Abstract
OBJECTIVE:
This study sought to outline the clinical and laboratory characteristics of minimal change disease in adolescents and adults and establish the clinical and laboratory characteristics of relapsing and non-relapsing patients.
METHODS:
We retrospectively evaluated patients with confirmed diagnoses of minimal change disease by renal biopsy from 1979 to 2009; the patients were aged >13 years and had minimum 1-year follow-ups.
RESULTS:
Sixty-three patients with a median age (at diagnosis) of 34 (23-49) years were studied, including 23 males and 40 females. At diagnosis, eight (12.7%) patients presented with microscopic hematuria, 17 (27%) with hypertension and 17 (27%) with acute kidney injury. After the initial treatment, 55 (87.3%) patients showed complete remission, six (9.5%) showed partial remission and two (3.1%) were nonresponders. Disease relapse was observed in 34 (54%) patients who were initial responders (n = 61). In a comparison between the relapsing patients (n = 34) and the non-relapsing patients (n = 27), only proteinuria at diagnosis showed any significant difference (8.8 (7.1-12.0) vs. 6.0 (3.6-7.3) g/day, respectively, p = 0.001). Proteinuria greater than 7 g/day at the initial screening was associated with relapsing disease.
CONCLUSIONS:
In conclusion, minimal change disease in adults may sometimes present concurrently with hematuria, hypertension, and acute kidney injury. The relapsing pattern in our patients was associated with basal proteinuria over 7 g/day.
Keywords: Minimal Change Disease, Adults, Relapse, Proteinuria
INTRODUCTION
Minimal change disease (MCD) is the leading cause of nephrotic syndrome in children under the age of 10, accounting for 90% of all cases. In adults, however, MCD accounts for only 9-15% of primary glomerulopathy cases (1)-(3), and studies in adults are scarce, likely due to the lower disease incidence in this age group. Waldman et al. observed that at the time of diagnosis, in addition to the classic nephrotic presentation of glomerulopathy, 43% of patients presented with hypertension, 17.8% had acute kidney injury, and 29% had microscopic hematuria (4). It was also observed that the treatment response was favorable. However, relapses are frequent and may range from 62.3 to 73.1%, with corticosteroid dependence in 12% of cases and corticosteroid resistance in 4.8 to 27% of cases (4)-(6). This relapsing characteristic has led some authors to seek diagnostic markers that can be used to differentiate relapsing from non-relapsing patients, and some studies have indicated that adults with early presentation of the disease and high serum levels of IgE are more likely to relapse (5),(6).
A study in adult Chinese patients also found elevated serum IgE levels in relapsing patients (7), and these findings were explained by the fact that serum IgE levels reflected immune dysfunction as well as B- and T-cell activation (7).
The absence of studies on this pathology in the Brazilian population led us to conduct a retrospective study aimed at identifying the clinical and laboratory characteristics of primary onset MCD in a patient population over the age of 13 and establishing the clinical and laboratory characteristics of relapsing versus non-relapsing patients.
MATERIALS AND METHODS
We retrospectively evaluated patients who had been diagnosed with nephrotic syndrome and MCD, as confirmed by renal biopsy, from 1979 to 2009 in two university centers in the State of São Paulo, Brazil (Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo and Universidade Estadual Paulista-School of Medicine-Hospital das Clínicas of Botucatu).
Inclusion Criteria
The following inclusion criteria were established: an initial clinical presentation of nephrotic syndrome; a diagnosis of MCD that was confirmed by renal biopsy using conventional criteria with normal light microscopy and negative immunofluorescence in a representative sample; age greater than 13 years; and follow-up time of at least 1 year.
Exclusion Criteria
Patients diagnosed with any systemic disease, such as systemic lupus erythematosus or other autoimmune diseases, diabetes, or previous hypertension were excluded, as were those with less than 1 year of follow-up. To confirm these data, all of the patients were tested for ANA, viral serology, and blood glucose, and they also received an ophthalmological review or an echocardiogram.
From 1979 to 2009, 102 renal biopsies confirmed the diagnoses of MCD in the proposed patient group. Thirty-nine patients were excluded from the analysis; ten were discharged to their original medical units and 29 missed their follow-up appointments during this period.
