Summary
Background and objectives
Podocyte loss is key in glomerulosclerosis. Activated parietal epithelial cells are proposed to contribute to pathogenesis of glomerulosclerosis and may serve as stem cells that can transition to podocytes. CD44 is a marker for activated parietal epithelial cells. This study investigated whether activated parietal epithelial cells are increased in early recurrent FSGS in transplant compared with minimal change disease.
Design, setting, participants, & measurements
CD44 staining in renal allograft biopsies from 12 patients with recurrent FSGS was performed and compared with native kidneys with minimal change disease or FSGS and normal control native and transplant kidneys without FSGS. CD44+ epithelial cells along Bowman’s capsule in the parietal epithelial cell location and over the glomerular tuft in the visceral epithelial cell location were assessed.
Results
Cases with early recurrent FSGS manifesting only foot process effacement showed significantly increased CD44+ visceral epithelial cells involving 29.0% versus 2.6% of glomeruli in minimal change disease and 0% in non-FSGS transplants. Parietal location CD44 positivity also was numerically increased in recurrent FSGS. In later transplant biopsies, glomeruli with segmental lesions had more CD44+ visceral epithelial cells than glomeruli without lesions.
Conclusions
Parietal epithelial cell activation marker is significantly increased in evolving FSGS versus minimal change disease, and this increase may distinguish early FSGS from minimal change disease. Whether parietal epithelial cell activation contributes to pathogenesis of sclerosis in idiopathic FSGS or is a regenerative/repair response to replace injured podocytes awaits additional study.
Introduction
FSGS is the most common cause of nephrotic syndrome in adults (1–3). Podocyte injury and loss play central roles in initial injury and progression to glomerulosclerosis (4–7). Podocytes are terminally differentiated postmitotic cells with very limited regenerative ability. Injury and detachment or apoptosis of podocytes may lead to denuded areas of glomerular basement membrane (GBM). Remaining podocytes hypertrophy, but their inability to proliferate may, ultimately, lead to sclerosis if the remaining podocytes cannot cover the GBM (2–10). Injured podocytes produce extracellular matrix proteins contributing to glomerulosclerosis. Injured podocytes may also release oxidants and proteinases, which may alter the matrix constituents of the underlying GBM, making the GBM less hospitable to anchor the podocytes, thus leading to podocyte apoptosis, detachment, and loss (11). Injury may also spread from injured podocytes to initially spared podocytes, perpetuating a local vicious cycle of injury (12).
Parietal epithelial cells (PECs) were previously considered to be innocent bystanders in noninflammatory glomerular diseases (13,14). However, varying degrees of epithelial cell hyperplasia are observed in all morphologic variants of FSGS. PECs may contribute to these activated epithelial cells (4,5). PECs not only have the potential to become highly active proliferating cells in crescentic glomerulonephritis, but also, a subset of parietal epithelial cells expresses stem cell markers and transcription factors and may represent a renal stem cell niche through which injured cells can be replaced (4,15).
De novo expression of CD44 in PECs has been detected in extracapillary proliferating cells in mouse models of collapsing glomerulopathy and crescentic glomerulonephritis (16). CD44 is a glycoprotein involved in cell adhesion, cell matrix interaction, and cell migration (17,18). Its expression by activated PECs points to a role for this adhesion molecule in the guided migration of PECs onto the glomerular tuft to replace injured/lost podocytes (16,19).
We, therefore, evaluated CD44 expression in human renal biopsies and investigated whether activated PECs can differentiate early recurrent FSGS in the transplant from minimal change disease (MCD).
Materials and Methods
Cases of recurrent FSGS in the transplant over the last 15 years at Vanderbilt University Medical Center were identified. Cases were selected for study if the patient had primary FSGS as the cause of end stage kidney disease and the first post-transplant biopsy showed extensive (>50%) foot process effacement as the morphologic evidence of recurrent FSGS without segmental sclerosis, thereby mimicking MCD morphologically, and in the setting of marked proteinuria developing shortly after transplant. Biopsies were performed for cause, with patients showing marked dipstick-positive proteinuria (not further quantified). Thus, patients with recurrent FSGS in whom the initial post-transplant biopsy showed segmental sclerotic lesions were excluded. A total of 25 transplant renal biopsies from 12 patients with recurrent FSGS met entry criteria; 11 cases of MCD and 4 cases of FSGS involving the native kidney selected from the same time period with adequate tissue for immunostaining were also assessed. These biopsies were compared with eight normal control kidneys specimens (three uninvolved normal kidneys from tumor nephrectomies and five baseline donor biopsies without significant lesions) and control transplant biopsies in patients without FSGS as primary disease with acute rejection without a diagnosis of FSGS (n=6) or with minimal histologic abnormalities (n=6) also from the same time period with adequate tissue remaining for immunostaining (Table 1). These control transplant biopsies were done to investigate the cause of increased serum creatinine.
