Abstract
Airway diseases are highly prevalent worldwide; however, the prevalence of these diseases is underestimated. Although these diseases present several common characteristics, they have different clinical outcomes. The differentiation between asthma, chronic obstructive pulmonary disease and bronchiectasis in the early stage of disease is extremely important for the adoption of appropriate therapeutic measures. However, because of the high prevalence of these diseases and the common pathophysiological pathways, some patients with different diseases may present with similar symptoms. The objective of this review is to highlight the similarities and differences between these diseases in terms of the risk factors, pathophysiology, symptoms, diagnosis and treatment.
Keywords: Asthma, Chronic Obstructive Pulmonary Disease, Bronchiectasis
INTRODUCTION
The prevalence of airway diseases has increased in recent decades despite therapeutic advances. Furthermore, the prevalence of these diseases is underestimated according to epidemiological surveys, which further increases the complexity of managing these diseases. In Brazil, acute asthma exacerbations and chronic obstructive pulmonary disease (COPD) are major causes of hospitalization (1),(2). Despite the presentation of similar symptoms, such as dyspnea, coughing, wheezing and expectoration, airway diseases have different underlying pathophysiological processes and must be distinguished to enable the administration of appropriate treatment. With an appropriate clinical history and objective diagnostic testing, the distinction between these diseases can be performed efficiently in most cases. However, many patients who are evaluated for respiratory symptoms are misdiagnosed due to an atypical case presentation, an insufficient etiological investigation or an overlapping of the diseases.
This review aims to present the similarities and differences between airway diseases and suggest a practical approach for the differentiation of the most common respiratory illnesses, i.e., asthma, COPD and bronchiectasis.
Epidemiology
Because of the variability in the definition of COPD in epidemiological studies, an accurate prevalence of this disease is difficult to determine. The prevalence is approximately 14% in smokers, 7% in former smokers and 3% in individuals who never smoked (3). Surveys on the prevalence of asthma suggest a prevalence of approximately 9% in the British population. In Brazil, an epidemiological study of the population in São Paulo revealed a COPD prevalence of 15.8% (4), whereas the prevalence of asthma was estimated to be approximately 10% of the general population (1). Several studies have suggested a similar prevalence of asthma among children and adult populations; however, extensive variability has been found depending on multiple factors that include geographic differences and socioeconomic status (5).
Because of a lack of well-conducted epidemiological studies, an accurate prevalence of bronchiectasis is more difficult to estimate than that of asthma and COPD. There has been a decrease in the incidence of this disease, which has been attributed to the increased use of antibiotics for infection control and immunization strategies in children. Tsang and Tipo (6) reported a hospital admission rate of 16.4 per 100,000 people and a mortality rate of 1 out of 100,000 people in Hong Kong.
The overlap in the terminology that is used to define asthma, chronic bronchitis, emphysema, COPD and bronchiectasis is the greatest cause of confusion in distinguishing these diseases and in accurately determining the prevalence of airway diseases. The prevalence of obstructive diseases in adults can vary by more than 200% in the general population and depends on the definition that is used (a self-reported diagnosis versus a diagnosis based on spirometry findings) (7). Furthermore, the actual prevalence of obstructive diseases is underestimated. When a spirometric evaluation was performed in the general population, approximately 58% of the patients with an obstructive disorder did not report a prior lung disease diagnosis (8).
Individual patients may have different combinations of airway diseases. Studies in an American population demonstrated that more than 15% of the patients with an obstructive disease received more than one diagnosis, and this rate reached 50% in a population older than 50 years of age (8). In Australia (9), this proportion was approximately 25% in individuals who were between 45 and 69 years of age. In Italy (10), approximately 20% of the asthmatic population had symptoms that included a productive cough, which is compatible with a diagnosis of chronic bronchitis. Patients who exhibit the coexistence of two or more obstructive diseases tend to be older and have spirometric data that indicate lower values of forced expiratory volume in the first second (FEV1) (8). Furthermore, the coexistence of asthma and COPD was associated with a higher mortality rate (11).
The high prevalence and morbidity of these diseases translates into a substantial cost to the healthcare system. Drug costs are the main expenses that are associated with the treatment of asthma, whereas COPD and bronchiectasis have a greater economic impact due to high hospitalization rates (12). The main findings from these epidemiological studies are as follows: (1) the prevalence of chronic obstructive pulmonary disease, which is closely correlated with the definition that is used, is an important social and economic problem; (2) the overlap between asthma, COPD and bronchiectasis is associated with an increase in clinical severity and mortality; and (3) approximately half of patients with obstructive findings on spirometry are not properly diagnosed; therefore, screening programs that include a pulmonary function evaluation (with an assessment of both spirometry and peak flow) should be adopted to decrease the proportion of patients without adequate monitoring (13),(14).
Risk factors
Patients with asthma and COPD can usually be distinguished according to the classic risk factors that are associated with each disease. However, certain risk factors may be common in both diseases. Regarding the illnesses that are associated with bronchiectasis, the identification of the risk factors is crucial and is complex because of the wide variety of conditions that predispose a patient to a permanent dilation of the airways (15) (Table 1).
Table 1.
Risk factors for asthma, COPD and bronchiectasis.
| Asthma | COPD | Bronchiectasis | |
| Environmental factors | Allergen exposure | Smoking | Respiratory infections |
| Occupational sensitizers | Occupational exposure | Bronchial obstructions | |
| Respiratory infections | Pollution | Transplantation | |
| Alcoholism | |||
| Low socio-economic condition | |||
| Host factors | Atopy | Alpha-1 antitrypsin deficiency | Alpha-1 antitrypsin deficiency |
| Gender | Low birth weight | Cystic fibrosis | |
| Low birth weight | Family history | Immunodeficiency | |
| Genetic predisposition | Autoimmune disease | ||
| Mucociliary dysfunction | |||
| Yellow nail syndrome | |||
| Congenital diseases (Mounier-Kuhn syndrome, Williams-Campbell syndrome) |
COPD: Chronic obstructive pulmonary disease.
In atopic individuals, the main risk factor for developing asthma is exposure to allergens. Consequently, many patients with asthma have high serum levels of IgE and eosinophils (16),(17). For individuals with non-atopic asthma, several risk factors have been found, including smoking, occupational exposure, advanced age, an unfavorable socioeconomic condition and housing in an urban center (18).
Asthma can be induced by either animal or plant proteins and by organic and inorganic chemical agents (19),(20). In addition, the development of asthma is correlated with the Western lifestyle, which is characterized by a high hygiene rate. Increased hygiene reduces exposure to allergens and decreases natural desensitization (21). The exposure to external agents can result in the development of COPD, which is mainly associated with occupational activities, such as coal and gold mining (22), cadmium mining (23) and exposure to smoke from burning wood (24),(25). Regarding bronchiectasis, exposure to fungi is a cause of exacerbated allergic responses, such as in allergic bronchopulmonary aspergillosis (15).
Smoking is the main etiological factor for the development of COPD. While it is known that quitting smoking is the only factor that can slow the progression of this disease, there is no evidence that there exists a reversion for the pulmonary impairment that has already began. Approximately 90% of COPD cases are related to smoking, whereas other less common risk factors include occupational exposure and biomass burning (26),(27),(28). The historical finding of a lower prevalence of COPD in the female population is associated with a lower proportion of smokers in this group. However, an increase in the number of women who smoke in recent decades has increased the prevalence of COPD in the female population and the mortality that is associated with this pathology (29)-(31).
In Brazil, data indicate a decline in the smoking prevalence (32); however, several factors are extremely relevant. There are an insufficient number of effective public policies that discourage smoking among young people who are usually influenced by alcohol consumption, media advertising and paternal smoking (33). In addition, a large portion of pneumologists in Brazil need to be trained because these physicians cannot effectively treat smoking (34). These steps are crucial in reducing the prevalence of COPD.
