Abstract
Initial denitration of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by Rhodococcus sp. strain DN22 produces CO2 and the dead-end product 4-nitro-2,4-diazabutanal (NDAB), OHCNHCH2NHNO2, in high yield. Here we describe experiments to determine the biodegradability of NDAB in liquid culture and soils containing Phanerochaete chrysosporium. A soil sample taken from an ammunition plant contained RDX (342 μmol kg−1), HMX (octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine; 3,057 μmol kg−1), MNX (hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine; 155 μmol kg−1), and traces of NDAB (3.8 μmol kg−1). The detection of the last in real soil provided the first experimental evidence for the occurrence of natural attenuation that involved ring cleavage of RDX. When we incubated the soil with strain DN22, both RDX and MNX (but not HMX) degraded and produced NDAB (388 ± 22 μmol kg−1) in 5 days. Subsequent incubation of the soil with the fungus led to the removal of NDAB, with the liberation of nitrous oxide (N2O). In cultures with the fungus alone NDAB degraded to give a stoichiometric amount of N2O. To determine C stoichiometry, we first generated [14C]NDAB in situ by incubating [14C]RDX with strain DN22, followed by incubation with the fungus. The production of 14CO2 increased from 30 (DN22 only) to 76% (fungus). Experiments with pure enzymes revealed that manganese-dependent peroxidase rather than lignin peroxidase was responsible for NDAB degradation. The detection of NDAB in contaminated soil and its effective mineralization by the fungus P. chrysosporium may constitute the basis for the development of bioremediation technologies.
The cyclic nitramine hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) (Fig. 1) is a widely used energetic compound which has caused severe soil and groundwater contamination (13, 24). RDX is toxic (27, 33), and substantial research has been done to evaluate its fate in the environment and to develop efficient bioremediation processes (19). The degradation of RDX under anaerobic conditions was extensively studied, and the studies revealed the involvement of an important microbial diversity (14, 17, 22, 23, 35-38). Aerobic degradation studies using RDX as a nitrogen source were more recently reported and have led to the isolation of three Rhodococcus strains, namely, Rhodococcus sp. strain DN22 (9), Rhodococcus sp. strain A (20), and Rhodococcus rhodochrous strain 11Y (29), and a strain of Stenotrophomonas maltophilia (7). Coleman et al. (9) reported the release of nitrite (NO2−) during RDX degradation with strain DN22, whereas Fournier et al. (12) reported the formation of several other products, including nitrous oxide (N2O), ammonia (NH3), formaldehyde (HCHO), and a dead-end product (C2H5N3O3) that was tentatively identified as either 4-nitro-2,4-diazabutanal (NDAB;O2NNHCH2NHCHO) or 2-nitro-3-amino-2-azapropanal(NH2CH2NNO2CHO). Subsequent work by Bhushan et al. (6) rigorously identified the metabolite as NDAB using a reference standard and nuclear magnetic resonance spectroscopy. It is clear that cytochrome P-450 catalyzes the initial denitration of RDX that leads to its decomposition in water (6, 10, 29).
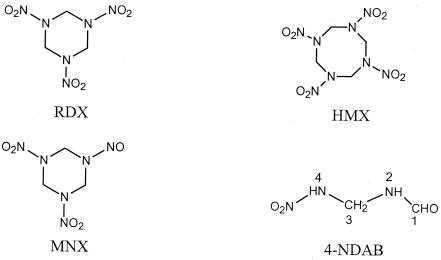
FIG. 1.
Structures of RDX, MNX, HMX, and NDAB.
We previously determined that 30% of the carbon content of RDX was released as CO2 and 64% was incorporated in NDAB (12). NDAB was also produced following denitration of RDX during alkaline hydrolysis at pH 10 (2) and during photolysis in aqueous solutions (16).
The universal production of NDAB via different biotic (12, 16) and abiotic (2) routes suggests that its detection at contaminated sites might provide an effective method of monitoring natural attenuation. On the other hand, since RDX degradation with strain DN22 led to the formation of NDAB as a dead-end product, the successful degradation of the latter could constitute the basis for the development of an effective remediation technology.
We tested the white rot fungus Phanerochaete chrysosporium for its ability to degrade NDAB because of the versatility of its ligninolytic enzymes and because it was reported to degrade and mineralize RDX effectively (3, 11, 30, 31). Fernando and Aust (11) reported that P. chrysosporium mineralizes RDX in liquid culture under ligninolytic conditions. The manganese-dependent peroxidase (MnP), not the lignin peroxidase (LiP), is responsible for the degradation of RDX (30, 31).
Therefore, the aims of the present study were to determine whether NDAB is present in RDX-contaminated soil as an indicator of natural attenuation and to test the potential of P. chrysosporium to mineralize NDAB in liquid culture and in different soils.
MATERIALS AND METHODS
Chemicals.