Table 1.
Study group and controls
| Group | Number of Patients | Total number Biopsies |
|---|---|---|
| Recurrent FSGS | 12 | 25 |
| Native kidney FSGS | 4 | 4 |
| Native MCD | 11 | 11 |
| Normal native kidneys | 8 | 8 |
| Transplant kidneys without FSGS | 12 | 12 |
MCD, minimal change disease.
All biopsies were stained for CD44 in 2-µm-thick formalin-fixed paraffin-embedded tissue sections, with the exception of one case of recurrent FSGS, where frozen tissue was used. Antigen retrieval was done by boiling in citrate buffer (pH 6.0) in a microwave for 15 minutes. After washing, sections were blocked with Power Block (PBS + 0.01% Triton; Biogenex) for 15 minutes. Sections were then incubated with anti-CD44 antibody (1:200, Ab 16728; Abcam, Cambridge, MA) for 30 minutes at room temperature followed by secondary biotinylated goat anti-mouse antibody (1:50; Southern Biotech 1010–05, Birmingham, AL) for 30 minutes. Staining for common leukocyte antigen (1:100, CD45; Dako, Carpinteria, CA) was also done in cases with available tissue on adjacent sections or sections close to the CD44-stained section.
Detection was carried out with the Ultravision Detection System (Thermo Shandon Limited; Astmoor, Runcorn Cheshire, United Kingdom) with peroxidase as label and 3,3′-diaminobenzidine as substrate. Sections were counterstained with hematoxylin. In each biopsy, all glomeruli present were evaluated individually for the number of epithelial cells staining for CD44 along the inside of Bowman’s capsule, in an anatomic PEC location, and over the glomerular tuft in an anatomic visceral epithelial cell (VEC) location (i.e., directly overlying the capillary tuft on the outside of the GBM), thus not counting endothelial cell location staining. Tissue was not available for double staining to further validate this anatomic approach. CD44-positive leukocytes, confirmed by CD45 staining, in the interstitium or mesangium or in capillary lumens were also not scored. The presence of any segmental adhesion and/or sclerosis was also noted. Positive controls (i.e., crescentic biopsies with activated parietal cells for CD44 and tonsil for leukocytes for CD44 and CD45) showed appropriate staining, and negative controls without primary antibody showed no staining. For each case, the percentage of positive staining was calculated, and then, the mean percentage for each group was determined.
Data are shown as mean ± SD. Comparisons were done by two-tailed t test with Welch correction and Mann–Whitney test for nonparametric data. Multigroup comparisons were done by one-way ANOVA with Tukey post-test and Kruskal–Wallis test with Dunns post-test as appropriate. A P value<0.05 was considered significant.
Results
Our study population of patients with recurrent FSGS included six males and six females, of which eight patients were Caucasian, two patients were African American, one patient was Hispanic, and one patient was multiracial. No patients had family history of FSGS, and no genetic testing was done for podocyte structural protein mutations or APOL1 allele variants. The age range was 12–59 years (27.3±13.8 years). Proteinuria was detected from 1 day to 2 months post-transplant. One patient underwent two successive renal transplants. Of 13 allograft kidneys, 9 allografts were from living donors, 3 allografts were from deceased donors, and 1 allograft had unknown donor status. The 11 cases of minimal change disease included seven male patients and four female patients ranging in age from 5 to 66 years (38.4±23.7 years). In the 12 patients with recurrent FSGS, 16 early initial and repeat biopsies displayed only extensive foot process effacement by electron microscopy without segmental sclerosis, and 9 later biopsies had segmental sclerosis. A total of 116 glomeruli were available for immunohistochemical study in the early recurrent FSGS biopsies with foot process effacement only. A total of 53 glomeruli were studied in the later biopsies with overt segmental sclerosis. These sclerosing lesions were manifest with overt focal segmental sclerosis and/or adhesion involving 11 of 53 glomeruli. In the MCD cases, a total of 263 glomeruli with no light microscopic abnormalities were available for study. Four native kidney FSGS cases (two male patients, two female patients [all Caucasian]; 43±27.6 years; range, 4–64 years) had proteinuria of 10–24 g/24 h and serum creatinine of 0.24–2.0 mg/dl with 47 glomeruli total. Six acute rejection transplant biopsies (four male patients, two female patients [five Caucasian and one ethnicity not specified], average age 29 years [19–57 years]) with 57 glomeruli total and six transplants with minor lesions (three male patients, three female patients, [three Caucasian, three African American], average age 54 years [29–70 years]) with 112 glomeruli total were also studied.
