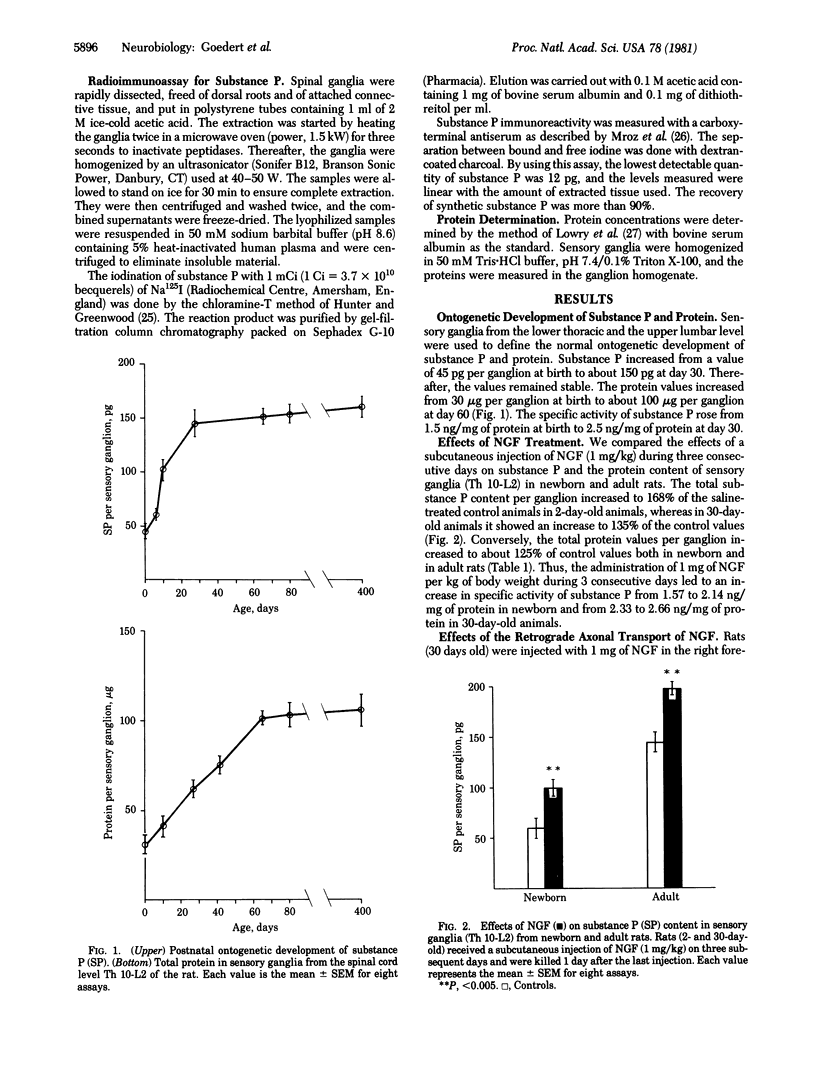
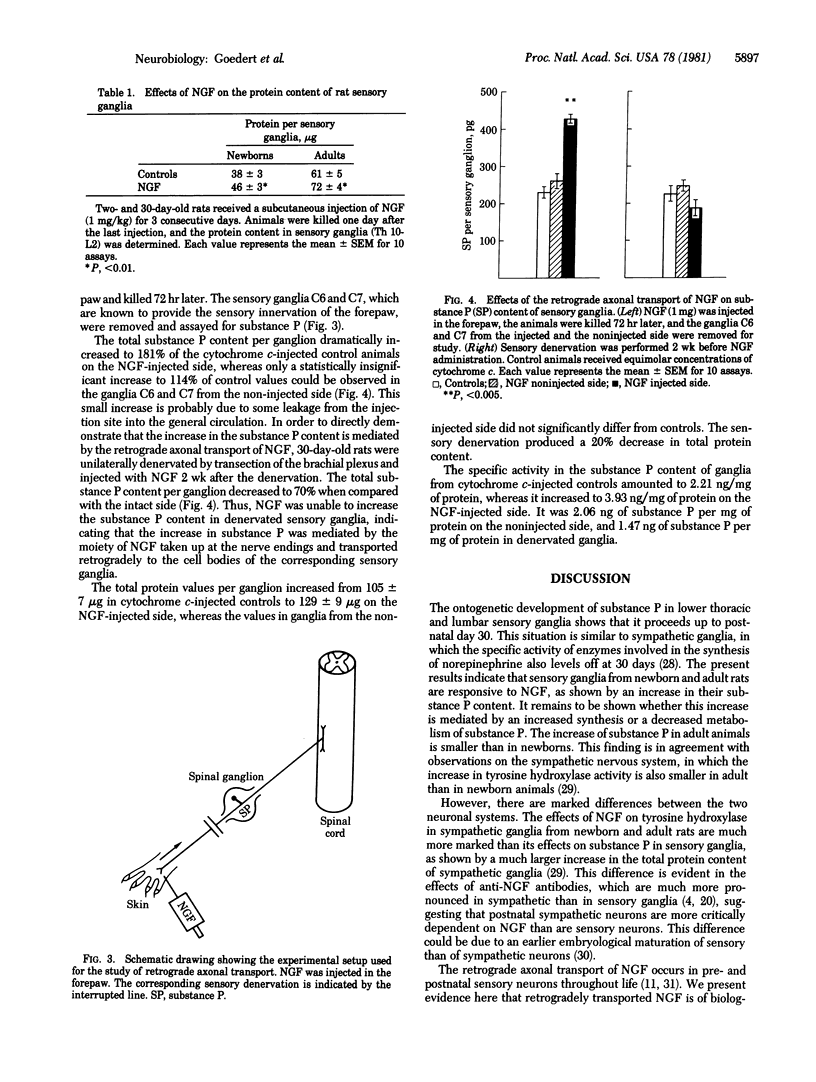
Abstract
Nerve growth factor is retrogradely transported in sympathetic and sensory neurons throughout life. Although this transport is known to be biologically significant in sympathetic neurons, such a function was not yet known in sensory ganglia. By using the neuropeptide substance P as a biochemical marker, we show that sensory ganglia from newborn and adult rats respond in nerve growth factor and that its retrograde axonal transport is biologically relevant, as indicated by an increase in substance P and in general protein content.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bill A., Stjernschantz J., Mandahl A., Brodin E., Nilsson G. Substance P: release on trigeminal nerve stimulation, effects in the eye. Acta Physiol Scand. 1979 Jul;106(3):371–373. doi: 10.1111/j.1748-1716.1979.tb06412.x. [DOI] [PubMed] [Google Scholar]
- Bocchini V., Angeletti P. U. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969 Oct;64(2):787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunso-Bechtold J. K., Hamburger V. Retrograde transport of nerve growth factor in chicken embryo. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1494–1496. doi: 10.1073/pnas.76.3.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebendal T., Olson L., Seiger A., Hedlund K. O. Nerve growth factors in the rat iris. Nature. 1980 Jul 3;286(5768):25–28. doi: 10.1038/286025a0. [DOI] [PubMed] [Google Scholar]
- Ennis M., Pearce F. L., Vernon C. A. Some studies on the mechanism of action of antibodies to nerve growth factor. Neuroscience. 1979;4(9):1391–1398. doi: 10.1016/0306-4522(79)90166-0. [DOI] [PubMed] [Google Scholar]
- Fenton E. L. Tissue culture assay of nerve growth factor and of the specific antiserum. Exp Cell Res. 1970 Mar;59(3):383–392. doi: 10.1016/0014-4827(70)90645-2. [DOI] [PubMed] [Google Scholar]
- Goedert M., Otten U., Schäfer T., Schwab M., Thoenen H. Immunosympathectomy: lack of evidence for a complement-mediated cytotoxic mechanism. Brain Res. 1980 Nov 17;201(2):399–409. doi: 10.1016/0006-8993(80)91043-4. [DOI] [PubMed] [Google Scholar]
- Goedert M., Otten U., Thoenen H. Biochemical effects of antibodies against nerve growth factor on developing and differentiated sympathetic ganglia. Brain Res. 1978 Jun 9;148(1):264–268. doi: 10.1016/0006-8993(78)90401-8. [DOI] [PubMed] [Google Scholar]
- Greene L. A. Quantitative in vitro studies on the nerve growth factor (NGF) requirement of neurons. II. Sensory neurons. Dev Biol. 1977 Jul 1;58(1):106–113. doi: 10.1016/0012-1606(77)90077-x. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hamburger V. Trophic interactions in neurogenesis: a personal historical account. Annu Rev Neurosci. 1980;3:269–278. doi: 10.1146/annurev.ne.03.030180.001413. [DOI] [PubMed] [Google Scholar]
