SUMMARY
Presenilin 1 (PS1) regulates environmental enrichment (EE)-mediated neural progenitor cell (NPC) proliferation and neurogenesis in the adult hippocampus. We now report that transgenic mice that ubiquitously express human PS1 variants linked to early-onset familial Alzheimer's disease (FAD) neither exhibit EE-induced proliferation, nor neuronal lineage commitment of NPCs. Remarkably, the proliferation and differentiation of cultured NPCs from standard-housed mice expressing wild-type PS1 or PS1 variants are indistinguishable. On the other hand, wild-type NPCs co-cultured with primary microglia from mice expressing PS1 variants exhibit impaired proliferation and neuronal lineage commitment, phenotypes that are recapitulated with mutant microglia conditioned media in which we detect altered levels of selected soluble signaling factors. These findings lead us to conclude that factors secreted from microglia play a central role in modulating hippocampal neurogenesis, and argue for non-cell-autonomous mechanisms that govern FAD-linked PS1-mediated impairments in adult hippocampal neurogenesis.
INTRODUCTION
Alzheimer's disease (AD), a progressive neurodegenerative disease, is characterized by deterioration of cognitive function, neuronal loss and deposition of β-amyloid (Aβ) peptides. Aβ peptides are liberated from larger transmembrane amyloid precursor proteins (APP) by the concerted action of BACE 1 and γ-secretase. Familial, early-onset, autosomal dominant forms of AD (FAD) are caused by expression of mutant genes encoding presenilin 1 (PS1), presenilin 2 (PS2) and APP. While the molecular mechanism(s) by which expression of mutant PS1 polypeptides cause FAD are not fully understood, expression of these polypeptides enhances the ratio of Aβ42/Aβ40 peptides, leading to selective deposition of the Aβ42 peptides in amyloid plaques (Selkoe, 1998).
PS1 is the catalytic component of a multi-subunit complex, termed γ-secretase, that is responsible for the intramembranous proteolysis of several type I membrane proteins, including APP and Notch-1 [for review, see (De Strooper, 2003; Sisodia and St George-Hyslop, 2002)]. PS1 also plays a role in aspects of neurogenesis both during development and in the adult: PS1-deficient mice exhibit precocious proliferation of neural progenitors in the central nervous system (CNS), leading to reductions in cortical neurons (Handler et al., 2000; Shen et al., 1997); and enrichment-mediated hippocampal neurogenesis is impaired in adult mice with conditionally-deleted PS1 alleles in the forebrain (Feng et al., 2001).
It is now well accepted that proliferation of neuronal progenitors occurs in the adult CNS, and primarily, if not exclusively, in the subgranular layer (SGL) of the dentate gyrus (DG) and the anterior part of the subventricular zone (SVZ). It has been suggested that neurogenesis in the adult CNS plays a role in learning and memory, adaptation to novel environments and injury or disease [For review, see (Lie et al., 2004)]. Understanding the role of neurogenesis in these settings is critical, particularly when considering the impact of aging and neurodegenerative diseases, such as AD. Numerous studies have suggested that the rate of neurogenesis in both SVZ and DG declines with age, raising the possibility that reduced neurogenesis may account, at least in part, for impaired learning and memory and cognitive deterioration in the elderly (Kempermann et al., 2002; Kempermann et al., 1998; Seki and Arai, 1995; Tropepe et al., 1997). Unfortunately, there is limited information regarding neurogenesis in AD and transgenic animal models [for review, see (van Praag, 2008)].
It has become clear that proliferation and differentiation properties of hippocampal NPCs are regulated by the combination of both cell intrinsic mechanisms and changes in local environment. For example, the levels or composition of chemokines and/or secreted factors within the microenvironment have been implicated in hippocampal NPC proliferation and differentiation [For review, see (Nakashima and Taga, 2002)]. Moreover, glial cells have been implicated in governing the factors within stem cell niches that influence cell fate commitment of hippocampal progenitors to a neuronal lineage (Lie et al., 2005; Nakajima and Kohsaka, 2001; Song et al., 2002).
It is now well established that exposure of adult rodents to an enriched environment (EE) induces neurogenesis in the DG (Kempermann et al., 1997; van Praag et al., 1999). In this paper, we employed EE to explore the role of wild-type or two independent FAD-linked mutant human PS1 variants in the regulation of dentate neurogenesis. We now report that EE-induced proliferation and neuronal differentiation of progenitor cells in the DG of mice harboring ubiquitously-expressed mouse prion protein (PrP) promoter-driven transgenes encoding the FAD-linked PS1ΔE9 or PS1M146L variants are significantly impaired, compared to mice expressing human wild-type PS1 (PS1hWT). Moreover, impaired neurogenesis in the DG of mice expressing FAD-linked PS1 variants is associated with decreased numbers of activated microglia that populate the hippocampus. Extending these observations, we show that primary microglia expressing FAD-linked PS1 or conditioned media from these cells markedly inhibits proliferation and neuronal lineage commitment of cultured NPCs from wild-type mice, and impairs the neurogenenic potential of newly-born progenitors in organotypic slices from adult wild-type mice. Indeed, we observe considerable differences in secreted soluble factor profiles between microglia expressing FAD-linked PS1 variants and PS1hWT, leading us to conclude that soluble factors released from microglia, at least in part, are responsible for the defective EE-induced neurogenesis observed in FAD-linked PS1 mutant mice.
EXPERIMENTAL PROCEDURES
See Supplemental Experimental Procedures for detailed methods on Enriched environment (EE), BrdU injections, tissue processing, BrdU immunostaining, immunofluorescence, stereology and organotypic slice cultures.
Transgenic mice
Male mice expressing human wild-type PS1 (PS1hWT) [line S8-4; (Thinakaran et al., 1996)], FAD-linked human PS1ΔE9 [line S9; (Lee et al., 1997)] or PS1M146L [line I5; (Lee et al., 1997)] were used in this study. Mice were weaned and either exposed to EE, or kept in standard housing conditions for 1 month. All mice are heterozygous for the transgene. Background strain for these mice are [C3H/HeJ X C57BL/6J F3] × C57BL/6J n1 (Lee et al., 1997). APP-deficient mice (Zheng et al., 1995) were maintained as C57BL/6JX 129sv hybrids and were crossed with mice expressing PS1hWT or PS1ΔE9 to generate PS1hWT/APPKO and PS1ΔE9/APPKO mice, respectively.
Neural progenitor and primary microglial cell culture studies
Neural progenitor cells (NPCs) that give rise to neurospheres were cultured from dissected hippocampal tissue of 4 to 8 weeks old male transgenic lines according to the described methods with minor modifications (Bull and Bartlett, 2005; Seaberg and van der Kooy, 2002). See Supplemental Experimental Procedures for enumeration of clonal neurosphere numbers, NPC proliferation and multipotency assays. Primary cultures of mixed glia cells were prepared from dissected hippocampal tissue of newborn transgenic mice lines and then weakly adherent microglia were enriched from these monolayers. See Supplemental Experimental Procedures for NPC and microglia co-culture studies, measurement of soluble factors in microglia conditioned media using high-density antibody arrays, quantitative real time-PCR (Q-PCR) analysis of transcripts encoding soluble factors and NPC proliferation assays with purified soluble factors.
Isolation of adult hippocampal microglia
One month old male offspring of PS1hWT and FAD-linked PS1 variant transgenic lines were housed in EE or maintained in standard housing conditions for 4 weeks. Following exposure to standard or enriched housing conditions, hippocampi were dissected, pooled (n = 6 animals per group) and microglia were isolated as described (Cardona et al., 2006). Total RNA from the adult microglial cells was prepared using Trizol Reagent and reverse transcribed using Superscript III synthesis kit (Invitrogen, Carlsbad, CA) and SYBR green Q-PCR was performed using certified RT2 Q-PCR primers [SuperArray BioSciences, Boston, MA].
Statistical Analysis
Data are expressed as mean values ± standard error of the mean (Mean ± SEM). All statistical analyses were performed with SPSS. T-test or Factorial ANOVA was performed for all comparisons of quantitative data followed by Scheffe or Tukey-Kramer post hoc tests, where appropriate. Values of P < 0.05 were considered to be significant.
