine samples (Alliance HT 2795, Waters, Milford, MA; type 861, Metrohm, respectively). Stability of both variables over repeated freeze-thaw cycles was analyzed in advance of the study; differences after thawing compared with the original mean values were insignificant. NAE, the gold standard for measuring metabolic acid load, was calculated in the conventional manner as TA + NH4+ − HCO3− (13).
Assessment of Diet and Acid Load
Diet was assessed using a semi-quantitative food-frequency questionnaire (FFQ) inquiring about intake of 177 items during the last month, taking seasonal variations into account. The FFQ was developed at Wageningen University (14) and has been updated several times. For the present study, the FFQ was slightly modified for accurate assessment of protein intake, including types and sources of protein. For each item, the frequency was recorded in times per day, week, or month. The number of servings was expressed in natural units (e.g., slice of bread or apple) or household measures (e.g., cup or spoon). The questionnaire was self-administered and filled out at home. All FFQs were checked for completeness by a trained researcher, and inconsistent answers were verified with the patients. Dietary data were converted into daily nutrient intake using the Dutch Food Composition Table of 2006 (15). The validity of the FFQ in RTRs was checked by comparing the estimated protein intake with calculations of protein intake according to the Maroni formula, based on 24-hour urinary urea excretion, reliably reflecting protein intake (16,17). On the basis of this formula, protein intake was 85±21 g/d (approximately 1.1±0.3 g/kg per day), similar to the estimates derived from the FFQ (83±20 g/d [approximately 1.1±0.3 g/kg per day]; P=0.3). Also, in a subgroup of 60 RTRs, we compared dietary data of FFQ with 3-day food records. Pearson correlations between FFQ and records were 0.72 for energy intake, 0.64 for protein intake (0.53 for animal protein; 0.73 for plant protein), 0.50 for fat intake, and 0.69 for carbohydrate intake, similar to values observed in previous studies analyzing validity of FFQs in population-based cohort studies (18). Regarding fruit and vegetable intake, correlation coefficients were 0.66 and 0.41, respectively.
Dietary acid load was assessed with two validated methods. First, potential renal acid load (PRAL) was calculated using the algorithm described by Remer and Manz (10):
 |
Second, we estimated dietary acid load using the algorithm described by Frassetto et al. (9):
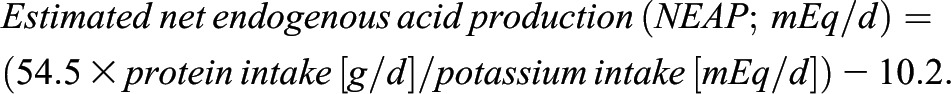 |
Outcome Measures
All measurements were performed after an 8- to 12-hour overnight fasting period. BP (mmHg) was measured semi-automatically according to strict protocol as described previously (19). Measurements were performed every minute for 15 minutes, and the last three measurements were averaged. Renal function was assessed by the eGFR calculated by the Chronic Kidney Disease-Epidemiology Collaboration equation (20). Serum creatinine was determined using a modified version of the Jaffé method (MEGA AU 510; Merck Diagnostica, Darmstadt, Germany). Blood was drawn in the morning after completion of the 24-hour urine collection. Concentrations of electrolytes and urea were measured using routine clinical laboratory methods, as were serum cholesterol, hemoglobin A1c, and N-terminal pro-brain natriuretic peptide (Nt-Pro-BNP) levels. Venous blood gas analyses were assessed photometrically. Acidosis was defined as serum [HCO3−] < 24 mmol/L.
Information on medical history and medication use was obtained from patient records. Data on smoking behavior (current, former, never) were obtained with an additional questionnaire. Body mass index was calculated as weight divided by height squared (kg/m2), and body surface area was estimated by applying the universally adopted formula of DuBois (21).
Statistical Analyses
Data were analyzed using SPSS software, version 18.0 (SPSS Inc., Chicago, IL). Skewed data were log-transformed for analyses (i.e., albuminuria, proteinuria, parathyroid hormone, Nt-pro-BNP, triglycerides, cholesterol). Data are presented as mean ± SD unless stated otherwise. Patient characteristics were calculated across sex-stratified tertiles of NAE, controlled for body surface area. P for trend was obtained using medians as continuous variables in univariate linear regression analysis. For nominal and ordinal variables, we applied the chi-squared test and the Jonckheere-Terpstra test, respectively.
Linear regression was applied for the associations of NAE with acidosis and cardiovascular risk factors. Regression coefficients are given as standardized β values, referring to the number of SDs the dependent variable changes, per SD increase of NAE. Adjustments were made for age, body surface area, and sex (model 1); use of medication (proliferation inhibitors, calcineurin inhibitors, renin-angiotensin system inhibitors, sodium bicarbonate, and diuretics) (model 2); and eGFR (ml/min per 1.73 m2), time since transplantation (years), and smoking behavior (categories; model 3). To separate effects of low eGFR and metabolic acid load on acidosis in RTRs, we performed regression analyses for (1) the association between eGFR and acidosis and (2) the association between NAE and acidosis.