Regarding genetic factors, several conditions are classically associated with the development of COPD. A deficiency of alpha-1 antitrypsin decreases the defense of the lungs against inhaled noxious agents, thus increasing the development of emphysema. Patients with this deficiency account for approximately 1-2% of COPD cases (35),(36). Other genetic influences on the development of COPD include a polymorphism in the promoter region of inflammatory mediators, such as tumor necrosis factor-alpha (TNF-alpha) (37), and polymorphic variants in the hydrolase-encoding genes (38).
Several genetic diseases are associated with the development of bronchiectasis. Cystic fibrosis is characterized by a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene and is the leading cause of genetic disease-related death among Caucasians, which is typically due to respiratory failure. In addition, immotile cilia syndrome (primary ciliary dyskinesia) and genetic anomalies that are associated with a humoral or cellular immunodeficiency are common causes of bronchiectasis (39).
Several studies have analyzed the development of asthma and specific genetic alterations but have not found an association. Because of the multifactorial presentation of this disease, the existence of a single genetic site associated with the development of this disease is questionable. However, evidence suggests that chromosomal regions may modulate the degree of disease severity, such as the relationship between chromosome 2q and the levels of IgE and bronchial hyperresponsiveness (40).
The influence of gender on the development of asthma varies with age. Childhood asthma is more common among boys, whereas women are more commonly diagnosed with asthma in adulthood (41),(42). COPD is more common among men than women, which is related to the gender difference in smoking intensities (43). However, women develop more severe airflow obstruction than men after an adjustment for the tobacco intake intensity (44),(45).
Additionally, other factors influence obstructive respiratory diseases. The presence of gastroesophageal reflux is correlated with increased inflammation in the airways of patients with bronchiectasis and is associated with increased asthma severity. However, because of a lack of well-conducted longitudinal studies, the relationship between gastroesophageal reflux disease and bronchial hyperresponsiveness may not be causal and may not be associated with severity (46). In addition, a low birth weight may predispose individuals to the development of asthma (47) or COPD (48). The proposed mechanism for this association is based on the normal respiratory functional decline with age that occurs from a lower peak in these individuals. Additionally, a history of viral or bacterial infections in childhood is correlated with the development of asthma, and these infections are well-established causes of bronchiectasis in adulthood (49),(50). Passive smoking and a deficiency of certain dietary elements, such as polyunsaturated fatty acids, are associated with the development of chronic airway inflammation in adulthood (51).
Physiopathology
Asthma, COPD and bronchiectasis are diseases that cause chronic inflammation of the airways but have distinct characteristics. In asthma, eosinophils, mast cells and CD4 T lymphocytes represent the predominant cell types in the inflammatory process. In contrast, COPD and bronchiectasis demonstrate a greater number of neutrophils, macrophages and CD8 T lymphocytes (52)-(54).
Asthmatic patients have airway obstructions that are predominantly characterized by bronchoconstriction through the activation of the smooth muscle and basal membrane thickening. These alterations are positively correlated with the frequency of asthma attacks and bronchial hyperresponsiveness (55),(56).
COPD patients exhibit a reduced airway caliber, which is associated with cell damage that is induced by external toxic agents, especially cigarette smoke, via reactive oxygen species (57),(58). The presence of goblet cells (mucous metaplasia) in the small airways and mucous hypersecretions results from the process of airway narrowing (59),(60). Despite the prevalence of inflammation in COPD, which occurs due to the presence of neutrophils and macrophages, several studies have demonstrated the presence of eosinophilic inflammation both in stable patients and in patients with acute exacerbations of the disease (61). This finding confirms the potential anti-inflammatory effect of inhaled corticosteroids in the treatment of COPD. Another interesting finding for this disease is the correlation between inflammation intensity and COPD severity. In the final stages of the disease, an intense inflammatory process occurs, which suggests that, even in these scenarios, treatment with anti-inflammatory drugs, such as inhaled corticosteroids, may be effective (62).
Bronchiectasis develops with recurrent damage to the airways, which generally occurs in individuals with mucociliary clearance that is altered by genetic susceptibility, thus leading to inflammation and destruction of the muscular and elastic components of the bronchial walls (63). Respiratory infections are the leading causes of bronchiectasis; however, other pro-inflammatory attacks can trigger or accelerate the process, such as a toxin inhalation, environmental exposure, smoking, aspiration of gastric contents or changes in immune responses (6).
Abnormally dilated airways are susceptible to bacterial colonization, which leads to a constant presence of inflammation that is mainly mediated by neutrophils (64). There is progressive impairment of ciliary function with a worsening of airway mucociliary clearance, which further facilitates the presence of bacterial colonization and the accumulation of thick mucus (65),(66). In addition, the perpetuation of inflammation leads to further damage to the mucosal integrity of the airways, thus promoting continuous bacterial invasion and permanence in the mucosa. This infectious and inflammatory cyclic process causes progressive damage to the bronchial wall with associated clinical deterioration. Increased arterial bronchial proliferation and arteriovenous malformations can occur as a result of the inflammatory process and bronchial wall alterations. This vicious cycle can produce significant bacterial proliferation and inflammation with increased suppuration and clinical worsening.
However, understanding the inflammatory patterns of each disease is important for distinguishing between airway diseases. In a study of 27 COPD patients and 19 asthma patients with similar degrees of pulmonary obstruction, several parameters were evaluated. Several functional (residual volume and diffusion capacity) and tomographical (emphysema score) parameters could differentiate between the individuals with COPD and those with asthma. However, the inflammatory and pathological features of basal membrane thickening, eosinophilia and the CD4/CD8 relationship in the bronchoalveolar lavages were the best predictors of a history of asthma (67).
Immunological differentiation may have an important prognostic role in patients. Sputum eosinophilia is associated with difficult-to-control asthma (68), and the normalization of this condition after treatment was correlated with a decrease in the number of exacerbations and hospitalizations. Neutrophilic inflammation in asthma is less sensitive to corticosteroid treatment and is associated with rapid functional loss (69).
Despite the distinct pathophysiological mechanisms of airway diseases, these diseases share several common features. Specific therapies, especially anti-inflammatory medications and bronchodilators, may be the most beneficial treatments for these diseases because these drugs control symptoms and improve the quality of life of the patients.
Diagnosis
Characterizing the physiological and phenotypic differences between patients with obstructive diseases is important for obtaining a greater understanding of the evolution of these diseases and the therapeutic implications. Most patients can be distinguished by a detailed clinical history and simple functional and imaging tests; however, many patients have atypical clinical profiles due to the heterogeneity of the airway diseases, which can lead to a misdiagnosis. This factor is particularly important for the elderly population in which the coexistence of asthma, COPD and bronchiectasis is common.
Symptoms
Asthmatic patients who exhibit bronchoconstriction are characterized by wheezing, breathlessness, chest tightness and coughing. The characteristic of the reversible inflammatory process in asthma and a good response to therapeutic measures characterize the evolution of the disease, which is marked by intermittent exacerbations (70)-(72). However, the lack of these symptoms can define a subgroup of patients who are considered hypoperceivers and who evolve with a worse prognosis for disease control (73).
The main clinical manifestation of patients with bronchiectasis is a chronic cough with sputum production, although patients may only have a dry cough. Patients often have dyspnea on exertion (75%), wheezing (75%) and pleuritic chest pain (50%). Additionally, patients may have systemic symptoms, such as fatigue or weight loss, and rhinosinusitis. A physical examination may identify crackles (70%), snoring (44%) and wheezing (34%). Clubbing appears in approximately 3% of the cases. The clinical evolution consists of a progressive functional loss and is marked by recurrent infectious exacerbations with the need for frequent antibiotics (15).
Patients with COPD have a cough that is frequently associated with chronic sputum production (74). Characteristically, these individuals present with dyspnea and effort limitations due to the fixed airflow obstruction (75)-(77). More advanced stages of the disease result in a worse quality of life for these patients. Respiratory exacerbations are associated with an increased underlying inflammatory process that requires appropriate therapy with antibiotics and systemic corticosteroids. These exacerbations are characterized by a worsening of the basal symptoms of the patient, such as cough, expectoration and dyspnea. According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD), an exacerbation is defined as “an event in the natural course of the disease characterized by a change in dyspnea, cough and/or basal expectoration of the patient who goes beyond the normal daily variation, and that can cause a change in regular medication of the patient” (78).