The patient data were obtained through an analysis of medical records and included clinical characteristics, laboratory parameters, response to medical treatment and relapses.
After an initial treatment, proteinuria <0.3 g/day was defined as complete remission; the reduction of baseline proteinuria by >50% with a final value <3 g/day was defined as partial remission; and resistance was defined as cases where no remission occurred after the use of immunosuppressive medication for up to 16 weeks. Relapse was defined as proteinuria values that returned to >3 g/day after a remission period; immunosuppressive medication dependence was defined as relapses occurring within four weeks after withdrawal; and acute kidney injury (AKI) was defined as an increase of 50% or more in the baseline creatinine level.
Hematuria was defined as more than eight red blood cells per high-power field. Hypertension was defined as systolic blood pressure or diastolic blood pressure >139 mmHg or 90 mmHg, respectively, in two sequential readings.
This study was approved by the Nephrology Department of the Universities of São Paulo and Botucatu as well as the university ethics committee.
Statistical Analysis
The continuous variable data were expressed as the median with quartile intervals and percentages for categorical variables. Differences between the two groups were evaluated using the unpaired Student's t-test; when the sample was not normally distributed, we applied the Mann-Whitney U-test. Categorical variables between groups were evaluated using the chi-squared test. Logistic regression was tested for a dependent variable (relapse) in relation to the independent variables (patient age at diagnosis, serum albumin and proteinuria). The level of statistical significance was set at p<0.05.
RESULTS
We studied 63 patients with a median age at diagnosis of 34 (23-49) years, including 23 males and 40 females. The biochemical data at diagnosis showed levels of serum creatinine of 0.8 (0.7-1.3) mg/dL, serum albumin of 1.8 (1.5-2.3) g/dL, proteinuria of 7.2 (5.3-10.3) g/day, total cholesterol of 423.0 (335.0-524.0) mg/dL and triglycerides of 214.5 (162.5-310.5) mg/dL. At the time of diagnosis, eight (12.7%) patients presented with microscopic hematuria, 17 (27%) had hypertension and 17 (27%) had acute kidney injury. The mean follow-up time for these patients was 24 (17-60) months.
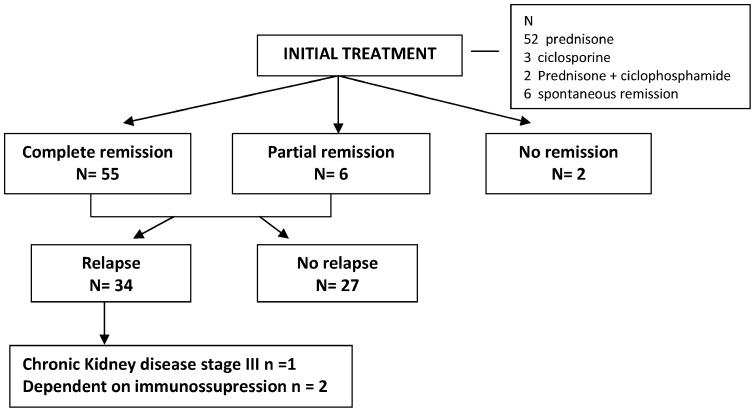
The decision to treat and the choice of the treatment regimen were made by the assistant nephrologist in accordance with clinical criteria. In the initial treatment, most patients (52, 82.5%) were treated only with prednisone at 1 mg/kg/day for eight weeks with gradual tapering thereafter; three (4.7%) patients received cyclosporine; two (3.1%) were given prednisone and cyclophosphamide; and six (9.5%) received no immunosuppression because they presented with spontaneous remission. After the initial treatment, 55 (87.3%) patients demonstrated complete remission, six (9.5%) presented with partial remission and two (3.1%) were nonresponders. In the patients with remission (n = 61), relapse occurred in 34 (54%) patients with a median of two (1)-(3) relapses per patient during the follow-up period. Two of these patients (3.1%) were considered dependent on immunosuppressive medication.
