Abstract
OBJECTIVE:
Postsurgical abdominal adhesions are common, serious postoperative complications. The present study compared the usefulness of 4% icodextrin and canola oil in preventing postoperative peritoneal adhesions.
METHODS:
Twenty-four Wistar albino rats were divided into three groups. Following a laparotomy, a serosal abrasion was made by brushing the cecum, and 3 mL of 0.9% NaCl, 4% icodextrin, or 3 mL of canola oil were intraperitoneally administered for the control, icodextrin, and canola oil groups, respectively. The abdomen was then closed. All of the rats were sacrificed at day 10. Macroscopic, histopathological, and biochemical evaluations were performed. The results were statistically analyzed using Kruskal–Wallis and ANOVA tests.
RESULTS:
Macroscopic analyses revealed that both canola oil and 4% icodextrin reduced adhesion formation, but the difference was not statistically significant (p = 0.17). The histopathological examinations revealed no significant differences in terms of giant cell, lymphocyte/plasmocyte, neutrophil, ICAM1, or PECAM1 scores. However, both canola oil and 4% icodextrin significantly reduced fibrosis (p = 0.025). In the canola oil group, the histiocytic reactions were significantly increased (p = 0.001), and the hydroxyproline levels were significantly lower than those in the other groups (p = 0.034).
CONCLUSIONS:
In the present study, canola oil was determined to be superior to 4% icodextrin in lowering hydroxyproline levels and increasing histiocytic reactions. Considering these results, we believe that canola oil is a promising agent for preventing adhesion formation.
Keywords: Peritoneum, Peritoneal Adhesion, Icodextrin, Canola Oil, Hydroxyproline, Experimental Study
INTRODUCTION
Postoperative peritoneal adhesions are major complications in abdominopelvic surgery, occurring in 60-93% of patients (1),(2). These adhesions can result in major postoperative complications, such as intestinal obstruction, infertility, and chronic pelvic pain and can require re-admission and further operations (1)-(3). In addition to medical problems, increased surgical costs are an additional concern. An American study reported a total cost of $1.3 billion annually for the treatment of postoperative peritoneal adhesions (4).
Peritoneal adhesions that occur following surgical trauma are caused by metabolic processes and the combination of a large number of inflammatory and anti-inflammatory processes. Peritoneal adhesions are initiated by tissue damage and can cause a coagulation cascade over the course of several hours. Following coagulation, the inflammation phase begins within the first few postoperative days. Cell seeding, proliferation, migration and matrix deposition occur in the first week postoperatively. Lastly, the matrix remodeling phase lasts over a period of weeks to months (5).
Several studies have aimed to reduce the frequency of this commonly encountered condition. Progesterone, soybean oil, aloe vera gel, vitamin E, methylene blue, and amniotic membrane have been frequently used in experimental studies of peritoneal adhesion prevention (5)-(12). These materials are most commonly instilled and/or lavaged into or around the peritoneal cavity in a liquid, gel or spray form. Some of these substances have been used to inhibit adhesion formation (i.e., coagulation, inflammation and matrix formation), and others separate the peritoneal surfaces.
A corn starch derivative, icodextrin is a water-soluble branched glucose polymer; its monomers are linked by alpha (1)-(4) and alpha (1-6 [<10%]) glucosidal bonds. When administered intraperitoneally in a 4% solution, icodextrin functions as a colloidal osmotic agent. The colloidal osmotic action of this polymer retains a reservoir of fluid within the peritoneal cavity for 3-4 days. Icodextrin provides a temporary physical separation of the peritoneal surfaces by hydroflotation as the result of maintaining a fluid reservoir. This effect minimizes tissue apposition during the critical period of fibrin formation and mesothelial regeneration following surgery, thereby providing a barrier to adhesion formation (13).
Canola oil, which is also referred to as low erucic acid rapeseed oil, is a vegetable oil that contains monounsaturated fatty acids, oleic acid (55%), and polyunsaturated fatty acids (PUFAs), which are composed of linoleic acid (ω-6) (25%) and alpha-linoleic acid (ω-3) (10%). Canola oil has the lowest concentration of saturated fatty acids (SFA, 4%) of all the commonly consumed oils, and it is a good source of vitamins E and K and phytosterols (14). Although erucic acid is a monounsaturated fatty acid and a member of the ω-9 FA family, it metabolizes to oleic acid and has anti-inflammatory effects (15). Ω-3 PUFAs are essential nutrients that play a beneficial role in several disease processes because of their anti-inflammatory, analgesic, anti-thrombotic, and anti-mutagenic effects. These fatty acids also modulate some forms of lipids and positively affect the central nervous system. In contrast, ω-6 fatty acids have inflammatory, nociceptive, thrombotic, and mutagenic effects.