In native kidney MCD cases, 2.6% of glomeruli stained positive for CD44 in a VEC location, and 0.7% of glomeruli showed positive staining involving PECs, with single cell positivity in nearly all of the glomeruli with staining. Cases with recurrent FSGS with only foot process effacement showed significantly increased CD44 positivity involving VECs (detected in 29.0% of glomeruli, P<0.01 versus MCD and normal control). There was also a numerical increase in CD44 positivity involving PEC (detected in 7.1% of glomeruli, P=0.09 versus MCD cases) (Figure 1). Native kidney FSGS cases showed CD44 positivity in VEC in 32.7% of glomeruli (P=0.56 versus transplant recurrence with only foot process effacement, P<0.05 versus MCD and normal control), and 41% of glomeruli were CD44-positive in PEC location (P<0.05 versus recurrent FSGS, MCD, and normal control).
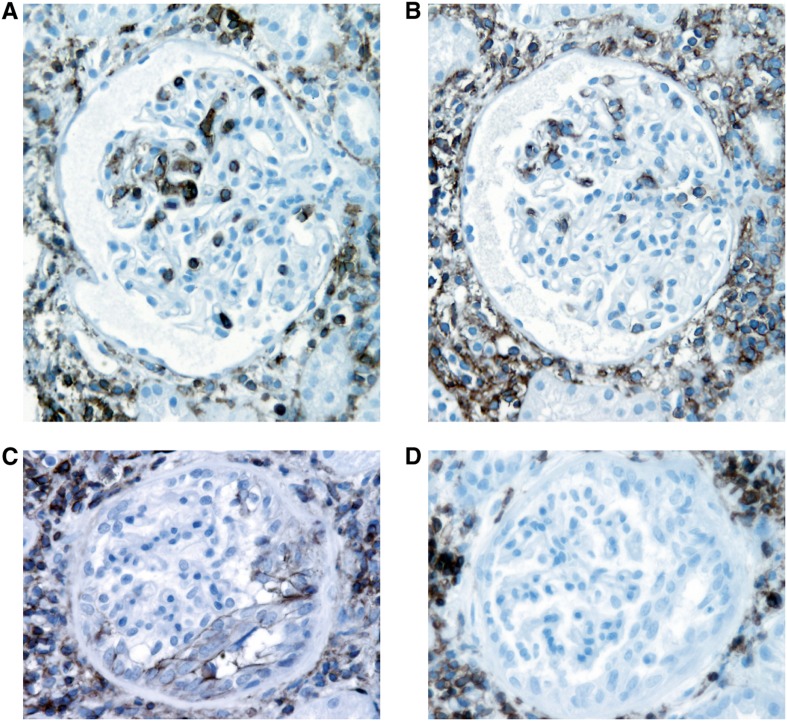
Figure 1.
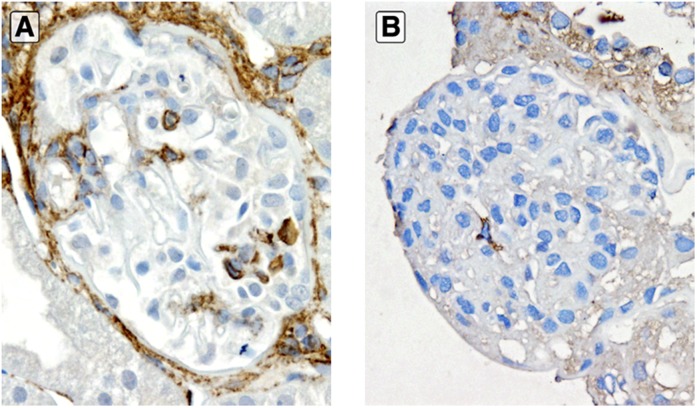
CD44 in early recurrent FSGS. Significantly increased CD44 staining in visceral epithelial cell location in early recurrent FSGS with extensive foot process effacement only and no segmental sclerosis (A) compared with minimal CD44 staining in visceral location in a case of native kidney minimal change disease (B; anti-CD44 stain). Original magnification, ×400.
In the later transplant biopsies with segmental sclerosis, CD44 positivity was increased in a visceral location in glomeruli with these lesions versus those glomeruli that were not yet affected by sclerosis or adhesion (4.5±9.0 CD44-positive cells in glomeruli with segmental lesions versus 0.9±1.2 in glomeruli without segmental lesions; P<0.05). Most of these CD44-positive visceral cells were located overlying the lesional area. In contrast, the average number of CD44+ PECs was similar in glomeruli with or without lesions (2.6±5.6 versus 1.8±5.2 CD44+ PECs per glomerulus; P=0.68) (Figure 2). Rare glomeruli had CD44 staining in both visceral and parietal locations (Figure 3). In addition, rare adhesions showed CD44+ cells forming a bridge between the glomerular tuft and Bowman’s capsule (Figure 4). In native kidney FSGS, the average number of CD44-positive cells in visceral location or parietal location was similar in glomeruli with or without lesions (0.6±0.8 versus 0.4±0.4 and 1.5±1.4 versus 0.5±0.2 for VEC and PEC in sclerosing versus intact glomeruli, respectively; P=0.69 and P=0.25, respectively).