Comparing the clinical presentation at diagnosis between the groups of patients with relapse (n = 34) and without relapse (n = 27), there was no difference in age (32.5 (22.5-46.0) vs. 34.0 (23.0-61.0) years, respectively), gender, clinical presentation (hematuria, hypertension and acute kidney injury) and follow-up time (Table 1). In the initial laboratory data, there was a statistically significant difference only in proteinuria, which was greater in the relapsing group than in the non-relapsing group (8.8 (7.1-12.0) vs. 6.0 (3.6-7.3) g/day, p = 0.001, respectively) (Table 1).
Table 1.
Clinical characteristics of relapsing and non-relapsing patients at diagnosis.
| Relapsing (n = 34) | Non-relapsing (n = 27) | p-value | |
| Age (years) | 32.5 (22.5-46.0) | 34.0 (23.0-61.0) | 0.27 |
| Gender (M/F) | 13/21 | 10/17 | 0.92 |
| Serum creatinine (mg/dL) | 0.9 (0.7-1.3) | 0.8 (0.7-1.2) | 1.00 |
| Serum albumin (g/dL) | 1.8 (1.4-2.2) | 1.8 (1.5-2.3) | 0.65 |
| Proteinuria (g/day) | 8.8 (7.1-12.0) | 6.0 (3.6-7.3) | 0.001 |
| Cholesterol (mg/dL) | 423.0 (344.5-569.0) | 439.0 (317.0-509.5) | 0.25 |
| Triglycerides (mg/dL) | 227 (177-371) | 198 (143.5-331.5) | 0.26 |
| Hematuria (n/%) | 6/17.6 | 2/7.4 | 0.23 |
| Hypertension (n/%) | 12/35.2 | 5/18.5 | 0.14 |
| Acute kidney injury (n/%) | 12/35.2 | 5/18.5 | 0.14 |
| Follow-up time (months) | 24 (12-72) | 24 (17-48) | 0.58 |
median (interval between quartiles).
AKI - acute kidney injury.
In a logistic regression, only proteinuria affected the relapse frequency, with an odds ratio of 1.23 (p = 0.01; 95% CI 1.04 to 1.46). However, the patient age at diagnosis and the serum albumin level did not show any effect on relapse (Table 2). For the patients with proteinuria >7 g/day (at diagnosis), 27 (44.2%) were in the relapse group, and 11 (18%) were in non-relapse group (p = 0.002). In contrast, only seven (11.4%) of the patients with proteinuria ≤7 g/day (at diagnosis) were in the relapse group.
Table 2.
Clinical variables influencing relapse.
| Β | SE | Odds Ratio | 95% CI | p-value | |
| Age (years) | -0.22 | 0.01 | 0.97 | 0.94-1.01 | 0.26 |
| Serum albumin (mg/dL) | -0.40 | 0.41 | 0.96 | 0.43-2.14 | 0.92 |
| Proteinuria (g/day) | 0.21 | 0.08 | 1.23 | 1.04-1.46 | 0.01 |
Sixty-five percent of patients received non-immunosuppressive agents for renin-angiotensin system-blocking therapy, and 45% received statin therapy. There was no difference in the use of these medications between the relapsing and non-relapsing patients.
At the end of the follow-up period, there were no differences between the groups with and without relapse regarding the levels of serum creatinine (0.8 (0.7-0.9) vs. 0.8 (0.6-0.9) mg/dL, respectively), proteinuria (0.1 (0.07-0.3) vs. 0.1 (0.06-0.3) g/day, respectively), triglycerides (97 (66-129) vs. 102 (70-141.5) mg/dL, respectively) and total cholesterol (193.0 (158.0-216.0) vs. 191.0 (168.5-223.0) mg/dL, respectively). During this period, one patient in the relapse group presented with loss of renal function but did not require dialysis.
The following complications occurred during the follow-up period. In the group without relapse, one patient developed diabetes mellitus, and another developed melanoma. In the group with relapse, three patients developed diabetes mellitus, two had osteoporosis, two had depression, and one developed obesity.
DISCUSSION
This study assessed retrospective data from two university centers over a 30-year period and found that minimal change disease in adults affected a patient population in their third decade of life (34 (23-49) years); only nine patients were aged 60 years or older (data not shown), and there was a slight predominance of females (1.7/1). The disease evolution was favorable; however, one patient developed chronic kidney disease, and one patient aged 70 years was diagnosed with melanoma close to glomerulopathy symptoms.
