Abstract
Acid adaptation of Streptococcus mutans UA159 involves several different mechanisms, including the ability to alter its proportion of long-chain, monounsaturated membrane fatty acids (R. G. Quivey, Jr., R. Faustoferri, K. Monahan, and R. Marquis, FEMS Microbiol. Lett. 189:89-92, 2000). In the present study, we examined the mechanism and timing of changes in fatty acid ratios and the potential benefit that an increased proportion of long-chained fatty acids has for the organism during growth at low pH. Cells taken from steady-state cultures at intermediate pH values of 6.5, 6, and 5.5 showed incremental changes from the short-chained, saturated membrane fatty acid profile normally seen in pH 7 cultures to the long-chained, monounsaturated fatty acids more typically observed in acidic cultures (pH 5). Our observations showed that the bacterium was capable of effecting the majority of changes in approximately 20 min, far less than one generation time. However, reversion to the distribution of fatty acids seen in cells growing at a pH of 7 required a minimum of 10 generations. Fatty acid composition analysis of cells taken from cultures treated with chloramphenicol suggested that the changes in fatty acid distribution did not require de novo protein synthesis. Cells treated with the fatty acid biosynthesis inhibitor cerulenin were unable to alter their membrane fatty acid profiles and were unable to survive severe acidification. Results presented here indicate that membrane fatty acid redistribution is important for low pH survival and, as such, is a component of the S. mutans acid-adaptation arsenal.
Streptococcus mutans is an etiologic agent of the disease dental caries. The organism resides in supragingival plaque, a biofilm formed above the gumline in the oral cavity. A major characteristic contributing to the organism's pathogenicity is its ability to metabolize many sugars, and as a consequence S. mutans produces various organic acids. Acid production consequently lowers the pH of the surrounding environment, and over time the drop in pH will lead to tooth demineralization and the development of caries. The organism, however, is capable of surviving in low-pH environments and can metabolize glucose and acidify the environment to a pH of 3.5 (3).
To withstand the acidification of its environment, S. mutans utilizes a series of adaptive mechanisms, including increases in both the activity and amounts of F1F0 ATPase and a low-pH-inducible DNA repair system (22). One of the main defenses against environmental challenges such as acid shock is the bacterial membrane itself. When the total membrane fatty acid content of S. mutans UA159 was examined after the organism had been grown to steady state at pH values of 7 and 5, a major shift in total membrane fatty acid composition was observed wherein cells grown at pH 5 produced a higher proportion of long-chained, monounsaturated fatty acids than cells grown at pH 7 (21). However, the causal relationship between membrane fatty acid changes and resistance to external acidification has not yet been established for S. mutans. In Escherichia coli, increased proportions of cyclopropane fatty acids were found in the membranes of acid-stressed organisms (6, 8). The cyclopropane fatty acid synthase gene (cfa) of E. coli is up-regulated during acid stress via the stress regulator rpoS. Mutant strains, deficient in cfa, were more susceptible to acid killing than wild-type organisms, suggesting that the shift to membranes with a higher percentage of cyclopropane fatty acids serves as a survival mechanism against low pH (8). Although S. mutans does not produce detectable levels of cyclopropane fatty acids, the shift to a more monounsaturated fatty acid profile at pH 5 suggests that this alteration may be an acid-stress survival mechanism.
Although it was demonstrated that S. mutans UA159 is capable of changing its membrane fatty acid content in response to acidification of the environment (21), there remained some unanswered questions. The previous report focused on steady-state pH 7 and 5 cells and did not examine intermediate pH levels. In this study, we investigated whether those intermediate pH values resulted in membrane profiles different than those of pH 7- and 5-grown organisms. Since the organism produces acid through the metabolism of sugars, we mimicked a glucose shock situation that may occur in vivo to determine how the bacterium responds to self-acidification. We investigated whether the underlying mechanism by which these changes occurred were due to de novo protein synthesis or de novo fatty acid biosynthesis. The underlying physiological role for such changes was also investigated.
(This research was conducted by Elizabeth M. Fozo in partial fulfillment of the requirements for a Ph.D. from the University of Rochester, Rochester, N.Y.)
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. mutans UA159 (20) was maintained on brain heart infusion (BHI; Difco) agar plates. Organisms were grown on solid medium at 37°C in an atmosphere containing 5% (vol/vol) CO2, 95% air. Continuous cultures were grown in either a BioFlo 2000 fermentor (New Brunswick Scientific) or a Sixfors multiple fermentor system (ATR, Laurel, Md.) as described previously (3, 21) in TY medium containing 3% tryptone, 0.1% yeast extract, 0.5% KOH, and 1 mM H3PO4. Cultures were grown at a dilution rate of 0.24 h−1, were limited by glucose (2.3 mM), and were maintained at steady-state pH levels by the addition of 2 N KOH. After steady-state growth had been maintained for a minimum of 10 generations, the cultures were treated with excess glucose (20 mM), referred to as glucose shock. The medium feed, pH control, and waste pump were discontinued. The culture pH was continuously monitored throughout the experiment by using an in-dwelling pH electrode. As the pH of the vessel decreased, 100-ml aliquots of culture were removed at pH values of 6.5, 6, 5.5, and 5. Cells were collected in 250-ml bottles by centrifugation at 7,600 × g for 10 min at 4°C. Cell pellets were washed with 30 ml of deionized water, recollected by centrifugation, and stored frozen at −80°C prior to fatty acid analysis. Typically, three or more independent chemostat runs were used to collect samples for analysis.
Experimental design.
Previous work using continuous-flow fermentors (operating as chemostats) to prepare steady-state cultures of S. mutans has shown that removing pH control from the cultures allows the organism to lower the pH value in the vessel from 7 to 5 (3). During the fall in pH, the organism responds to acidification in several ways (22). In the present study, the experimental approach was to measure changes in membrane fatty acid proportions in cells treated with specific reagents. Steady-state cultures, initially held at pH 7 for 10 generations, were allowed to fall to a pH of 5 by removal of pH control following addition of excess glucose. The main variable that was followed was the change in the proportion of C14:0 plus C16:0 fatty acids versus C18:1 plus C20:1 fatty acids in samples taken from cultures as the pH fell. This ratio was compared to the ratio seen in control cultures treated with excess glucose alone.
Initial experiments were conducted to determine the time required to detect changes in the proportions of membrane fatty acids. Later experiments were conducted to determine the effects on membrane fatty acid profiles by agents known to reduce protein synthesis (chloramphenicol) or inhibit fatty acid elongation (cerulenin). The ability of exogenously supplied fatty acids to block the effect of cerulenin was also determined.