Canola oil is a lipid that can separate the traumatic peritoneal surfaces, and it contains fatty acids that can inhibit adhesion formation; therefore, it may successfully prevent peritoneal adhesions. Thus, the aim of this experimental study is to compare the macroscopic, histopathological and biochemical effects of icodextrin and canola oil in preventing postoperative peritoneal adhesions.
MATERIALS AND METHODS
This study was performed at the Experimental Animal Laboratory of Marmara University Medical Faculty after obtaining approval from the Animal Ethics Committee. All of the protocols followed the declaration of Helsinki guidelines concerning the care and use of laboratory animals.
Twenty-four Wistar albino outbred female rats (mean weight 250±30 g, mean age seven months) were divided into three groups and were housed in standard rat cages, each containing a maximum of five rats. The rats were housed using a 12-hr light/12-hr dark cycle at stable temperatures (between 19 and 22 °C). The animals were provided with standard rat pellet and tap water ad libitum.
Operation and adhesion model
Following a 12-hour starvation, the rats were anaesthetized with IM ketamine hydrochloride (Ketalar™, Eczacıbaşı, Istanbul-Turkey) at 40 mg/kg and xylazine (ROMPUN™, Bayer, Berlin Germany) at 10 mg/kg body weight. The rats were placed in a supine position, and their extremities were affixed to the operating table with plaster. All of the operations were performed using powder-free, non-latex gloves to prevent the anticipated peritoneal adhesions caused by foreign body reactions. After abdominal skin shaving, antisepsis was maintained using povidone iodine (Betadine™, Kurtsan, Istanbul-Turkey).
A laparotomy was performed via a 3 cm midline incision. The cecum was pulled from the abdomen and scrubbed five times with a sterile toothbrush to induce a subserosal hemorrhage on an area that was equivalent to the toothbrush surface. The cecum was then returned to its normal position.
Prior to closing the abdomen, 3 mL of 0.9% saline solution (Eczacibasi-Baxter, Istanbul-Turkey), 3 mL of 4% icodextrin (ADEPT™, Baxter, Deerfield, USA), or 3 mL of canola oil (Yudum™ Canola Oil, Balikesir-Turkey), which was sterilized in an autoclave and cultured prior to use, were intraperitoneally administered to the three groups of rats (n = 8 each).
To overcome fluid leakage from the peritoneal cavity, the wound edges were held together by four clamps immediately following the application of the protocol material. The abdominal incision was subsequently closed with 4-0 polypropylene running sutures (Prolene™, Ethicon, Cornelia, GA, USA). Then, 100 mL/kg of paracetamol (Perfalgan™, Bristol Myers Squibb, Park Avenue, NY, USA) was injected subcutaneously for analgesia. Normal feeding was allowed after 6 hours. Wound healing and abdominal wall integrity were assessed daily over the first three days following the surgery. All of the rats were sacrificed 10 days postoperatively using a high dose (100-150 mg/kg) of sodium thiopental (Pentotal™, Abott, Illinois, USA).
Macroscopic assessment
The peritoneal cavity was entered via a “reverse U” incision without damaging the formed adhesions. Retracting the anterior abdominal wall caudally, the peritoneal cavity, the small bowels and the cecum were carefully inspected and assessed according to the Blauer staging scale (16) (Table 1). Following a macroscopic evaluation, a 2-cm ileocecal segment and its neighboring mesenteric root (0.5×0.5 cm) were resected for the histopathological and biochemical examinations. The sacrificed animals were placed in the Marmara University Experimental Animal Laboratory's medical waste, and the study was completed.
Table 1.
The scoring system used for the macroscopic and microscopic evaluations of the inflammatory reactions on serosal surfaces (16).
| SCORE | Macroscopic findings | Cellular reaction*) | Fibrosis | ICAM1 and PECAM1 staining |
| 0 | No adhesions | None | None | None |
| 1 | Thin and narrow, easily separable adhesions | Rare | Rare | <10% |
| 2 | Thick adhesions, limited to one area | Mild | Mild | 10-50 % |
| 3 | Thick and wide adhesions | Severe | Severe | >50% |
| 4 | Thick and wide adhesions between the organs and the abdominal wall | NA**) | NA**) | NA**) |
: Giant cell, lymphocyte/plasmocyte, neutrophil, and histiocyte reactions. **NA: not available.