Our patients were similar to those observed in other studies in terms of age at the onset of disease, gender and the presence of hematuria at diagnosis (4),(8). However, as compared to other study protocols, our study evaluated fewer patients with hypertension and proteinuria at diagnosis and a higher frequency of patients with acute kidney injury at diagnosis (4),(8).
The clinical presentation of acute kidney injury in MCD is associated with acute tubular necrosis in most cases and occurs more frequently in older hypertensive patients (4),(9). In the present study, the AKI patients were older than those without AKI (48.0 (34.0-60.0) vs. 29.0 (22.0-41.0) years, p = 0.015), but there was no difference in hypertension, proteinuria and serum albumin level between these groups (data not shown).
The response to initial treatment with glucocorticosteroids for this pathology is generally high, approximately 80% (4),(10), and our data revealed a total 96.8% remission rate, including complete remissions (87.3%) and partial remissions (9.5%). However, relapse is an important characteristic of this disease, as it occurred in 54% of the cases in our study. Nine patients (14.2%) experienced more than two relapses during the follow-up period, and two of these patients were considered dependent on immunosuppressive medication (Figure 1). Other studies have also shown a high frequency of relapse, with values between 67.1 and 73.1% (4),(5),(11).
Figure 1.
Outcome after initial treatment.
It would be beneficial to establish clinical and laboratory characteristics to differentiate relapsing from non-relapsing patients, with the aim of using steroids for a shorter period of time and calcineurin inhibitors in the long term. In our study, the patients who presented with proteinuria >7 g/day at the time of diagnosis were more likely to experience relapse. The clinical parameter most related to relapse in other studies was age, indicating that younger patients relapse more frequently than older patients (6),(12); however, this finding was not made in our sample population.
Glucocorticosteroids remain the first-line treatment for relapse; however, in frequent relapsing and corticosteroid-dependent patients, it is necessary to use other immunosuppressants to minimize the side effects of the prolonged use of glucocorticosteroids. Eguchi A et al. demonstrated that the combined use of low-dose cyclosporine and prednisone was as effective at inducing remission as the use of prednisone (1 mg/kg/day) alone (13). Even without proper knowledge of the pathophysiology of MCD, treatment with glucocorticosteroids or calcineurin inhibitors can be effective at inducing remission, but these drugs do not prevent relapses in most cases. One of the theories regarding the pathophysiology of this disease is the dysfunction of podocytes with an overexpression of angiopoetin-like-4 (ANGPTL4) and CD80 (14), the latter of which contributes to T-cell dysfunction (15). In an experimental model, it was found that interleukin-13 acts as a potent stimulator of CD80 expression (16), and this interleukin is also known for its role in allergic processes (17).
In summary, minimal change disease in adults, in addition to its nephrotic characteristics, may present with hematuria, hypertension, and AKI. The treatment response and disease evolution are generally favorable, but there is a major risk of relapse. According to the current study, patients with proteinuria >7 g/day were at risk for relapse. Although these findings are clinically relevant, interpretations should be made with caution because this was a retrospective study and more trials are needed to support the results.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Cameron JS. Nephrotic syndrome in the elderly. Semin Nephrol. 1996;16(4):319–29. [PubMed] [Google Scholar]