Membrane fatty acid reversion experiments were conducted to determine how quickly the fatty acid profile of acidic cultures returned to the profile of neutral pH cultures. Cultures that had been treated with excess glucose (glucose shock) were allowed to fall to a pH of 5. When the culture reached pH 5, the medium feed, waste pump, and pH control were restored. The pH of the culture was raised to 7 by the addition of 2 N KOH, and time zero was designated when the culture pH returned to 7. Cells were then harvested at 1 h, 2.9 h (1 generation), 14.5 h (5 generations), and 29 h (10 generations). A 100-ml aliquot was then processed as described above and stored at −80°C to be used for membrane fatty acid determinations. Comparisons were made between the fatty acid compositions of samples taken from steady-state pH 7 cultures, cells taken from cultures at pH 5 following glucose shock, and samples that were in the process of reverting to growth at neutral pH.
Using cells initially grown at steady state at a pH of 7, we determined whether growth was necessary for membrane fatty acid alteration. A glucose-shock experiment was performed, and in addition to glucose, chloramphenicol (150 μg ml−1; Sigma), a protein synthesis inhibitor, was added. Culture growth was followed by determination of absorbance at 600 nm. A 100-ml aliquot of cells was harvested at pH 6.5, 6, and 5.5, washed with deionized water, repelleted, and stored frozen at −80°C for membrane fatty acid analysis. As above, three separate chemostat runs were performed. Fatty acids of these cells were compared to the compositions of cells from control cultures treated with glucose alone.
The necessity of de novo fatty acid biosynthesis, leading to membrane fatty acid changes, was determined using cultures grown at steady-state pH 7 and subjected to glucose shock as described above. In addition to glucose, cerulenin (Sigma) was added to a final concentration of 10 μg ml−1. Cells were harvested as described above at pH 6.5, 6, 5.5, and 5 and stored frozen at −80°C prior to membrane fatty acid determination. Three separate chemostat runs were performed. Cells from glucose-shock cultures were used as the standard for comparison of fatty acid profiles of cells treated with cerulenin.
Experiments were conducted to determine the ability of exogenous fatty acids to block the effect of cerulenin. Cultures subjected to glucose shock and cerulenin treatment (10 μg ml−1) were simultaneously supplemented with either cis-vaccenic acid (C18:1) at 10 μg ml−1 or cis-eicosenoic acid (C20:1) at 10 μg ml−1 (Sigma). When the culture pH reached 5, 100-ml aliquots were harvested as described above and stored frozen at −80°C for membrane fatty acid analysis. In these experiments, two separate chemostats were prepared for each culture, using the C18:1 or C20:1 fatty acids. Three glucose-shock chemostat cultures were used as controls. As a control for the impact of exogenously supplied fatty acids alone, on glucose-shocked organisms, cultures were treated with glucose plus 10 μg of either cis-vaccenic acid or cis-eicosenoic acid ml−1. Two independent chemostat experiments, per exogenously supplied fatty acid, were performed as controls.
Statistical evaluation of the data.
Student's t test was used to make pair-wise comparisons between the (C14:0 + C16:0)/(C18:1 +C20:1) ratios found in cells taken from glucose-shocked cultures, at specific pH values of 6.5, 6.0, 5.5, and 5.0 and ratios for the corresponding samples from each of the other conditions tested (steady-state growth at each pH value, chloramphenicol-treated cultures, cerulenin-treated cultures, the reversion cultures, and cultures supplied with exogenous fatty acids). The confidence interval for a difference in the means was set at 95% (P ≤ 0.05) for all comparisons.
Membrane fatty acid determination.
The membrane fatty acid content of the cultures was determined by Avanti Polar Lipids Inc. (Alabaster, Ala.) as previously described (21). Total lipids were extracted by the method of Bligh and Dyer (5). Approximately 5 mg of lipid was extracted per sample (100-ml aliquot of culture). Each preparation was used to prepare membrane fatty acid esters by the addition of 0.2 ml of toluene and 0.4 ml of 1% H2SO4 in methanol. The mixture was heated for 30 min; samples were cooled and fatty acids were extracted via addition of 1 ml of hexane and 1 ml of H2O. The hexane phase was then evaporated under nitrogen gas, and the fatty acid methyl esters were reconstituted in hexane. Gas chromatography was performed on a Hewlett Packard model 5890 chromatograph equipped with a J & W DB-225 capillary column (30 m by 0.25 mm by 0.25 μm). The column was kept at 15 lb/in2 and 220°C, and nitrogen was used as the carrier gas. A Nu-Chek Prep standard no. 68A was used to determine retention times and the identity of fatty acids derived from S. mutans.
Acid survival.
We employed an established acid sensitivity assay to determine how culture conditions and membrane fatty acid composition may affect the ability of S. mutans to survive low-pH conditions (3). Glucose-shock culture conditions were used to prepare cells for acid sensitivity tests (n = 3), with the following additional modifications: glucose shock alone, glucose plus cerulenin, glucose plus cis-vaccenic acid, glucose plus cis-eicosenoic acid, glucose plus cerulenin plus cis-vaccenic acid, and glucose plus cerulenin plus cis-eicosenoic acid. Once the cultures reached pH 5, 10 ml was harvested in 15-ml plastic, conical tubes by centrifugation at 2,500 × g for 10 min. Cell pellets were resuspended in 3 ml of 0.1 M glycine-HCl, pH 2.5, and were continuously stirred for 1 h at room temperature. Aliquots (0.1 ml) of the cell suspension were removed at 0, 15, 30, and 60 min, serially diluted into BHI medium (Difco), and plated in duplicate on BHI agar. Plates were incubated for 48 h at 37°C, 5% CO2-95% air. Viable cell counts were enumerated and used to calculate log n/n0. Glucose-shocked pH 5 cells were used as the standard for survival under severe acidic conditions. In order to determine whether the presence of cerulenin itself served to decrease the organism's ability to survive severe acidic conditions, steady-state pH 7 cells were also treated with 10 μg of cerulenin ml−1 for 20 min and harvested for acid sensitivity testing. Untreated steady-state pH 7 organisms were used as the standard.
RESULTS
Membrane fatty acid changes occur incrementally.
Previously, our investigators showed that S. mutans UA159 alters its membrane fatty acid profile when grown at pH 7 versus pH 5 (21). The membrane composition shifted from a short-chained, saturated fatty acid profile at neutral pH to a long-chained, monounsaturated profile at acidic pH. Specifically, when grown at pH 7, 68% of the membrane consisted of C14:0 plus C16:0, whereas at pH 5 the membrane consisted of 48% C18:1 plus C20:1 (21). S. mutans UA159 was grown at pH values of 6.5, 6, and 5.5 in a chemostat to determine whether there were shifts in the membrane composition when the organism was grown under steady-state conditions intermediate to pH 7 and 5. The levels of C14:0 decreased from approximately 13% at pH 6.5 to 9% at pH 6, about 5% at pH 5.5, and 1% at pH 5 (Table 1). A similar decrease was seen in the proportion of C16:0, with a drop from 43% of total membrane fatty acid content to 38% and then to 28% between the pH values of 6, 5.5, and 5, respectively.