Histopathological assessment
The resected adhesion model specimen was fixed in a 10% formalin solution. Following dehydration, the samples were paraffin-embedded using tissue processing equipment (Leica ASP300S, Newcastle-UK). Four 3 μm-thick slices from each intestinal segment were obtained using a microtome (Leica RM2255, Newcastle-UK).
As defined by the producers of the standard protocol, the first section was stained with hematoxylin and eosin (HE) (Bio optica, Milano-Italy, Bio stain, Manchester-UK) to assess giant cell, lymphocyte/plasmocyte, neutrophil, and histiocyte reactions. The second section was stained with Masson Trichrome (Sigma Aldrich, St. Louis, MO-ABD) to assess fibrosis. The third section was stained for CD54/ICAM1 (Novocastra™ Leica, Newcastle-UK, 23G12 clone) and CD31/PECAM1 (Novocastra™ Leica, Newcastle-UK, 1A10 clone).
The slides that were stained with HE to assess the giant cell, lymphocyte/plasmocyte, neutrophil, and histiocyte reactions, as well as the slides that were stained with Masson Trichrome for the fibrosis analysis, were scored from zero to three based on the criteria that were used by Delaco et al. (17). The two slides that were stained for CD54 and CD31 were also stratified from zero to three, as shown in Table 1. The microscopic assessment was made using light microscopy (Nikon E600, Tokyo-Japan) under 100x and 200x magnifications. Images were obtained of the observed samples (Nikon E5400, Tokyo-Japan). The pathologist was blinded to the study group.
Biochemical assessment
The mesenteric tissue samples that were used for the biochemical assessment were kept in dry tubes and taken to the biochemistry laboratory. After collecting all of the tissues, 70 mg of tissue were homogenized in a 1 mL 0.9% NaCl solution (Janke & Kunkel Ultra-Turrax T25, Staufen-Germany). After adding equal volumes of HCl, the homogenized tissues were incubated for 24 hours in a 95°C water bath.
The compounds that were used for the analysis, i.e., acetate citrate buffer (pH 6.5), chloramine T and Erlich reactive, were all purchased from Sigma Aldrich St. Louis, MO, USA, and were freshly prepared. A standard hydroxyproline study was prepared. After completing the study, the absorbency values from the samples and the standards were quantified using a spectrophotometer (Beckman Coulter DU-530, Brea, CA, USA).
Statistical analyses
All of the statistical analyses were performed using the SPSS statistical software package (version 16.0, IBM, USA). The numerical data were expressed as the mean and standard derivation, unless otherwise stated. Because the values were nonparametric and the number of rats in the groups was under 30, the Kruskal–Wallis test was used for the statistical analysis of the giant cell, lymphocyte/plasmocyte, neutrophil, and histiocyte reactions and for the fibrosis, ICAM1 and PECAM1 scores. The statistical significance of the hydroxyproline levels was assessed using an ANOVA test given that all of the values that were obtained from the three groups were parametric. The results were evaluated with a confidence interval of 95%, and p-values below 0.05 were considered to be statistically significant.
RESULTS
No postoperative complications, such as bowel obstructions and peritonitis, or mortality was observed in any of the groups during the study. At necropsy assessment, no intraperitoneal fluid was found at day 10.