RESULTS
Expression of FAD-linked PS1 variants impairs enrichment-induced proliferation of hippocampal progenitor cells
It is well-established that exposure of adult rodents to an enriched environment (EE) induces neurogenesis in the dentate gyrus (DG) (Kempermann et al., 1997; van Praag et al., 1999). To determine whether FAD-linked mutant PS1 affects proliferation of progenitor cells, we examined transgenic mice harboring mouse PrP promoter-driven transgenes that encode either human wild-type PS1 (PS1hWT), or the FAD-linked PS1ΔE9 and PS1M146L variants (Borchelt et al., 1996b; Lee et al., 1997). We established that the polypeptides expressed by each transgene were ubiquitously expressed and overlapping patterns in brain (Figure S1).
Mice were either exposed to EE (termed “enriched” mice) for 3 hours a day for 1 month, or maintained in standard housing conditions (termed “standard” mice) for 1 month. At the end of 1 month, standard housed mice or their “enriched” counterparts were injected with a single dose of BrdU. Mice were sacrificed 24 h post injection and brain sections were subject to immunocytochemical analysis using a BrdU-specific antibody; the number of BrdU-positive cells in the subgranular (SGL) and granule cell layers (GCL) was quantified by stereological methods.
We failed to observe any significant difference in the numbers of BrdU-positive cells in the DG of mice expressing PS1hWT or either of the FAD-linked PS1 variants that were maintained in standard housing conditions (Figure 1, compare panels A versus B and C, respectively; quantified in Figure 1G, first left panel). As expected, we observed a marked increase in the number of BrdU-positive cells in the enriched PS1hWT mice (Figure 1D) compared to standard PS1hWT mice (Figure 1A). Remarkably, we observed that enriched mice expressing the human PS1 variants failed to elicit an enhancement in the number of BrdU-positive cells in the DG (Compare Figures 1B and 1E, and Figures 1C and 1F). These data indicate that EE-mediated enhancement of progenitor cell proliferation in the DG is significantly attenuated by the expression of two independent FAD-linked PS1 variants.
Figure 1. Expression of FAD-linked PS1 variants impairs enrichment-induced proliferation of hippocampal neural progenitors.
(A - F) Photomicrographs of BrdU (green or yellow)+ cells in the DG of PS1hWT (A, D), PS1ΔE9 (B, E) and PS1M146L (C, F). SH, standard housing conditions (A, B, C); EE, enriched conditions (D, E, F). NeuN, red. Scale bar: 100 μm.
(G) Quantification of BrdU+ cells (mean ± SEM; N = 10 - 15 per group). * P < 0.05.
(H) Quantification of granule cell number. * P < 0.05.
(I) Photomicrograph of primary neurospheres. Scale bar: 50 μm.
(J) Image of nestin (green)+ neurosphere. Scale bar: 25 μm.
(K) Nuclei were stained with DAPI (blue).
(L) Percentage of multipotent neurospheres obtained in clonogenic neurosphere assay (mean ± SEM, N = 5 animals per group). ** P < 0.01.
We extended the latter observations to quantify the absolute number of granule cells in the DG of mice housed in either standard conditions or exposed to EE (Figure 1H). We did not find significant differences in granule cell numbers between the three transgenic lines maintained in standard housing or enriched conditions (Figure 1H). However, and in view of earlier studies showing that running is critical for enhancing neural progenitor cell (NPC) proliferation (van Praag et al., 1999), we examined granule cell numbers in the DG of mice that spent over 40% of their enrichment time on the running wheels (“highly active mice”), and observed that these PS1hWT mice contained, on average, greater than 270,000 granule cells, as opposed to approximately 240,000 granule cells in standard PS1hWT mice. On the other hand, mice expressing the PS1 variants that were “highly active” showed only a modest increase in total number of granule cells compared to standard housed mice and significantly lower than the numbers of dentate granule cells in “highly active” PS1hWT mice (Figure 1H).
To assess the specificity of the enrichment paradigm in mediating hippocampal NPC proliferation, we assessed the impact of expressing PS1hWT and the FAD-linked PS1 mutants on NPC proliferation in the SVZ, a neurogenic niche that is not subject to enrichment, or running-mediated proliferation of NPCs. NPC proliferation in the SVZ of PS1hWT or FAD-linked PS1 mutant mice is unchanged following enrichment (Figure S2A).
Finally, we tested the possibility that the impact of the PS1 variants on NPC proliferation may be mediated by the presence of Aβ peptides or toxic Aβ oligomers that might be generated as a consequence of PS1 mutant-mediated elevation in Aβ42/40 ratios. For these studies, we crossed the PS1hWT or PS1ΔE9 mice with APP-deficient mice [APP KO mice; (Zheng et al., 1995)]. We did not observe any significant differences in NPC proliferation amongst the PS1hWT/APPKO or PS1ΔE9/APPKO strains that were exposed to standard housing conditions (Figure S2B). More importantly, we neither observed significant differences in NPC proliferation between enriched PS1hWT and enriched PS1hWT/APPKO mice, nor between enriched PS1ΔE9 and enriched PS1ΔE9/APPKO mice. We conclude that the impaired NPC proliferation in mice expressing PS1 variants is independent of APP expression.
Expression of FAD-linked PS1 variants impairs EE-induced clonal proliferation of hippocampal progenitor cells in in vitro neurosphere assays
NPCs from hippocampus and other neurogenic regions of the adult mammalian brain can be isolated and grown in vitro as clonal sphere-forming cell colonies, termed neurospheres (Bull and Bartlett, 2005; Hitoshi et al., 2002). We cultured hippocampal NPCs from 4 to 8 weeks old male transgenic lines expressing PS1hWT or PS1 variants exposed to standard or enriched housing conditions (Figures 1I-1K). Western blot analysis of detergent-solubilized lysates confirmed that the transgenes expressed the human PS1 polypeptides in NPCs (Figure S3A).
No significant changes were observed in the percentage (~0.14%; 1 in every 6-8 × 103 cells) of neurospheres that appeared in cultures derived from each standard housed transgenic line (Figure 1L, left panel). However, a significant increase in the percentage of neurospheres was observed in the cell preparations derived from enriched PS1hWT mice (Figure 1L, right panel), findings fully consistent with the in vivo BrdU labeling studies (Figure 1G). More importantly, we did not observe an elevation in the fraction of neurospheres derived from plated populations of cells from hippocampi of enriched mice expressing the PS1 variants compared to their standard housed littermates (Figure 1L, right panel).
Collectively, our studies argue that the enrichment paradigm induces proliferation of progenitor cells in the hippocampus of PS1hWT mice and that this phenomenon is attenuated by expression of two independent FAD-linked PS1 variants.
FAD-linked PS1 mutants affect neuronal differentiation of progenitor cells in the DG
To determine whether FAD-linked PS1 variants affect the differentiation of newly-born progenitors, we performed double or triple labeling studies using anti-BrdU antibodies combined with antibodies specific for neuronal or glial lineages, two weeks after BrdU injection (Figure 2). We examined expression of the following markers: doublecortin (DCX), a marker of early neuronal differentiation; βIII-tubulin (TUJ1), a marker of both early and mature neurons; NeuN, a neuron-specific nuclear antigen associated with mature granule cells; glial fibrillary acidic protein (GFAP) and s100β, astrocyte-specific markers. The numbers of new DCX-, βIII-tubulin-, NeuN-, GFAP- or s100β-labeled cells were determined by multiplying the number of surviving BrdU-labeled cells by the fraction of cells that expressed each marker (Figures 2G, 2I, 2K, 2M and 2O). Standard mice expressing either PS1hWT or FAD-linked PS1 variants did not show any differences in the levels of differentiated cell types derived from newly-born progenitors. Quantification of BrdU-positive cells that are also DCX-positive revealed that both the fraction (Figure 2F) and the total number (Figure 2G) of colabeled cells were significantly lower in enriched mice expressing either of the FAD-linked PS1 variants compared to PS1hWT mice. Moreover, there appears to be a significantly lower fraction (Figure 2H) and number (Figure 2I) of BrdU-labeled cells that are immunopositive for βIII-tubulin within the DG of mice expressing the PS1 variants compared to PS1hWT mice. While we did not observe any difference in the fraction of BrdU- and NeuN-positive neurons between mice expressing PS1hWT and PS1ΔE9 (Figure 2J), the number of BrdU- and NeuN-positive neurons in the DG of mice expressing PS1ΔE9 is significantly reduced compared to PS1hWT (Figure 2K). Collectively, these data indicate that both the mutant PS1 lines produced significantly fewer neurons from newly-generated NPCs compared to the PS1hWT mice. We also observed that only a minor fraction of BrdU-positive cells were colabeled with GFAP (Figure 2L) or s100β (Figure 2N), and we found no significant differences between the groups. However, while there appears to be more GFAP and s100β cells produced in the enriched PS1hWT mice compared to the other five groups (Figures 2M, 2O, respectively), only the differences in GFAP cell numbers reached statistical significance (Figure 2M).