TABLE 1.
Membrane fatty acid proportions in S. mutans UA159 cultures grown at steady state at the indicated pH values
| Fatty acid(s) | % of totala at:
|
||
|---|---|---|---|
| pH 6.5 | pH 6 | pH 5.5 | |
| C14:0 | 12.7 ± 2.5 | 8.7 ± 1.1 | 4.8 ± 2.2 |
| C14:1 | 0.2 ± 0.3 | NDb | 0.1 ± 0.1 |
| C16:0 | 47.1 ± 3.0 | 43.3 ± 2.0 | 38.4 ± 5.6 |
| C16:1 | 0.4 ± 0.3 | 2.1 ± 2.7 | 0.7 ± 0.2 |
| C18:0 | 5.5 ± 1.2 | 7.4 ± 1.2 | 9.1 ± 2.1 |
| C18:1 | 17.6 ± 1.7 | 20.1 ± 1.9 | 24.0 ± 1.8 |
| C20:0 | 0.1 ± 0.2 | 0.5 ± 0.5 | 1.0 ± 0.5 |
| C20:1 | 5.3 ± 1.9 | 8.5 ± 1.5 | 13.7 ± 4.0 |
| Others | 11.1 ± 4.8 | 9.3 ± 3.3 | 8.3 ± 0.6 |
| C14:0+C16:0 | 59.7 ± 2.3 | 52.0 ± 2.5 | 43.3 ± 7.8 |
| C18:1+C20:1 | 22.9 ± 3.3 | 28.6 ± 3.1 | 37.7 ± 5.6 |
| (C14:0+C16:0)/(C18:1+C20:1) | 2.6 ± 0.4c | 1.8 ± 0.3 | 1.2 ± 0.4 |
The values represent percentages of total fatty acids and are means ± standard deviations for three independent cultures.
ND, not detected.
Statistically different (P ≤ 0.05) from the ratio in cultures held at pH 7 (3.8 ± 0.7) (21).
Concomitant with the decrease in the proportion of short-chained, saturated fatty acids, a gradual increase in the proportion of long-chained, monounsaturated fatty acids in the membrane was observed. Levels of C18:1 increased from approximately 18% at pH 6.5 to 24% at pH 5.5. A similar increase was seen in the proportion of C20:1 over the same pH values, with an increase from 5.3% to 14% at pH 5.5. Thus, over the pH range examined here, the incremental shift from a short-chain, saturated fatty acid membrane profile to that of a long-chain, monounsaturated profile was observed as environmental pH decreased.
Acid generation by S. mutans UA159 leads to rapid membrane fatty acid changes.
As S. mutans metabolizes sugars, it produces organic acids and lowers the environmental pH. Thus, it was important to determine whether the production of acid by the organism itself was sufficient to cause membrane fatty acid shifts. Using a glucose-shock chemostat system (described in Materials and Methods), culture pH values fell from 7 to 5 in 2 h (less than one generation), with the culture reaching pH 6.5 within 20 min on average (data not shown). The levels of C14:0 plus C16:0 fatty acids reported previously (21) comprised approximately 68% of the total membrane fatty acid content at pH 7; however, at pH 6.5, this value was approximately 46% (Table 2) and continued to drop to a combined level of nearly 40% at pH 5.
TABLE 2.
Membrane fatty acid proportions in samples taken from glucose-shocked cultures of S. mutans UA159
| Fatty acid(s) | % of totala at:
|
|||
|---|---|---|---|---|
| pH 6.5 | pH 6 | pH 5.5 | pH 5 | |
| C14:0 | 7.4 ± 1.4 | 7.1 ± 0.6 | 5.9 ± 1.0 | 5.5 ± 1.2 |
| C14:1 | NDb | ND | ND | ND |
| C16:0 | 38.1 ± 6.8 | 37.6 ± 2.3 | 35.3 ± 5.4 | 34.4 ± 5.7 |
| C16:1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.0 | 0.5 ± 0.0 |
| C18:0 | 7.9 ± 0.5 | 8.7 ± 0.6 | 9.2 ± 0.7 | 9.7 ± 0.5 |
| C18:1 | 23.2 ± 3.1 | 22.9 ± 0.9 | 24.2 ± 3.1 | 24.7 ± 3.0 |
| C20:0 | 1.3 ± 0.8 | 1.6 ± 0.3 | 1.8 ± 0.3 | 1.9 ± 0.5 |
| C20:1 | 13.9 ± 4.4 | 14.2 ± 0.7 | 16.3 ± 3.7 | 17.3 ± 3.9 |
| Others | 7.7 ± 1.0 | 7.5 ± 1.0 | 6.4 ± 0.2 | 6.0 ± 0.2 |
| C14:0 + C16:0 | 45.5 ± 8.2 | 43.7 ± 0.4 | 41.1 ± 6.4 | 39.9 ± 6.9 |
| C18:1 + C20:1 | 37.1 ± 7.5 | 37.0 ± 1.4 | 40.9 ± 6.7 | 42 ± 6.8 |
| (C14:0 + C16:0)/(C18:1 + C20:1) | 1.3 ± 0.4 | 1.2 ± 0.1 | 1.0 ± 0.3 | 1.0 ± 0.3 |
The values represent percentages of total fatty acids and are means ± standard deviations for three independent cultures.
ND, not detected.