- Harmar A., Schofield J. G., Keen P. Cycloheximide-sensitive synthesis of substance P by isolated dorsal root ganglia. Nature. 1980 Mar 20;284(5753):267–269. doi: 10.1038/284267a0. [DOI] [PubMed] [Google Scholar]
- Hendry I. A., Stöckel K., Thoenen H., Iversen L. L. The retrograde axonal transport of nerve growth factor. Brain Res. 1974 Mar 15;68(1):103–121. doi: 10.1016/0006-8993(74)90536-8. [DOI] [PubMed] [Google Scholar]
- Herrup K., Shooter E. M. Properties of the beta-nerve growth factor receptor in development. J Cell Biol. 1975 Oct;67(1):118–125. doi: 10.1083/jcb.67.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T., Elde R., Johansson O., Luft R., Nilsson G., Arimura A. Immunohistochemical evidence for separate populations of somatostatin-containing and substance P-containing primary afferent neurons in the rat. Neuroscience. 1976;1(2):131–136. doi: 10.1016/0306-4522(76)90008-7. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Johansson O., Ljungdahl A., Lundberg J. M., Schultzberg M. Peptidergic neurones. Nature. 1980 Apr 10;284(5756):515–521. doi: 10.1038/284515a0. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Kellerth J. O., Nilsson G., Pernow B. Substance p: localization in the central nervous system and in some primary sensory neurons. Science. 1975 Nov 28;190(4217):889–890. doi: 10.1126/science.242075. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Jr, Gorin P. D., Brandeis L. D., Pearson J. Dorsal root ganglion neurons are destroyed by exposure in utero to maternal antibody to nerve growth factor. Science. 1980 Nov 21;210(4472):916–918. doi: 10.1126/science.7192014. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Black I. B. Nerve growth factor stimulates development of substance P in the embryonic spinal cord. Brain Res. 1981 Mar 9;208(1):135–145. doi: 10.1016/0006-8993(81)90626-0. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Black I. B. Nerve growth factor stimulates the development of substance P in sensory ganglia. Proc Natl Acad Sci U S A. 1980 Jan;77(1):649–652. doi: 10.1073/pnas.77.1.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Second symposium on catecholamines. Modification of sympathetic function. Immunosympathectomy. Pharmacol Rev. 1966 Mar;18(1):619–628. [PubMed] [Google Scholar]
- Levi A., Shechter Y., Neufeld E. J., Schlessinger J. Mobility, clustering, and transport of nerve growth factor in embryonal sensory cells and in a sympathetic neuronal cell line. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3469–3473. doi: 10.1073/pnas.77.6.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten U., Goedert M., Mayer N., Lembeck F. Requirement of nerve growth factor for development of substance P-containing sensory neurones. Nature. 1980 Sep 11;287(5778):158–159. doi: 10.1038/287158a0. [DOI] [PubMed] [Google Scholar]