Figure 2. FAD-linked PS1 mutants alter differentiation pattern of neural progenitors.
(A - E) Images of BrdU+ cells that are colabeled with either DCX (A), βIII-tubulin (B), NeuN (C), GFAP (D) or s100β (E). Scale bar: 50 μm (A, B, D, and E) or 100 μm (C). (F, H, J, L, N) The fraction of BrdU+ cells that expressed DCX (F), βIII-tubulin (H), NeuN (J), GFAP (L), or s100β (N).
(G, I, K, M, O) The number of new DCX (G)-, βIII-tubulin (I)-, NeuN (K)-, GFAP (M)-, or s100β (O)-cells. * P < 0.05; ** P < 0.01. N = 8 per group.
FAD-linked PS1 mutant mice fail to exhibit high activity-induced increases in microglial cell numbers
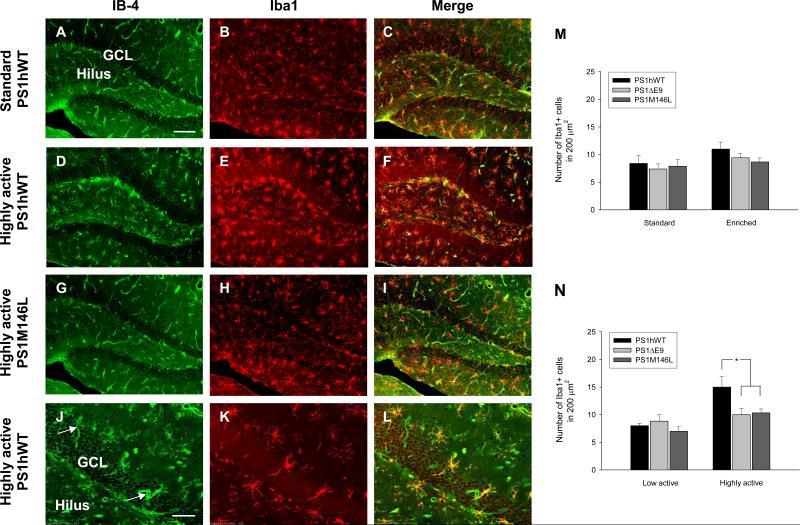
Recent studies have revealed a pivotal role for microglia in activation and maintenance of adult hippocampal neurogenesis in rodents following exposure to EE conditions (Ziv et al., 2006). The fact that the PrP promoter-driven PSEN1 transgenes are ubiquitously expressed in neuronal and non-neuronal cells (Figure S1) in the CNS prompted us to examine the influence of EE on microglia expressing FAD-linked PS1 variants. For these studies, we employed the microglial markers, Isolectin B-4 (IB-4; Figures 3A, 3D, and 3G) and Iba1 (Figures 3B, 3E, and 3H). Notably, IB-4 detects microglia and endothelial cells (Streit and Kreutzberg, 1987); see our Figure 3J (arrow) and compare to Iba1 immunoreactive profile in Figure 3K. Further, the signal observed with IB-4 was very weak compared to Iba1 staining (compare Figures 3D and 3E) and was prominent in samples that showed strong Iba1 immunoreactivity (compare Figures 3D with 3A or 3G). To avoid issues with regard to specificity and signal to noise, we chose to count Iba1-positive microglial cells in the hilus, SGL, and GCL (Figures 3B, 3E, and 3H). We did not find any significant differences in microglia numbers between the standard housed or enriched lines expressing either PS1hWT or PS1 variants (Figure 3M). However, we observed a significant increase in the number of Iba1-positive microglia in the DG of highly active PS1hWT mice in the enriched group compared to enriched, but low active, PS1hWT mice (Figure 3N). More importantly, the number of Iba1-positive microglia in the DG of highly active FAD-linked PS1 variants was significantly lower than highly active PS1hWT mice (Figure 3N). We then examined whether there might be potential differences in proliferation of microglia in the DG by performing double-labeling for BrdU and Iba1, but found no significant differences amongst all the groups (data not shown).
Figure 3. FAD-linked PS1 mutant mice fail to exhibit high activity-induced increase in microglial cell numbers.
(A - L) Images of microglia in DG of standard PS1hWT (A, B, and C), highly active PS1hWT (D, E, and F), highly active PS1M146L (G, H, and I), and highly active PS1hWT (high magnification, J, K, and L). Microglia are labeled with IB-4 (green; A, D, G, and J) and Iba1 (red; B, E, H, and K). Merged images are shown in (C, F, I, and L). Scale bars: 100 μm in A; 50 μm in J.
(M) Quantification of Iba1+ cells in standard or enriched PS1hWT and FAD-linked PS1 variants (mean ± SEM; N = 10 - 15 per group).
(N) Quantification of Iba1+ cells in highly active or low active PS1hWT and FAD-linked PS1 variants. * P < 0.05.
Microglia expressing FAD-linked PS1 variants impairs proliferation of NPCs in in vitro co-culture assays
We considered the possibility that microglia from mice expressing mutant PS1 may have differential responses to pro- or anti-inflammatory processes that occur as a consequence of EE that may, in turn, affect the proliferation or cell fate determination of neural progenitors in vivo. Thus, we chose to extend our in vivo observations by testing the ability of cultured primary microglia from mice expressing PS1hWT or PS1 variants to differentially regulate the proliferation of hippocampal-derived NPCs, in vitro. We first measured the basal proliferation of NPCs cultured from the hippocampi of 4 to 8 week old mice expressing PS1hWT or PS1 variants. Single cell suspensions of primary neurospheres were incubated with BrdU and proliferation was determined by measuring BrdU incorporation over 6 days using an ELISA-based assay. We failed to observe any significant differences in the proliferation rates between NPCs expressing PS1hWT or PS1 variants (Figure 4A). In view of these findings, the observed increase in percentage of neurosphere forming cells from PS1hWT mice following enrichment (Figures 1L, above) is not because of inherent differences in the rates of proliferation of NPCs, in vitro, but rather reflects an overall increase in the size of the neural progenitor pool, in vivo.
Figure 4. Microglia expressing FAD-linked PS1 variants impair proliferation of NPCs in in vitro co-culture assays.
(A) Proliferation achieved by NPCs, expressing PS1hWT, PS1ΔE9 or PS1M146L, grown in SFM supplemented with BrdU (mean ± SEM from a minimum of three experiments per group).
(B - D) IB-4+ microglial cells (B, green) and nestin+ NPCs (C, red) under co-culture conditions. The merged image is shown in D. Fibrillar staining of the intermediate filament protein, Nestin, is evident on NPCs (arrow head) but not on microglial cells (arrow). Scale bar: 10 μm.
(E - F) Proliferation of PS1hWT NPCs when co-cultured with AraC-treated resting (E) or IL4-activated (F) microglia (MG) expressing PS1hWT, PS1ΔE9 or PS1M146L as measured by BrdU labeling. ** indicates significant difference from PS1hWT MG at P < 0.01 on day 3 or 6.
(G - J) Confocal z-series reconstructed image of cells stained with IB-4 (G), anti-nestin (H), anti-BrdU antibody (I) and DAPI (J), following 6 days under co-culturing conditions.