Concurrent with the decrease in the proportion of short-chained, saturated fatty acids, there was an increase in the proportion of long-chain, monounsaturated fatty acids in relation to the total membrane fatty acid composition of glucose-shocked cells. The levels of C18:1 nearly doubled with acidification of the environment, from approximately 12% (21) to 23% as the pH decreased from 7 to 6.5. The proportion continued to increase as the pH reached 5, with C18:1 comprising approximately 25% of total membrane fatty acid content. The proportion of C20:1 also rose sharply as the pH decreased from the reported steady-state pH 7 levels to 6.5, with an increase of approximately threefold from 4.5% to nearly 14%, respectively, of total membrane fatty acid. The percentage of C20:1 increased to 17% at pH 5, a fourfold increase over the proportion found at pH 7 (21). The results indicated that the major membrane fatty acid changes occurred early in the acidification of the culture. By the time the pH decreased to 6.5, in a typical time span of approximately 20 min, the proportion of C14:0 and C16:0 decreased from 68 to 45.5%. In the same time and pH span, the ratio of C18:1 and C20:1 increased from 16.9 to 37.1%. Comparison of the changes in the (C14:0 + C16:0)/C18:1 + C20:1) ratios showed that the only statistical difference existed between the ratios seen previously in fatty acids from the pH 7 samples, where the ratio was 3.8 (±0.72), and those from pH 6.5-grown cells (P ≤ 0.03). There were no statistical differences in the comparisons made between pH values of 6.5 and 6.0, between 6.0 and 5.5, or between 5.5 and 5.0, suggesting that the bulk of changes, and possibly the most significant changes, occurred quickly as the cells moved from steady state at pH 7 to pH 6.5. Interestingly, the corresponding (C14:0 + C16:0)/C18:1 + C20:1) ratios from the incrementally controlled cultures (Table 1) and the glucose-shocked cultures (Table 2) were statistically different (P ≤ 0.008) when comparing the values at pH 6.5 and pH 6. The differences between the two types of cultures disappeared, however, as the pH came to 5.5, presumably because the glucose-shocked cultures were becoming more fully adjusted to the external pH.
Reversion of the acidic membrane fatty acid profile occurs gradually.
We determined whether the organism could reverse its fatty acid content, from the pH 5 profile to that of the pH 7 profile, by performing a reversion experiment, described in Materials and Methods. Here, the pH in cultures growing at steady state was elevated from pH 5 to pH 7 by the addition of KOH. The change in membrane fatty acid composition was monitored over time to see how rapidly the composition reverted to levels seen in steady-state cultures growing at a pH of 7. We observed that the levels of C14:0 and C16:0 rose gradually, with a 3% increase in C14:0 after five generations (14.5 h) and an approximately 7% increase in C16:0 over the same time span (Table 3). The decrease in the proportion of long-chained, monounsaturated fatty acids found in the membrane was also gradual over time. For example, there was an approximate 5% decrease in the amount of C18:1 in cells that had been growing for 14.5 h (five generation times) compared to cells held at pH 5. The ratio of C20:1 found in the membrane also decreased from 17% (the pH 5 value) to 5% in five generations. The data from the reversion experiments also had statistical significance after one generation (P ≤ 0.002), but not in the first hour, supporting the idea that reversion takes considerably longer than acid adaptation itself, perhaps to protect against future episodes of acidification. Thus, although the membrane profile of S. mutans changed quickly with a decrease in environmental pH, the reverse shift took much longer.
TABLE 3.
Reversion of the fatty acid profile of S. mutans UA159 from its composition at acidic pH to neutral pH
| Fatty acid(s) | % of totala after:
|
|||
|---|---|---|---|---|
| 1 h | 2.9 hb | 14.5 h | 29 h | |
| C14:0 | 5.1 ± 0.9 | 7.1 ± 1.9 | 8.9 ± 1.4 | 11.1 ± 2.0 |
| C14:1 | 0.3 ± 0.2 | 0.4 ± 0.2 | 0.4 ± 0.3 | 0.6 ± 0.4 |
| C16:0 | 32.5 ± 2.7 | 37.2 ± 2.6 | 41.5 ± 2.4 | 41.9 ± 3.8 |
| C16:1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 |
| C18:0 | 8.2 ± 1.2 | 7.1 ± 0.9 | 7.1 ± 0.8 | 6.4 ± 0.9 |
| C18:1 | 26.2 ± 2.7 | 24.3 ± 3.0 | 20.4 ± 1.3 | 17.8 ± 1.0 |
| C20:0 | 1.5 ± 0.4 | 0.9 ± 0.3 | 0.7 ± 0.2 | 0.5 ± 0.2 |
| C20:1 | 16.0 ± 8.7 | 5.9 ± 6.6 | 5.1 ± 5.4 | 3.7 ± 4.0 |
| Others | 9.8 ± 7.7 | 16.8 ± 8.6 | 15.4 ± 7.7 | 17.6 ± 7.8 |
| C14:0 + C16:0 | 38.9 ± 3.6 | 45.4 ± 4.5 | 51.5 ± 3.7 | 53.5 ± 5.7 |
| C18:1 + C20:1 | 36.3 ± 10.7 | 27.6 ± 5.4 | 23.6 ± 4.7 | 23.7 ± 7.1 |
| (C14:0 + C16:0)/(C18:1 + C20:1) | 1.2 ± 0.5 | 1.8 ± 0.3c | 2.2 ± 0.4c | 2.3 ± 0.3c |
The values represent percentages of total fatty acids and are means ± standard deviations for three independent chemostat cultures.
One generation = 2.9 h.
Statistically different (P ≤ 0.05) from the ratio in glucose-shocked cultures at pH 5.0.
Membrane fatty acid shifts do not require new growth.
Previous studies have shown that de novo protein synthesis is required for acid adaptation of S. mutans (25). In the present study, we wished to determine whether an inhibitor of cell growth and protein synthesis would also inhibit the organism's ability to make membrane fatty acid changes in response to external acidification. The standard chemostat conditions, described in Materials and Methods, were employed with the addition of chloramphenicol, a de novo protein synthesis inhibitor. Cell growth, as measured by absorbance at 600 nm, ceased (data not shown), suggesting that de novo protein synthesis was disrupted. Four hours following the addition of glucose and chloramphenicol, the pH of the cultures had not fallen to 5; thus, samples were not collected beyond this time point. The total amount of C14:0 plus C16:0 at pH 6, following the addition of glucose and chloramphenicol, was 46% of total membrane fatty acid composition (Table 4). When the organisms were treated with glucose alone, the percentage of C14:0 plus C16:0 at pH 6 was approximately 44% (Table 2). Thus, the proportion of total membrane composition comprised of saturated fatty acids, C14:0 and C16:0, remained relatively constant in the presence of a de novo protein synthesis inhibitor and the lack of cell growth (data not shown). The relative proportions of the dominant monounsaturated fatty acids, i.e., C18:1 and C20:1, also did not vary significantly at a culture pH of 6 despite the lack of measurable cell growth (data not shown). In the presence of chloramphenicol, the amount of C18:1 plus C20:1 was approximately 35% (Table 4), whereas without chloramphenicol the membrane fatty acid composition included approximately 37% C18:1 and C20:1 (Table 2). Chloramphenicol treatment of the cultures showed modest differences from the control cultures at pH 6 and no differences elsewhere, indicating that the antibiotic had no major impact on the changes in the fatty acid composition and supporting the contention that acid-mediated changes in the membrane fatty acid profile of S. mutans probably do not require growth.
TABLE 4.