Figure 2.
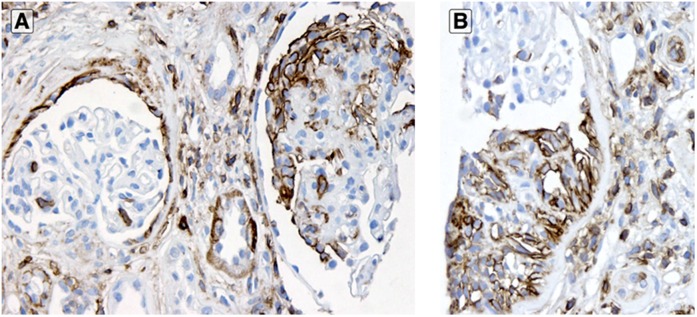
CD44 in established FSGS. In cases with established segmental sclerosis, glomeruli with lesions (A; right glomerulus) showed more visceral epithelial cell location CD44 staining than non-sclerosed glomeruli (A; left glomerulus). Parietal epithelial cells stained for CD44 equally in glomeruli with (B) or without (A; left glomerulus) segmental sclerosis (anti-CD44 stain). Original magnification, ×200 in A; ×400 in B.
Figure 3.
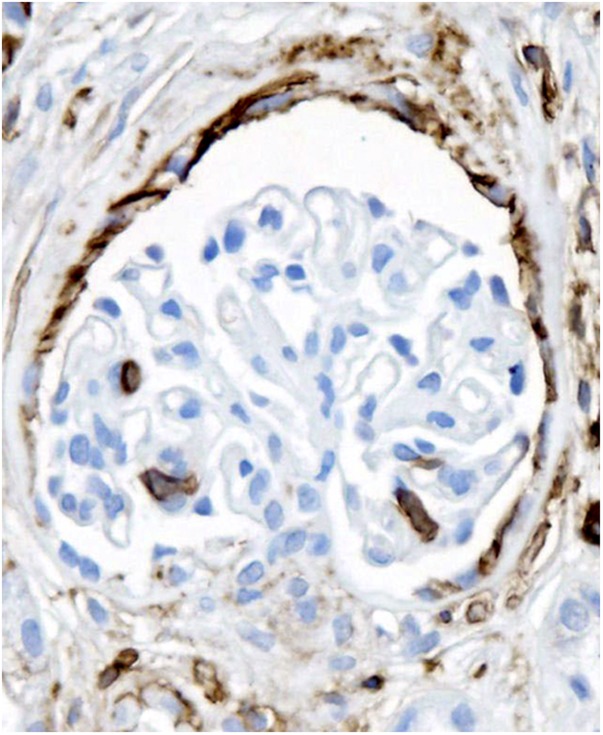
CD44 staining in both podocyte and parietal location in a glomerulus without segmental lesion in a biopsy with FSGS (anti-CD44 stain). Original magnification, ×200.
Figure 4.
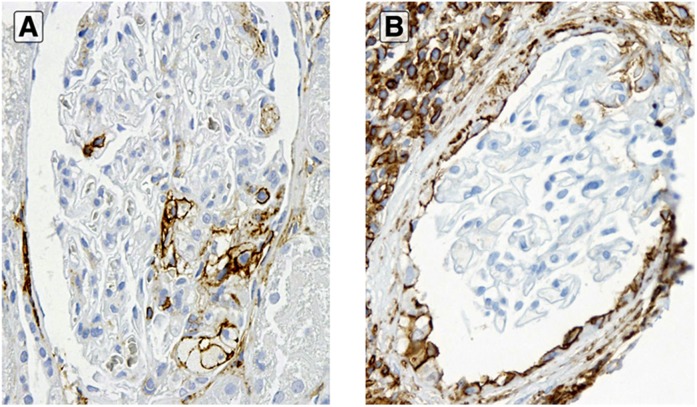
Cellular bridges in recurrent FSGS. Glomeruli in two cases (A and B) of recurrent FSGS in the transplant with cellular bridges with CD44+ cells along these adhesions (anti-CD44 stain). Original magnification, ×3800 in A; ×3400 in B.