(K - N) Confocal images of a single z-plane within nestin+ neural progenitor cluster (K; red, arrow head) reveals several BrdU+ progenitors (K; cyan blue, enclosed within circle) and an IB-4+ MG (K; green, arrow). Insert shows the zoom-in area marked within dotted square (L). Scale bar: 50 μm.
(M - N) Zoomed images of cells that are co-labeled with BrdU/nestin (M) or BrdU/IB-4 (N). Scale bar: 10 μm.
(O - P) Quantification of BrdU and nestin (O) or BrdU and IB-4 (P) co-labeled cells in the co-culture assay. The asterisk indicates significant difference from PS1hWT MG at * P < 0.05 and ** P < 0.01.
We then chose to examine the potential role of microglia in NPC proliferation using a co-culture assay that employed primary microglial cultures from hippocampi of neonatal transgenic pups (Figures S4A and S4B). The expression of PrP promoter-driven human PS1 transgenes were confirmed in microglia cultures (Figure S3B) and these findings mirrored the results observed with primary NPC cultures (Figure S3A). Previous studies have suggested that depending on the physiological stimuli, microglia can differentially influence the fate of NPCs, in culture (Butovsky et al., 2006). In particular, priming cultured microglia with a T-helper cell type 2 (TH-2)-derived cytokine, interlukin-4 (IL4), promotes neuroprotective and anti-inflammatory phenotype of microglia. Protection by IL4 has been partly attributed to increased production of insulin-like growth factor 1 (IGF-1), a growth factor that has been linked with adult neurogenesis (Anderson et al., 2002; Trejo et al., 2001). Notably, the phenotype of neuroprotective microglia observed in the DG of rodents housed in the EE resembles that of IL4-primed cultured microglia that express IGF-1 (Ziv et al., 2006). With these observations in mind, we examined the effects of microgila expressing PS1hWT and PS1 variants on proliferation of neural progentiors cultured under resting conditions or after priming with IL4.
Treatment of PS1hWT microglia with IL4 led to their activation, as confirmed by an increase in the Iba1 expression (Figure S4B, compare panels iii and iv). In addition, IL4-treated microglia also induced IGF-1 transcript levels (Figure S4C), providing evidence that the activated microglia used in our studies exhibit neuroprotective, anti-inflammatory microglial phenotypes (Ziv et al., 2006). Induction of IGF-1 was accompanied by an increase in transcripts encoding arginase-1 (Figure S4D), previously shown to be induced by IL4-mediated activation of the IL4 receptor (Gray et al., 2005; Pauleau et al., 2004; Wynes and Riches, 2003).
In co-culture assays, we used the BrdU-uptake assay to examine the proliferation of NPC expressing PS1hWT in the presence of resting (Figure 4E) or IL4-activated (Figure 4F) microglia derived from mice expressing either PS1hWT or PS1 variants (Figure 4A). Prior to co-culture, the enriched microglial cell preparations were treated with irreversible cell cycle inhibitor, AraC, in order to arrest their growth. Representative photomicrographs, shown in Figures 4B-4D, reveals the existence of nestin-positive NPCs with IB-4-positive microglia in these co-culture assays. While the extent of BrdU incorporation was similar across all NPC-microglia co-culture combinations at 1 day, the proliferation of PS1hWT expressing NPC was significantly retarded at days 3 and 6 in the presence of resting or IL4-treated microglia that were derived from neonatal pups expressing PS1 variants (Figures 4E and 4F). To verify that BrdU incorporation was selective for NPCs, we immunostained fixed co-cultures with IB-4, nestin and BrdU antibodies. Immunofluorescence microscopy revealed that the majority of BrdU-positive cells within the neural progenitor cluster following 6 days in co-culture conditions were within the neurosphere (Figure S5). Confocal laser z- series image reconstruction of these co-cultures revealed distinct staining of IB-4-positive microglial cells (Figures 4G) within the nestin (Figure 4H) and BrdU-positive (Figure 4I) neural progenitor cluster. Neural progenitors co-labeled with nestin and BrdU (Figures 4K and 4M), and microglia co-labeled with IB-4 and BrdU (Figures 4N) were identified from each focal plane and quantified by sampling a minimum of five size matched neural progenitor clusters and 200 IB-4-positive microglial cells, respectively. Quantification of cells co-labeled with BrdU and nestin revealed that proliferation of PS1hWT NPC was significantly inhibited on days 3 and 6 when co-cultured with microglia expressing PS1 variants (Figure 4O). BrdU and IB-4 staining revealed no significant differences across the genotypes over the 6 day period (Figure 4P). Thus, our microscopic co-labeling studies reveal that the vast majority of BrdU-labeled cells in the NPC-microglial co-cultures are the NPCs. More importantly, these data fully support our ELISA-based BrdU-incorporation assays (Figures 4E and 4F) and argue that expression of FAD-linked PS1 variants impair proliferation of NPCs expressing PS1hWT.
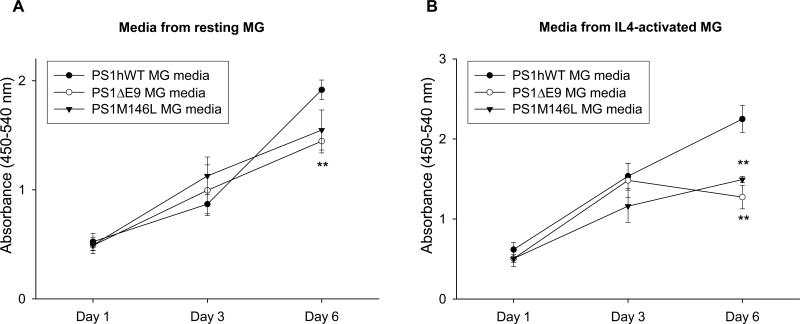
Conditioned media from microglia expressing FAD-linked PS1 variants impairs proliferation of NPCs in in vitro assays and in organotypic slice cultures
Extending the co-culture studies, we chose to ask whether factors secreted by microglia may be involved in regulating proliferation of NPC. For these studies, we measured the growth rate of NPCs expressing PS1hWT that were exposed to conditioned media (CM) collected from resting (Figure 5A) or IL4-activated (Figure 5B) microglial cells derived from neonatal mice expressing PS1hWT or PS1 variants. By day 1 and 3, the extent of BrdU incorporation was similar across NPCs treated with CM collected from resting microglia from each of the lines, but by day 6, proliferation of NPC was significantly retarded in the presence of CM from microglia that express PS1ΔE9 (Figure 5A). Likewise, significant retardation in NPC proliferation was observed by day 6 when exposed to CM collected from IL4-activated microglia expressing both PS1 variants, but not PS1hWT (Figure 5B). These studies lead us to conclude that the pronounced effects of microglia expressing PS1 mutants on NPC proliferation can be attributed to factors secreted by the mutant PS1 microglia. To investigate whether secreted factors from PS1 mutant microglia could affect NPC proliferation in a more physiological setting, we employed organotypic slice cultures (Figure S6) (Namba et al., 2007; Raineteau et al., 2004; Tozuka et al., 2005). In this setting, we demonstrate that in contrast to slices treated with resting or IL4-activated PS1hWT microglia CM, the percentage of BrdU- and DCX-labeled NPCs per total BrdU-labeled cells were significantly reduced when the slices were exposed to resting or IL4-activated CM collected from PS1ΔE9 microglia (Figure S6). Collectively, these data indicate that soluble factors secreted from PS1ΔE9 microglia impair proliferation of PS1hWT NPCs in in vitro primary culture studies and in ex vivo organotypic slice cultures.
Figure 5. Conditioned media from microglia expressing FAD-linked PS1 variants impair proliferation of neural progenitors.
(A - B) Proliferation achieved by PS1hWT NPCs following treatment with conditioned media collected from resting (A) or IL4-activated (B) microglia (MG) expressing mentioned PS1 transgenes, as measured by BrdU labeling. ** indicates significant difference from PS1hWT MG media at P < 0.01 on day 3 or 6.