Lung function
In asthma, the typical finding of airflow obstruction that is characterized by a decrease of VEF1/FVC (forced vital capacity) with a disorder reversal after the administration of a bronchodilator is the mainstay in the diagnostic confirmation of the disease (79). However, many patients may present with a reversal after the use of a bronchodilator without exhibiting normalization in pulmonary function tests, thus indicating signs of bronchial remodeling. In addition, clinically stable patients may present with normal spirometry but a positive bronchial provocation test (1). Other functional variables can be used for the diagnosis of obstructive lung disease, such as the forced expiratory flow at 50% of the FVC FEF50%/0.5FVC (80) ratio. However, the use of the FEV1/FVC ratio remains the most widely accepted parameter for diagnostic confirmation.
In COPD, a post-bronchodilator FEV1<80% that is associated with a FEV1/FVC ratio <70% confirms the obstructive disorder, which is characterized by a lack of complete reversibility of airflow. However, a significant response to the bronchodilator does not exclude a COPD diagnosis (78).
More accurate functional tests can differentiate between these two diseases through measures that assess lung hyperinflation and the diffusion capacity of the lungs for carbon monoxide (DLCO). Hyperinflation, which is confirmed by plethysmography, is more commonly found in COPD that is characterized by a greater residual volume than is found in asthma. Another characteristic of COPD is a decrease in the DLCO (70).
Early in the course of the disease, patients with bronchiectasis usually present with lung function tests that are characteristic of an obstructive disorder, thus confirming the inflammatory nature of this disease and the initial involvement of the small airways. However, with the progression of the disease, a mixed functional disorder or a restrictive disorder can be found due to the progressive destruction of the pulmonary parenchyma, which is characterized by recurrent infectious exacerbations and massive destruction of the small airways (81).
Imaging tests
Chest radiography is not sufficiently sensitive for the diagnosis of airway diseases, and this test is recommended for the differential diagnosis of a patient with respiratory symptoms. In more severe cases of patients with bronchiectasis, the dilation of large airways can be visualized as a thickening of the peribronchovascular interstitium using this method. A universal radiographic finding in obstructive diseases is lung hyperinflation, which is characterized as follows: (1) an increase in the lung volume, (2) an increase in the intercostal spaces, (3) a rectification of the diaphragmatic domes, (4) an accentuation of the retrosternal space and (5) the presence of air below the inferior border of the heart (82).
A thoracic CT scan is a more sensitive test than chest radiography and is useful in the management of airway diseases. This test is considered the gold standard in the diagnosis of bronchiectasis. Common findings in obstructive diseases include bronchial wall thickening, centrilobular nodules and mosaic attenuation by air trapping (82).
The following tomographic criteria are used for the diagnosis of CT bronchiectasis: (1) a bronchial internal diameter that is larger than 1.5 times the diameter of the adjacent pulmonary artery (signet ring sign), (2) an absence of a gradual decrease in the bronchial diameter from the central regions to the periphery and (3) bronchial visualization in the periphery 1-2 cm from the parietal pleura (6). According to CT scan findings, we can classify bronchiectasis as cylindrical (bronchial dilation), varicose (with focal constrictions along the airways) or cystic (saccular dilations at the end of a bronchus). In addition, we can classify the bronchiectasis as localized (i.e., confined to one lobe) or generalized (83).
In asthma patients, bronchial wall thickening and air trapping are commonly found; however, patients with mild asthma may have completely normal thoracic tomography findings. However, current analyses with quantitative techniques and high-resolution scans can distinguish between normal and mildly controlled asthmatic individuals based on the degree of air trapping and bronchial thickening. Moreover, severe asthma is associated with a greater degree of bronchial thickening in these individuals. Another interesting finding of these studies is that a greater degree of air trapping was associated with an increased risk of exacerbation. Because CT scan is noninvasive, this technique may be used to monitor patients with severe asthma, especially for the evaluation of bronchial remodeling (84),(85).
In patients with COPD, the most important tomographic finding is the presence of centrilobular emphysema in the superior lung fields (smoking-related) or diffuse panlobular emphysema (alpha-1 antitrypsin deficiency). The intensity of emphysema, which is characterized by the amount of area with low attenuation, and bronchial thickening correlate with the degree of airflow obstruction that is measured in functional tests (86)-(88).
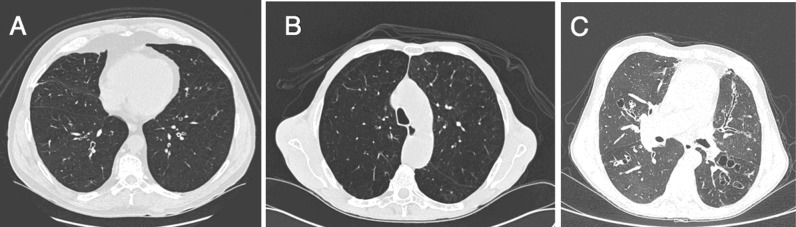
Therefore, thoracic tomography is an important tool that can be used in the differential diagnosis of airway diseases (Figure 1). However, many of these CT findings are common among several obstructive diseases, and more advanced stages of asthma and COPD may lead to the development of bronchiectasis.
Figure 1.
Tomographic cross-sections. A) Asthmatic patient with diffuse bronchial wall thickening. B) COPD patient with extensive areas of centrilobular emphysema predominantly in the superior lung fields. C) Patient with cystic and varicose bronchiectasis characterized by dilation and thickening of airways.
The diagnosis of an airway disease in patients should begin with a detailed anamnesis, and complementary exams should be performed as appropriate. No single test can completely differentiate between these diseases. The coexistence of more than one disease in the same patient should always be considered when a patient presents with an unfavorable clinical evolution or when their laboratory tests are not consistent with the initial clinical hypothesis (Table 2).
Table 2.
Important clinical characteristics in the differentiation of patients with asthma, COPD and bronchiectasis.
| Clinical characteristic | Asthma | COPD | Bronchiectasis | Potential overlap |
| Risk factors | Family history Allergies | Smoking | Repeated infections Immunodeficiency | Asthmatics Smokers have an increased risk of developing COPD |
| Age | Children and young people | Advanced age | Variable | Asthma and bronchiectasis are misdiagnosed in the elderly and are commonly mistaken for COPD |
| Symptoms | Wheezing Outbreaks of dyspnea | Chronic dyspnea Productive cough | Productive cough | Patients with bronchiectasis are diagnosed late because they are first treated for COPD due to productive cough symptoms |
| Spirometry | Reversibility | Absence of reversibility | Absence of reversibility May present restrictive pattern | Asthmatic patients may lose reversibility over time |
| Computerized tomography | Bronchial thickening | Central lobular emphysema | Bronchial dilations | Bronchial thickening can occur in patients with COPD and bronchiectasis, and bronchiectasis may appear in asthmatics and individuals with COPD |
COPD: Chronic obstructive pulmonary disease.
Treatment
Asthma treatment guidelines aim for the appropriate control of symptoms through a strategy of phased measures that focus on the severity of the disease and the daily complaints of the patient. Once this goal has been achieved, the treatment should be maintained at the lowest possible dosage to reduce side effects and the associated costs (1),(5). In addition, the guidelines for the treatment of COPD use a phased strategy according to the severity of the disease. However, the guidelines emphasize the prevention of disease progression. Moreover, once the treatment goal has been achieved, a reduction in the medication dosage is uncommon (2),(78),(89). Regarding bronchiectasis, there is a lack of literature on the guidelines for the treatment of this pathology. British guidelines were recently published (90); however, there are few data on the therapeutic recommendations for bronchiectasis due to a lack of well-conducted randomized clinical trials. Many measures are still being extrapolated from studies of cystic fibrosis, but physicians must be aware of the differences between the various diseases that may be similar to bronchiectasis (91).