Six patients underwent multiple biopsies, in which only the later biopsies showed segmental lesions (Table 2). In most patients, CD44 positivity increased over time, and was further increased when sclerosis developed, particularly in VEC location. In control non-FSGS transplants with acute rejection, CD44 positivity was present in 0% of glomeruli in VEC location and 0% of glomeruli in PEC location (P<0.05 versus native kidney FSGS and recurrent FSGS with foot process effacement only; P<0.05 versus native kidney FSGS, respectively). In control non-FSGS transplants without specific lesions, CD44 positivity was present in 0% of glomeruli in VEC location and 0.7% in PEC location (both P<0.05 versus native kidney FSGS). CD45 staining aided in confirming epithelial cell staining and excluding leukocyte staining (Figure 5).
Table 2.
CD44 staining in transplants with recurrent FSGS and multiple biopsies
| Biopsy 1 | Biopsy 2 | Biopsy 3 | ||||
|---|---|---|---|---|---|---|
| VEC (per Glomerulus) | PEC (per Glomerulus) | VEC (per Glomerulus) | PEC (per Glomerulus) | VEC (per Glomerulus) | PEC (per Glomerulus) | |
| Patient 1 (first transplant) | 0.2 | 0.2 | 0.8 | 0 | 2.7 | 4.5 |
| No sclerosis | No sclerosis | No sclerosis | No sclerosis | Sclerosis | Sclerosis | |
| Patient 1 (second transplant) | 0 | 0 | 0.5 | 0.1 | ||
| No sclerosis | No sclerosis | No sclerosis | No sclerosis | |||
| Patient 2 | N/Aa | N/A | 2.0 | 0 | ||
| No sclerosis | No sclerosis | No sclerosis | No sclerosis | |||
| Patient 3 | 0 | 0 | 0.3 | 0 | 1.0 | 0 |
| No sclerosis | No sclerosis | No sclerosis | No sclerosis | Sclerosis | Sclerosis | |
| Patient 4 | 0.6 | 0 | 0.3 | 0 | 1.8 | 0 |
| No sclerosis | No sclerosis | Sclerosis | Sclerosis | Sclerosis | Sclerosis | |
| Patient 5 | 2.0 | 0 | 1.0 | 0 | ||
| No sclerosis | No sclerosis | Sclerosis | Sclerosis | |||
| Patient 8 | 0.3 | 0 | 0 | 0 | 1.6 | 3.0 |
| No sclerosis | No sclerosis | No sclerosis | No sclerosis | No sclerosis | No sclerosis | |
Average CD44-positive cells per glomerulus in VEC or PEC location. Patient 1 had a fourth biopsy with only global sclerosis, and thus, staining was not performed. VEC, visceral epithelial location; PEC, parietal epithelial cell location.
No glomeruli remaining for immunostaining.
Figure 5.
Specific identification of CD44 staining in glomerular epithelial cells was done by anatomic location and aided by CD45 staining on adjacent sections to identify leukocyte staining. In a case of rejection, intraglomerular (within capillary lumens) and interstitial leukocytes were stained for CD44 and CD45 (A and B). In a case of FSGS recurrence in the transplant, activated parietal epithelial cells along the inside of Bowman’s capsule were stained with CD44 (C) but not CD45 (D). Interstitial leukocytes were stained with both CD44 and CD45 (C and D). A and C, anti-CD44; B and D, anti-CD45 (same glomeruli from each case in adjacent sections). Original magnification, ×200.
Normal controls, including three noninvolved portions of tumor nephrectomies and five donor baseline kidneys (all without morphologic abnormalities other than rare global glomerulosclerosis), showed four VEC and one PEC CD44 positives in a total of 607 glomeruli. Thus, 0.8% of glomeruli stained positive for CD44 in a VEC location (P<0.05 versus native FSGS and recurrent FSGS), and 0.2% of glomeruli showed positive staining involving PECs (P<0.05 versus native FSGS).
Discussion
Our study indicates that glomerular epithelial cells are activated with significantly increased CD44 staining, a marker of activated PECs, in the earliest stage of recurrent FSGS, which is characterized by extensive foot process effacement by electron microscopy and no segmenta lesion by light microscopy. In contrast, patients with MCD showed only very rare glomerular epithelial cell positivity for CD44. These findings suggest that CD44 positivity may help distinguish recurrent early FSGS in the transplant and potentially, early FSGS in native kidneys, from MCD.
FSGS is caused by podocyte injury, and in some cases, recurrence seems to be mediated by a circulating factor (20). The initial injury is, thus, only podocyte effacement associated with marked proteinuria and overt sclerosis then develops over the subsequent weeks (21). The diagnosis of recurrent FSGS in the transplant can, therefore, be made based solely on the presence of extensive foot process effacement in this clinical setting. However, in the native kidney, the distinction of MCD versus early FSGS may be difficult, particularly when biopsies are small and diagnostic segmental lesions are not adequately sampled or when glomerular injury is in an early presclerotic stage.