Total membrane fatty acid composition of S. mutans UA159 cultures treated with glucose and chloramphenicol
| Fatty acid(s) | % of totala at:
|
||
|---|---|---|---|
| pH 6.5 | pH 6.0 | pH 5.5 | |
| C14:0 | 8.6 ± 0.8 | 7.5 ± 0.7 | 6.7 ± 0.4 |
| C14:1 | NDb | ND | ND |
| C16:0 | 40.5 ± 0.3 | 38.5 ± 1.2 | 41.5 ± 3.3 |
| C16:1 | 0.6 ± 0.3 | 0.05 ± 0.1 | 0.6 ± 0.2 |
| C18:0 | 9.1 ± 0.4 | 11.1 ± 0.4 | 9.6 ± 3.2 |
| C18:1 | 20.6 ± 0.9 | 21.0 ± 0.8 | 20.4 ± 0.5 |
| C20:0 | 1.7 ± 0.1 | 2.8 ± 0.2 | 2.3 ± 0.8 |
| C20:1 | 11.9 ± 1.4 | 13.6 ± 1.1 | 12.1 ± 1.6 |
| Others | 7.0 ± 1.3 | 6.3 ± 0.9 | 5.3 ± 0.4 |
| C14:0 + C16:0 | 49.2 ± 0.9 | 46.1 ± 1.5 | 43.7 ± 3.3 |
| C18:1 + C20:1 | 32.4 ± 2.3 | 34.6 ± 1.5 | 36.1 ± 4.1 |
| (C14:0 + C16:0)/(C18:1 + C20:1) | 1.5 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.2 |
The values represent percentages of total fatty acids and are means ± standard deviations for three independent chemostat cultures for all but the pH 5.5 sample, where n = 2.
ND, not detected.
Changes in membrane fatty acid composition can be blocked by the fatty acid biosynthesis inhibitor cerulenin.
The data obtained from the glucose-shock-plus-chloramphenicol experiment made clear that the membrane fatty acid profile shifted despite the presence of an inhibitor of de novo protein synthesis. The results suggested that the mechanism for the increased production of longer, monounsaturated fatty acids occurred not at the protein synthesis level, but elsewhere. Both E. coli and Bacillus subtilis contain membrane fatty acid-modifying enzymes that become active following environmental stress (1, 9, 10). Genomic analysis of UA159 has not indicated the presence of such modifying enzymes (2). However, a homologue to the E. coli fatty acid biosynthesis gene fabF was located. The fabF gene encodes β-ketoacyl synthase II, which is responsible for elongation of fatty acids (17). The activity of FabF can be inhibited by the antibiotic cerulenin (7, 10, 26). If the observed membrane fatty acid shifts were due to changes in the activities of fatty acid biosynthesis enzymes, then blocking a step in the fatty acid biosynthesis cycle could prevent the shifts. We tested this possibility by the addition of excess glucose and cerulenin to steady-state cultures.
The results of the cerulenin treatment indicated that amounts of C14:0 and C16:0 remained relatively stable over the pH levels observed (Table 5). However, when the organisms were treated with glucose alone, the proportion of C14:0 and C16:0 decreased significantly, from approximately 68% at pH 7 (21) to 45.5% at pH 6.5 (Table 2). Combined, the amount of C14:0 and C16:0 at pH 5 comprised over 60% of total membrane fatty acids when cultures were treated with cerulenin. This was in contrast to organisms treated with glucose alone, for which the amount of C14:0 and C16:0 comprised approximately 40% of total membrane fatty acids at pH 5.
TABLE 5.
Total membrane fatty acid composition of S. mutans UA159 cultures treated with glucose and cerulenin
| Fatty acid(s) | % of totala at:
|
|||
|---|---|---|---|---|
| pH 6.5 | pH 6.0 | pH 5.5 | pH 5 | |
| C14:0 | 15.7 ± 1.8 | 14.0 ± 5.4 | 18.1 ± 1.0 | 18.0 ± 1.6 |
| C14:1 | 0.2 ± 0.4 | NDb | ND | ND |
| C16:0 | 46.0 ± 2.5 | 48.0 ± 3.1 | 46.3 ± 1.1 | 45.1 ± 1.6 |
| C16:1 | 2.6 ± 3.5 | 2.5 ± 3.8 | 2.5 ± 3.5 | 2.4 ± 3.2 |
| C18:0 | 5.4 ± 1.0 | 5.7 ± 1.3 | 5.1 ± 0.8 | 5.3 ± 0.8 |
| C18:1 | 16.7 ± 1.9 | 17.1 ± 2.2 | 15.9 ± 1.7 | 15.9 ± 1.5 |
| C20:0 | 0.2 ± 0.3 | ND | ND | 0.2 ± 0.3 |
| C20:1 | 6.3 ± 1.5 | 6.5 ± 1.7 | 6.1 ± 1.5 | 6.1 ± 1.5 |
| Others | 6.8 ± 1.2 | 6.2 ± 0.4 | 5.9 ± 1.1 | 7.1 ± 1.6 |
| C14:0 + C16:0 | 62.4 ± 1.1 | 62.1 ± 3.1 | 64.5 ± 1.5 | 63.1 ± 2.8 |
| C18:1 + C20:1 | 23.3 ± 3.3 | 23.6 ± 3.9 | 22.0 ± 3.1 | 22.0 ± 3.0 |
| (C14:0 + C16:0)/(C18:1 + C20:1) | 2.7 ± 0.4c | 2.7 ± 0.5c | 3.0 ± 0.4c | 2.9 ± 0.4c |
The values represent percentages of total fatty acids and are means ± standard deviations for three independent cultures.
ND, not detected.
Statistically different (p ≤ 0.05) from the corresponding ratios in the glucose-shock experiments (Table 2).
The proportions of long-chained, monounsaturated fatty acids also did not change over the pH values examined in the cerulenin-treated cultures. There was a slight increase in C18:1 as the pH was lowered to 6; however, this appears to be a fluctuation in the data, as the levels dropped again at pH 5.5 and 5. In cells treated with glucose alone, the amount of C18:1 comprised approximately 25% of total membrane fatty acid content at pH 5 (Table 2), whereas the total membrane composition of those organisms treated with glucose and cerulenin was approximately 16% C18:1. The levels of C20:1 also remained steady in cerulenin-treated organisms at about 6% of total membrane fatty acid content. This was unlike the cells treated with glucose alone, where the amount of C20:1 composed approximately 14% of total membrane fatty acid content at pH 6 and rose to over 17% of the total proportion of membrane fatty acids at pH 5 (Table 2). Statistical differences were observed at every pH value compared with the corresponding values from the glucose-shock cultures (Table 2) and of those treated with cerulenin (Table 5) (P ≤ 0.008 at each pH value). These data clearly showed that cerulenin had a considerable impact on the ability of the cells to elongate fatty acid chains.