RDX (>99% purity) and uniformly 14C-labeled RDX [U-14C]RDX (98% purity) were provided by the Defense Research and Development Canada, (Valcartier, Québec, Canada) (1). The specific activity and radiochemical purity of the radioactive RDX were 28.7 mCi/mmol and 97%, respectively. All other chemicals used were of reagent grade.
Synthesis of NDAB.
Methylene(bis)formamide was prepared by the method of Sauer and Follett (28). Acetyl nitrate was prepared by adding 7 g (100 mmol) of nitric acid (90%) to 15 g of acetic anhydride in 15 ml of acetic acid with ice cooling. Methylene(bis)formamide (6.1 g; 50 mmol) was added, and the mixture was stirred at room temperature for 24 h. Chlorobenzene (100 ml) was added, and the solution was concentrated carefully (>40°C) in vacuo to remove excess nitric acid, acetic acid, and acetyl nitrate. The residue was dissolved in 100 ml of ethanol, and the solution was kept at 45 to 50°C for 24 h to achieve ethanolysis of the initially formed mononitromethylene(bis)formamide by cleaving the undesired formyl group. The concentrate was flash chromatographed with a 4- by 1-in. silica gel column. The compound that eluted at an Rf of 0.4 (ethyl acetate [EtOAc]-SiO2) was collected and crystallized from EtOAc-toluene to yield 1.5 g of crystalline product (40%). As previously done by Bhushan et al. (6), the structure of the product was determined by 1H nuclear magnetic resonance spectroscopy (Bruker; AV-400) in d6-dimethyl sulfoxide (δ 12.45 [s, 1H, NHNO2]; δ 8.93 [s, 1H, NHCHO]; δ 8.06 [d, 1H, CHO]; δ 4.70 [d, 2H, CH2]), 13C NMR (δ 165.05 [CHO]; δ 47.73 [CH2]), and elemental analysis (C2H5N3O3; calculated percentages: C, 20.17%; N, 35.28%; H, 4.23%; found percentages: C, 20.48%; N, 34.25%; H, 4.19%).
Organisms and growth conditions.
Rhodococcus sp. strains DN22, 11Y, and A were kindly provided by Nicholas Coleman (9), Neil Bruce (29), and Charles W. Greer (20). The three strains were isolated from aerobic enrichments prepared from soils contaminated with explosives. In this study, each of the three strains (DN22, 11Y, and A) was cultivated in a mineral salt medium supplemented with succinate (m-succinate medium) as previously described (9).
P. chrysosporium ATCC 24725 was provided by Ian Reid (Paprican, Montreal, Canada) and was maintained on YPD (yeast extract, 5 g liter−1; peptone, 10 g liter−1; dextrose, 20 g liter−1; agar, 20 g liter−1; pH 5.5, adjusted with H2SO4) at 37°C. Conidiospores were harvested from 10-day-old cultures in a sterile aqueous solution with 0.2% Tween 80 and kept at 4°C. Degradation assays were performed in 120-ml sealed serum bottles with 10 ml of ligninolytic P. chrysosporium cultures. The ligninolytic cultures are referred to as 7-day-old cultures incubated in a medium deficient in nitrogen (MC1 medium; 1.2 mM ammonium). The composition of the low-nitrogen MC1 medium was modified from that described by Capdevila et al. (8). Glycerol was replaced by glucose (10 g liter−1). Yeast extract and veratryl alcohol were omitted, and diammonium tartrate was added at 0.22 g liter−1. The cultures were inoculated at a concentration of 2 × 105 conidiospores/ml, and then they were incubated in the dark at 37°C under static conditions. The headspace of the microcosms was flushed for 30 s with air every 3 to 5 days. Unless otherwise specified, NDAB was added after 7 days of incubation. A similar procedure was used for the degradation experiments performed in soils. P. chrysosporium conidiospores were inoculated into 20% (wt/vol) soil slurries prepared in MC1 medium (2 g of soil and 10 ml of MC1). After a preincubation period of 7 days, NDAB (126 μmol liter−1) was added to the slurries.
Microtox assay of NDAB.
The toxicity of NDAB was assessed using the 15-min Microtox (Vibrio fischeri) test for acute microbial toxicity (Microbics Corp., Microtox manual: Microtox basic test procedures, 1992). Reconstituted cells of V. fischeri were exposed in triplicate to different dilutions of NDAB. The detailed procedure is described by Sunahara et al. (32), and relative toxicity levels were expressed as the concentrations that inhibit bioluminescence by 20% (IC20s).
Soil characteristics.
Three soils were used in the present study: (i) an agricultural topsoil (VT) originating from Varennes, Quebec, Canada; (ii) a Sassafras sandy loam (SSL) obtained from Aberdeen Proving Ground, Aberdeen, Md.; and (iii) a soil sample collected from an ammunition plant in Valleyfield, Quebec, Canada. The last soil was analyzed according to U.S. Environmental Protection Agency method 8330 (34) and found to contain RDX (342 μmol kg−1), MNX (hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine; 155 μmol kg−1), and HMX (octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine; 3,057 μmol kg−1). Other characteristics of the soils are presented in Table 1. Prior to usage, each soil was passed through a 2-mm sieve and residual moisture was removed by air drying in a fume hood.