In addition to this practical diagnostic consideration, the early stage of podocyte injury is also important to understand pathogenesis of MCD versus recurrent FSGS in the transplant. Some have suggested that MCD and FSGS are pathogenetically similar in early phases and that FSGS evolves from MCD. However, others have suggested that, even at early presclerotic phases of injury, FSGS is distinct from MCD. We showed that glomerular hypertrophy is one such distinguishing feature present in early presclerotic FSGS (22). More recent studies have shown altered proteomic signals distinguishing MCD from FSGS (23,24). Measurement of increased urinary CD80 to distinguish MCD from FSGS seems promising. However, the study supporting this approach was based on a limited number of the patients, and this approach needs to be tested on a larger scale. Several additional studies on individual podocyte-associated or profibrotic proteins, such as nephrin, podocin, synaptopodin, and TGF-β, have attempted to distinguish FSGS from MCD, but they were inconclusive (25–28).
In this small study, we did not have genetic information, and thus, we do not know if our findings are relevant for patients with genetic FSGS. Recurrent nephrotic syndrome in patients with FSGS caused by podocyte structural gene mutations is less common than idiopathic FSGS, and thus, it is likely that our patients mostly had idiopathic FSGS.
In the past, we have proposed that α-dystroglycan staining may be a useful marker for distinguishing unsampled FSGS from MCD in nephrotic patients with extensive foot process effacement as the only morphologic abnormality. Our initial small study showed decreased α-dystroglycan expression in cases without segmental sclerosis followed by a complete clinical remission, thus behaving like MCD, contrasting with preserved dystroglycan expression in cases with FSGS (29). However, our later and larger study showed normal α-dystroglycan staining in MCD compared with slightly decreased staining in uninvolved glomeruli in FSGS variants and markedly decreased staining in involved segments, mostly in collapsing glomerulopathy (30). Thus, we speculate that decreased dystroglycan may not be specifically or uniquely related to the podocyte perturbations underlying MCD.
Recently, the concept has evolved that PECs are not simply innocent bystanders but may play a pivotal role in the development of FSGS. We, therefore, used CD44, a marker for activated PECs, as a tool to potentially distinguish early FSGS from MCD. We compared CD44 expression in epithelial cells in MCD cases with early FSGS to see whether PEC activation could be a morphologic marker of this early presclerotic stage of recurrent FSGS in the transplant. CD44 positivity was also increased in native kidney FSGS cases in both VEC and PEC locations compared with MCD. Our data support the proposition that CD44 may also be useful in analyzing diagnostically challenging native renal biopsies with extensive foot process effacement but without diagnostic segmental sclerosing lesions, and it may aid in distinguishing early FSGS in the transplant or possibly, native FSGS from MCD. Extensive study and follow-up of nephrotic patients without sclerosis in the native kidney and possible unsampled or early FSGS will be necessary to determine the positive predictive value of CD44 expression in diagnosing progressive podocytopathies such as FSGS. Current data, including immunohistochemical and genetic studies, support that PECs are the predominant extracapillary proliferating cells in the collapsing and other specific variants of FSGS. Previously, these proliferating epithelial cells were thought to represent dysregulated and/or transdifferentiated podocytes. These epithelial cells may have a very basic role in the pathogenesis of FSGS (5,16,19,31–36). Whether these cells in human biopsies represent podocytes that have transdifferentiated to a parietal phenotype in situ or parietal epithelial cells that have migrated to a visceral location has not been proven. The work by Kriz and LeHir (37) proposed the following mechanism through which glomerular epithelial cell hyperplasia may lead to glomerulosclerosis: (1) cellular bridges between the glomerular and parietal basement membranes form; (2) cellular bridges develop into tuft adhesion to Bowman’s capsule, which initiates extracapillary cell proliferation; and (3) repair of extracapillary lesion with tuft adhesion by formation of segmental adherent scar occurs (i.e., segmental glomerulosclerosis) (37). However, whether this mechanism applies to all or a subgroup of human FSGS awaits additional study.