Blocking shifts in membrane fatty acid composition leads to increased acid sensitivity.
The shifting of the membrane fatty acid profile of UA159 in response to acidification demonstrates a common adaptation technique utilized by bacteria in response to environmental stresses (1, 6, 8), although why S. mutans would alter its membrane in such a manner was unclear. S. mutans is known to be more acid resistant when grown at pH 5 than at pH 7 (3); therefore, it may be that the change in membrane fatty acid profile in response to pH offers protection against acid damage. To determine whether the shift in membrane fatty acid content offers protection against acid stress, acid sensitivity experiments were performed on glucose-treated organisms and those treated with glucose plus cerulenin.
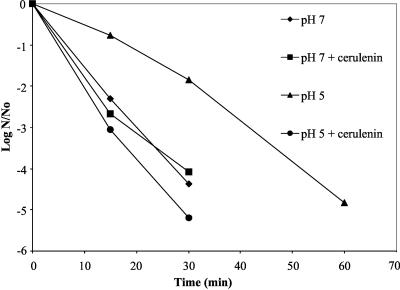
Glucose-only treated control cells were still viable after 1 h at pH 2.5 (Fig. 1). Cells that were treated with glucose plus cerulenin, however, were not detectable past 30 min at pH 2.5 (Fig. 1). There was no difference in the viability of pH 7-grown cells versus pH 7-grown cells treated with cerulenin, demonstrating that the presence of cerulenin alone did not cause a decrease in viability (Fig. 1). Hence, by blocking the change of membrane fatty acids from a short-chained, saturated profile to that of a long-chained, monounsaturated profile, the organism was more susceptible to acid-mediated killing.
FIG. 1.
Cerulenin-treated cultures of S. mutans are sensitive to acidic environments. Cells were treated as described in Materials and Methods. Representative results are shown from samples that were plated and enumerated in duplicate from three independent experiments. ⧫, steady-state pH 7-grown cells; ▪, pH 7 samples treated with cerulenin; ▴, glucose-shocked cells grown at pH 5; •, glucose-shocked cerulenin-treated pH 5-grown cells.
Cerulenin-treated cells cannot be rescued from acid stress via addition of long-chained, monounsaturated fatty acids.
We performed a series of rescue experiments to determine whether wild-type levels of acid resistance could be restored to cerulenin-treated cells. Previous work has shown that S. mutans can incorporate exogenous fatty acids into its membrane (23). Other studies have demonstrated that growth medium supplemented with various fatty acids can affect the proton permeabilities of S. mutans (16). We hypothesized that the addition of long-chain, monounsaturated fatty acids, specifically C18:1 or C20:1, to cerulenin-treated bacteria may restore their ability to survive low-pH environments.
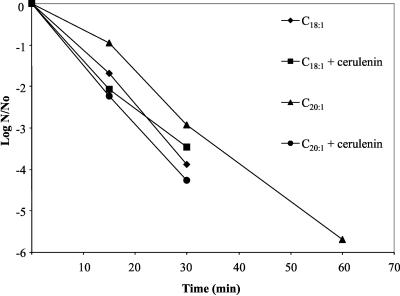
Interestingly, the addition of long-chain, monounsaturated fatty acids did not increase the organism's ability to survive low-pH conditions in the presence of cerulenin (Fig. 2). The addition of C18:1 or C20:1 had no apparent effect on acid survival. The addition of fatty acids to cells shocked with glucose did not produce acid-resistant bacteria. In fact, unlike glucose-treated cells, organisms treated with C18:1 were not detectable in samples past the 30-min time point. Cells treated with C20:1 did survive until the 60-min time point, suggesting that the addition of C18:1 had different effects on the membrane than the addition of C20:1.
FIG. 2.
Addition of exogenous fatty acids does not rescue cerulenin-treated organisms from their acid-sensitive phenotype. Cultures were treated as described in Materials and Methods. Steady-state pH 7 cells were subjected to glucose shock with the addition of the indicated fatty acid with or without cerulenin. Each sample was plated and enumerated in duplicate from three independent chemostat cultures for each condition. A representative graph of the data is shown. ⧫, glucose-shocked pH 5 cells plus cis-vaccenic acid; ▪, glucose-shocked cerulenin-treated pH 5 cells supplemented with cis-vaccenic acid; ▴, glucose-shocked pH 5 cells supplemented with cis-eicosenoic acid; •, glucose-shocked cerulenin-treated pH-5 cells supplemented with cis-eicosenoic acid.
Although it has been demonstrated that S. mutans can incorporate exogenous fatty acids (23), our results suggest that exogenous fatty acids alone do not make the cell more likely to survive acid shock. To verify that S. mutans did take up the exogenous fatty acids and incorporate them into its membrane, fatty acid analysis was performed. The presence of the exogenous fatty acids resulted in increases in the proportions of membrane C18:1 and C20:1 when each was added to the cultures (Table 6). It appeared that the proportions of each fatty acid were essentially equal regardless of whether the cultures were treated with cerulenin or not, suggesting that the presence of cerulenin had no effect on the uptake of fatty acids. Also, the addition of fatty acids led to changes in the proportions of the membrane fatty acid content. For example, at pH 5, the membranes of glucose-treated cells were approximately 25% C18:1. However, by adding C18:1 with glucose, the value rose to approximately 35% C18:1, as might be expected based on previous studies (23).
TABLE 6.