To identify dietary factors contributing to NAE, we calculated dietary intake across tertiles of NAE, controlling for BSA. A two-sided P value less than 0.05 was considered to represent a statistically significant difference.
Results
Patient Characteristics
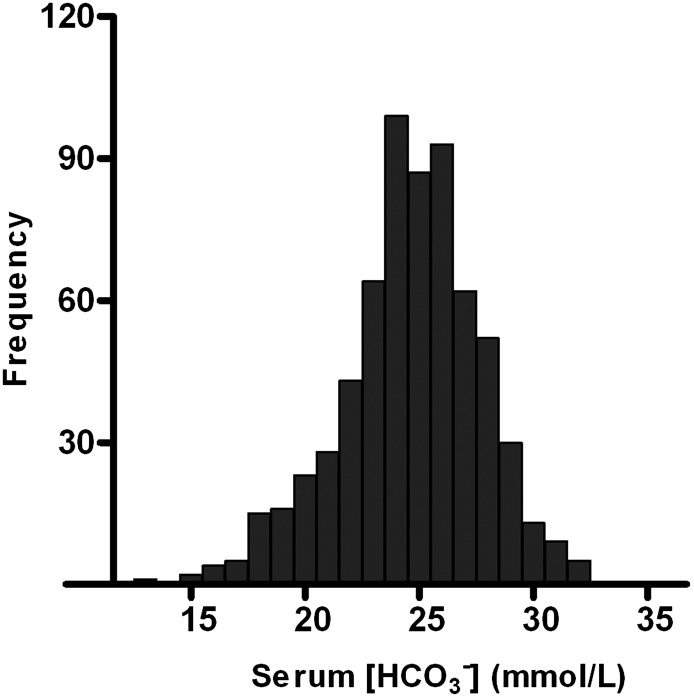
Mean participant age was 53±13 years; 57% of participants were male. Body mass index was 26.7±4.8 kg/m2, and 59.4% of RTRs were overweight (i.e., BMI≥25 kg/m2). Mean BP was 136±17 mmHg systolic and 83±11 mmHg diastolic; 91% of RTRs had hypertension (i.e., BP≥140/90 mmHg or use of antihypertensive medication). Of 707 RTRs, 637 (89%) used antihypertensive drugs; 198 (30%) used one, 231 (35%) used two, and 159 (24%) used three or more different antihypertensive drugs. Calcineurin and proliferation inhibitors were used in 57% and 83% of RTRs, respectively. Median prednisolone dose was 10 (interquartile range, 7.5–10.0) mg/d. Only 3 RTRs (0.4%) used sodium bicarbonate. Acidosis (i.e., serum HCO3− < 24 mmol/L) was present in 31% of RTRs, and about half of these patients had a systemic pH < 7.35 (Figure 1). RTRs with acidosis had a significantly lower eGFR compared with nonacidotic RTRs (44±20 ml/min per 1.73 m2 versus 56±19 ml/min per 1.73 m2, respectively; P<0.001).
Figure 1.
Histogram of serum bicarbonate concentration in a single-center cohort of 707 renal transplant recipients with a functioning graft for at least 1 year. Metabolic acidosis (i.e., [HCO3−] < 24mmol/L) was present in 31% of renal transplant recipients.
Mean NAE was 40.7±18.1 mEq/d, ranging from 22.2±10.2 to 60.0±11.8 mEq/d across tertiles of NAE (Table 1). NAE was positively associated with eGFR; use of mycophenolate; serum chloride; and urinary excretion of ammonia, TA, phosphorus, and sulfate. NAE was inversely related to time since transplantation, use of azathioprine, serum and urine pH, and serum and urine bicarbonate. No significant differences were found regarding smoking behavior over tertiles of NAE.
Table 1.