TABLE 1.
Characteristics of soils used during this study
| Soil | Granulometry result (%)
|
% Total organic C | Total N concn (mg kg−1) | Total P concn (mg kg−1) | pH | Nitramine concn(s) (μmol kg−1) | ||
|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | ||||||
| VT | 83 | 12 | 4 | 8.4 | 1,100 | 400 | 5.6 | None |
| SSL | 71 | 18 | 11 | 0.3 | 420 | 100 | 5.1 | None |
| Ammunition plant | 14 | 44 | 42 | 0.8 | 1,100 | 400 | 6.5 | 342 (RDX), 155 (MNX), 3,057 (HMX), 3.8 (NDAB) |
Biodegradation of NDAB in soil.
To distinguish the role of P. chrysosporium from that of the soil microorganisms, the soils were sterilized by gamma irradiation from a cobalt 60 source at the Canadian Irradiation Center (Laval, Quebec, Canada) with a dose of 50 kGy over 2 h. Twenty percent (wt/vol) soil slurries were prepared with 2 g of soil and 10 ml of MC1 medium in 120-ml serum bottles. Spores of P. chrysosporium were added, and cultures were grown as described above. On the seventh day of incubation, NDAB (126 μmol liter−1) was added to the soil slurries. Uninoculated soil slurries served as controls.
Mineralization assay.
We prepared [14C]NDAB in situ by incubating [14C]RDX with strain DN22 as previously described (12). When the mineralization reached a plateau of 30%, representing maximum mineralization by DN22 (12), the culture medium containing NDAB (10 ml) was directly transferred to 10 ml of a 7-day-old culture of P. chrysosporium. A KOH trap was placed in the serum bottle to capture generated 14CO2.
Enzyme assays.
MnP (provided by M. Paice [Paprican]) assays were performed as previously described by Hofrichter et al. (18). Briefly, the MnP enzyme was added to 50 mM sodium malonate buffer (pH 4.5) containing 1 mM MnCl2 (1 mM), 15 mM glucose, 0.04 U of glucose oxidase (from Aspergillus niger, low in catalase activity) (Sigma-Aldrich), and 1 U of MnP. The reaction was conducted following the addition of NDAB (30 mg liter−1) in the dark at 37°C under agitation.
LiP (Sigma-Aldrich) assays were performed as described previously by Stahl et al. (31). Briefly, NDAB (30 mg liter−1) was incubated in 15 mM sodium tartrate buffer (pH 4.5) containing 15 mM glucose, 0.033 U of glucose oxidase, 2.5 mM veratryl alcohol, and 1 U of LiP. The reaction was performed as described for MnP.
Analytical procedures.
The concentrations of NDAB and nitramide (H2NNO2) in the supernatant of centrifuged samples (16,000 × g) were determined with a high-pressure liquid chromatography (HPLC) system. The HPLC system (Waters Associates, Milford, Mass.) consisted of a W600 pump, a 717 Plus autosampler, a model 996 photodiode array (PDA) detector, and Millenium data acquisition software. The samples were injected into an AnionSep Ice-Ion-310 Fast organic acid column (6.5 by 150 mm; Cobert Associates Chromatography Products, St. Louis, Mo.) kept at 35°C. The mobile phase was composed of 1.73 mM sulfuric acid with a flow rate of 0.6 ml/min. Under these conditions NDAB eluted at 8 min. The detection was at 225 nm. NDAB in soil was extracted by washing with deionized water for 1 h. Soil extract was filtered through a 3-aminopropyl-functionalized silica gel (Aldrich) column to remove interfering ions. NDAB was also analyzed with a Bruker (Billerica, Mass.) benchtop ion trap mass detector attached to a Hewlett Packard 1100 series HPLC system equipped with a PDA detector. The samples were injected into a 5-μm-pore-size Zorbax SB-C18 capillary column (0.5 mm [internal diameter] by 150 mm; Agilent Technologies, Mississauga, Ontario, Canada) at 25°C. The solvent system was composed of 20% (vol/vol) acetonitrile-water and was isocratic at a flow rate of 12 μl min−1. For mass analysis, ionization was performed in a negative electrospray ionization mode, ES(−), producing mainly the deprotonated molecular mass ions [M-H]. The mass range was scanned from 40 to 400 Da. Analyses of nitrite (NO2−), nitrate (NO3−), formaldehyde (HCHO), formic acid (HCOOH), nitrous oxide (N2O), and ammonium (NH4+) were performed as described in Hawari et al. (16).
RESULTS AND DISCUSSION
Detection of NDAB in contaminated soil; evidence of natural attenuation.