The exact mechanisms leading to PEC proliferation and possible migration to a visceral location are not known. PEC activation seems to occur after podocyte injury, possibly in an attempt to replenish/replace injured podocytes, which is in accordance with the concept put forward in the work by Sagrinati et al. (15). These studies from human tissue support that PECs are niche stem cells/multipotent progenitor cells that proliferate in an attempt to replace injured cells. Whether some of these proliferating cells succeed in differentiating and replacing podocytes is unknown. Our mouse lineage tracing studies describe transitional cells at the glomerular vascular pole bearing morphologic and immunohistochemical features of both PECs and podocytes and a recruitment of podocytes from PECs during development (4). More recent lineage tracing studies found no expression of podocyte markers in proliferating cells in Bowman’s capsule, thus arguing against a differentiation of the cells to a mature podocyte phenotype (16,19,38). In our most recent study, we showed that glomerular injuries that trigger secondary FSGS result in an activation of resident parietal cells, resulting in PEC proliferation and invasion of the glomerular tuft through an adhesion that, ultimately, results in sclerosis (19). Cell bridges between Bowman’s capsule and glomerular tuft were present as an early lesion, suggesting a nidus for sclerosis (19). Interestingly, in this study, we also observed very early adhesions between the glomerular tuft and Bowman’s capsule, with strong staining of PECs for CD44 along Bowman’s capsule in the area of adhesion preceding epithelial cell proliferation. Our study also showed significantly increased staining of PECs in the segmentally sclerotic tuft in the visceral location, supporting the concept that the PECs have a significant role in sclerosis. Our recent studies showed that activated PECs are associated with increased matrix within the sclerotic lesions, supporting a prosclerotic role in that setting (19). However, whether this PEC proliferation always results in sclerosis or could also, in some settings, contribute to repair is not clear. In our opinion, these two processes may not be mutually exclusive and may represent two ends of the spectrum of injury response. The ultimate balance may then determine whether progressive sclerosis develops.
In summary, CD44 is a marker of PEC activation and may be helpful in differentiating early FSGS from MCD in the transplant or native kidney. Whether the PEC activation is causal and always contributes to sclerosis in the pathogenesis of idiopathic FSGS or could also be a regenerative/repair response to replace injured podocytes awaits additional study.
Disclosures
None.
Acknowledgment
This study was supported, in part, by American Recovery and Reinvestment Act Grant DK080095 from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (to A.B.F, V.D.D., and C.E.A.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Fogo AB: Minimal change disease and focal segmental glomerulosclerosis. In: Fundamentals of Renal Pathology, edited by Fogo AB, New York, Springer Science, 2005, pp 40–52 [Google Scholar]
- 2.Haas M, Meehan SM, Karrison TG, Spargo BH: Changing etiologies of unexplained adult nephrotic syndrome: A comparison of renal biopsy findings from 1976-1979 and 1995-1997. Am J Kidney Dis 30: 621–631, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Braden GL, Mulhern JG, O’Shea MH, Nash SV, Ucci AA, Jr, Germain MJ: Changing incidence of glomerular diseases in adults. Am J Kidney Dis 35: 878–883, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ: Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20: 333–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dijkman H, Smeets B, van der Laak J, Steenbergen E, Wetzels J: The parietal epithelial cell is crucially involved in human idiopathic focal segmental glomerulosclerosis. Kidney Int 68: 1562–1572, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Kriz W, Gretz N, Lemley KV: Progression of glomerular diseases: Is the podocyte the culprit? Kidney Int 54: 687–697, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Kriz W, Lemley KV: The role of the podocyte in glomerulosclerosis. Curr Opin Nephrol Hypertens 8: 489–497, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Kriz W: Progressive renal failure—inability of podocytes to replicate and the consequences for development of glomerulosclerosis. Nephrol Dial Transplant 11: 1738–1742, 1996 [PubMed] [Google Scholar]
- 9.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Matsusaka T, Xin J, Niwa S, Kobayashi K, Akatsuka A, Hashizume H, Wang QC, Pastan I, Fogo AB, Ichikawa I: Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol 16: 1013–1023, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Shankland SJ: The podocyte’s response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Matsusaka T, Sandgren E, Shintani A, Kon V, Pastan I, Fogo AB, Ichikawa I: Podocyte injury damages other podocytes. J Am Soc Nephrol 22: 1275–1285, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magil AB: Histogenesis of glomerular crescents. Immunohistochemical demonstration of cytokeratin in crescent cells. Am J Pathol 120: 222–229, 1985 [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison DJ, MacDonald MK: The origin of cells in the glomerular crescent investigated by the use of monoclonal antibodies. Histopathology 10: 945–952, 1986 [DOI] [PubMed] [Google Scholar]