Characteristics of 707 renal transplant recipients across sex-stratified tertiles of urinary net acid excretion controlled for body surface area
| Variables | Sex-Stratified Tertiles of Net Acid Excretion/BSA × 1.73 (mEq/d) | P Value for Trenda | ||
|---|---|---|---|---|
| 1 (n=236) | 2 (n=235) | 3 (n=236) | ||
| Net acid excretion (mEq) | 22.2±10.2 | 39.8±5.3 | 60.0±11.8 | |
| Demographic characteristics | ||||
| Age (yr) | 54±13 | 53±13 | 52±12 | 0.07 |
| BSA (m2) | 1.94±0.22 | 1.95±0.22 | 1.93±0.22 | 0.62 |
| Current smokers (%) | 11 | 13 | 14 | 0.43 |
| Diabetes mellitus (%)b | 27 | 22 | 23 | 0.34 |
| Hypertensives (%) | 91 | 92 | 89 | 0.50 |
| Systolic BP (mmHg) | 136±18 | 137±17 | 136±17 | 0.89 |
| Diastolic BP (mmHg) | 83±11 | 84±10 | 83±11 | 0.99 |
| Renal variables | ||||
| Time since renal transplantation (yr) | 7.7 (3.2–15.2) | 5.8 (2.0–11.9) | 4.3 (1.3–10.0) | <0.001 |
| Serum creatinine (μmol/L) | 139 (107–176) | 121 (98–153) | 116 (97–152) | 0.006 |
| eGFR (ml/min per 1.73 m2) | 48±20 | 54±20 | 56±20 | <0.001 |
| Albumin excretion (mg/24 hr) | 58 (11–202) | 35 (9–200) | 36 (12–124) | 0.26 |
| Proteinuria (≥0.5 g/24 hr) (%) | 23 | 25 | 20 | 0.52 |
| Medication users | ||||
| Sodium bicarbonate (n) | 1 | 2 | 0 | 0.9 |
| Antihypertensive drugs (%) | 88 | 90 | 86 | 0.65 |
| Proliferation inhibitor (%) | 77 | 84 | 90 | 0.001 |
| Mycophenolate (%) | 47 | 71 | 80 | <0.001 |
| Azathioprine (%) | 30 | 12 | 10 | <0.001 |
| Calcineurin inhibitor (%) | 57 | 58 | 57 | 0.88 |
| Ciclosporin (%) | 37 | 44 | 37 | 0.95 |
| Tacrolimus (%) | 17 | 16 | 20 | 0.53 |
| Diuretics (%) | 49 | 36 | 37 | 0.01 |
| Antidiabetic drugs (%) | 15 | 16 | 14 | 0.66 |
| Serum measures | ||||
| pH | 7.38±0.04 | 7.37±0.05 | 7.36±0.04 | 0.001 |
| HCO3− (mmol/L) | 25.1±3.0 | 24.7±3.0 | 24.1±3.2 | 0.004 |
| v-pCO2 (kPa) | 5.85±0.80 | 5.86±0.83 | 5.82±0.82 | 0.71 |
| Base excess | 0.5 (−1.3 to 1.9) | 0.2 (−1.9 to 1.5) | −0.5 (−2.9 to 1.1) | 0.001 |
| Anion gap (mmol/L) | 11.1±2.2 | 10.8±2.1 | 10.7±2.1 | 0.25 |
| Chloride (mmol/L) | 104.5±3.4 | 105.3±3.2 | 106.2±3.7 | <0.001 |
| Phosphate (mmol/L) | 1.01±0.22 | 0.97±0.22 | 0.92±0.19 | <0.001 |
| PTH (pmol/L) | 9.1 (5.9–15.6) | 8.5 (5.0–13.9) | 9.1 (6.3–14.4) | 0.64 |
| Albumin (mmol/L) | 42.5±3.2 | 43.2±2.9 | 43.4±2.8 | 0.002 |
| Cholesterol (mmol/L) | 5.30 (4.50–6.00) | 5.00 (4.40–5.70) | 4.90 (4.20–5.70) | 0.04 |
| HDL cholesterol (mmol/L) | 1.30 (1.10–1.76) | 1.30 (1.10–1.60) | 1.30 (1.00–1.60) | 0.53 |
| Triglycerides (mmol/L) | 1.70 (1.29–2.30) | 1.63 (1.22–2.34) | 1.69 (1.16–2.21) | 0.58 |
| HbA1c (%) | 6.0±0.7 | 6.0±0.9 | 6.0±0.8 | 0.91 |
| hsCRP | 1.9 (0.9–6.0) | 1.6 (0.7–3.0) | 1.4 (0.6–4.7) | 0.06 |
| Nt-pro-BNP | 340 (143–787) | 239 (96–594) | 185 (85–442) | 0.006 |
| Urinary measures | ||||
| Ammonium (mEq/24 hr) | 13.3±7.2 | 20.0±7.6 | 32.2±12.3 | <0.001 |
| Titratable acid (mEq/24 hr) | 21.0±7.6 | 30.2±7.9 | 39.4±11.4 | <0.001 |
| HCO3− (mEq/24 hr) | 7.2 (3.9–12.8) | 4.2 (2.3–6.9) | 3.3 (2.2–4.9) | <0.001 |
| pH | 6.36±0.46 | 6.00±0.42 | 5.76±0.45 | <0.001 |
| Urea (mmol/24 hr) | 341±99 | 384±101 | 452±119 | <0.001 |
| Creatinine (mmol/24 hr) | 11.1±7.9 | 11.7±3.4 | 12.7±3.4 | 0.003 |
| Phosphorus (mmol/24 hr) | 21.0±7.8 | 25.1±8.0 | 29.4±8.5 | <0.001 |
| Sulfate (mmol/24 hr) | 15.2±5.8 | 17.6±5.4 | 20.9±6.9 | <0.001 |
| Sodium (mmol/24 hr) | 147±65 | 155±58 | 170±60 | <0.001 |
| Potassium (mmol/24 hr) | 73.0±25.1 | 72.9±24.4 | 73.9±24.0 | 0.72 |
| Calcium (mmol/24 hr) | 2.1 (0.9–3.4) | 2.5 (1.2–4.2) | 2.5 (1.2–4.2) | 0.08 |
Data are presented as mean ± SD, percentage, or median (interquartile range). BSA, body surface area; eGFR, estimated GFR; HCO3−, bicarbonate; v-pCO2, venous partial pressure of CO2; PTH, parathyroid hormone; HbA1c, glycosylated hemoglobin; hsCRP, high-sensitivity C-reactive protein; Nt-pro-BNP, N-terminal pro-brain natriuretic peptide.