Fatty acid profiles for S. mutans UA159 cultures grown in the presence of exogenous fatty acids and cerulenin
| Fatty acid(s) | % of totala with supplement(s)
|
|||
|---|---|---|---|---|
| C18:1 | C18:1 + cerulenin | C20:1 | C20:1 + cerulenin | |
| C14:0 | 6.6 ± 0.4 | 9.2 ± 5.5 | 6.1 ± 1.7 | 7.2 ± 4.0 |
| C14:1 | NDb | 0.1 ± 0.1 | 0.1 ± 0.2 | 0.1 ± 0.2 |
| C16:0 | 33.2 ± 7.5 | 33.4 ± 6.2 | 26.7 ± 7.1 | 28.4 ± 5.3 |
| C16:1 | 0.5 ± 0.1 | 0.2 ± 0.3 | 0.5 ± 0.1 | 2.6 ± 2.0 |
| C18:0 | 6.9 ± 3.3 | 6.3 ± 0.3 | 6.2 ± 3.4 | 5.4 ± 0.5 |
| C18:1 | 35.4 ± 8.2 | 34.1 ± 5.2 | 15.4 ± 7.5 | 15.2 ± 3.7 |
| C20:0 | 0.9 ± 0.9 | 0.7 ± 1.0 | 1.4 ± 1.1 | 0.6 ± 0.3 |
| C20:1 | 5.4 ± 7.4 | 4.8 ± 2.3 | 32.6 ± 15.4 | 31.2 ± 9.7 |
| Others | 11 ± 10.2 | 11.3 ± 7.4 | 10.9 ± 3.3 | 9.1 ± 4.8 |
| C14:0 + C16:0 | 39.8 ± 7.1 | 42.6 ± 11.7 | 32.9 ± 6.8 | 35.7 ± 9.3 |
| C18:1 + C20:1 | 40.8 ± 0.8 | 38.9 ± 2.9 | 48.0 ± 8.8 | 46.5 ± 12.5 |
| (C14:0 + C16:0)/(C18:1 + C20:1) | 1.0 ± 0.2 | 1.1 ± 0.4c | 0.7 ± 0.3c | 0.8 ± 0.4c |
The values represent percentages of total fatty acids and are means ± standard deviations for two independent cultures.
ND, not detected.
No statistical differences.
DISCUSSION
S. mutans possesses an acid survival strategy consisting of a variety of components that allow it to survive the low-pH environment of supragingival plaque (22). The ability to shift its membrane fatty acid profile from a short-chained, saturated profile at pH 7 to that of the long-chained, monounsaturated profile at pH 5 is another survival component of the organism. By blocking this ability, the organism becomes more acid sensitive.
As a response to glucose shock, the organism shifted its membrane fatty acid profile quite dramatically when the pH was lowered to 6.5. Although the organism continued to reduce the amount of saturated fatty acids and increase the proportion of C18:1 and C20:1 as the environmental pH decreased, the bulk of the changes appeared to occur between pH 7 and pH 6.5. This suggests that UA159 is capable of a rapid response to pH changes and makes adjustments quickly. What remains to be determined is whether the changes observed at pH 6.5 are sufficiently similar to those of fully acid-adapted cells (pH 5) to confer survival under severe acid stress. Because the reverse shift from the acidic membrane profile to the neutral profile was so gradual, this may offer protection to the organism from recurrent acid challenges, as the pH of dental plaque rises and falls with the consumption of carbohydrates (24). Currently, it is not known how membrane fatty acid shifts may protect S. mutans from acidic conditions. However, it is known that other organisms undergo shifts in their membrane fatty acid and phospholipid profiles in response to environmental stress. Although S. mutans UA159 does not produce appreciable amounts of cyclopropane fatty acids, as observed in E. coli, blocking the organism's ability to increase the proportion of long-chain, monounsaturated fatty acids sensitized the organism to low-pH conditions. In addition to acid-mediated changes in the membranes of S. mutans and E. coli, it has been demonstrated that B. subtilis and Bacillus stearothermophilus alter the degree of membrane saturation in response to temperature shifts (1, 19). Moreover, in response to temperature changes, the mycoplasma Acholeplasma laidlawii also alters its polar lipid composition (4, 15, 18).
The ability of S. mutans to regulate its membrane fatty acid composition in response to external pH may be an important element of its pathogenic capability. By blocking membrane fatty acid changes, the organism is more sensitive to severe acidic conditions. Thus, it appears that an increase in the proportion of long-chain, monounsaturated fatty acids may be necessary for survival in dental plaque, where there are repeated cycles of acidification (24). It is interesting that clinical isolates of Helicobacter pylori from the gut produce large amounts of cyclopropane fatty acids, whereas isolates from the intestine do not (12). Consequently, the ability to produce a different membrane fatty acid profile may render strains of Helicobacter more virulent. Work is currently in progress to determine whether blocking the increase in long-chain, monounsaturated fatty acids in S. mutans renders the organism less virulent.
Although de novo protein synthesis is required for optimal acid survival of S. mutans (25), the results here suggest it may not be needed for the pH-induced membrane changes. In response to cold shock, B. subtilis produces a desaturase which modifies existing saturated fatty acids in the membrane to form monounsaturated fatty acids (1). The increase in monounsaturated fatty acids in the membrane leads to increased membrane fluidity, thereby preventing the organism from freezing. Genomic analysis of UA159 did not indicate the presence of any homologues to the B. subtilis des gene product (2); however, it is possible that the organism produces a membrane-modifying enzyme that does not have homology to known enzymes. Further analysis of the UA159 genome suggested that the organism does not produce the range of enzymes involved with fatty acid biosynthesis and additional membrane modification enzymes of E. coli and B. subtilis (2). The results shown here and the genomic data suggest that S. mutans may use its fatty acid biosynthesis enzymes to generate membrane fatty acid changes. Using cerulenin, which stalls the cycle of fatty acid biosynthesis (7, 10, 26), we were able to prevent changes in membrane composition and demonstrate that the biosynthetic enzymes were involved in membrane fatty acid changes.
The mechanism by which the fatty acid biosynthetic enzymes contribute to pH-induced membrane changes remains to be determined. It is known that, despite the ability of F1F0 ATPase to pump protons from the cytoplasm of the organism under low-pH conditions, thereby maintaining a ΔpH across the membrane, there are still changes in the pH of the cytoplasm (11, 13, 14). It is possible that acidification of the cytoplasm affects the biochemical property of a particular enzyme or enzymes and consequently leads to the formation of longer, monounsaturated fatty acids. However, this remains to be determined experimentally.
The addition of fatty acids to cerulenin-treated cultures did not rescue those organisms from their weakened acid survival (when compared to organisms treated with glucose alone), although it did lead to shifts in the fatty acid composition of those cells. However, these changes were insufficient to prolong low-pH survival. It has been demonstrated that supplementing the growth medium of S. mutans strain GS-5 with various fatty acids can lead to changes in proton permeability (16), which may affect the ability of the organism to withstand internal acidification. The correlation between proton permeability and total membrane fatty acid content remains to be determined for S. mutans UA159. These combined experiments may help to clarify why the organism changes its membrane composition and how uncontrolled shifts can lead to poor survival under low pH conditions.
Our findings are a demonstration of an organism that appears to alter its membrane composition in response to acidification of the environment. We speculate that other organisms that encounter low-pH environments may also shift their membrane profiles to survive acidification. Other organisms that inhabit dental plaque with S. mutans may specifically use membrane adaptation to protect themselves from the acid by-products of fermentation. We are currently investigating other oral bacteria to determine whether membrane compositional shifting is common or is an adaptation specific to S. mutans.
Acknowledgments
We thank Robert Marquis for helpful discussion throughout. We also thank Roberta Faustoferri for her technical expertise.