Macroscopic assessment
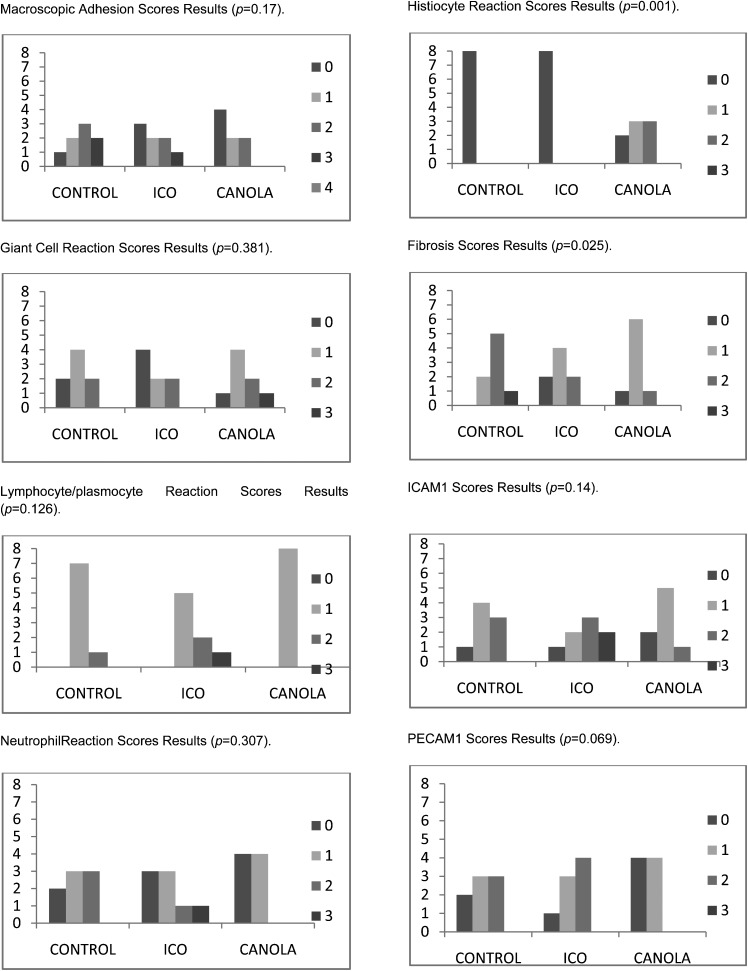
The rats in both the canola and the icodextrin groups, nearly half of which were free from adhesions, appeared to have lower macroscopic scores than those in the control group. However, this difference was not statistically significant (p = 0.17) (Table 2, Figure 1).
Table 2.
The mean scores that were obtained from the gross evaluation and the histopathological and biochemical assessments of each study group.
| Scores | Control | Icodextrin | Canola | p-value |
| Macroscopic assessment | 1.75±1.04 | 1.12±1.13 | 0.75±1.06 | 0.17 |
| Giant cell | 1.00±0.76 | 0.75±0.89 | 1.38±0.92 | 0.381 |
| Lymphocyte / plasmocyte | 1.12±0.35 | 1.50±0.76 | 1.00±0.00 | 0.126 |
| Neutrophil | 1.12±0.84 | 1.00±1.11 | 0.50±0.54 | 0.307 |
| Histiocyte | 0.00±0.00 | 0.00±0.00 | 1.12±0.84 | 0.001 |
| Fibrosis | 1.88±0.64 | 1.00±0.76 | 1.00±0.54 | 0.025 |
| ICAM1 | 1.25±0.70 | 1.75±1.04 | 0.88±0.64 | 0.14 |
| PECAM1 | 1.12±0.83 | 1.38±0.74 | 0.50±0.54 | 0.069 |
| Hydroxyproline | 302.65±147.69 | 273.40±118.56 | 151.14 ±53.30 | 0.034 |
Figure 1.
The results of the macroscopic and histopathological assessments of the study groups.
Histopathological assessment
The microscopic assessment of the giant cell (p = 0.381), lymphocyte/plasmocyte (p = 0.126), and neutrophil reactions (p = 0.307) revealed no statistically significant difference in comparison between the control, icodextrin and canola groups.
Although no histiocytic reaction was observed in the sham or the icodextrin-treated groups, a significant histiocytic reaction was observed in the rats in the canola oil-treated group: two rats had scores of zero; three rats had scores of one; and three rats had scores of two (p = 0.001) (Table 2, Figure 1).
The fibrosis assessment revealed that both the icodextrin- and the canola oil-treated groups exhibited significantly decreased fibrotic reactions compared to the control group; none of the icodextrin- or canola-treated rats received a score of three (p = 0.025) (Table 2, Figure 1).
The immunohistochemistry studies revealed that the rats in the control and icodextrin groups expressed higher ICAM and PECAM1 levels in the cellular membranes: the mean ICAM scores were 1.25±0.70, 1.75±1.04 and 0.88±0.64 for the control, icodextrin-treated and canola oil-treated groups, respectively; the mean PECAM1 scores were 1.12±0.83, 1.38±0.74 and 0.54±0.50 for the control, icodextrin-treated and canola oil-treated groups, respectively. However, there was no statistically significant difference between the three groups (p = 0.14 and p = 0.069) (Table 2, Figure 1).