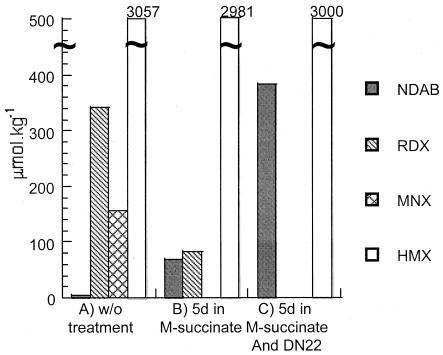
Analysis of a field soil sample from the ammunition plant showed the presence of traces of NDAB (3.8 μmol kg−1) in addition to RDX (342 μmol kg−1), MNX (155 μmol kg−1), and HMX (3,057 μmol kg−1) (Fig. 2). The detection of the ring cleavage product NDAB represents the first experimental evidence of the occurrence of natural attenuation of cyclic nitramines. Liquid chromatography-mass spectrometry analysis confirmed that the mass spectrum of the compound was similar to that previously reported (12). Previously only the nitroso derivatives of RDX (and HMX) were reported to coexist with the cyclic nitramines at contaminated sites (R. J. Spanggord, W. R. Mabey, T.-W. Chou, S. Lee, P. L. Aferness, and T. Mill, Environmental fate studies of HMX, phase II, detailed studies, final report, SRI International, Menlo Park, Calif.). However, RDX and HMX are often contaminated with their nitroso derivatives, making the use of the latter as markers for natural attenuation uncertain. In contrast, NDAB can be formed only from the degradation of the cyclic nitramines and has never been detected during the synthesis of RDX or HMX. Zhao et al. (37) reported the formation of NDAB in sterilized controls during the degradation of MNX with Clostridium bifermentans strain HAW-1. Balakrishnan et al. (2) found that hydrolysis of RDX and MNX at pH 10 can also lead to the formation of NDAB.
FIG. 2.
Distribution of cyclic nitramines and products in soil collected from an ammunition plant containing RDX (342 μmol kg−1), HMX (3,057 μmol kg−1), MNX (155 μmol kg−1), and traces of NDAB (3.8 μmol kg−1) with no added reagents (A), after 5 days of incubation in m-succinate medium (20%, wt/vol) (B), and after 5 days of incubation in m-succinate medium with the RDX-induced DN22 strain (20%, wt/vol) (C). DN22 was induced with RDX, harvested at mid-log phase, and concentrated to an optical density at 530 nm of approximately 4.0. The bacterial suspension (0.6 ml) was used to inoculate the slurries. Incubation was performed at 30°C with agitation at 180 rpm.
The above observations are in sharp contrast to findings for the ring cleavage product methylenedinitramine (MDNA), which we detected earlier during degradation of RDX (and HMX) with anaerobic sludge (14, 17, 25), Klebsiella pneumoniae SCZ-1 (36), or nitrate reductase (5). MDNA is unstable in water at pH 7 and decomposes to N2O and HCHO. Beller and Tiemeier (4) searched for MDNA in groundwater contaminated with RDX but were unable to detect it.
Biotransformation of RDX and MNX to NDAB.
Figure 2 shows the formation of NDAB as a major product during incubation of the nonsterilized ammunition plant soil with and without the inoculation of RDX-induced DN22. In the absence of DN22, all of MNX and 76% of RDX in the soil degraded, with the concurrent formation of NDAB (69 μmol kg−1). Previously, we isolated Rhodococcus sp. strain A, which was found to degrade RDX (20) and to form NDAB (15) from the site. Likewise, the presence of DN22 led to the degradation of both MNX and RDX completely, with concurrent production of NDAB (384 μmol kg−1) (Fig. 2). In contrast, HMX did not degrade.
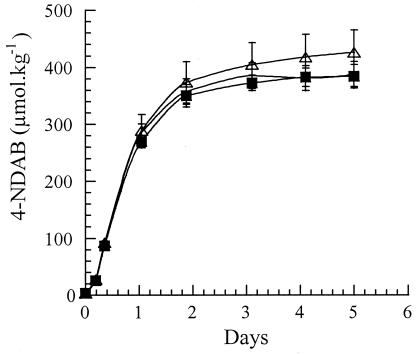
When the three Rhodococcus strains, from widely different locations, were added separately to the ammunition plant soil described above, similar amounts (388 ± 22, 426 ± 39, and 384 ± 21 μmol kg−1) of NDAB were produced (Fig. 3). Although Seth-Smith et al. (29) were unable to detect NDAB from the degradation of RDX by 11Y, we were able to observe the accumulation of the dead-end product. As was the case with Rhodococcus sp. strains A and DN22, the addition of supplementary carbon and nitrogen sources to 11Y culture did not promote any further degradation of NDAB. In addition, when we incubated MNX (200 μmol) with any of the three Rhodococcus strains in m-succinate liquid culture medium, MNX degraded rapidly to produce NDAB (90 μmol), which persisted. Once again, HMX was found to be recalcitrant under these conditions.
FIG. 3.