This work was supported by a grant from the National Institute for Dental and Craniofacial Research (DE-11549). E.M.F. was supported by the Rochester Training Program in Oral Infectious Diseases (T32-DE07165).
REFERENCES
- 1.Aguilar, P. S., P. Lopez, and D. de Mendoza. 1999. Transcriptional control of the low-temperature-inducible des gene, encoding the Δ5 desaturase of Bacillus subtilis. J. Bacteriol. 181:7028-7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 99:14434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belli, W. A., and R. E. Marquis. 1991. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl. Environ. Microbiol. 57:1134-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhakoo, M., and R. N. McElhaney. 1988. The effect of variations in growth temperature, fatty acid composition and cholesterol content on the lipid polar head-group composition of Acholeplasma laidlawii B membranes. Biochim. Biophys. Acta 945:307-314. [DOI] [PubMed] [Google Scholar]
- 5.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:912-917. [DOI] [PubMed] [Google Scholar]
- 6.Brown, J. L., T. Ross, T. A. McMeekin, and P. D. Nichols. 1997. Acid habituation of Escherichia coli and the potential role of cyclopropane fatty acids in low pH tolerance. Int. J. Food Microbiol. 37:163-173. [DOI] [PubMed] [Google Scholar]
- 7.Buttke, T. M., and L. O. Ingram. 1978. Inhibition of unsaturated fatty acid synthesis in Escherichia coli by the antibiotic cerulenin. Biochemistry 17:5282-5286. [DOI] [PubMed] [Google Scholar]
- 8.Chang, Y. Y., and J. E. Cronan, Jr. 1999. Membrane cyclopropane fatty acid content is a major factor in acid resistance of Escherichia coli. Mol. Microbiol. 33:249-259. [DOI] [PubMed] [Google Scholar]
- 9.de Mendoza, D., A. Klages Ulrich, and J. E. Cronan, Jr. 1983. Thermal regulation of membrane fluidity in Escherichia coli. Effects of overproduction of beta-ketoacyl-acyl carrier protein synthase I. J. Biol. Chem. 258:2098-2101. [PubMed] [Google Scholar]
- 10.Garwin, J. L., A. L. Klages, and J. E. Cronan, Jr. 1980. Beta-ketoacyl-acyl carrier protein synthase II of Escherichia coli. Evidence for function in the thermal regulation of fatty acid synthesis. J. Biol. Chem. 255:3263-3265. [PubMed] [Google Scholar]
- 11.Hamilton, I. R. 1990. Maintenance of proton motive force by Streptococcus mutans and Streptococcus sobrinus during growth in continuous culture. Oral Microbiol. Immunol. 5:280-287. [DOI] [PubMed] [Google Scholar]
- 12.Haque, M., Y. Hirai, K. Yokota, N. Mori, I. Jahan, H. Ito, H. Hotta, I. Yano, Y. Kanemasa, and K. Oguma. 1996. Lipid profile of Helicobacter spp.: presence of cholesteryl glucoside as a characteristic feature. J. Bacteriol. 178:2065-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwami, Y., S. Hata, C. F. Schachtele, and T. Yamada. 1995. Simultaneous monitoring of intracellular pH and proton excretion during glycolysis by Streptococcus mutans and Streptococcus sanguis: effect of low pH and fluoride. Oral Microbiol. Immunol. 10:355-359. [DOI] [PubMed] [Google Scholar]
- 14.Kashket, S., and E. R. Kashket. 1985. Dissipation of the proton motive force in oral streptococci by fluoride. Infect. Immun. 48:19-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindblom, G., G. Oradd, L. Rilfors, and S. Morein. 2002. Regulation of lipid composition in Acholeplasma laidlawii and Escherichia coli membranes: NMR studies of lipid lateral diffusion at different growth temperatures. Biochemistry 41:11512-11515. [DOI] [PubMed] [Google Scholar]
- 16.Ma, Y., and R. E. Marquis. 1997. Thermophysiology of Streptococcus mutans and related lactic-acid bacteria. Antonie Leeuwenhoek 72:91-100. [DOI] [PubMed] [Google Scholar]
- 17.Magnuson, K., S. Jackowski, C. O. Rock, and J. E. Cronan, Jr. 1993. Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol. Rev. 57:522-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McElhaney, R. N. 1974. The effect of alterations in the physical state of the membrane lipids on the ability of Acholeplasma laidlawii B to grow at various temperatures. J. Mol. Biol. 84:145-157. [DOI] [PubMed] [Google Scholar]
- 19.McElhaney, R. N., and K. A. Souza. 1976. The relationship between environmental temperature, cell growth and the fluidity and physical state of the membrane lipids in Bacillus stearothermophilus. Biochim. Biophys. Acta 443:348-359. [DOI] [PubMed] [Google Scholar]
- 20.Murchison, H. H., J. F. Barrett, G. A. Cardineau, and R. Curtiss III. 1986. Transformation of Streptococcus mutans with chromosomal and shuttle plasmid (pYA629) DNAs. Infect. Immun. 54:273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quivey, R. G., Jr., R. Faustoferri, K. Monahan, and R. Marquis. 2000. Shifts in membrane fatty acid profiles associated with acid adaptation of Streptococcus mutans. FEMS Microbiol. Lett. 189:89-92. [DOI] [PubMed] [Google Scholar]
- 22.Quivey, R. G., Jr., W. L. Kuhnert, and K. Hahn. 2000. Adaptation of oral streptococci to low pH. Adv. Microb. Physiol. 42:239-274. [DOI] [PubMed] [Google Scholar]
- 23.Sato, M., H. Tsuchiya, H. Tani, K. Yamamoto, R. Yamaguchi, H. Nitta, N. Kanematsu, I. Namikawa, and N. Takagi. 1991. Incorporation of fatty acids by Streptococcus mutans. FEMS Microbiol. Lett. 65:117-121. [DOI] [PubMed] [Google Scholar]
- 24.Stephan, R. M. 1944. Intra-oral hydrogen ion concentrations associated with dental caries activity. J. Dent. Res. 23:257-266. [Google Scholar]
- 25.Svensater, G., U. B. Larsson, E. C. Greif, D. G. Cvitkovitch, and I. R. Hamilton. 1997. Acid tolerance response and survival by oral bacteria. Oral Microbiol. Immunol. 12:266-273. [DOI] [PubMed] [Google Scholar]
- 26.Vance, D., I. Goldberg, O. Mitsuhashi, and K. Bloch. 1972. Inhibition of fatty acid synthetases by the antibiotic cerulenin. Biochem. Biophys. Res. Commun. 48:649-656. [DOI] [PubMed] [Google Scholar]