- 2.Zech P, Colon S, Pointet P, Deteix P, Labeeuw M, Leitienne P. The nephritic syndrome in adults aged over 60: Etiology, evolution and treatment of 76 cases. Clin Nephrol. 1982;17(5):232–6. [PubMed] [Google Scholar]
- 3.Malafronte P, Mastroianni-Kirsztajn G, Betônico GN, Romão JE, Junior, Alves MA, Carvalho MF, et al. Paulista Registry of glomerulonephritis: 5- year data report. Nephrol Dial Transplant. 2006;21(11):3098–105. doi: 10.1093/ndt/gfl237. [DOI] [PubMed] [Google Scholar]
- 4.Waldman M, Crew RJ, Valeri A, Busch J, Stokes B, Markowitz G, et al. Adult Minimal-Change Disease: Clinical Characteristics, Treatment, and Outcomes. Clin J Am Soc Nephrol. 2007;2(3):445–53. doi: 10.2215/CJN.03531006. [DOI] [PubMed] [Google Scholar]
- 5.Takei T, Koike M, Suzuki K, Shirota S, Itabashi M, Ohtsubo S, et al. The characteristics of relapse in adult-onset minimal-change nephritic syndrome. Clin Exp Nephrol. 2007;11(3):214–7. doi: 10.1007/s10157-007-0484-5. [DOI] [PubMed] [Google Scholar]
- 6.Nakayama M, Katafuchi R, Yanase T, Ikeda K, Tanaka H, Fujimi S. Steroid Responsiveness and Frequency of Relapse in Adult-Onset Minimal Change Nephrotic Syndrome. Am J Kidney Dis. 2002;39(3):503–12. doi: 10.1053/ajkd.2002.31400. [DOI] [PubMed] [Google Scholar]
- 7.Tan Y, Yang D, Fan J, Chen Y. Elevated Levels of Immunoglobulin E May Indicate Steroid Resistance or Relapse in Adult Primary Nephrotic Syndrome, Especially in Minimal Change Nephrotic Syndrome. J Int Med Research. 2011;39(6):2307–13. doi: 10.1177/147323001103900629. [DOI] [PubMed] [Google Scholar]
- 8.Mak SK, Short CD, Mallick NP. Long-term outcome of adult onset minimal-change nephropathy. Nephrol Dial Transplant. 1996;11(11):2192–201. doi: 10.1093/oxfordjournals.ndt.a027136. [DOI] [PubMed] [Google Scholar]
- 9.Jennette JC, Falk RJ. Adult minimal change glomerulopathy with acute renal failure. Am J Kidney Dis. 1990;16(5):432–7. doi: 10.1016/s0272-6386(12)80055-2. [DOI] [PubMed] [Google Scholar]
- 10.Palmer SC, Nand K, Strippoli GFM. Interventions for minimal change disease in adults with nephrotic syndrome. Cochrane Database Syst Rev. 2008;(1):CD001537. doi: 10.1002/14651858.CD001537.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korbet SM, Schwartz MM, Lewis EJ. Minimal-change glomerulopathy of adulthood. Am J Nephrol. 1988;8(4):291–297. doi: 10.1159/000167603. [DOI] [PubMed] [Google Scholar]
- 12.Tse KC, Lam MF, Yip PS, Li FK, Choy BY, Lai KN, et al. Idiopathic minimal change nephrotic syndrome in older adults: steroid responsiveness and pattern of relapses. Nephrol Dial Transplant. 2003;18(7):1316–20. doi: 10.1093/ndt/gfg134. [DOI] [PubMed] [Google Scholar]
- 13.Eguchi A, Takei T, Yoshida T, Tsuchiya T, Nitta K. Combined cyclosporine and prednisolone therapy in adults patients with the first relapse of minimal-change nephrotic syndrome. Nephrol Dial Transplant. 2010;25(1):124–9. doi: 10.1093/ndt/gfp422. [DOI] [PubMed] [Google Scholar]
- 14.Wei C Reiser J. Minimal change disease as a modifiable podocyte paracrine disorder. Neprol Dial Transplant. 2011;26(6):1776–7. doi: 10.1093/ndt/gfr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garin EH, Mu W, Arthur JM, Rivard CJ, Araya CE, Shimada M, et al. Urinary CD80 is elevated in minimal change disease but not in focal segmental glomerulosclerosis. Kidney Int. 2010;78(3):296–302. doi: 10.1038/ki.2010.143. [DOI] [PubMed] [Google Scholar]
- 16.Lai KW, Wei CL, Tan LK, Tan PH, Chiang GS, Lee CG, et al. Overexpression of Interleukin-13 Induces Minimal-Change-Like Nephropathy in Rats. J Am Soc Nephrol. 2007;18(5):1476–85. doi: 10.1681/ASN.2006070710. [DOI] [PubMed] [Google Scholar]
- 17.Abdel-Hafez M, Shimada M, Lee PY, Johnson RJ, Garin EH. Idiopathic Nephrotic Syndrome and Atopy: Is There a Common Link. Am J Kidney Dis. 2009;54(5):945–53. doi: 10.1053/j.ajkd.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]