P trend was tested by entering the median values within tertiles into the model as covariate, by chi-squared test or by Jonckheere-Terpstra test.
Diabetes mellitus was defined as serum glucose level ≥7 mmol/L or use of antidiabetic drugs.
NAE, Acidosis, and Cardiovascular Risk Profile
The associations between NAE, serum HCO3−, and various cardiovascular risk factors are shown in Table 2. After adjustment for age, sex, body surface area, use of medication, eGFR, time since transplantation, and smoking behavior, NAE was inversely associated with serum bicarbonate and serum pH (standardized β=−0.16; P<0.001 and standardized β=−0.18; P<0.001, respectively). Similar associations were found between PRAL and NEAP and acidosis: Adjusted regression coefficients (model 3) for the association of PRAL and NEAP with serum HCO3− were −0.13 and −0.12, respectively (both P=0.001). Standardized β values for the associations of PRAL and NEAP with serum pH were −0.07 (P=0.07) and −0.09 (P=0.02), respectively. In secondary analyses, the association of NAE with HCO3− and serum pH appeared to be significant in RTRs with and without metabolic acidosis (all P<0.01; data not shown).
Table 2.
Regression coefficients for the association of urinary net acid excretion with metabolic acidosis and related cardiovascular risk factors in 707 renal transplant recipients
| Dependent Variable | SD | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| Standardized β for NAE (95% CI) | P Value | Standardized β for NAE (95% CI) | P Value | Standardized β for NAE (95% CI) | P Value | ||
| Systemic acidosis | |||||||
| Serum HCO3− in mmol/L | 3.01 | −0.08 (−0.14 to −0.02) | 0.005 | −0.17 (−0.28 to −0.05) | <0.001 | −0.16 (−0.23 to −0.10) | <0.001 |
| Cardiovascular risk factors | |||||||
| Serum pH | 0.04 | −0.10 (−0.15 to −0.03) | 0.001 | −0.20 (−0.30 to −0.07) | <0.001 | −0.18 (−0.24 to −0.12) | <0.001 |
| Systolic BP in mmHg | 17 | 0.009 (−0.51 to 0.46) | 0.97 | −0.2 (−2.13 to 2.52) | 0.87 | −0.01 (−0.05 to 0.03) | 0.75 |
| Diastolic BP in mmHg | 11 | 0.04 (−0.14 to 0.22) | 0.66 | 0.02 (−0.04 to 0.08) | 0.52 | 0.02 (−0.09 to 0.13) | 0.57 |
| Mean arterial pressure in mmHg | 12 | 0.03 (−0.21 to 2.72) | 0.80 | 0.004 (−0.01 to 0.02) | 0.64 | 0.008 (−0.29 to 0.27) | 0.84 |
| Cholesterol in mmol/L | 1.12 | −0.12 (−0.19 to −0.05) | 0.002 | −0.11 (−0.19 to −0.03) | 0.006 | −0.10 (−0.17 to −0.03) | 0.02 |
| HDL cholesterol in mmol/L | 0.48 | −0.02 (−0.06 to 0.02) | 0.28 | −0.05 (−0.13 to 0.03) | 0.20 | −0.03 (−0.06 to 0.00) | 0.46 |
| LDL cholesterol in mmol/L | 0.93 | −0.09 (−0.17 to −0.02) | 0.02 | −0.08 (−0.16 to −0.01) | 0.05 | −0.07 (−0.14 to 0.00) | 0.10 |
| Triglycerides in mmol/La | 0.47 | −0.10 (−0.19 to −0.01) | 0.03 | −0.09 (−0.20 to 0.02) | 0.12 | −0.08 (−0.19 to 0.03) | 0.06 |