Factors Secreted from Activated microglia expressing FAD-linked PS1 mutants impairs in vitro neuronal differentiation of NPCs
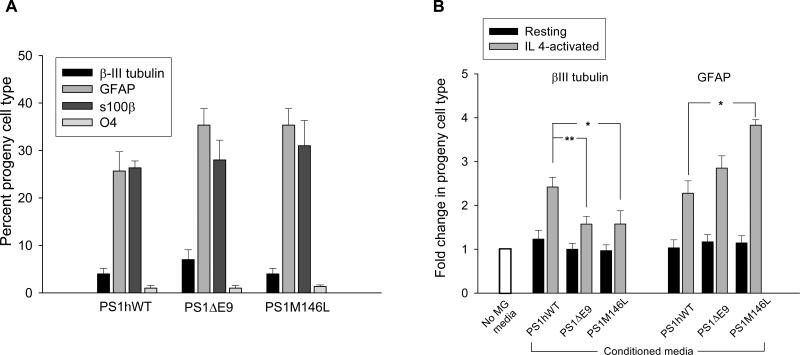
We sought to determine whether FAD-linked PS1 variants affect the intrinsic differentiation potential of cultured neural progenitors by differentiating single cell suspensions of primary neurospheres derived from mice expressing PS1hWT or PS1 variants by supplementing the medium with 3% fetal bovine serum. After 10 days, fixed cells were immunostained with antibodies that detect neurons (βIII-tubulin), oligodendrocytes (O4), astrocytes (GFAP), or astroglia (s100β) (Figures S7A-S7D). Across all the tested genotypes, quantitative analysis revealed 4.5 to 6%, 1 to 2%, 24 to 27%, and 27 to 30% of the sampled cells in each culture were positive for βIII-tubulin, O4, GFAP, and s100β lineage markers, respectively (Figure 6A). Importantly, we did not observe any significant differences in distinct lineage specific differentiation of NPCs that express either PS1hWT or FAD-linked PS1 variants.
Figure 6. Differentiation pattern of cultured NPCs is modulated by FAD-linked PS1 mutant expressing microglia.
(A) Progeny cell types generated by NPCs in the multipotency assay were quantified by sampling a minimum of 300 cells as marked by DAPI or propidium iodide+ nuclei (mean ± SEM of the results obtained from independent cultures established from 6 animals per group).
(B) Fold changes in the number of βIII-tubulin+ neuronal or GFAP+ astrocyte progeny cell type derived from PS1hWT NPCs exposed to CM collected from resting or IL4-activated MG, over differentiated PS1hWT NPC cultures in the absence of MG CM (mean ± SEM; N = 6). The asterisk indicates significant difference from PS1hWT MG CM at * P < 0.05 and ** P < 0.01.
We then investigated whether the in vitro differentiation pattern of NPCs expressing PS1hWT could be modulated by soluble factors secreted by resting or IL4-activated microglia from mice expressing either PS1hWT or PS1 variants. PS1hWT NPCs were treated with CM for 12 days, fixed and subject to indirect immunofluorescence microscopy assess commitment towards βIII-tubulin-positive or GFAP-positive lineages (see Figure S7E). Quantitative analysis showed no significant differences in the differentiation pattern of PS1hWT NPCs when exposed to CM collected from resting microglia that express either PS1hWT or PS1 variants (Figure 6B). On the other hand, we observed an ~2.4-fold increase in the number of βIII-tubulin-positive cells when NPCs were exposed to CM collected from IL4-activated PS1hWT microglia (Figure 6B, left panel), but only an ~1.5-fold increase in βIII-tubulin-positive cells when NPCs were exposed to CM from IL4-activated microglia expressing the PS1 variants. Interestingly, in comparison with CM collected from PS1hWT microglia, PS1M146L expressing microglia media were significantly more potent in driving NPC differentiation towards astrocytes (Figure 6B, right panel).The data obtained from CM studies indicate that IL4-activated FAD-linked PS1 expressing microglia fail to sustain differentiation of NPCs towards the neurogenic fate, but promote differentiation towards the astrocyte lineage in in vitro multipotency assays.
Differences in the profile of secreted signaling proteins in PS1hWT and FAD-linked PS1 variant microglia
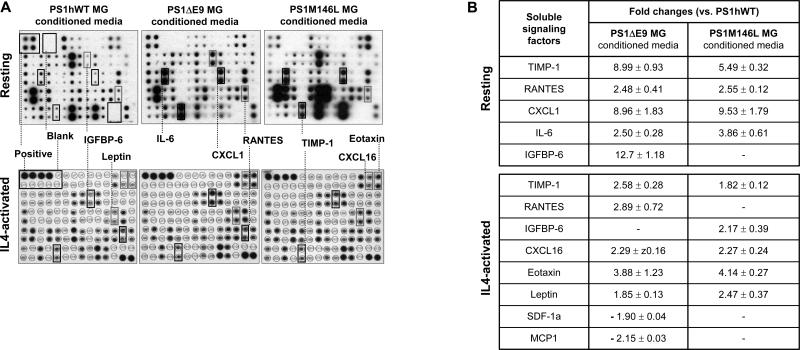
As it is well established that microglia secrete a variety of cytokines and other soluble factors, including endotoxins and chemokines, in response to physiological stimuli, we then examined the relative levels of 62 different cytokines and secreted signaling factors in the CM collected from resting or IL4-activated microglial cultures using a biotin-label based protein antibody array [Figure 7A; (Lin et al., 2003)]. Analysis of protein array profiles revealed that the signal intensity was below detectable threshold for about 24% of candidate proteins. Of the detectable candidates, 58% revealed no change in signal intensity. Interestingly, in comparison with the levels observed in resting or IL4-activated PS1hWT microglia CM, less than 11% of the candidate proteins revealed reproducible increases or decreases in expression levels of at least 1.4-fold in the CM from the PS1 variants (Figure 7B; see list in Table S1).
Figure 7. Quantitative changes in signaling factors secreted by PS1hWT versus FAD-linked PS1 variant expressing microglia.
(A) Images of biotin-label based protein antibody arrays probed with CM collected from resting or IL4-activated microglia (MG).
(B) Soluble factors whose levels were consistently altered (up or down ‘-’; mean ± SEM of fold changes observed across three experiments). - denotes, no detectable change in mean signal intensity.
TIMP-1 (a protease inhibitor - Tissue inhibitor of metalloprotease), RANTES (a chemokine - Regulated upon activation, Normal T cell expressed, and secreted, or CCL5), CXCL1 (a small cytokine - Chemokine CXC ligand 1), IL-6 (a pro-inflammatory cytokine - Interlukin 6) and IGFBP-6 (Insulin like growth factor binding protein) were consistently elevated in CM collected from resting microglia expressing PS1 variants (Figure 7B, upper panel). Interestingly, in addition to TIMP-1, RANTES and IGFBP-6, several other soluble signaling factors like CXCL16 (a cytokine - Chemokine CXC ligand 16), Eotaxin (a chemokine) and Leptin (a protein hormone) were increased in CM from IL4-activated microglia expressing both PS1 variants (Figure 7B, lower panel).
Increased levels of Fas L and decreased levels of MCP-1 (a chemokine - Monocyte chemoattractant protein-1), SDF-1a (a chemokine - Stromal derived factor-1 alpha) and Lymphotactin were exclusively altered in CM collected from IL4-primed PS1ΔE9 microglia (Table S1B). Likewise, elevated levels of IL (interleukin)-1α and IGFBP-6, and lower levels of MCP-5 and IL-17 were unique to CM collected from IL4-primed PS1M146L microglia. Several of these factors have been attributed to the activated status of microglia. It is also worth noting that MCP-1, SDF-1a, Eotaxin, and TIMPs have been linked to NPC biology (Belmadani et al., 2006; Cacci et al., 2008; Edman et al., 2008; Krathwohl and Kaiser, 2004; Nakanishi et al., 2007; Perez-Martinez and Jaworski, 2005; Tran et al., 2004; Vallieres et al., 2002; Zhang et al., 2007).
We then asked whether we could assess the contributions of specific factors that exhibited altered levels in the medium of IL4-activated microglia expressing PS1 variants on proliferation of NPCs in vitro. Since the absolute levels of each factor in microglia CM were not known, PS1hWT NPCs were treated with 1, 5 or 10 ng/ml of purified, biologically active factors, and proliferation was measured over 6 days. Factors were tested individually, or in combination and we identified several factors that strongly inhibited NPC proliferation (see Figures S8 and S9). Further studies using blocking antibodies or shRNA approaches are required to fully establish the identity of the critical factors responsible for the observed outcomes.