- 15.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P: Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol 17: 2443–2456, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Smeets B, Uhlig S, Fuss A, Mooren F, Wetzels JF, Floege J, Moeller MJ: Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells. J Am Soc Nephrol 20: 2604–2615, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourguignon LY, Zhu H, Shao L, Chen YW: CD44 interaction with c-Src kinase promotes cortactin-mediated cytoskeleton function and hyaluronic acid-dependent ovarian tumor cell migration. J Biol Chem 276: 7327–7336, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Besse-Eschmann V, Le Hir M, Endlich N, Endlich K: Alteration of podocytes in a murine model of crescentic glomerulonephritis. Histochem Cell Biol 122: 139–149, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Smeets B, Kuppe C, Sicking EM, Fuss A, Jirak P, van Kuppevelt TH, Endlich K, Wetzels JF, Gröne HJ, Floege J, Moeller MJ: Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis. J Am Soc Nephrol 22: 1262–1274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savin VJ, Sharma R, Sharma M, McCarthy ET, Swan SK, Ellis E, Lovell H, Warady B, Gunwar S, Chonko AM, Artero M, Vincenti F: Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med 334: 878–883, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Verani RR, Hawkins EP: Recurrent focal segmental glomerulosclerosis. A pathological study of the early lesion. Am J Nephrol 6: 263–270, 1986 [DOI] [PubMed] [Google Scholar]
- 22.Fogo A, Hawkins EP, Berry PL, Glick AD, Chiang ML, MacDonell RC, Jr, Ichikawa I: Glomerular hypertrophy in minimal change disease predicts subsequent progression to focal glomerular sclerosis. Kidney Int 38: 115–123, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Hodgin JB, Borczuk AC, Nasr SH, Markowitz GS, Nair V, Martini S, Eichinger F, Vining C, Berthier CC, Kretzler M, D’Agati VD: A molecular profile of focal segmental glomerulosclerosis from formalin-fixed, paraffin-embedded tissue. Am J Pathol 177: 1674–1686, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garin EH, Mu W, Arthur JM, Rivard CJ, Araya CE, Shimada M, Johnson RJ: Urinary CD80 is elevated in minimal change disease but not in focal segmental glomerulosclerosis. Kidney Int 78: 296–302, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Patrakka J, Ruotsalainen V, Ketola I, Holmberg C, Heikinheimo M, Tryggvason K, Jalanko H: Expression of nephrin in pediatric kidney diseases. J Am Soc Nephrol 12: 289–296, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Horinouchi I, Nakazato H, Kawano T, Iyama K, Furuse A, Arizono K, Machida J, Sakamoto T, Endo F, Hattori S: In situ evaluation of podocin in normal and glomerular diseases. Kidney Int 64: 2092–2099, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Srivastava T, Garola RE, Whiting JM, Alon US: Synaptopodin expression in idiopathic nephrotic syndrome of childhood. Kidney Int 59: 118–125, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Strehlau J, Schachter AD, Pavlakis M, Singh A, Tejani A, Strom TB: Activated intrarenal transcription of CTL-effectors and TGF-beta1 in children with focal segmental glomerulosclerosis. Kidney Int 61: 90–95, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Giannico G, Yang H, Neilson EG, Fogo AB: Dystroglycan in the diagnosis of FSGS. Clin J Am Soc Nephrol 4: 1747–1753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giannico G, Phillips S, Shyr Y, Alpers CE, D’Agati VD, Fogo AB: Dystroglycan patterns in FSGS variants. Mod Pathol 24[Suppl 1]: 344A–345A, 2011 [Google Scholar]
- 31.Smeets B, Te Loeke NA, Dijkman HB, Steenbergen ML, Lensen JF, Begieneman MP, van Kuppevelt TH, Wetzels JF, Steenbergen EJ: The parietal epithelial cell: A key player in the pathogenesis of focal segmental glomerulosclerosis in Thy-1.1 transgenic mice. J Am Soc Nephrol 15: 928–939, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Ohse T, Vaughan MR, Kopp JB, Krofft RD, Marshall CB, Chang AM, Hudkins KL, Alpers CE, Pippin JW, Shankland SJ: De novo expression of podocyte proteins in parietal epithelial cells during experimental glomerular disease. Am J Physiol Renal Physiol 298: F702–F711, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barisoni L, Kriz W, Mundel P, D’Agati V: The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 10: 51–61, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Ohtaka A, Ootaka T, Sato H, Ito S: Phenotypic change of glomerular podocytes in primary focal segmental glomerulosclerosis: developmental paradigm? Nephrol Dial Transplant 17[Suppl 9]: 11–15, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Asano T, Niimura F, Pastan I, Fogo AB, Ichikawa I, Matsusaka T: Permanent genetic tagging of podocytes: Fate of injured podocytes in a mouse model of glomerular sclerosis. J Am Soc Nephrol 16: 2257–2262, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Zhong J, Zuo Y, Ma J, Fogo AB, Jolicoeur P, Ichikawa I, Matsusaka T: Expression of HIV-1 genes in podocytes alone can lead to the full spectrum of HIV-1-associated nephropathy. Kidney Int 68: 1048–1060, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Kriz W, LeHir M: Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 67: 404–419, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Dijkman HB, Weening JJ, Smeets B, Verrijp KC, Van Kuppevelt TH, Assmann KK, Steenbergen EJ, Wetzels JF: Proliferating cells in HIV and pamidronate-associated collapsing focal segmental glomerulosclerosis are parietal epithelial cells. Kidney Int 70: 338–344, 2006 [DOI] [PubMed] [Google Scholar]