Much of the therapeutic arsenal is common between obstructive diseases, especially bronchodilators (beta2-agonists and anticholinergics) and inhaled corticosteroids. However, the treatment goal for each disease may vary. COPD therapy is directed primarily to the relief of symptoms and the prevention of disease progression. In bronchiectasis, the primary goal of treatment is to prevent disease progression and improve the quality of life and symptoms. In asthma, the primary goal of treatment is to control the underlying inflammatory process with the consequent control of symptoms.
Beta2-agonists
Bronchodilators with a direct action on beta-adrenergic receptors can be classified as short- or long-term depending on the half-life.
Short-acting beta2-agonists (SABAs) are the first-line treatment for COPD; however, the use of SABAs as a rescue medication in asthma is appropriate. In patients with bronchiectasis, SABAs are generally used for symptom relief despite the lack of evidence for the use of these drugs.
In contrast, long-acting beta2-agonists (LABAs) are used in combination with anti-inflammatory medications in asthma. LABAs may be used alone in patients with COPD. The isolated use of a beta2-agonist in asthma is contraindicated and is associated with a poor prognosis (92).
These drugs act by relaxing the bronchial smooth muscle, increasing the mucociliary clearance, decreasing the vascular permeability and possibly reducing inflammatory mediators (5).
In COPD, the use of LABAs is associated with functional improvement (increased FEV1), symptom control and improved quality of life (93),(94). In asthmatic patients, the combination therapy of a LABA with inhaled corticosteroids (ICs) is more efficient in controlling symptoms than an isolated increase in the IC dosage (95). For patients with bronchiectasis, the combination of a LABA with a conventional IC improved the symptom scores and quality of life (96).
Anticholinergics
The release of acetylcholine by vagal stimulation triggers a bronchoconstrictor response and an increased production of pulmonary secretions. The use of short-acting anticholinergic drugs (ipratropium) in patients with COPD yielded a dose-dependent functional improvement with better responses than those that were achieved using a SABA. For asthma, ipratropium is usually only used for the management of severe asthma attacks, and there is no evidence of benefits in patients with bronchiectasis (97).
Long-acting anticholinergics (LAMAs) have a prolonged half-life (36 hours) with an action peak of approximately 1-2 hours (98). Tiotropium is the most studied pharmacological agent, and COPD studies have indicated satisfactory results, including a reduction in the number of exacerbations, an improvement in quality of life and an increase in the FEV1 (99). There is no evidence that supports the superiority of LAMAs over LABAs; however, the combination of these drugs may be used with an additive effect in patients with accentuated lung function or significant functional limitations (100). Recently, the use of tiotropium in asthma has been studied as an alternative in patients with contraindications to the use or in combination with LABAs (101). Similar to ipratropium, the use of tiotropium for the treatment of bronchiectasis has not been adequately studied.
Corticosteroids
The underlying inflammatory process in obstructive diseases has always been the focus of therapeutic interventions that aim to reduce disease progression, improve lung function and reduce symptoms and exacerbations. In asthma, inhaled corticosteroids are the mainstay of disease treatment and are considered the first-line treatment in patients with persistent asthma. ICs can reduce the number of exacerbations, improve lung function and quality of life, control respiratory symptoms and decrease the bronchial hyperresponsiveness (102)-(104). Current guidelines emphasize the use of an IC in the initial therapy of patients with asthma symptoms. The combination of an IC with a LABA is recommended as the next step in therapy despite the potential need to increase the IC dosage (1),(5). However, the use of measures that increase patient adherence to treatment and the adequate use of inhalers are fundamental to achieving good clinical control of symptoms (105)-(108).
The use of an IC to treat COPD was effective in reducing airway inflammation, although the IC did not affect the rate of functional decline. There are conflicting data on the ability of an IC to improve lung function, whereas there are concrete data that associate the use of an IC with a significant reduction in the exacerbation rate of COPD and improvements in the quality of life of COPD patients (109). The discontinuation of treatment results in a poor quality of life and a faster exacerbation recurrence (110). Current guidelines recommend the use of an IC in patients with advanced pulmonary disease that is characterized by a severe obstructive disorder or a large number of exacerbations (78).
In patients with bronchiectasis, the use of an IC was effective in reducing the inflammatory markers and sputum volume. The amount of daily sputum in patients with bronchiectasis is an important severity marker that correlates with the number of lung exacerbations. However, studies have not demonstrated a significant impact of ICs on the lung function and exacerbation rate in individuals with bronchiectasis (111).
Other pharmacological agents
Xanthines, such as theophylline, have moderate bronchodilator effects, immunomodulatory properties and anti-inflammatory effects, which increase sensitivity to corticoids in the nucleus of inflammatory cells through the histone deacetylase pathway. However, the clinical effects of xanthines, which are associated with a reduced therapeutic range and an increased risk of severe side effects, have reduced the clinical applicability of these drugs. The use of xanthines is increasingly reserved for severe cases, especially in patients with COPD (78).
Anti-leukotrienes, such as montelukasts, can reduce eosinophilic inflammation through inhibition of the lipoxygenase pathway. The bronchodilator effect of anti-leukotrienes is discrete, and the use of these drugs is reserved for asthmatic patients; however, there is no concrete clinical applicability of these drugs in COPD (5).
Other nonpharmacological therapies
Nonpharmacological measures are important in the management of patients with an obstructive disease.
Smoking cessation in patients with COPD or who are at risk for the development of COPD (such as individuals with asthma) must be encouraged. Although lung damage can continue to progress, smoking cessation was effective in reducing the rate of lung function decline (112).
Viral infections are a major cause of exacerbations in patients with asthma, COPD and bronchiectasis and are associated with a higher mortality rate. Vaccination against influenza is demonstrably linked to a reduction in severe exacerbations and mortality. Therefore, all patients with an obstructive disease should be directed to receive an annual flu shot. Conversely, the efficacy of the pneumococcal vaccine is not yet fully understood. Patients with a respiratory disease are at an increased risk of hospitalization for pneumonia, especially the elderly; therefore, the use of this vaccine should be considered (5),(78).
The use of bronchial thermoplasty has been considered an option in patients with severe asthma. Studies in individuals with severe persistent asthma demonstrated a reduction in the exacerbation rate and an improvement in symptom control. The finding of hypertrophied smooth bronchial muscles in asthma patients justifies the use of this approach. The use of a probe by bronchoscopy that releases heat into the airway and leads to the destruction and/or atrophy of bronchial smooth muscle supports the biological plausibility for the use of this technique (113),(114).
Pulmonary rehabilitation and an increase in physical activity interventions are useful for the improvement of respiratory symptoms and fitness. These measures are effective in the treatment of COPD patients (115). In addition, data in the literature suggest that asthma and bronchiectasis patients may benefit from these measures (116).
The prevalence of obstructive diseases continues to increase worldwide, with a considerable social and economic impact. Understanding the pathophysiological processes that underlie the various diseases that cause airflow limitations is essential for differentiating between these diseases. Therefore, specific diagnostic methods can be used, and adequate therapeutic interventions can be applied. However, the possibility that more than one condition coexists in the same patient should not be underestimated, especially due to the high prevalence of these diseases in the elderly population.
ACNOWLEDGMENTS
I would like to thank my colleagues Maria Cecília Nieves Teixeira Maiorano and Daniel Antunes Pereira for their contribution in reviewing this work.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Diretrizes da Sociedade Brasileira de Pneumologia e Tisiologia para o Manejo da Asma – 2012. J Bras Pneumol. 2012;38(1):1–58. [Google Scholar]
- 2.II Consenso Brasileiro sobre Doença Pulmonar Obstrutiva Crônica – DPOC. J Bras Pneumol. 2004;30(5):1–52. [Google Scholar]
- 3.Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000;160:1683–9. doi: 10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- 4.Menezes AM, Jardim JR, Pérez-Padilla R, Camelier A, Rosa F, Nascimento O, et al. Prevalence of chronic obstructive pulmonary disease and associated factors: the PLATINO Study in São Paulo, Brazil. Cad Saude Publica. 2005;21(5):1565–73. doi: 10.1590/s0102-311x2005000500030. [DOI] [PubMed] [Google Scholar]
- 5.National Heart, Lung, and Blood Institute. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention National Heart, Lung, and Blood Institute; updated 2010.