| Phosphate in mmol/l | 0.21 | −0.20 (−0.29 to −0.11) | <0.001 | −0.11 (−0.16 to −0.06) | <0.001 | −0.12 (−0.21 to −0.02) | 0.003 |
| Albumin in g/L | 2.98 | 0.13 (0.03 to 0.23) | 0.01 | 0.07 (−0.01 to 0.14) | 0.06 | 0.06 (−0.06 to 0.18) | 0.13 |
| Nt-Pro-BNP in ng/La | 1.42 | −0.16 (−0.26 to −0.06) | 0.002 | −0.07 (−0.13 to −0.02) | 0.01 | −0.05 (−0.24 to 0.14) | 0.17 |
| HbA1c in %a | 0.13 | 0.05 (−0.01 to 0.12) | 0.15 | 0.03 (0.01 to 0.06) | 0.17 | 0.04 (−0.01 to 0.10) | 0.32 |
| Albuminuria in mg/24 hra | 1.92 | −0.08 (−0.26 to 0.14) | 0.39 | 0.02 (−0.13 to 0.09) | 0.72 | 0.01 (−0.01 to 0.02) | 0.75 |
| Proteinuria in g/24 hr | 1.72 | −0.07 (−0.17 to 0.03) | 0.52 | 0.04 (−0.13 to 0.05) | 0.59 | 0.02 (−0.07 to 0.11) | 0.70 |
Association of net acid excretion as independent variable, adjusted for age (years), body surface area (m2), and sex (model 1); renin-angiotensin system blockade, diuretics, calcineurin inhibitors, proliferation inhibitors, and sodium bicarbonate (yes/no) (model 2); and renal function (estimated GFR), time since renal transplantation (years), and smoking behavior (model 3). Standardized βs refer to the number of SDs the dependent variable changes per SD increase of NAE. CI, confidence intervals; HCO3−, bicarbonate; Nt-Pro-BNP, N-terminal pro-brain natriuretic peptide; HbA1c, glycosylated hemoglobin.
Log-transformed for analyses.
Further analyses revealed standardized β values of 0.33 and 0.31 for the association of eGFR with serum [HCO3−] and serum pH, respectively (both P<0.001), whereas standardized β values were −0.08 (P=0.005) and −0.10 (P=0.001) for the association of NAE with serum [HCO3−] and serum pH, respectively. With respect to cardiovascular risk factors, NAE was inversely associated with total cholesterol (standardized β=−0.10; P=0.02) and serum phosphate (standardized β=−0.12; P=0.003) (model 3). Initially significant associations between NAE and serum albumin (inverse) and serum Nt-Pro-BNP (direct) disappeared after adjustment for eGFR, time since renal transplantation, and smoking behavior (Table 2). NAE was not related to BP or insulin resistance (Table 2).
Dietary Factors and NAE
Both formulas estimating dietary acid load were positively correlated with NAE: r=0.42 (P<0.001) for the correlation between PRAL and NAE and r=0.32 (P<0.001) for the correlation between NEAP and NAE. Dietary intake across sex-stratified tertiles of NAE, controlled for body surface area, is shown in Table 3. Across tertiles, NAE was positively associated with total protein, animal protein, phosphorus, and calcium (all P trend<0.05). Regarding food groups, NAE was directly related to cheese intake (P trend=0.001) and inversely related to fruit intake (P trend=0.05). Additional data on dietary factors and NAE are provided in Supplemental Table 1.
Table 3.