Formation of NDAB in aerobic soil slurries (20%, wt/vol) prepared with an ammunition plant soil contaminated with RDX (342 μmol kg−1), HMX (3,057 μmol kg−1), MNX (155 μmol kg−1), and traces of NDAB (3.8 μmol kg−1) in m-succinate medium inoculated with either RDX-induced Rhodococcus sp. strain DN22 (▪), Rhodococcus sp. strain A (○), or R. rhodochrous sp. strain 11Y (▵). The strains were induced with RDX, harvested at mid-log phase and concentrated to an optical density at 530 nm of approximately 4.0. The bacterial suspensions (0.6 ml) were used to inoculate the slurries. Incubation was performed at 30°C with agitation at 180 rpm. Values represent the averages and standard deviations of triplicate experiments.
Biodegradation of NDAB.
Experimental evidence gathered thus far indicated that aerobic bacteria are able to biotransform RDX to NDAB, which resisted further degradation. To assess the toxicity of NDAB, we chose the rapid and simple Microtox assay. The results showed NDAB (IC20, 191.0 μM) to be toxic but that its toxicity was less than that previously reported for RDX (IC20, 104.6 μM), HMX (IC20, 21.7 μM), or 2,4,6-trinitrotoluene (IC20, 0.5 μM) (32). Standard Microtox tests can be performed on aqueous solutions and soil leachates. In this work, the NDAB toxicity was assessed by using a pure solution, which cannot be compared directly with NDAB toxicity assessment in soil. Several factors may interact in the soil medium, such as sorption and synergistic and antagonistic effects with other compounds present in the soil. Because of its extreme solubility in water, NDAB can migrate through subsurface soil and cause groundwater contamination. Therefore, its degradability should be investigated by using a well-studied microorganism such as P. chrysosporium. This fungus has already been found to effectively mineralize RDX (30, 31) without NDAB being detected. We were unable to detect NDAB during RDX incubation with P. chrysosporium because of its potential degradation. For instance, when we incubated RDX with a crude extract obtained from the culture supernatant of the fungus, only trace amounts of NDAB were observed and these did not accumulate, indicating that the extracellular enzymes degraded NDAB.
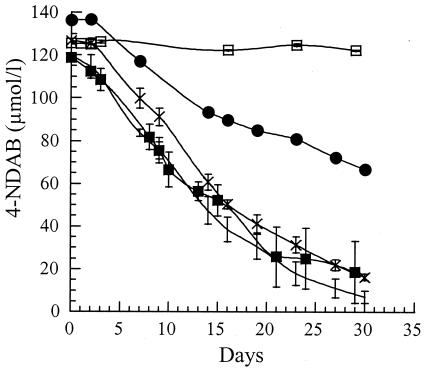
We tested the degradation of NDAB with P. chrysosporium in an artificially contaminated liquid culture and in soil slurries (Fig. 4). The ligninolytic cultures of P. chrysosporium in MC1 medium began to degrade NDAB after 3 to 4 days of incubation. More than 80% of the initial concentration of NDAB (118 μmol liter−1) was degraded within 29 days of incubation at a rate of 3.4 μmol day−1. In the uninoculated control NDAB did not degrade. Using ligninolytic cultures of P. chrysosporium, Stahl et al. (31) demonstrated that RDX began to degrade after 3 to 4 days of incubation and attributed degradation to the presence of both MnP and LiP. The concentrations of both enzymes reached optimal values after 5 days of incubation and persisted until RDX ceased to degrade. However, when they used pure peroxidases, only MnP was able to degrade RDX. In the present study, the incubation of NDAB (252 μmol liter−1) with pure MnP resulted in 18% (46 μmol liter−1) degradation of the nitramine after 18 h of incubation (data not shown). In control mixtures prepared without MnP or without glucose oxidase, no degradation of NDAB occurred. Pure LiP did not catalyze the degradation of NDAB (data not shown), but in vivo, the enzyme could play an indirect catalytic role in the degradation of the compound (21).
FIG. 4.
Degradation of NDAB added to uninoculated MC1 medium (□), to ligninolytic P. chrysosporium in MC1 (▪), and to P. chrysosporium grown in 20% (wt/vol) slurries prepared with VT (○), with SSL (×), and with an ammunition plant soil contaminated with RDX, MNX, HMX, and traces of NDAB (•). Values represent the averages and standard deviations of triplicate experiments.
The degradation of NDAB by P. chrysosporium cultures in MC1 medium led to the production of trace amounts of nitramide (H2NNO2) and nitrous oxide (N2O). Previously we demonstrated that the production of N2O during RDX degradation with DN22 (12) and the fungus (30) originated from the ring cleavage product H2NNO2. We calculated that the molar ratio of N2O produced to NDAB degraded was 0.89 ± 0.10. The detection of N2O and traces of H2NNO2 and the absence of NO2− suggest the cleavage of a 4N-3C bond in NDAB (Fig. 1).
P. chrysosporium was incubated in gamma-irradiated soils to determine its ability to degrade NDAB. Degradation rates of NDAB in VT and SSL soils and in MC1 cultures were closely similar (Fig. 4) despite the fact that SSL and VT contained different amounts of C and N (Table 1). The fungus degraded NDAB added to the ammunition plant soil but at a rate (2.0 μmol day−1) about 55% less than the rates in the two artificially contaminated soils. The above results suggest that, although not all forms of organic matter are repressive for the production of ligninolytic enzymes (21), the high nitrogen content of VT soil did not repress the enzyme(s) responsible for NDAB degradation.