- 6.Tsang KW, Tipoe GL. Bronchiectasis: not an orphan disease in the East. Int J Tuberc Lung Dis. 2004;8(6):691–702. [PubMed] [Google Scholar]
- 7.Celli BR, Halbert RJ, Isonaka S, Schaul B. Population impact of different definitions of airway obstruction. Eur Respir J. 2003;22(2):268–73. doi: 10.1183/09031936.03.00075102. [DOI] [PubMed] [Google Scholar]
- 8.Soriano JB, Davis KJ, Coleman B, Visick G, Mannino D, Pride NB. The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United Kingdom. Chest. 2003;124(2):474–81. doi: 10.1378/chest.124.2.474. [DOI] [PubMed] [Google Scholar]
- 9.Abramson M, Matheson M, Wharton C, Sim M, Walters EH. Prevalence of respiratory symptoms related to chronic obstructive pulmonary disease and asthma among middle aged and older adults. Respirology. 2002;7(4):325–31. doi: 10.1046/j.1440-1843.2002.00408.x. [DOI] [PubMed] [Google Scholar]
- 10.Cerveri I, Accordini S, Corsico A, Zoia MC, Carrozzi L, CazzolettiL, et al. Chronic cough and phlegm in young adults. Eur Respir J. 2003;22(3):413–7. doi: 10.1183/09031936.03.00121103. [DOI] [PubMed] [Google Scholar]
- 11.Meyer PA, Mannino DM, Redd SC, Olson DR. Characteristics of adults dying with COPD. Chest. 2002;122(6):2003–8. doi: 10.1378/chest.122.6.2003. [DOI] [PubMed] [Google Scholar]
- 12.Andersson F, Borg S, Jansson SA, Jonsson AC, Ericsson A, Prütz C. The costs of exacerbations in chronic obstructive pulmonary disease (COPD) Respir Med. 2002;96(9):700–8. doi: 10.1053/rmed.2002.1334. [DOI] [PubMed] [Google Scholar]
- 13.Buffels J, Degryse J, Heyrman J, Decramer M. Office spirometry significantly improves early detection of COPD in general practice: the DIDASCO Study. Chest. 2004;125(4):1394–9. doi: 10.1378/chest.125.4.1394. [DOI] [PubMed] [Google Scholar]
- 14.Jackson H, Hubbard R. Detecting chronic obstructive pulmonary disease using peak flow rate: cross sectional survey. BMJ. 2003;327(7416):653–4. doi: 10.1136/bmj.327.7416.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barker AF. Bronchiectasis. N Engl J Med. 2002;346(18):1383–93. doi: 10.1056/NEJMra012519. [DOI] [PubMed] [Google Scholar]
- 16.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320(5):271–7. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 17.Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med. 1991;325(15):1067–71. doi: 10.1056/NEJM199110103251504. [DOI] [PubMed] [Google Scholar]
- 18.Court CS, Cook DG, Strachan DP. Comparative epidemiology of atopic and non-atopic wheeze and diagnosed asthma in a national sample of English adults. Thorax. 2002;57(11):951–7. doi: 10.1136/thorax.57.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Association Asthme & Allergies. Occupational asthma dicitionnaire des allerge'nes. Palmare's 2002 des Hôpitaux. Available from http://www.asmanet.com. [Google Scholar]
- 20.Bezerra GF, Soares MD, Costa MR, Viana GM, Sousa MD. Environmental assessment of an asthma education program: Relationship between airborne fungi and IgE levels in children and adults. J Bras Pneumol. 2011;37(2):281–2. doi: 10.1590/s1806-37132011000200020. [DOI] [PubMed] [Google Scholar]
- 21.Peden DB. Influences on the development of allergy and asthma. Toxicology. 2002;181-182:323–8. doi: 10.1016/s0300-483x(02)00301-3. [DOI] [PubMed] [Google Scholar]
- 22.Oxman AD, Muir DC, Shannon HS, Stock SR, Hnizdo E, Lange HJ. Occupational dust exposure and chronic obstructive pulmonary disease. A systematic overview of the evidence. Am Rev Respir Dis. 1993;148(1):38–48. doi: 10.1164/ajrccm/148.1.38. [DOI] [PubMed] [Google Scholar]
- 23.Davison AG, Fayers PM, Taylor AJ, Venables KM, Darbyshire J, Pickering CA, et al. Cadmium fume inhalation and emphysema. 8587:663–7. doi: 10.1016/s0140-6736(88)91474-2. 1. [DOI] [PubMed] [Google Scholar]
- 24.Dennis RJ, Maldonado D, Norman S, Baena E, Martinez G. Woodsmoke exposure and risk for obstructive airways disease among women. Chest. 1996;109(1):115–9. doi: 10.1378/chest.109.1.115. [DOI] [PubMed] [Google Scholar]
- 25.Desalu OO, Adekoya AO, Ampitan BA. Increased risk of respiratory symptoms and chronic bronchitis in women using biomass fuels in Nigeria. J Bras Pneumol. 2010;36(4):441–6. doi: 10.1590/s1806-37132010000400008. [DOI] [PubMed] [Google Scholar]
- 26.American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) Am J Respir Crit Care Med. 1995;152(5Pt2):S77–S121. [PubMed] [Google Scholar]
- 27.Chatkin G, Chatkin JM, Aued G, Petersen GO, Jeremias ET, Thiesen FV. Evaluation of the exhaled carbon monoxide levels in smokers with COPD. J Bras Pneumol. 2010;36(3):332–8. doi: 10.1590/s1806-37132010000300011. [DOI] [PubMed] [Google Scholar]
- 28.Rondon EN, Silva RM, Botelho C. Respiratory symptoms as health status indicators in workers at ceramics manufacturing facilities. J Bras Pneumol. 2011;37(1):36–45. doi: 10.1590/s1806-37132011000100007. [DOI] [PubMed] [Google Scholar]
- 29.Chapman KR, Tashkin DP, Pye DJ. Gender bias in the diagnosis of COPD. Chest. 2001;119:1691–5. doi: 10.1378/chest.119.6.1691. [DOI] [PubMed] [Google Scholar]
- 30.Lombardi EM, Prado GF, Santos UP, Fernandes FL. Women and smoking: Risks, impacts, and challenges. J Bras Pneumol. 2011;37(1):118–28. doi: 10.1590/s1806-37132011000100017. [DOI] [PubMed] [Google Scholar]
- 31.Castro MR, Matsuo T, Nunes SO. Clinical characteristics and quality of life of smokers at a referral center for smoking cessation. J Bras Pneumol. 2010;36(1):67–74. doi: 10.1590/s1806-37132010000100012. [DOI] [PubMed] [Google Scholar]
- 32.Malta DC, Moura EC, Silva SA, Oliveira PP, Silva VL. Prevalence of smoking among adults residing in the Federal District of Brasília and in the state capitals of Brazil, 2008. J Bras Pneumol. 2010;36(1):75–83. doi: 10.1590/s1806-37132010000100013. [DOI] [PubMed] [Google Scholar]
- 33.Neto AS, Andrade TM, Napoli C, Abdon LC, Garcia MR, Bastos FI. Determinants of smoking experimentation and initiation among adolescent students in the city of Salvador, Brazil. J Bras Pneumol. 2010;36(6):674–82. doi: 10.1590/s1806-37132010000600003. [DOI] [PubMed] [Google Scholar]
- 34.Viegas CA, Valentim AG, Amoras AP, Nascimento EJ. Attitudes of Brazilian pulmonologists toward nicotine dependence: a national survey. J Bras Pneumol. 2010;36(2):239–42. doi: 10.1590/s1806-37132010000200013. [DOI] [PubMed] [Google Scholar]
- 35.Larsson C. Natural history and life expectancy in severe alpha1- antitrypsin deficiency, Pi Z. Acta Med Scand. 1978;204:345–51. doi: 10.1111/j.0954-6820.1978.tb08452.x. [DOI] [PubMed] [Google Scholar]