Dietary intake of 625 renal transplant recipients across sex-stratified tertiles of net acid excretion controlled for body surface area
| Variable | Sex-Stratified Tertiles of Net Acid Excretion/BSA × 1.73 (mEq/d) | P Value for Trenda | ||
|---|---|---|---|---|
| Model 1 (n=208) | Model 2 (n=209) | Model 3 (n=208) | ||
| Nutrients | ||||
| Energy intake (kcal/d) | 2141±607 | 2252±608 | 2174±700 | 0.61 |
| Protein intake (g/d) (energy %) | 79±20 (15.2) | 83±19 (14.7) | 85±20 (16.1) | 0.02 |
| Animal protein (g/d) (energy %) | 50±15 (9.6) | 52±15 (9.5) | 54±15 (10.4) | 0.01 |
| Vegetable protein (g/d) (energy %) | 30±9 (5.6) | 32±10 (5.8) | 31±10 (5.7) | 0.48 |
| Fat (g/d) (energy %) | 87±32 (36.0) | 92±31 (36.3) | 89±39 (36.6) | 0.56 |
| Carbohydrates (g/d) (energy %) | 247±75 (46.5) | 259±78 (46.1) | 244±80 (44.8) | 0.67 |
| Fiber (g/d) | 22±7 | 24±7 | 22±7 | 0.54 |
| Calcium (mg/d) | 1009±353 | 1058±358 | 1109±420 | 0.01 |
| Magnesium (mg/d) | 324±86 | 345±91 | 333±91 | 0.34 |
| Potassium (mg/d) | 3496±867 | 3667±912 | 3499±906 | 0.34 |
| Phosphorus (mg/d) | 1477±387 | 1559±374 | 1568±420 | 0.02 |
| Food groups (g/d) | ||||
| Meat | 94±40 | 97±40 | 97±40 | 0.47 |
| Fish | 11.4 (3.0–22.3) | 11.7 (4.7–19.4) | 10.7 (4.2–20.9) | 0.17 |
| Milk | 116±83 | 120±81 | 114±89 | 0.81 |
| Cheese | 32±25 | 34±26 | 41±32 | 0.001 |
| Fruit | 161±122 | 156±117 | 140±105 | 0.05 |
| Vegetables | 95±58 | 99±63 | 88±52 | 0.23 |
| Potatoes | 134±77 | 135±72 | 125±80 | 0.28 |
| Bread | 123±54 | 145±67 | 136±58 | 0.03 |
| Dietary acid load formulas (mEq/d) | ||||
| PRAL | 0.32 (−8.1 to 7.2) | 0.95(−5.8 to 6.8) | 5.1 (−3.2 to 11.1) | <0.001 |
| NEAP | 39±9 | 40±8 | 42±8 | <0.001 |
Data are presented as mean ± SD, percentage, or median (interquartile range). BSA, body surface area; PRAL, potential renal acid load; NEAP, net endogenous acid production. P trend was tested by entering the median values within tertiles into the model as covariates, by chi-squared test or by Jonckheere-Terpstra test.
P trend was tested by entering the median values within tertiles into the model as covariate, by chi-squared test, or by Jonckheere-Terpstra test.
Discussion
In this study, we examined the relation between diet and NAE (reflecting metabolic acid load). Additionally, we studied the association of NAE with acidosis and various cardiovascular risk factors in 707 RTRs. Acidosis was present in 31% of RTRs. NAE was positively associated with acidosis, whereas no associations were observed with insulin resistance and high BP. NAE was higher in patients with higher intake of animal protein, (presumably from cheese, meat, and fish) and calcium and was lower in patients with higher intakes of fruits and vegetables.
Strengths of our study include the large sample size and the novelty of our findings in this specific patient group. Furthermore, collection of 24-hour urine samples allowed direct measurement of NAE as a marker of metabolic acid load in addition to estimation of dietary acid load based on dietary recall (PRAL and NEAP). This enabled us to verify internal consistency of our data and made our conclusions more reliable. Limitations are the observational and cross-sectional study design, which does not allow for proving causality. However, although reverse causality should be considered, it seems unlikely that RTR with higher NAE would have adapted dietary habits because the extent of urinary acid excretion goes unnoticed. Second, NAE is a marker of the total amount of acids ingested, and therefore reflects both acidity of the diet and use of acidifying (or alkalinizing) drugs. Nevertheless, both PRAL and NEAP were significantly associated with NAE, which seems to validate NAE as marker of dietary acid load. Third, almost all RTR were white, which calls for prudence in extrapolation of our findings to patients of other ethnicities. Furthermore, although extensive data collection allowed controlling for many confounders, residual confounding could have remained even in multivariable analysis because it is hard to adjust completely for the severity of each factor. Therefore, prospective cohort and intervention studies in RTRs are needed to confirm our results.
Previously, Ashurst et al. showed that CKD patients with persistent low serum bicarbonate levels had a larger annual decline in eGFR than patients with normal bicarbonate levels (22). In another study, they observed that CKD patients supplemented with sodium bicarbonate were less likely to develop ESRD. That finding is in line with findings of de Brito-Ashurst (23) and Phisitkul (24) and colleagues, who showed that treatment of metabolic acidosis with sodium citrate in patients with low GFR reduced progression of kidney disease. Interestingly, a recently published study showed that reduction of dietary aid by increasing intake of fruits and vegetables led to reduced kidney injury in patients with CKD stage 2, which is in line with our findings in RTRs (25).
Acidosis has long been recognized as a risk factor in RTRs, and several potential causes have been suggested (26–30). Well known contributors to acidosis are reduced nephron mass, resulting in decreased acid excretion, and use of pharmacologic agents, such as calcineurin inhibitors, that directly influence acid-base status but also play a significant role in renal acid handling. Thus far, no studies have been performed on the contribution of diet to acid-base status in RTRs, despite the vast body of evidence showing that food intake does affect acid-base balance in both the general population and CKD patients (8,10,25). We found protein intake to be directly associated with NAE in RTRs. This finding was confirmed by significant associations between urinary urea, phosphorus, and sulfate excretion, which all have been suggested as reliable markers of protein intake (31,32).