- Paravicini U., Stoeckel K., Thoenen H. Biological importance of retrograde axonal transport of nerve growth factor in adrenergic neurons. Brain Res. 1975 Feb 7;84(2):279–291. doi: 10.1016/0006-8993(75)90982-8. [DOI] [PubMed] [Google Scholar]
- Schwab M. E. Ultrastructural localization of a nerve growth factor-horseradish peroxidase (NGF-HRP) coupling product after retrograde axonal transport in adrenergic neurons. Brain Res. 1977 Jul 8;130(1):190–196. doi: 10.1016/0006-8993(77)90857-5. [DOI] [PubMed] [Google Scholar]
- Stoeckel K., Schwab M., Thoenen H. Specificity of retrograde transport of nerve growth factor (NGF) in sensory neurons: a biochemical and morphological study. Brain Res. 1975 May 16;89(1):1–14. doi: 10.1016/0006-8993(75)90129-8. [DOI] [PubMed] [Google Scholar]
- Stöckel K., Schwab M., Thoenen H. Comparison between the retrograde axonal transport of nerve growth factor and tetanus toxin in motor, sensory and adrenergic neurons. Brain Res. 1975 Nov 28;99(1):1–16. doi: 10.1016/0006-8993(75)90604-6. [DOI] [PubMed] [Google Scholar]
- Suda K., Barde Y. A., Thoenen H. Nerve growth factor in mouse and rat serum: correlation between bioassay and radioimmunoassay determinations. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4042–4046. doi: 10.1073/pnas.75.8.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Otsuka M. Regional distribution of substance P in the spinal cord and nerve roots of the cat and the effect of dorsal root section. Brain Res. 1975 Apr 4;87(1):1–11. doi: 10.1016/0006-8993(75)90774-x. [DOI] [PubMed] [Google Scholar]
- Theriault E., Otsuka M., Jessell T. Capsaicin-evoked release of substance P from primary sensory neurons. Brain Res. 1979 Jul 6;170(1):209–213. doi: 10.1016/0006-8993(79)90957-0. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Angeletti P. U., Levi-Montalcini R., Kettler R. Selective induction by nerve growth factor of tyrosine hydroxylase and dopamine- -hydroxylase in the rat superior cervical ganglia. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1598–1602. doi: 10.1073/pnas.68.7.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H., Barde Y. A. Physiology of nerve growth factor. Physiol Rev. 1980 Oct;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Kettler R., Saner A. Time course of the development of enzymes involved in the synthesis of norepinephrine in the superior cervical ganglion of the rat from birth to adult life. Brain Res. 1972 May 26;40(2):459–468. doi: 10.1016/0006-8993(72)90146-1. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Shooter E. M. Nerve growth factor in the nucleus: interaction with receptors on the nuclear membrane. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1269–1273. doi: 10.1073/pnas.76.3.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]