- 36.Barnes PJ. Genetics and pulmonary medicine. 9.Molecular genetics of chronic obstructive pulmonary disease. Thorax. 1999;54(3):245–52. doi: 10.1136/thx.54.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang SL, Su CH, Chang SC. Tumor necrosis factor-alpha gene polymorphism in chronic bronchitis. Am J Respir Crit Care Med. 1997;156(5):1436–9. doi: 10.1164/ajrccm.156.5.9609138. [DOI] [PubMed] [Google Scholar]
- 38.Smith CA, Harrison DJ. Association between polymorphism in gene for microsomal epoxide hydrolase and susceptibility to emphysema. Lancet. 1997;350(9078):630–3. doi: 10.1016/S0140-6736(96)08061-0. [DOI] [PubMed] [Google Scholar]
- 39.Pasteur MC, Helliwell SM, Houghton SJ, Webb SC, Foweraker JE, Coulden RA, et al. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med. 2000;162(4 Pt1):1277–84. doi: 10.1164/ajrccm.162.4.9906120. [DOI] [PubMed] [Google Scholar]
- 40.Sherrill DL, Lebowitz MD, Halonen M, Barbee RA, Burrows B. Longitudinal evaluation of the association between pulmonary function and total serum IgE. Am J respir Crit Care Med. 1995;152(1):98–102. doi: 10.1164/ajrccm.152.1.7599870. [DOI] [PubMed] [Google Scholar]
- 41.Yunginger JW, Reed CE, O'Connell EJ, Melton LJ, III, O'Fallon WM, Silverstein MD. A community-based study of the epidemiology of asthma. Incidence rates, 1964–1983. Am Rev Respir Dis. 1992;146(4):888–94. doi: 10.1164/ajrccm/146.4.888. [DOI] [PubMed] [Google Scholar]
- 42.Bjornson CL, Mitchell I. Gender differences in asthma in childhood and adolescence. J Gend Specif Med. 2000;3(8):57–61. [PubMed] [Google Scholar]
- 43.Feenstra TL, van Genugten ML, Hoogenveen RT, Wouters EF, Rutten-van Molken MP. The impact of aging and smoking on the future burden of chronic obstructive pulmonary disease: a model analysis in the Netherlands. Am J Respir Crit Care Med. 2001;164(4):590–6. doi: 10.1164/ajrccm.164.4.2003167. [DOI] [PubMed] [Google Scholar]
- 44.Silverman EK, Weiss ST, Drazen JM, Chapman HA, Carey V, Campbell EJ. Gender-related differences in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162(6):2152–8. doi: 10.1164/ajrccm.162.6.2003112. [DOI] [PubMed] [Google Scholar]
- 45.Ferrari R, Tanni SE, Lucheta PA, Faganello MM, Amaral RA, Godoy I. Gender differences in predictors of health status in patients with COPD. J Bras Pneumol. 2010;36(1):37–43. doi: 10.1590/s1806-37132010000100008. [DOI] [PubMed] [Google Scholar]
- 46.Ratier JC, Pizzichini E, Pizzichini M. astroesophageal reflux disease and airway hyperresponsiveness: concomitance beyond the realm of chance? J Bras Pneumol. 2011;37(5):680–8. doi: 10.1590/s1806-37132011000500017. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz J, Gold D, Dockery DW, Weiss ST, Speizer FE. Predictors of asthma and persistent wheeze in a national sample of children in the United States. Association with social class, perinatal events, and race. Am Rev Respir Dis. 1990;142(3):555–62. doi: 10.1164/ajrccm/142.3.555. [DOI] [PubMed] [Google Scholar]
- 48.Barker DJ, Godfrey KM, Fall C, Osmond C, Winter PD, Shaheen SO. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ. 1991;303(6804):671–5. doi: 10.1136/bmj.303.6804.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gold DR, Tager IB, Weiss ST, Tosteson TD, Speizer FE. Acute lower respiratory illness in childhood as a predictor of lung function and chronic respiratory symptoms. Am Rev Respir Dis. 1989;140(4):877–84. doi: 10.1164/ajrccm/140.4.877. [DOI] [PubMed] [Google Scholar]
- 50.Forster J, Tacke U, Krebs H Streckter HJ, Werchau H, Bergmann RL. Respiratory syncytial virus infection: its role in aeroallergen sensitization during the first two years of life. Pediatr Allergy Immunol. 1996;7(2):55–60. doi: 10.1111/j.1399-3038.1996.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 51.Shahar E, Folsom AR, Melnick SL, Tockman MS, Comstock GW, Gennaro V, et al. Dietary n-3 polyunsaturated fatty acids and smoking-related chronic obstructive pulmonary disease. Atherosclerosis Risk in Communities Study Investigators. N Engl J Med. 1994;331(4):228–33. doi: 10.1056/NEJM199407283310403. [DOI] [PubMed] [Google Scholar]
- 52.Sutherland ER, Martin RJ. Airway inflammation in chronic obstructive pulmonary disease: comparisons with asthma. J Allergy Clin Immunol. 2003;112(5):819–27. quiz 828. doi: 10.1016/S0091. [DOI] [PubMed] [Google Scholar]
- 53.Picinin IF, Camargos PA, Marguet C. Cell profile of BAL fluid in children and adolescents with and without lung disease. J Bras Pneumol. 2010;36(3):372–91. doi: 10.1590/s1806-37132010000300016. [DOI] [PubMed] [Google Scholar]
- 54.Veras TN, Pizzichini E, Steidle LJ, Rocha CC, Moritz P, Pizzichini MM. Cellular composition of induced sputum in healthy adults. J Bras Pneumol. 2011;37(3):348–53. doi: 10.1590/s1806-37132011000300011. [DOI] [PubMed] [Google Scholar]
- 55.Hoshino M, Nakamura Y, Sim JJ. Expression of growth factors and remodelling of the airway wall in bronchial asthma. Thorax. 1998;53(1):21–7. doi: 10.1136/thx.53.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ward C, Pais M, Bish R, Reid D, Feitis B, Johns D, et al. Airway inflammation, basement membrane thickening and bronchial hyperresponsiveness in asthma. Thorax. 2002;57(4):309–16. doi: 10.1136/thorax.57.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stankiewicz W, Dabrowski MP, Chcialowski A, Plusa T. Cellular and cytokine immunoregulation in patients with chronic obstructive pulmonary disease and bronchial asthma. Mediat Inflamm. 2002;11(5):307–12. doi: 10.1080/09629350210000015719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burrows B, Knudson RJ, Cline MG, Lebowitz MD. Quantitative relationships between cigarette smoking and ventilatory function. Am Rev Respir Dis. 1977;115(2):195–205. doi: 10.1164/arrd.1977.115.2.195. [DOI] [PubMed] [Google Scholar]
- 59.Mitsunobu F, Ashida K, Hosaki Y, Tsugeno H, Okamoto M, Nishida N, et al. Influence of long-term cigarette smoking on immunoglobulin E-mediated allergy, pulmonary function, and highresolution computed tomography lung densitometry in elderly patients with asthma. Clin Exp Allergy. 2004;34(1):59–64. doi: 10.1111/j.1365-2222.2004.01844.x. [DOI] [PubMed] [Google Scholar]
- 60.Petays T, von Hertzen L, Metso T, Rytillä P, Jousilahti P, Helenius I, Varitiainen E, et al. Smoking and atopy as determinants of sputum eosinophilia and bronchial hyper-responsiveness in adults with normal lung function. Respir Med. 2003;97(8):947–54. doi: 10.1016/s0954-6111(03)00122-7. [DOI] [PubMed] [Google Scholar]