Transcripts encoding soluble factors in cultured neonatal and adult microglia
To assess potential mechanisms that underlie the alterations in secreted signaling proteins, we first asked whether these changes might be reflected in levels of transcripts encoding these polypeptides. Total RNA, prepared from resting or IL4-activated microglia expressing PS1hWT or PS1 variants were subject to Q-PCR analysis. Consistent with the protein array studies, the levels of transcripts encoding TIMP-1, RANTES, IL-6 and CXCL1 were elevated in resting microglia expressing FAD-linked PS1 mutants (Table 1A, upper panel). In the case of IL4-primed microglia, we were able to detect an increase in TIMP-1, CXCL16 and Eotaxin transcript levels (Table 1A, lower panel). The levels of MCP-1 transcripts were remarkably reduced in IL4-activated microglia expressing PS1ΔE9. We then investigated whether the alterations in a subset of transcripts observed in cultured microglia could be extrapolated to microglia from hippocampi of standard or enriched adult transgenic mice. Microglia were purified using a discontinuous percoll gradient method (Cardona et al., 2006), and transcript levels were examined by Q-PCR analysis. In comparison to PS1hWT microglia, an increase in TIMP-1 and CXCL1 transcripts were detected in standard housed PS1ΔE9 animals (Table 1B, upper panel). Remarkably, in addition to TIMP-1 and CXCL1, we also detected elevated levels of transcripts encoding Eotaxin, Leptin, CXCL16, RANTES and MCP-1 in microglia isolated from enriched PS1ΔE9 animals (Table 1B, lower panel). A subset of the transcripts shown to be elevated in microglia from enriched PS1ΔE9 mice were also elevated in microglia from enriched PS1M146L animals (Table 1B, lower panel). Notably, we demonstrate that while enrichment appears to induce IL4 signaling in adult microglia expressing PS1hWT, as revealed by elevated levels of transcripts encoding arginase-1 and IGF-1 (Figure S10), there appears to be no significant difference in levels compared to levels in microglia isolated from enriched PS1 mutant animals. These data indicate that IL4 receptor signaling is intact in adult microglia from enriched mice expressing either PS1hWT or PS1 mutants, findings that confirm our earlier analysis of IL4-activated cultured neonatal microglia (Figures S4C and S4D).
Table 1. Differences in the levels of transcripts encoding soluble factors in cultured and adult microglia derived from PS1hWT and FAD-linked PS1 variant transgenic mice.
(A) Threshold PCR cycle Ct value for the genes examined in resting or IL4-activated cultured neonatal microglia (MG) (mean ± SEM of Ct values obtained from three independent Q-PCR experiments). Relative fold changes are mentioned within brackets; up ‘+’ or down ‘-’. (B) Fold change in transcript levels for mentioned genes in MG isolated from the hippocampus of adult mutant PS1 over PS1hWT transgenic animals following exposure to standard (SH) or enriched environment (EE) (mean ± SEM of fold change obtained from three independent Q-PCR experiments). - denotes, not determined.
| A | ||||
|---|---|---|---|---|
| Genes examined | Threshold cycle Ct-value | |||
| PS1hWT MG | PS1ΔE9 MG | PS1M146L MG | ||
| Resting | Actin | 15.63 ± 0.29 | 20.60 ± 0.25 | 16.36 ± 0.44 |
| TIMP-1 | 32.66 ± 0.27 | 34.13 ± 0.12 (+ 11.30 fold) | 32.83 ± 0.14 (+ 1.47 fold) | |
| RANTES | 26.7 ± 0.11 | 22.63 ± 0.12 (+15.6 fold) | 17.90 ± 0.05 (+22.3 fold) | |
| IL-6 | 23.73 ± 0.06 | 22.56 ± 0.08 (+4 fold) | 21.66 ± 0.06 (+ 6.9 fold) | |
| CXCL1 | 26.36 ± 0.12 | 20.90 ± 0.30 (+10.56 fold) | 20.86 ± 0.51 (+8.66 fold) | |
| IL4-activated | GAPDH | 22.2 ± 0.11 | 22.76 ± 0.06 | 22.26 ± 0.37 |
| TIMP-1 | 32.66 ±0.14 | 29.20 ± 0.55 (+11.4 fold) | 26.50 ± 0.11 (+3.3 fold) | |
| CXCL16 | 28.46 ± 0.66 | 24.96 ± 0.14 (+13.9 fold) | 23.26 ± 0.06 (+32 fold) | |
| Eotaxin | 34.50 ±0.14 | 30.26 ± 0.06 (+8 fold) | 30.70 ± 0.10 (+11 fold) | |
| MCP1 | 27.63 ± 0.08 | 33.6 ± 0.15 (-32 fold) | - |
| B | |||
|---|---|---|---|
| Genes examined | Fold change in transcript levels (vs. PS1hWT MG) | ||
| PS1ΔE9 MG | PS1M146L MG | ||
| MG from SH adult mouse hippocampus | TIMP-1 | 6.35 ± 2.82 | 9.50 ± 2.25 |
| CXCL1 | 5.43 ±2.16 | 14.45 ± 2.03 | |
| MG from EE adult mouse hippocampus | TIMP-1 | 18.05 ± 8.15 | 33.31 ± 10.97 |
| RANTES | 2.23 ± 0.31 | - | |
| CXCL1 | 4.01 ± 1.25 | 24.05 ± 8.91 | |
| Eotaxin | 2.28 ± 0.86 | 1.92 ± 0.29 | |
| Leptin | 4.04 ±1.15 | 14.54 ± 6.56 | |
| CXCL16 | 2.33 ± 0.62 | 9.61 ± 3.64 | |
| MCP-1 | -1.30 ± 0.06 | - |
DISCUSSION
We have examined transgenic mice raised in either standard conditions, or exposed to an enriched environment (EE) to explore the role of wild-type or two independent FAD-linked mutant human PS1 variants in the regulation of hippocampal neurogenesis. Emerging from these efforts are several novel insights. First, we report that expression of mouse PrP promoter-driven transgenes expressing FAD-linked mutant PS1 impairs EE-induced neural progenitor cell (NPC) proliferation and neuronal differentiation in the adult hippocampus. Second, we show that the proliferation and differentiation of purified NPCs from adult transgenic mice expressing either PS1hWT or PS1 variants are indistinguishable, findings which suggest that these properties of NPCs are dictated by local cues within the stem cell niche, in vivo. Third, we show that microglia isolated from neonatal transgenic mice expressing FAD-linked PS1 mutants inhibit proliferation and impair neuronal lineage commitment of cultured NPCs in vitro, and impairs neuronal differentiation of progenitors in ex vivo organotypic slices. Fourth, we report significant changes in the levels of secreted factors released by microglia expressing FAD-linked PS1 variants compared to microglia expressing PS1hWT. Indeed, a subset of purified factors that are elevated in medium of IL4-activated mutant microglia resulted in a strong inhibition of NPC proliferation. Fifth, we demonstrate that the elevated levels of secreted signaling molecules in medium of PS1 mutant microglia is paralleled by elevated steady-state levels of transcripts encoding these polypeptides. Finally, we document that many of the transcripts observed to be elevated in cultured neonatal microglia from PS1 mutant mice are also elevated in adult microglia obtained from PS1 mutant mice following EE. We conclude that alterations in levels of signaling molecules secreted by microglia may, at least in part, be responsible for the defective neurogenesis observed with FAD-linked mutant PS1 in vivo, findings that support a non-cell-autonomous role for mutant PS1 in hippocampal neurogenesis.