Although robust associations between NAE and acidosis in RTRs were observed, no direct associations were found between NAE and cardiovascular risk factors, which is in contrast with findings of previous studies (33–36). Differences in outcomes might be explained by differences in study populations because previous studies were done in healthy persons without use of medication. Because 16% and 88% of RTRs used antidiabetic and antihypertensive drugs, respectively, this might have interfered in the potentially existing associations between NAE and cardiovascular risk factors, such as insulin resistance and hypertension. Another explanation might be that potential adverse effects of acidosis on such variables as BP were not detected in this cross-sectional study because both measures were determined at the same time. However, long-term unfavorable effects of acidosis might become visible if prospective data are obtained.
If confirmed by prospective and intervention studies, our findings might have several implications for clinical practice. Because acidosis is highly prevalent among RTRs, venous blood gas analysis should be performed occasionally—it provides important information on acid-base status relatively easily. If acidosis is confirmed, attention should be paid not only to known risk factors such as graft function but also to dietary habits. Higher intake of fruits and vegetables or lower intake of animal protein should be advised. On the basis of our results, daily enrichment of the diet with 100 g of vegetables and 100 g of fruits, while eliminating 50 g of meat and 20 g of cheese, would decrease NAE by about 15 mEq/d. Accordingly, serum bicarbonate levels would increase by about 0.5 mmol/l. This increase might seem marginal; however, it would imply a reduction in the prevalence of acidosis of 5%, or 40 RTRs in our cohort achieving appropriate bicarbonate levels by simple dietary modification.
In conclusion, acidosis is highly prevalent in RTRs. In addition to conventional factors contributing to acidosis in RTRs, diet appeared to be associated with acid-base homeostasis. Modification of the diet by increasing fruit and vegetable intake and decreasing intake of animal protein might improve acid-base balance in RTRs.
Disclosures
None.
Supplementary Material
Acknowledgments
We would like to thank Bettine Haandrikman and Twan Storteboom for their conscientious contributions.
The current manuscript was supported by Top Institute Food and Nutrition, which is a public-private partnership that generates vision on scientific breakthroughs in food and nutrition, resulting in the development of innovative products and technologies. Partners are major Dutch food companies and research organizations. This does not alter the authors' adherence to all the policies of Clinical Journal of the American Society of Nephrology on sharing data and materials.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04590512/-/DCSupplemental.
References
- 1.Lysaght MJ: Maintenance dialysis population dynamics: current trends and long-term implications. J Am Soc Nephrol 13[Suppl 1]: S37–S40, 2002 [PubMed] [Google Scholar]
- 2.Aakhus S, Dahl K, Widerøe TE: Cardiovascular morbidity and risk factors in renal transplant patients. Nephrol Dial Transplant 14: 648–654, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Kreis HA, Ponticelli C: Causes of late renal allograft loss: chronic allograft dysfunction, death, and other factors. Transplantation 71[Suppl]: SS5–SS9, 2001 [PubMed] [Google Scholar]
- 4.Ambühl PM: Posttransplant metabolic acidosis: a neglected factor in renal transplantation? Curr Opin Nephrol Hypertens 16: 379–387, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Yakupoglu HY, Corsenca A, Wahl P, Wüthrich RP, Ambühl PM: Posttransplant acidosis and associated disorders of mineral metabolism in patients with a renal graft. Transplantation 84: 1151–1157, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Adeva MM, Souto G: Diet-induced metabolic acidosis. Clin Nutr 30: 416–421, 2011 [DOI] [PubMed] [Google Scholar]
- 7.van den Berg E, Hospers FA, Navis G, Engberink MF, Brink EJ, Geleijnse JM, van Baak MA, Gans RO, Bakker SJ: Dietary acid load and rapid progression to end-stage renal disease of diabetic nephropathy in Westernized South Asian people. J Nephrol 24: 11–17, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Remer T: Influence of nutrition on acid-base balance—metabolic aspects. Eur J Nutr 40: 214–220, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Frassetto LA, Todd KM, Morris RC, Jr, Sebastian A: Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr 68: 576–583, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Remer T, Manz F: Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr 59: 1356–1361, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Frassetto L, Morris RC, Jr, Sellmeyer DE, Todd K, Sebastian A: Diet, evolution and aging—the pathophysiologic effects of the post-agricultural inversion of the potassium-to-sodium and base-to-chloride ratios in the human diet. Eur J Nutr 40: 