- 61.Brightling CE, Monteiro W, Ward R, Birring S, Green R, Siva R, et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2000;356(9240):1480–5. doi: 10.1016/S0140-6736(00)02872-5. [DOI] [PubMed] [Google Scholar]
- 62.Hogg JC, Chu F, Utokaparch S, Woods R, Elliot WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–53. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 63.Tsang KW, Bilton D. Clinical challenges in managing bronchiectasis. Respirology. 2009;14(5):637–50. doi: 10.1111/j.1440-1843.2009.01569.x. [DOI] [PubMed] [Google Scholar]
- 64.King PT. The pathophysiology of bronchiectasis. Int J Chron Obstruct Pulmon Dis. 2009;4:411–9. doi: 10.2147/copd.s6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morrissey BM. Pathogenesis of bronchiectasis. Clin Chest Med. 2007;28(2):289–96. doi: 10.1016/j.ccm.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 66.Tambascio J, Lisboa RM, Passarelli RC, Martinez JA, Gastaldi AC. Adhesiveness and purulence of respiratory secretions: implications for mucociliary transport in patients with bronchiectasis. J Bras Pneumol. 2010;36(5):545–53. doi: 10.1590/s1806-37132010000500005. [DOI] [PubMed] [Google Scholar]
- 67.Fabbri LM, Romagnoli M, Corbetta L, Casoni G, Busljetic K, Turato G, et al. Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167(3):418–24. doi: 10.1164/rccm.200203-183OC. [DOI] [PubMed] [Google Scholar]
- 68.Romagnoli M, Vachier I, Tarodo de la Fuente P, Meziane H, Chavis C, Bousquet J, et al. Eosinophilic inflammation in sputum of poorly controlled asthmatics. Eur Respir J. 2002;20(6):1370–7. doi: 10.1183/09031936.02.00029202. [DOI] [PubMed] [Google Scholar]
- 69.Keatings VM, Jatakanon A, Worsdell YM, Barnes PJ. Effects of inhaled and oral glucocorticoids on inflammatory indices in asthma and COPD. Am J Respir Crit Care Med. 1997;155(2):542–8. doi: 10.1164/ajrccm.155.2.9032192. [DOI] [PubMed] [Google Scholar]
- 70.Magnussen H, Richter K, Taube C. Are chronic obstructive pulmonary disease (COPD) and asthma different diseases. Clin Exp Allergy. 1998;28(Suppl. 5):187–94. discussion 203–5. doi: 10.1046/j.1365-2222.1998.028s5187.x. [DOI] [PubMed] [Google Scholar]
- 71.Ponte EV, Souza-Machado A, Souza-Machado C, Franco R, Cruz AA. Clinical characteristics and prognosis in near-fatal asthma patients in Salvador, Brazil. J Bras Pneumol. 2011;37(4):431–7. doi: 10.1590/s1806-37132011000400004. [DOI] [PubMed] [Google Scholar]
- 72.Vieira AA, Santoro IL, Dracoulakis S, Caetano LB, Fernandes AL. Anxiety and depression in asthma patients: impact on asthma control. J Bras Pneumol. 2011;37(1):13–8. doi: 10.1590/s1806-37132011000100004. [DOI] [PubMed] [Google Scholar]
- 73.Reck CL, Fiterman-Molinari D, Barreto SS, Fiterman J. Poor perception of dyspnea following methacholine challenge test in patients with asthma. J Bras Pneumol. 2010;36(5):539–44. doi: 10.1590/s1806-37132010000500004. [DOI] [PubMed] [Google Scholar]
- 74.Rogers DF. Mucus pathophysiology in COPD. differences to asthma, and pharmacotherapy. Monaldi Arch Chest Dis. 2000;55(4):324–32. [PubMed] [Google Scholar]
- 75.Ryu JH, Scanlon PD. Obstructive lung diseases: COPD, asthma, and many imitators. Mayo Clin Proc. 2001;76:1144–53. doi: 10.4065/76.11.1144. [DOI] [PubMed] [Google Scholar]
- 76.Camargo LA, Pereira CA. Dyspnea in COPD: Beyond the modified Medical Research Council scale. J Bras Pneumol. 2010;36(5):571–8. doi: 10.1590/s1806-37132010000500008. [DOI] [PubMed] [Google Scholar]
- 77.Araujo ZT, Holanda G. Does the BODE index correlate with quality of life in patients with COPD? J Bras Pneumol. 2010;36(4):447–52. doi: 10.1590/s1806-37132010000400009. [DOI] [PubMed] [Google Scholar]
- 78.NIH/NHLBI. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (GOLD). WHO/NHLBI Workshop Report. National Institutes for Health/National Heart, Lung and Blood Institute 2011. [Google Scholar]
- 79.Araújo FB, Corrêa RA, Pereira LF, Silveira DC, Mancuso EV, Rezende NA. Spirometry with bronchodilator test: effect that the use of large-volume spacers with antistatic treatment has on test response. J Bras Pneumol. 2011;37(6):752–8.. doi: 10.1590/s1806-37132011000600008. [DOI] [PubMed] [Google Scholar]
- 80.Rodrigues MT, Fiterman-Molinari D, Barreto SS, Fiterman J. The role of the FEF50%/0.5FVC ratio in the diagnosis of obstructivelung diseases. J Bras Pneumol. 2010;36(1):44–50. doi: 10.1590/s1806-37132010000100009. [DOI] [PubMed] [Google Scholar]
- 81.Hamutcu R, Rowland JM, Horn MV, Kaminsky C, MacLaughlin EF, Starnes VA, et al. Clinical findings and lung pathology in children with cystic fibrosis. Am J Respir Crit Care Med. 2002;165(8):1172–5. doi: 10.1164/ajrccm.165.8.2104090. [DOI] [PubMed] [Google Scholar]
- 82.Wells AU. Computed tomographic imaging of bronchiolar disorders. Curr Opin Pulm Med. 1998;4(2):85–92. doi: 10.1097/00063198-199803000-00005. [DOI] [PubMed] [Google Scholar]
- 83.Silva CI, Marchiori E, Júnior AS, Müller NL, et al. Illustrated Brazilian consensus of terms and fundamental patternsin chest CT scans. J Bras Pneumol. 2010;36(1):99–123. doi: 10.1590/s1806-37132010000100016. [DOI] [PubMed] [Google Scholar]
- 84.Walker C, Gupta S, Hartley R, Brightling CE. Computed tomography scans in severe asthma: utility and clinical implications. Curr Opin Pulm Med. 2012;18(1):42–47. doi: 10.1097/MCP.0b013e32834db255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Castro M, Fain SB, Hoffman EA, Gierada DS, Erzurum SC, Wenzel S, et al. Lung imaging in asthmatic patients: the picture is clearer. J Allergy Clin Immunol. 2011;128(3):467–78. doi: 10.1016/j.jaci.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers: correlation with lung function. Am J Respir Crit Care Med. 162:(3 Pt 1):1102–8. doi: 10.1164/ajrccm.162.3.9907120. [DOI] [PubMed] [Google Scholar]
- 87.Cerveri I, Dore R, Corsico A, Zoia MC, Pellegrino R, Brusasco V, et al. Assessment of emphysema in COPD: a functional and radiologic study. Chest. 2004;125(5):1714–8. doi: 10.1378/chest.125.5.1714. [DOI] [PubMed] [Google Scholar]
- 88.Boschetto P, Miniati M, Miotto D, Braccioni F, De Rosa E, Bononi I, et al. Predominant emphysema phenotype in chronic obstructive pulmonary. Eur Respir J. 2003;21(3):450–4. doi: 10.1183/09031936.03.00048703. [DOI] [PubMed] [Google Scholar]
- 89.Menezes AM, Macedo SE, Noal RB, Fiterman J, Cukier A, Chatkin JM, et al. Pharmacological treatment of COPD. J Bras Pneumol. 2011;37(4):527–43. doi: 10.1590/s1806-37132011000400016. [DOI] [PubMed] [Google Scholar]