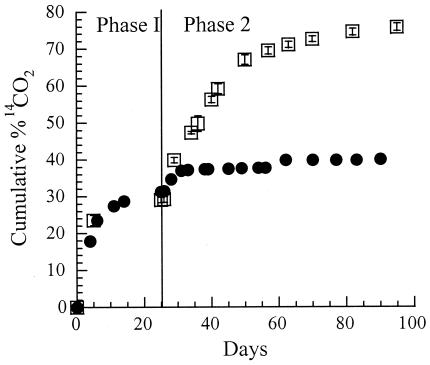
Mineralization of [14C]RDX in liquid medium took place in two distinct phases (Fig. 5). The first phase, which lasted from 0 to 26 days, involved incubation with DN22. In the second phase, the 26-day-old DN22 cultures were added to P. chrysosporium pregrown in MC1 medium. In phase I, approximately 30% of the RDX was converted to CO2 and the remainder of the energetic chemical accumulated as NDAB (see above). In phase II, the [14C]NDAB generated in situ was mineralized to 14CO2 (75.8% ± 1.0%) in 95 days (Fig. 5). The addition of the acidic MC1 medium (pH 4.5) alone following DN22 treatment (phase I) led to a slight increase (from 30 to 37%) in the concentration of 14CO2 due to the reduction in the solubility of carbon dioxide. Since no other products were detected, we assumed that the 76% accumulated mineralization in 100 days also represents the C mass balance.
FIG. 5.
Sequential biodegradation of [14C]RDX (40 ppm). Phase 1 was incubation with Rhodococcus sp. strain DN22 in 10 ml of m-succinate medium (for in situ production of [14C]NDAB); phase 2 was the period after the addition of MC1 medium (10 ml) to 10 ml of DN22 culture (•) and after the addition of P. chrysosporium culture (10 ml) to 10 ml of DN22 culture (□). Values represent the averages and standard deviations of triplicate experiments.
Conclusion.
The detection of the ring cleavage product NDAB in RDX-contaminated soil provides clear evidence of RDX natural attenuation. It is not clear whether the low concentrations of NDAB indicate low rates of RDX degradation or subsequent transformation of the NDAB. Because abiotic transformation and biological transformation of RDX both yield NDAB, it is not possible to judge the relative contributions of the processes in the soil collected from the contaminated site. It is clear, however, from our results that aerobic biodegradation in soil produces NDAB almost exclusively and that the metabolite is biodegradable by fungi. Our current goal is to determine the relative contributions of biodegradation and abiotic processes during natural attenuation of RDX and to provide insight about the factors that determine the rates of NDAB production and degradation.
Acknowledgments
We thank Stéphane Deschamps, Louise Paquet, Chantale Beaulieu, Alain Corriveau, and Dominic Manno for their technical assistance; Claude Masson for soil sampling; and Sylvie Rocheleau for providing the toxicity data on NDAB. We are grateful to Charles W. Greer, Nicholas V. Coleman, and Neil C. Bruce for providing the Rhodococcus strains and to Sandra Trott and Manish Bhatt for the revision of the manuscript. We also thank Defense Research and Development Canada, Valcartier, Canada, for providing samples of RDX and HMX.
We thank the United States Strategic Environmental Research and Development Program (CU-1213) and ONR, U.S. Navy (N0001140310269), for financial support.