Biochemical assessment
The mean hydroxyproline levels were 302.65±147.69, 273.40±118.56 and 151.14±53.30 in the control, icodextrin-treated, and canola oil-treated groups, respectively. The statistical analysis revealed a significant decrease in hydroxyproline levels in the canola oil-treated group (p = 0.034) (Table 2).
DISCUSSION
Postoperative peritoneal adhesions can lead to readmissions and reoperations caused by mechanical small bowel obstruction, which can increase clinical workloads and hospital costs. Many preventive measures have been proposed to overcome this problem, including meticulous surgical techniques, excellent intraoperative hemostasis, avoiding unnecessary handling of the bowel, creating devitalized or ischemic tissue, minimizing the risk of foreign body granulomas (primarily surgical glove powder and excessive suture material), and preventing peritoneal contamination; even certain serosal plication techniques have been proposed to overcome this problem (18).
In addition to these techniques, a wide range of biologically active substances in the form of simple fluids, gels and solids, either combined or alone, have been investigated both clinically and experimentally to reduce or prevent abdominal adhesions (19). These novel approaches aim to prevent adhesion formation by physically separating the surgically manipulated areas via irrigation and instillation or by covering the serosal surfaces with liquid agents or barriers in the form of films, sprays or gels. Carboxymethyl cellulose + hyaluronic acid is the most examined material (20)-(26).
Oxidized regenerated cellulose and polytetrafluoroethylene are two other synthetic absorbable barriers that have been shown to effectively reduce the incidence of surgical adhesions (27).
An ideal barrier should be biodegradable, biocompatible and surgically easy to handle and should act locally to avoid side effects. However, only certain materials meet all of these requirements, and no large prospective, randomized double-blind human studies have demonstrated their efficacy. Furthermore, none of these materials have been widely adopted by surgeons, indicating that the materials only decrease adhesion severity, not incidence (28),(29).
The effect of a 4% icodextrin solution, which has received limited approval by the FDA for use in laparoscopic gynecological surgery, was largely investigated using ARIEL registry data. These results indicated that this solution was widely accepted by both gynecological surgeons and patients (30). This clinical evaluation had been conducted in patients who underwent a routine gynecological surgery via either laparoscopy or laparotomy in six European countries. The participating surgeons were asked to use 4% icodextrin solutions for the lavage and instillation of the peritoneal cavity. A questionnaire was used to assess the patients' experiences, and it revealed a high acceptability rate of this method, with low rates of adverse events (7.5% and 13.9% of the patients who underwent laparoscopy and laparotomy, respectively) (31). The same group achieved similar results in general surgery patients but observed a substantial number of adverse effects (16.7% and 30.6% of the patients who underwent laparoscopy and laparotomy, respectively) (32). Nevertheless, in the context of laparoscopic pelvic surgery, the use of this solution is avoided in bowel resections and peritoneal inflammatory conditions, which may require more robust adhesion prevention techniques.
It is believed that other agents and pharmaceutics in the form of gels, sprays or liquids might be rapidly absorbed by the peritoneum. Studies of these compounds, which were performed primarily in animals, demonstrated conflicting results (5-12),(17). Thus, more evidence is needed.
The mechanism of peritoneal healing is similar to wound healing. However, regardless of the size of the peritoneal trauma, healing requires approximately 7-10 days. Peritoneal leukocytes, histiocytes, and tissue-consolidating mature macrophages are components of the monocyte-phagocytic system. These cell types are the most important chronic inflammation-mediating cells with respect to the subsequent response of other immune cells and the cellular mediators to injury. A fibrin matrix is exuded from inflammatory cells, and this matrix is gradually organized into fibrin bands that contain fibroblasts, macrophages and giant cells that bridge the two injured peritoneal surfaces (33). Several animal studies have revealed the cellular response to peritoneal damage, and many therapeutics have been designed to prevent adhesion formation by interacting with the extracellular matrix and cellular mediators. Thus, it appears that the complex relationship between peritoneal healing and adhesion formation will continue to be investigated until the pathogenesis of adhesions is completely understood.