- 90.Pasteur MC, Bilton D, Hill AT. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65(1):1–58. doi: 10.1136/thx.2010.142778. [DOI] [PubMed] [Google Scholar]
- 91.Athanazio RA, Rached SZ, Rohde C, Pinto RC, Fernandes FL, Stelmach R. Should the bronchiectasis treatment given to cystic fibrosis patients be extrapolated to those with bronchiectasis from other causes? J Bras Pneumol. 2010;36(4):425–31. doi: 10.1590/s1806-37132010000400006. [DOI] [PubMed] [Google Scholar]
- 92.Lazarus SC, Boushey HA, Fahy JV, Chinchili VM, Lemanske RF, Jr, Sorkness CA, et al. Long-acting β2-agonist monotherapy vs. continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA. 2001;285(20):2583–93. doi: 10.1001/jama.285.20.2583. [DOI] [PubMed] [Google Scholar]
- 93.Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 164(10 Pt 2):S28–38. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- 94.Appleton S, Poole P, Smith B, Veale A, Bara A. Long-acting β2-agonists for chronic obstructive pulmonary disease patients with poorly reversible airflow limitation (Cochrane Review) Cochrane Database Syst Rev. 2002;(3):CD001104. doi: 10.1002/14651858.CD001104. [DOI] [PubMed] [Google Scholar]
- 95.Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting b2-agonists and corticosteroids. Eur Respir J. 2002;19(1):182–91. doi: 10.1183/09031936.02.00283202. [DOI] [PubMed] [Google Scholar]
- 96.Martínez-García MÁ, Soler-Cataluña JJ, Catalán-Serra P, Román-Sánchez P, Tordera MP. Clinical efficacy and safety of budesonide-formoterol in non-cystic fibrosis bronchiectasis. Chest. 2012;141(2):461–8. doi: 10.1378/chest.11-0180. [DOI] [PubMed] [Google Scholar]
- 97.Gross NJ, Petty TL, Friedman M, Skorodin MS, Silvers GW, Donohue JF. Dose response to ipratropium as a nebulized solution in patients with chronic obstructive pulmonary disease: a three-center study. Am Rev Respir Dis. 1989;139(5):1188–91. doi: 10.1164/ajrccm/139.5.1188. [DOI] [PubMed] [Google Scholar]
- 98.Barnes PJ. The pharmacological properties of tiotropium. Chest. 2000;117(2 Suppl):63S–6S. doi: 10.1378/chest.117.2_suppl.63s. [DOI] [PubMed] [Google Scholar]
- 99.Barr RG, Bourbeau J, Camargo CA, Ram FS. Inhaled tiotropium for stable chronic obstructive pulmonary disease: a meta analysis: . Thorax. 2006;61(10):854–62. doi: 10.1136/thx.2006.063271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fernandes FL, Pavezi VA, Dias SA, Pinto RM, Stelmach R, Cukier A. Short-term effect of tiotropium in COPD patients being treated with a β2 agonist. J Bras Pneumol. 2010;36(2):181–9. doi: 10.1590/s1806-37132010000200005. [DOI] [PubMed] [Google Scholar]
- 101.Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium in Asthma Poorly Controlled with Standard Combination Therapy. N Engl J Med. 2012;367(13):1198–207. doi: 10.1056/NEJMoa1208606. [DOI] [PubMed] [Google Scholar]
- 102.Barnes PJ, Pedersen S, Busse WW. Efficacy and safety of inhaled corticosteroids. New developments. Am J Respir Crit Care Med. 1998;157(3 Pt 2:S1–53. doi: 10.1164/ajrccm.157.3.157315. [DOI] [PubMed] [Google Scholar]
- 103.Djukanovic R, Wilson JW, Britten KM, Wilson SJ, Walls AF, Roche WR, et al. Effect of an inhaled corticosteroid on airway inflammation and symptoms in asthma. Am Rev Respir Dis. 1992;145(3):669–674. doi: 10.1164/ajrccm/145.3.669. [DOI] [PubMed] [Google Scholar]
- 104.Pereira CA, Vianna FF, Cukier A, Stelmach R, Oliveira JC, Carvalho EV, et al. Efficacy and safety of two dry-powder inhalers for the administration of mometasone furoate in asthma patients. J Bras Pneumol. 2010;36(4):410–6. doi: 10.1590/s1806-37132010000400004. [DOI] [PubMed] [Google Scholar]
- 105.Santos DO, Martins MC, Sipriano SL, Pinto RM, Cukier A, Stelmach R. Pharmaceutical care for patients with persistent asthma: assessment of treatment compliance and use of inhaled medications. J Bras Pneumol. 2010;36(1):14–22. doi: 10.1590/s1806-37132010000100005. [DOI] [PubMed] [Google Scholar]
- 106.Dalcin PT, Grutcki DM, Laporte PP, Lima PB, Viana VP, Konzen GL, et al. Impact of a short-term educational intervention on adherence to asthma treatment and on asthma control. J Bras Pneumol. 2011;37(1):19–27. doi: 10.1590/s1806-37132011000100005. [DOI] [PubMed] [Google Scholar]
- 107.Coelho AC, Souza-Machado A, Leite M, Almeida P, Castro L, Cruz CS, et al. Use of inhaler devices and asthma control in severe asthma patients at a referral center in the city of Salvador, Brazil. J Bras Pneumol. 2011;37(6):720–8. doi: 10.1590/s1806-37132011000600004. [DOI] [PubMed] [Google Scholar]
- 108.Pereira ED, Cavalcante AG, Pereira EN, Lucas P, Holanda MA. Asthma control and quality of life in patients with moderate or severe asthma. J Bras Pneumol. 2011;37(6):704–11. doi: 10.1590/s1806-37132011000600002. [DOI] [PubMed] [Google Scholar]
- 109.Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone proprionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320(7245):1297–303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van der Valk P, Monninkhof E, van der Palen J, Zielhuis G, van Herwaarden C. Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: the COPE study. Am J Respir Crit Care Med. 2002;166:1358–63. doi: 10.1164/rccm.200206-512OC. [DOI] [PubMed] [Google Scholar]
- 111.Kapur N, Bell S, Kolbe J, Chang AB. Inhaled steroids for bronchiectasis. Cochrane Database Syst Rev. 2009;21;(1):CD000996. doi: 10.1002/14651858.CD000996.pub2. Review. [DOI] [PubMed] [Google Scholar]
- 112.Decramer M, Gosselink,R, Bartsch P, Lofdahl CG, Vincken W, Dekhuijzen R, et al. Effect of treatments on the progression of COPD: Report of a workshop held in Leuven, March 11–12 2004. Thorax. 2005;60(4):343–9. doi: 10.1136/thx.2004.028720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rubin AS, Cardoso PF. Bronchial thermoplasty in asthma. J Bras Pneumol. 2010;36(4):506–12. doi: 10.1590/s1806-37132010000400018. [DOI] [PubMed] [Google Scholar]
- 114.Cox G, Thomson NC, Rubin AS, Niven RM, Corris PA, Siersted HC, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356(13):1327–37. doi: 10.1056/NEJMoa064707. [DOI] [PubMed] [Google Scholar]
- 115.Wehrmeister FC, Knorst M, Jardim JR, Macedo SE, Noal RB, Martínez-Mesa J, et al. Pulmonary rehabilitation programs for patients with COPD. J Bras Pneumol. 2011;37(4):544–55. doi: 10.1590/s1806-37132011000400017. [DOI] [PubMed] [Google Scholar]
- 116.Bradley J, Moran F, Greenstone M. Physical training for bronchiectasis. Cochrane Database Syst Rev. 2002;(3):CD002166. doi: 10.1002/14651858.CD002166. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]