Despite the strengths of our conclusions, it is notable that our data on the impact of FAD-linked PS1 on EE-mediated NPC proliferation and neurogenesis are not fully consistent with reports in the literature that have offered rather differing outcomes. As an example, Wen et al (2004) reported that enriched mice expressing the PS1M117L variant exhibited elevated levels of cell proliferation in the DG, compared to PS1hWT mice (Wen et al., 2004). The differences in outcomes observed between our laboratory and those of earlier reports may be a reflection of differences in methodology, including the enrichment protocol and BrdU injection schedule [for review, see (van Praag, 2008)]. However, we feel the most salient differences relates to the promoter elements that drive expression of the PSEN1 transgenes. In the case of the studies of Wen et al (2004), the promoter elements were derived from NSE genes that are preferentially expressed in mature neurons but not NPCs, or nonneuronal cells. In contrast, we have employed a mouse prion promoter that with the exception of Purkinje cells, is ubiquitously expressed in both neuronal and non neuronal cells in the brain in a pattern that very closely reflects the distribution of endogenous mouse PS1 (Borchelt et al., 1996a). In this regard, it has become increasingly evident that microglia play a critical role in hippocampal neurogenesis; in response to physiological stimuli, microglia get activated, proliferate (Ehninger and Kempermann, 2003) and localize in close proximity to the subgranular zone (stem cell niche) of the DG (Ziv et al., 2006) where they produce soluble factors that promote differentiation of neural progenitors (Aarum et al., 2003; Battista et al., 2006; Nakanishi et al., 2007; Walton et al., 2006). Hence, we felt it was critical to examine the role of microglia expressing either PS1hWT or mutant PS1 variants in adult neurogenesis. Using an established co-culture paradigm, we observed that proliferation of cultured PS1hWT NPC was strongly inhibited when co-cultured with resting or IL4-activated microglia that express the PS1 variants. Indeed, the conditioned media (CM) from IL-4 activated microglia expressing PS1 variants also inhibits NPC proliferation and neuronal differentiation in vitro.
Using a cytokine antibody array assay, we identified quantitative differences in the expression profiles of several secreted signaling molecules in the CM of IL4-activated PS1hWT versus FAD-linked PS1 microglia. For example, RANTES and Eotaxin, that are elevated in mutant PS1 microglia medium, have been shown to promote quiescence of cultured human NPCs and progenitor cells in hippocampal slices (Krathwohl and Kaiser, 2004), while decreased levels of the MCP family members, MCP1 and MCP5 could result in the impaired infiltration and migration of microglia into the hippocampus following exposure to enriched housing conditions. Indeed, we readily observed significant differences in the numbers of Iba1-positive microglia in the hippocampus of highly active enriched mice expressing PS1hWT compared to highly active enriched mice expressing PS1 variants. Notwithstanding the important roles for microglia in regulating NPC proliferation and differentiation, in vivo, it should be noted that other cell populations in the neural stem cell niche are likely to contribute to the observed phenotypes. For example, endothelial cells have been shown to release soluble factors that stimulate the self-renewal of neural stem cells and alter their differentiation (Shen et al., 2004). Furthermore, it has become evident that astrocytes in the subgranular layer express Wnt3, a factor that plays a critical role in promoting neurogenesis of hippocampal NPCs by activating the Wnt/β-catenin pathway, components of which are present in NPCs in vivo (Lie et al., 2005). Indeed, while it is clear that mutant PS1 microglia CM-drives NPC differentiation towards astrocytic lineages in vitro, this appears not to be the case in vivo, leading us to suggest that the differences in these two settings is a reflection of differences in factors present within microglia culture medium versus those derived from other cells within the hippocampal niche.
The mechanism(s) by which expression of FAD-linked mutant PS1 leads to impairments in proliferation and neuronal differentiation of NPCs in vivo are not fully established. PS1 is the catalytic subunit of the γ-secretase complex responsible for intramembranous processing of a variety of type I membrane proteins, including Notch 1, and processing of these substrates are largely compromised in cells expressing FAD-linked PS1 variants (Moehlmann et al., 2002; Song et al., 1999). It should be noted that while the Notch signaling pathway has been implicated in microglial activation and cytokine secretion, we have neither detected the presence of transcripts encoding the Notch ligand, Jagged 1, nor have we observed any increase in levels of transcripts encoding the Notch target gene product, Hes1, following IL4 activation of purified microglia from PS1hWT or PS1 mutant mice (data not shown), and hence, we are quite certain that the effects we have observed are independent of Notch signaling.
While the bulk of our data point to alterations in levels of microglial-secreted signaling factors that are responsible for the differences in NPC proliferation and differentiation, it is equally plausible that cell-intrinsic signaling within NPCs also contribute to the observed phenotypes in vivo. In this regard, hippocampal NPCs express receptors and signaling components for the Wnt/β-catenin pathway, and Wnt3 is sufficient to increase neurogenesis from these cells in vitro and in vivo (Lie et al., 2005). In this regard, studies have revealed that both wild-type and FAD mutant PS1 interact with β-catenin in transfected cells and brains of transgenic mice (Kang et al., 1999) and wild-type PS1 regulates β-catenin stability in vitro and in vivo (Kang et al., 2002). Finally, it should be noted that Notch and components of the Notch signaling pathway, including Jagged 1, Delta 1, CSL (CBF 1/Su(H)/Lag 2), Hes 1 and Hes 5 are expressed in cultured NPCs and it is conceivable that cross-talk between the Notch and Wnt/β-catenin signaling pathways (Carlson and Conboy, 2007) could have an impact on the proliferative and differentiative properties of hippocampal NPCs, in vivo.
In summary, our data offer strong support for the view that expression of FAD-linked mutant PS1 impairs proliferation and neuronal differentiation of hippocampal NPCs by non-cell-autonomous mechanism(s). Future investigations of the role of FAD-linked PS1 on adult hippocampal neurogenesis will have broad implications for understanding the cellular and biochemical substrates that underlie the signaling events in the hippocampal neurogenic niche during normal aging and in the setting of age-associated neurodegenerative disorders, such as AD.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank John Robinson and Yi Huang for excellent technical assistance, and Dr. Linda Van Eldik and Ling Guo (Northwestern University School of Medicine, Chicago, IL) for help with neonatal microglial cultures. The authors also thank Dr. Vytas Bindokas and Dr. Christine Labno for expert technical assistance with confocal microscopy. This work was supported by National Institute of Health grants AG021494 (SSS) and AG027854 (SSS), the Edward H. Levi Fund (SHC), Alzheimer's Association New Investigator Research Grant (OL), Cure Alzheimer's Fund (SSS), Brain Research Foundation (SSS), and the Adler Foundation (KV). The corresponding author (SSS) discloses that he is a paid Consultant of Neuropharma, Inc., Torrey Pines Therapeutics and Eisai Research Labs Inc, but does not a shareholder in any company that is a maker or owner of a FDA-regulated drug or device.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci U S A. 2003;100:15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MF, Aberg MA, Nilsson M, Eriksson PS. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Brain Res Dev Brain Res. 2002;134:115–122. doi: 10.1016/s0165-3806(02)00277-8. [DOI] [PubMed] [Google Scholar]
- Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23:83–93. doi: 10.1111/j.1460-9568.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- Belmadani A, Tran PB, Ren D, Miller RJ. Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J Neurosci. 2006;26:3182–3191. doi: 10.1523/JNEUROSCI.0156-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchelt DR, Davis J, Fischer M, Lee MK, Slunt HH, Ratovitsky T, Regard J, Copeland NG, Jenkins NA, Sisodia SS, Price DL. A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genet Anal. 1996a;13:159–163. doi: 10.1016/s1050-3862(96)00167-2. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, et al. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996b;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- Bull ND, Bartlett PF. The adult mouse hippocampal progenitor is neurogenic but not a stem cell. J Neurosci. 2005;25:10815–10821. doi: 10.1523/JNEUROSCI.3249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Cacci E, Ajmone-Cat MA, Anelli T, Biagioni S, Minghetti L. In vitro neuronal and glial differentiation from embryonic or adult neural precursor cells are differently affected by chronic or acute activation of microglia. Glia. 2008;56:412–425. doi: 10.1002/glia.20616. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Huang D, Sasse ME, Ransohoff RM. Isolation of murine microglial cells for RNA analysis or flow cytometry. Nat Protoc. 2006;1:1947–1951. doi: 10.1038/nprot.2006.327. [DOI] [PubMed] [Google Scholar]
- Carlson ME, Conboy IM. Regulating the Notch pathway in embryonic, adult and old stem cells. Current opinion in pharmacology. 2007;7:303–309. doi: 10.1016/j.coph.2007.02.004. [DOI] [PubMed] [Google Scholar]
- De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin Generate an Active gamma- Secretase Complex. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- Edman LC, Mira H, Arenas E. The beta-chemokines CCL2 and CCL7 are two novel differentiation factors for midbrain dopaminergic precursors and neurons. Exp Cell Res. 2008 doi: 10.1016/j.yexcr.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Kempermann G. Regional effects of wheel running and environmental enrichment on cell genesis and microglia proliferation in the adult murine neocortex. Cereb Cortex. 2003;13:845–851. doi: 10.1093/cercor/13.8.845. [DOI] [PubMed] [Google Scholar]
- Feng R, Rampon C, Tang YP, Shrom D, Jin J, Kyin M, Sopher B, Miller MW, Ware CB, Martin GM, et al. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron. 2001;32:911–926. doi: 10.1016/s0896-6273(01)00523-2. [DOI] [PubMed] [Google Scholar]
- Gray MJ, Poljakovic M, Kepka-Lenhart D, Morris SM., Jr. Induction of arginase I transcription by IL-4 requires a composite DNA response element for STAT6 and C/EBPbeta. Gene. 2005;353:98–106. doi: 10.1016/j.gene.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Handler M, Yang X, Shen J. Presenilin-1 regulates neuronal differentiation during neurogenesis. Development. 2000;127:2593–2606. doi: 10.1242/dev.127.12.2593. [DOI] [PubMed] [Google Scholar]
- Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16:846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DE, Soriano S, Frosch MP, Collins T, Naruse S, Sisodia SS, Leibowitz G, Levine F, Koo EH. Presenilin 1 facilitates the constitutive turnover of beta-catenin: differential activity of Alzheimer's disease-linked PS1 mutants in the beta-catenin-signaling pathway. J Neurosci. 1999;19:4229–4237. doi: 10.1523/JNEUROSCI.19-11-04229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DE, Soriano S, Xia X, Eberhart CG, De Strooper B, Zheng H, Koo EH. Presenilin couples the paired phosphorylation of beta-catenin independent of axin: implications for beta-catenin activation in tumorigenesis. Cell. 2002;110:751–762. doi: 10.1016/s0092-8674(02)00970-4. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krathwohl MD, Kaiser JL. Chemokines promote quiescence and survival of human neural progenitor cells. Stem Cells. 2004;22:109–118. doi: 10.1634/stemcells.22-1-109. [DOI] [PubMed] [Google Scholar]
- Lee MK, Borchelt DR, Kim G, Thinakaran G, Slunt HH, Ratovitski T, Martin LJ, Kittur A, Gandy S, Levey AI, et al. Hyperaccumulation of FAD-linked presenilin 1 variants in vivo. Nat Med. 1997;3:756–760. doi: 10.1038/nm0797-756. [DOI] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- Lin Y, Huang R, Cao X, Wang SM, Shi Q, Huang RP. Detection of multiple cytokines by protein arrays from cell lysate and tissue lysate. Clin Chem Lab Med. 2003;41:139–145. doi: 10.1515/CCLM.2003.023. [DOI] [PubMed] [Google Scholar]
- Moehlmann T, Winkler E, Xia X, Edbauer D, Murrell J, Capell A, Kaether C, Zheng H, Ghetti B, Haass C, Steiner H. Presenilin-1 mutations of leucine 166 equally affect the generation of the Notch and APP intracellular domains independent of their effect on Abeta 42 production. Proc Natl Acad Sci U S A. 2002;99:8025–8030. doi: 10.1073/pnas.112686799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Kohsaka S. Microglia: activation and their significance in the central nervous system. J Biochem (Tokyo) 2001;130:169–175. doi: 10.1093/oxfordjournals.jbchem.a002969. [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Niidome T, Matsuda S, Akaike A, Kihara T, Sugimoto H. Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur J Neurosci. 2007;25:649–658. doi: 10.1111/j.1460-9568.2007.05309.x. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Taga T. Mechanisms underlying cytokine-mediated cell-fate regulation in the nervous system. Mol Neurobiol. 2002;25:233–244. doi: 10.1385/MN:25:3:233. [DOI] [PubMed] [Google Scholar]
- Namba T, Mochizuki H, Onodera M, Namiki H, Seki T. Postnatal neurogenesis in hippocampal slice cultures: early in vitro labeling of neural precursor cells leads to efficient neuronal production. J Neurosci Res. 2007;85:1704–1712. doi: 10.1002/jnr.21295. [DOI] [PubMed] [Google Scholar]
- Pauleau AL, Rutschman R, Lang R, Pernis A, Watowich SS, Murray PJ. Enhancer-mediated control of macrophage-specific arginase I expression. J Immunol. 2004;172:7565–7573. doi: 10.4049/jimmunol.172.12.7565. [DOI] [PubMed] [Google Scholar]
- Perez-Martinez L, Jaworski DM. Tissue inhibitor of metalloproteinase-2 promotes neuronal differentiation by acting as an anti-mitogenic signal. J Neurosci. 2005;25:4917–4929. doi: 10.1523/JNEUROSCI.5066-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineteau O, Rietschin L, Gradwohl G, Guillemot F, Gahwiler BH. Neurogenesis in hippocampal slice cultures. Mol Cell Neurosci. 2004;26:241–250. doi: 10.1016/j.mcn.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport. 1995;6:2479–2482. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer's disease. Trends Cell Biol. 1998;8:447–453. doi: 10.1016/s0962-8924(98)01363-4. [DOI] [PubMed] [Google Scholar]
- Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Sisodia SS, St George-Hyslop PH. gamma-Secretase, Notch, Abeta and Alzheimer's disease: where do the presenilins fit in? Nat Rev Neurosci. 2002;3:281–290. doi: 10.1038/nrn785. [DOI] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Song W, Nadeau P, Yuan M, Yang X, Shen J, Yankner BA. Proteolytic release and nuclear translocation of Notch-1 are induced by presenilin-1 and impaired by pathogenic presenilin-1 mutations. Proc Natl Acad Sci U S A. 1999;96:6959–6963. doi: 10.1073/pnas.96.12.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Kreutzberg GW. Lectin binding by resting and reactive microglia. J Neurocytol. 1987;16:249–260. doi: 10.1007/BF01795308. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Borchelt DR, Lee MK, Slunt HH, Spitzer L, Kim G, Ratovitsky T, Davenport F, Nordstedt C, Seeger M, et al. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Tran PB, Ren D, Veldhouse TJ, Miller RJ. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J Neurosci Res. 2004;76:20–34. doi: 10.1002/jnr.20001. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropepe V, Craig CG, Morshead CM, van der Kooy D. Transforming growth factor-alpha null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J Neurosci. 1997;17:7850–7859. doi: 10.1523/JNEUROSCI.17-20-07850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22:486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular medicine. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, 2nd, Scheffler B, Steindler DA. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–825. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- Wen PH, Hof PR, Chen X, Gluck K, Austin G, Younkin SG, Younkin LH, DeGasperi R, Gama Sosa MA, Robakis NK, et al. The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice. Exp Neurol. 2004;188:224–237. doi: 10.1016/j.expneurol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Wynes MW, Riches DW. Induction of macrophage insulin-like growth factor-I expression by the Th2 cytokines IL-4 and IL-13. J Immunol. 2003;171:3550–3559. doi: 10.4049/jimmunol.171.7.3550. [DOI] [PubMed] [Google Scholar]
- Zhang J, Moats-Staats BM, Ye P, D'Ercole AJ. Expression of insulin-like growth factor system genes during the early postnatal neurogenesis in the mouse hippocampus. J Neurosci Res. 2007;85:1618–1627. doi: 10.1002/jnr.21289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Jiang M, Trumbauer ME, Sirinathsinghji DJ, Hopkins R, Smith DW, Heavens RP, Dawson GR, Boyce S, Conner MW, et al. beta-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell. 1995;81:525–531. doi: 10.1016/0092-8674(95)90073-x. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.