200–213, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Frassetto LA, Morris RC, Jr, Sebastian A: Effect of age on blood acid-base composition in adult humans: role of age-related renal functional decline. Am J Physiol 271: F1114–F1122, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Frassetto LA, Lanham-New SA, Macdonald HM, Remer T, Sebastian A, Tucker KL, Tylavsky FA: Standardizing terminology for estimating the diet-dependent net acid load to the metabolic system. J Nutr 137: 1491–1492, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Feunekes GI, Van Staveren WA, De Vries JH, Burema J, Hautvast JG: Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol. Am J Clin Nutr 58: 489–496, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Voorlichtingsbureau voor de Voeding, The Hague: NEVO-tabel (dutch food composition table): Nederlands voedingsstoffenbestand. 2006. Available at: http://www.fao.org/infoods/tables_europe_en.stm#nethe
- 16.Masud T, Manatunga A, Cotsonis G, Mitch WE: The precision of estimating protein intake of patients with chronic renal failure. Kidney Int 62: 1750–1756, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Maroni BJ, Steinman TI, Mitch WE: A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int 27: 58–65, 1985 [DOI] [PubMed] [Google Scholar]
- 18.Klipstein-Grobusch K, den Breeijen JH, Goldbohm RA, Geleijnse JM, Hofman A, Grobbee DE, Witteman JC: Dietary assessment in the elderly: Validation of a semiquantitative food frequency questionnaire. Eur J Clin Nutr 52: 588–596, 1998 [DOI] [PubMed] [Google Scholar]
- 19.van den Berg E, Geleijnse JM, Brink EJ, van Baak MA, Homan van der Heide JJ, Gans RO, Navis G, Bakker SJ: Sodium intake and blood pressure in renal transplant recipients. Nephrol Dial Transplant 27: 3352–3359, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubois DDE: Clinical calorimetry. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med17: 863–871, 1916 [Google Scholar]
- 22.Ashurst I, Varagunam M, Yaqoob MM: The effect of metabolic acidosis on the rate of decline of glomerular filtration rate in patients with stage 4 CKD [Abstract]. Presented at the World Congress of Nephrology, Singapore, June 26–30, 2005 [Google Scholar]
- 23.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM: Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20: 2075–2084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phisitkul S, Khanna A, Simoni J, Broglio K, Sheather S, Rajab MH, Wesson DE: Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int 77: 617–623, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Goraya N, Simoni J, Jo C, Wesson DE: Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int 81: 86–93, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Györy AZ, Stewart JH, George CR, Tiller DJ, Edwards KD: Renal tubular acidosis, acidosis due to hyperkalaemia, hypercalcaemia, disordered citrate metabolism and other tubular dysfunctions following human renal transplantation. Q J Med 38: 231–254, 1969 [PubMed] [Google Scholar]
- 27.Better OS, Chaimowitz C, Alroy GG, Sisman I: Spontaneous remission of the defect in urinary acidification after cadaver kidney homotransplantation. Lancet 1: 110–112, 1970 [DOI] [PubMed] [Google Scholar]
- 28.Batlle DC, Mozes MF, Manaligod J, Arruda JA, Kurtzman NA: The pathogenesis of hyperchloremic metabolic acidosis associated with kidney transplantation. Am J Med 70: 786–796, 1981 [DOI] [PubMed] [Google Scholar]
- 29.Massry SG, Preuss HG, Maher JF, Schreiner GE: Renal tubular acidosis after cadaver kidney homotransplantation. Studies on mechanism. Am J Med 42: 284–292, 1967 [DOI] [PubMed] [Google Scholar]
- 30.Better OS, Chaimowitz C, Naveh Y, Stein A, Nahir AM, Barzilai A, Erlik D: Syndrome of incomplete renal tubular acidosis after cadaver kidney transplantation. Ann Intern Med 71: 39–46, 1969 [DOI] [PubMed] [Google Scholar]
- 31.Simmons WK: Use of the inorganic sulfate sulfur-creatinine ratio in field studies. Am J Clin Nutr 26: 72–76, 1973 [DOI] [PubMed] [Google Scholar]
- 32.Simmons WK: Urinary urea nitrogen-creatinine ratio as indicator of recent protein intake in field studies. Am J Clin Nutr 25: 539–542, 1972 [DOI] [PubMed] [Google Scholar]
- 33.Farwell WR, Taylor EN: Serum bicarbonate, anion gap and insulin resistance in the National Health and Nutrition Examination Survey. Diabet Med 25: 798–804, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Abate N, Chandalia M, Cabo-Chan AV, Jr, Moe OW, Sakhaee K: The metabolic syndrome and uric acid nephrolithiasis: Novel features of renal manifestation of insulin resistance. Kidney Int 65: 386–392, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Taylor EN, Forman JP, Farwell WR: Serum anion gap and blood pressure in the national health and nutrition examination survey. Hypertension 50: 320–324, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Forman JP, Rifas-Shiman SL, Taylor EN, Lane K, Gillman MW: Association between the serum anion gap and blood pressure among patients at Harvard Vanguard Medical Associates. J Hum Hypertens 22: 122–125, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.