In certain studies, polyunsaturated fatty acids and their derivatives, eicosapentaenoic acid and docosahexaenoic acid, have been observed to promote peritoneal wound healing by activating the inflammatory cascade via peroxisome proliferator activated receptors (PPARs). This activation, which mediates lipid metabolism, fatty acid oxidation, and cytokine production, can induce the anti-adhesogenic effects of ω-3 fatty acids. This effect is mediated by reducing the levels of type 1 collagen, vascular endothelial growth factor, and transforming growth factor β-1 (34). This tremendous modulation of the inflammatory response indicates the potential anti-adhesive effects of PUFAs. However, whether this action is beneficial or detrimental in clinical wound healing is unknown.
PUFAs affect numerous physiological processes that modulate the physical properties of the lipid bilayer composition and the fluidity of the cell membrane. Our study focused on the cellular response to peritoneal damage and revealed a significant decrease in fibrosis in the canola oil-treated group compared to the icodextrin-treated and control groups. Although both icodextrin and canola oil reduced adhesions macroscopically, this alteration did not reach statistical significance. Meanwhile, cellular reactions, such as giant cells, mononuclear and polymorph nuclear leukocyte reactions, did not differ in any of the groups. This result may be explained as the neutral effect of both of the tested anti-adhesion materials on normal wound healing. In contrast, a very prominent histiocytic reaction was observed in the canola oil-treated group (1.12±0.84), whereas no such reaction was observed in the other two groups. This result suggests that tissue macrophages also contributed to the inflammatory process in animals following canola oil treatment.
Soybean oil, which contains linoleic acid (51%), oleic acid (25%), methyl methacrylate, palmitic acid, linolenic acid, and stearic acid, have been experimentally tested for their abilities to decrease the severity of postoperative peritoneal adhesions and have been reported to decrease adhesion formation when applied prior to the peritoneal trauma (6). Vitamin E, when applied just prior to the closing of the incision, was effective in reducing adhesion formation (8). In agreement with this previous study, Durmus et al. demonstrated that vitamin E and selenium, which are believed to be commonly used antioxidants, thoroughly decreased fibrosis and intra-abdominal adhesions by reducing hydrogen peroxides and lipid hydroperoxides to nontoxic elements (35). Canola oil, a good source of vitamin E, may also prevent peritoneal adhesions.
The fact that no dose adjustments were performed in the canola oil-treated group is an important limitation of the present study. The effect of intraperitoneally administering canola oil has, to our knowledge, never been examined. Further investigations are required to determine the most effective dose and form in which canola oil can optimally prevent adhesions.
Given the benefits of canola oil in inducing the histiocytic reaction and lowering hydroxyproline levels, the data that are presented here demonstrate that intraperitoneally administered canola oil decreases collagen synthesis and has no detrimental effect on the wound healing process. Compared with icodextrin, canola oil may be a promising agent in the prophylaxis of adhesion formation.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Menzies D. Postoperative adhesions: their treatment and relevance in clinical practice. Ann R Coll Surg Engl. 1993;75(3):147–53. [PMC free article] [PubMed] [Google Scholar]
- 2.Monk BJ, Berman ML, Montz FJ. Adhesions after extensive gynecologic surgery: clinical significance, etiology, and prevention. Am J Obstet Gynecol. 1994;170(5 Pt 1:1396–403. doi: 10.1016/s0002-9378(94)70170-9. [DOI] [PubMed] [Google Scholar]