REFERENCES
- 1.Ampleman, G., S. Thiboutot, J. Lavigne, A. Marois, J. Hawari, A. M. Jones, and D. Rho. 1995. Synthesis of 14C-labelled hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX), 2,4,6-trinitrotoluene (TNT), nitrocellulose (NC) and glycidylazide polymer (GAP) for use in assessing the biodegradation potential of these energetic compounds. J. Label. Compd. Radiopharm. 36:559-577. [Google Scholar]
- 2.Balakrishnan, V., A. Halasz, and J. Hawari. 2003. Alkaline hydrolysis of the cyclic nitramine explosives RDX, HMX, and CL-20: new insights into degradation pathways obtained by the observation of novel intermediates. Environ. Sci. Technol. 37:1838-1843. [DOI] [PubMed] [Google Scholar]
- 3.Bayman, P., S. D. Ritchey, and J. W. Bennett. 1995. Fungal interactions with the explosive RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine). J. Ind. Microbiol. 15:418-423. [Google Scholar]
- 4.Beller, H. R., and K. Tiemeier. 2002. Use of liquid chromatography/tandem mass spectrometry to detect distinctive indicators of in situ RDX transformation in contaminated groundwater. Environ. Sci. Technol. 36:2060-2066. [DOI] [PubMed] [Google Scholar]
- 5.Bhushan, B., A. Halasz, J. Spain, S. Thiboutot, G. Ampleman, and J. Hawari. 2002. Biotransformation of hexahydro-1,3,5-trinitro-1,3,5-triazine catalyzed by a NAD(P)H: nitrate oxidoreductase from Aspergillus niger. Environ. Sci. Technol. 36:3104-3108. [DOI] [PubMed] [Google Scholar]
- 6.Bhushan, B., S. Trott, J. C. Spain, A. Halasz, L. Paquet, and J. Hawari. 2003. Biotransformation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by a rabbit liver cytochrome P450: insight into the mechanism of RDX biodegradation by Rhodococcus sp. strain DN22. Appl. Environ. Microbiol. 69:1347-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binks, P. R., S. Nicklin, and N. C. Bruce. 1995. Degradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by Stenotrophomonas maltophilia PB1. Appl. Environ. Microbiol. 61:1318-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capdevila, C., S. Moukha, M. Ghyczy, J. Theilleux, B. Gelie, M. Delattre, G. Corrieu, and M. Asther. 1990. Characterization of peroxidase secretion and subcellular organization of Phanerochaete chrysosporium INA-12 in the presence of various soybean phospholipid fractions. Appl. Environ. Microbiol. 56:3811-3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman, N. V., D. R. Nelson, and T. Duxbury. 1998. Aerobic degradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) as a nitrogen source by a Rhodococcus sp., strain DN22. Soil Biol. Biochem. 30:1159-1167. [Google Scholar]
- 10.Coleman, N. V., J. C. Spain, and T. Duxbury. 2002. Evidence that RDX biodegradation by Rhodococcus strain DN22 is plasmid-borne and involves a cytochrome p-450. J. Appl. Microbiol. 93:463-472. [DOI] [PubMed] [Google Scholar]
- 11.Fernando, T., and S. D. Aust. 1991. Biodegradation of municipal waste, TNT (2,4,6-trinitrotoluene) and RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine) by Phanerochaete chrysosporium, p. 214-231. In D. W. Tedder and F. G. Pohland (ed.), Emerging technologies in hazardous waste management. American Chemical Society, Washington, D.C.
- 12.Fournier, D., A. Halasz, J. C. Spain, P. Fiurasek, and J. Hawari. 2002. Determination of key metabolites during biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine with Rhodococcus sp. strain DN22. Appl. Environ. Microbiol. 68:166-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas, R., I. Schreiber, E. von Löw, and G. Stork. 1990. Conception for the investigation of contaminated munitions plants. 2. Investigation of former RDX-plants and filling stations. Fresenius' J. Anal. Chem. 338:41-45. [Google Scholar]
- 14.Halasz, A., J. Spain, L. Paquet, C. Beaulieu, and J. Hawari. 2002. Insights into the formation and degradation mechanisms of methylenedinitramine during the incubation of RDX with anaerobic sludge. Environ. Sci. Technol. 36:633-638. [DOI] [PubMed] [Google Scholar]
- 15.Hawari, J. 2000. Biodegradation of RDX and HMX: from basic research to field application, p. 277-310. In J. C. Spain, J. B. Hughes, and H.-J. Knackmuss (ed.), Biodegradation of nitroaromatic compounds and explosives. CRC Press, Boca Raton, Fla.
- 16.Hawari, J., A. Halasz, C. Groom, S. Deschamps, L. Paquet, C. Beaulieu, and A. Corriveau. 2002. Photodegradation of RDX in aqueous solution: a mechanistic probe for biodegradation with Rhodococcus sp. Environ. Sci. Technol. 36:5117-5123. [DOI] [PubMed] [Google Scholar]
- 17.Hawari, J., A. Halasz, S. Beaudet, L. Paquet, G. Ampleman, and S. Thiboutot. 2001. Biotransformation routes of octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine by municipal anaerobic sludge. Environ. Sci. Technol. 35:70-75. [DOI] [PubMed] [Google Scholar]
- 18.Hofrichter, M., K. Scheibner, I. Schneegaß, and W. Fritsche. 1998. Enzymatic combustion of aromatic and aliphatic compounds by manganese peroxidase from Nematoloma frowardii. Appl. Environ. Microbiol. 64:399-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jerger, D. E., and P. Woodhull. 2000. Applications and costs for biological treatment of explosives-contaminated soils in the US, p. 395-423. In J. C. Spain, J. B. Hughes, and H.-J. Knackmuss (ed.), Biodegradation of nitroaromatic compounds and explosives. CRC Press, Boca Raton, Fla.
- 20.Jones, A. M., C. W. Greer, G. Ampleman, S. Thiboutot, J. Lavigne, and J. Hawari. 1995. Biodegradability of selected highly energetic pollutants under aerobic conditions, p. 251-257. In R. E. Hinchee, D. B. Anderson, and R. E. Hoeppel (ed.), Bioremediation of recalcitrant organics. Battelle Press, Columbus, Ohio.