- 3.Ellis H, Moran B, Thompson JN, Parker MC, Wilson MS, Menzies D, et al. Adhesion-related hospital readmissions after abdominal and pelvic surgery: a retrospective cohort study. Lancet. 1999;353(9163):1476–80. doi: 10.1016/S0140-6736(98)09337-4. [DOI] [PubMed] [Google Scholar]
- 4.Ray NF, Denton WG, Thamer M, Henderson SC, Perry S. Abdominal adhesiolysis: inpatient care and expenditures in the United States in 1994. J Am Coll Surg. 1998;186(1):1–9. doi: 10.1016/s1072-7515(97)00127-0. [DOI] [PubMed] [Google Scholar]
- 5.Boland GM, Weigel RJ. Formation and prevention of postoperative abdominal adhesions. J Surg Res. 2006;132(1):3–12. doi: 10.1016/j.jss.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Aysan E, Bektas H, Kaygusuz A, Huq GE. A new approach for decreasing postoperative peritoneal adhesions: preventing peritoneal trauma with soybean oil. J Invest Surg. 2009;22(4):275–80. doi: 10.1080/08941930903040148. [DOI] [PubMed] [Google Scholar]
- 7.Aysan E, Bektas H, Ersoz F. A new approach to postoperative peritoneal adhesions: prevention of peritoneal trauma by aloe vera gel. Eur J Obstet Gynecol Reprod Biol. 2010;149(2):195–8. doi: 10.1016/j.ejogrb.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 8.de la Portilla F, Ynfante I, Bejarano D, Conde J, Fernández A, Ortega JM, et al. Prevention of peritoneal adhesions by intraperitoneal administration of vitamin E: an experimental study in rats. Dis Colon Rectum. 2004;47(12):2157–61. doi: 10.1007/s10350-004-0741-6. [DOI] [PubMed] [Google Scholar]
- 9.Yildiz H, Durmus AS, Simsek H, Yaman I. The comparison of methylene blue and vitamin E in prevention of abdominal postoperative adhesion formation in rat uterine horn model. Biochemical and histopathological evaluation. Acta Cir Bras. 2011;26(1):51–7. doi: 10.1590/s0102-86502011000100010. [DOI] [PubMed] [Google Scholar]
- 10.Mahdy T, Mohamed G, Elhawary A. Effect of methylene blue on intra-abdominal adhesion formations in rats. Int J Surg. 2008;6(6):452–5. doi: 10.1016/j.ijsu.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Yetkin G, Uludag M, Citgez B, Karakoc S, Polat N, Kabukcuoglu F. Prevention of peritoneal adhesions by intraperitoneal administration of vitamin E and human amniotic membrane. Int J Surg. 2009;7(6):561–5. doi: 10.1016/j.ijsu.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Washburn S, Jennell JL, Hodges SJ. Halofuginone- and chitosan-coated amnion membranes demonstrate improved abdominal adhesion prevention. ScientificWorldJournal. 2010;10:2362–6. doi: 10.1100/tsw.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FDA. U.S. Adept Adhesion Reduction Solution (4% Icodextrin) P050011. http://www.accessdata.fda.gov/cdrh_docs/pdf5/p050011a.pdf 2006. Accessed at July 23, 2010.
- 14.Omidi H, Tahmasebi Z, Naghdi Badi HA, Torabi H, Miransari M. Fatty acid composition of canola (Brassica napus L.), as affected by agronomical, genotypic and environmental parameters. C R Biol. 2010;333(3):248–54. doi: 10.1016/j.crvi.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Bozcali E, Süzer O, Gürsoy HN, Atukeren P, Gümüstas KM. Effects of erucic acid supplemented feeding on chronic doxorubucin toxicity inrats. Int J Clin Exp Med. 2009;2(4):337–47. [PMC free article] [PubMed] [Google Scholar]
- 16.Blauer KL, Collins RL. The effect of intraperitoneal progesterone on postoperative adhesion formation in rabit. Fertil Steril. 1988;49(1):144–9. [PubMed] [Google Scholar]
- 17.Delaco PA, Stefanetti M, Pressato D, Piana S, Dona M, Pavesio A, et al. A novel hyaluronan-based gel in laparoscopic adhesion prevention: preclinical evaluation in an animal model. Fertil Steril. 1998;69(2):318–23. doi: 10.1016/s0015-0282(98)00496-8. [DOI] [PubMed] [Google Scholar]
- 18.Holmdahl L, Risberg B. Adhesions: prevention and complications in general surgery. Eur J Surg. 1997;163(3):169–74. [PubMed] [Google Scholar]
- 19.Ward BC, Panitch A. Abdominal adhesions: current and novel therapies. J Surg Res. 2011;165(1):91–111. doi: 10.1016/j.jss.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66(6):904–10. [PubMed] [Google Scholar]
- 21. Sheldon HK, Gainsbury ML, Cassidy MR, Chu DI, Stucchi AF, Becker JM.A sprayable hyaluronate/carboxymethylcellulose adhesion barrier exhibits regional adhesion reduction efficacy and does not impair intestinal healing J Gastrointest Surg; http://www.springerlink.com/content/r7q06100w7074588/fulltext.pdf 2011 Accessed at December 2, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Beck DE. The role of Seprafilm bioresorbable membrane in adhesion prevention. Eur J Surg Suppl. 1997;(577):49–55. [PubMed] [Google Scholar]
- 23.Tsapanos VS, Stathopoulou LP, Papathanassopoulou VS, Tzingounis VA. The role of Seprafilm bioresorbable membrane in the prevention and therapy of endometrial synechiae. J Biomed Mater Res. 2002;63(1):10–4. doi: 10.1002/jbm.10040. [DOI] [PubMed] [Google Scholar]
- 24.Khaitan L, Scholz S, Houston HL, Richards WO. Results after laparoscopic lysis of adhesions and placement of seprafilm for intractable abdominal pain. Surg Endosc. 2003;17(3):365–70. doi: 10.1007/s00464-002-8845-3. [DOI] [PubMed] [Google Scholar]
- 25.Kusunoki M, Ikeuchi H, Yanagi H, Noda M, Tonouchi H, Mohri Y, et al. Bioresorbable hyaluronate-carboxymethylcellulose membrane (Seprafilm) in surgery for rectal carcinoma: A prospective randomized clinical trial. Surg Today. 2005;35(11):940–5. doi: 10.1007/s00595-005-3061-0. [DOI] [PubMed] [Google Scholar]
- 26.Salum M, Wexner SD, Nogueras JJ, Weiss E, Koruda M, Behrens K, et al. Does sodium hyaluronate- and carboxymethylcellulose-based bioresorbable membrane (Seprafilm) decrease operative time for loop ileostomy closure. Tech Coloproctol. 2006;10(3):187–90. doi: 10.1007/s10151-006-0278-x. [DOI] [PubMed] [Google Scholar]
- 27.Farquar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Review Syst. doi: 10.1002/14651858.CD000475. Rev 2000 (2) CD 000475. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD000475.pub2/pdf. Accessed at July 23, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Mohri Y, Uchida K, Araki T, Inoue Y, Tonouchi H, Miki C, et al. Hyaluronic acid-carboxycellulose membrane (Seprafilm) reduces early postoperative small bowel obstruction in gastrointestinal surgery. Am Surg. 2005;71(10):861–3. [PubMed] [Google Scholar]
- 29.Vrijland WW, Tseng LN, Eijkman HJ, Hop WC, Jakimowicz JJ, Leguit P, et al. Fewer intraperitoneal adhesions with use of hyaluronic acid-carboxymethylcellulose membrane: a randomized clinical trial. Ann Surg. 2002;235(2):193–9. doi: 10.1097/00000658-200202000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.diZerega GS, Verco SJ, Young P, Kettel M, Kobak W, Martin D, et al. A randomized, controlled pilot study of the safety and efficacy of 4% icodextrin solution in the reduction of adhesions following laparoscopic gynaecological surgery. Hum Reprod. 2002;17(4):1031–8. doi: 10.1093/humrep/17.4.1031. [DOI] [PubMed] [Google Scholar]
- 31.Sutton C, Minelli L, Garcia E, Korell M, Pouly JL, Pados G, et al. Use of icodextrin 4% solution in the reduction of adhesion formation after laparoscopic surgery. Gynecol Surg. 2005;2:287–96. [Google Scholar]
- 32.Menzies D, Pascual MH, Walz MK, Duron JJ, Tonelli F, Crowe A, et al. Use of icodextrin 4% solution in the prevention of adhesion formation following general surgery: from the multicentre ARIEL registry. Ann R Coll Surg Engl. 2006;88(4):375–82. doi: 10.1308/003588406X114730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheong YCC, Laird SM, Li TC, Shelton JB, Ledger WL, Cooke ID. Peritoneal healing and adhesion formation/reformation. Hum Reprod Update. 2001;7(6):556–66. doi: 10.1093/humupd/7.6.556. [DOI] [PubMed] [Google Scholar]
- 34.Victory R, Saed GM, Diamond MP. Antiadhesion effects of docosahexaenoic acid on normal human peritoneal and adhesion fibroblasts. Fertil Steril. 2007;88(6):1657–62. doi: 10.1016/j.fertnstert.2007.01.123. [DOI] [PubMed] [Google Scholar]
- 35.Durmus AS, Yildiz H, Yaman I, Simsek H. Efficacy of vitamin E and selenium for the prevention of intra-abdominal adhesions in rats: uterine horn models. Clinics. 2011;66(7):1247–51. doi: 10.1590/S1807-59322011000700021. [DOI] [PMC free article] [PubMed] [Google Scholar]