- 21.Kirk, T. K., and R. L. Farrell. 1987. Enzymatic “combustion”: the microbial degradation of lignin. Annu. Rev. Microbiol. 41:465-505. [DOI] [PubMed] [Google Scholar]
- 22.Kitts, C. L., D. P. Cunningham, and P. J. Unkefer. 1994. Isolation of three hexahydro-1,3,5-trinitro-1,3,5-triazine-degrading species of the family Enterobacteriaceae from nitramine explosive-contaminated soil. Appl. Environ. Microbiol. 60:4608-4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCormick, N. G., J. H. Cornell, and A. M. Kaplan. 1981. Biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine. Appl. Environ. Microbiol. 42:817-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myler, C. A., and W. Sisk. 1991. Bioremediation of explosives contaminated soils (scientific questions/engineering realities), p. 137-146. In G. S. Sayler, R. Fox, and J. W. Blackburn (ed.), Environmental bio/technology for waste treatment. Plenum Press, New York, N.Y.
- 25.Oh, B.-T., C. L. Just, and P. J. J. Alvarez. 2001. Hexahydro-1,3,5-trinitro-1,3,5-triazine mineralization by zerovalent iron and mixed anaerobic cultures. Environ. Sci. Technol. 35:4341-4346. [DOI] [PubMed] [Google Scholar]
- 26.Pennington, J. C., and J. M. Brannon. 2002. Environmental fate of explosives. Thermochim. Acta 384:163-172. [Google Scholar]
- 27.Robidoux, P. Y., J. Hawari, S. Thiboutot, G. Ampleman, and G. I. Sunahara. 2001. Chronic toxicity of octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) in soil determined using the earthworm (Eisenia andrei) reproduction test. Environ. Pollut. 111:283-292. [DOI] [PubMed] [Google Scholar]
- 28.Sauer, C. W., and R. P. Follett. 1955. Nitramines. I. Methylenedinitramine. J. Am. Chem. Soc. 77:2560-2561. [Google Scholar]
- 29.Seth-Smith, H. M. B., S. J. Rosser, A. Basran, E. R. Travis, E. R. Dabbs, S. Nicklin, and N. C. Bruce. 2002. Cloning, sequencing, and characterization of the hexahydro-1,3,5-trinitro-1,3,5-triazine degradation gene cluster from Rhodococcus rhodochrous. Appl. Environ. Microbiol. 68:4764-4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheremata, T. W., and J. Hawari. 2000. Mineralization of RDX by the white rot fungus Phanerochaete chrysosporium to carbon dioxide and nitrous oxide. Environ. Sci. Technol. 34:3384-3388. [Google Scholar]
- 31.Stahl, J. D., B. Van Aken, M. D. Cameron, and S. D. Aust. 2001. Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) biodegradation in liquid and solid-state matrices by Phanerochaete chrysosporium. Bioremediation J. 5:13-25. [Google Scholar]
- 32.Sunahara, G. I., S. Dodard, M. Sarrazin, L. Paquet, G. Ampleman, S. Thiboutot, J. Hawari, and A. Y. Renoux. 1998. Development of a soil extraction procedure for ecotoxicity characterization of energetic compounds. Ecotoxicol. Environ. Safety 39:185-194. [DOI] [PubMed] [Google Scholar]
- 33.Talmage, S. S., D. M. Opresko, C. J. Maxwel, C. J. E. Welsh, F. M. Cretella, P. H. Reno, and F. B. Daniel. 1999. Nitroaromatic munition compounds: environmental effects and screening values. Rev. Environ. Contam. Toxicol. 161:1-156. [DOI] [PubMed] [Google Scholar]
- 34.U.S. Environmental Protection Agency. 1997. Method 8330 SW-846, update III, part 4. 1 (B), Nitroaromatics and nitramines by high performance liquid chromatography (HPLC). Office of Solid Waste, Washington, D.C.
- 35.Young, D. M., P. J. Unkefer, and K. L. Ogden. 1997. Biotransformation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by a prospective consortium and its most effective isolate, Serratia marcescens. Biotechnol. Bioeng. 53:515-522. [DOI] [PubMed] [Google Scholar]
- 36.Zhao, J.-S., A. Halasz, L. Paquet, C. Beaulieu, and J. Hawari. 2002. Biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine and its mononitroso derivative hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine by Klebsiella pneumoniae strain SCZ-1 isolated from an anaerobic sludge. Appl. Environ. Microbiol. 68:5336-5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao, J.-S., L. Paquet, A. Halasz, and J. Hawari. 2003. Metabolism of hexahydro-1,3,5-trinitro-1,3,5-triazine through initial reduction to hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine followed by denitration in Clostridium bifermentans HAW-1. Appl. Microbiol. Biotechnol. 63:187-193. [DOI] [PubMed] [Google Scholar]
- 38.Zhao, J.-S., J. Spain, and J. Hawari. 2003. Phylogenetic and metabolic diversity of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX)-transforming bacteria in strictly anaerobic mixed cultures enriched on RDX as nitrogen source. FEMS Microbiol. Ecol. 46:189-196. [DOI] [PubMed] [Google Scholar]