Abstract
Background
Cigarette smoking is associated with esophageal adenocarcinoma (EAC), esophagogastric junctional adenocarcinoma (EGJA) and esophageal squamous cell carcinoma (ESCC), and alcohol consumption with ESCC. However, no analyses have examined how delivery rate modifies the strength of odds ratio (OR) trends with total exposure, i.e., the impact on the OR for a fixed total exposure of high exposure rate for short duration compared with low exposure rate for long duration.
Methods
The authors pooled data from 12 case-control studies from the Barrett’s Esophagus and Esophageal Adenocarcinoma Consortium (BEACON), including 1,242 (EAC), 1,263 (EGJA) and 954 (ESCC) cases and 7,053 controls, modeled joint ORs for cumulative exposure and exposure rate for cigarette smoking and alcohol consumption, and evaluated effect modification by sex, body mass index (BMI), age and self-reported acid reflux.
Results
For smoking, all sites exhibited inverse delivery rate effects, whereby ORs with pack-years increased, but trends weakened with increasing cigarettes/day. None of the examined factors modified associations, except for ESCC where younger ages at diagnosis enhanced smoking effects (P<0.01). For EAC and EGJA, ORs with drink-years exhibited inverse associations in <5 drinks/day consumers and no association in heavier consumers. For ESCC, ORs with drink-years increased, with trends strengthening with greater drinks/day. There was no significant effect modification, except for EAC and EGJA where acid reflux mitigated the inverse associations (P=0.02). For ESCC, younger ages at diagnosis enhanced drinking-related ORs (P<0.01).
Conclusions
Patterns of ORs by pack-years and drink-years, delivery rate effects and effect modifiers revealed common as well as distinct etiologic elements for these diseases.
Keywords: alcohol drinking, risk model, smoking
1. Introduction
Cigarette smoking is an established risk factor for esophageal adenocarcinoma (EAC), esophagogastric junctional adenocarcinoma (EGJA) and esophageal squamous cell carcinoma (ESCC), while alcohol consumption is a risk factor only for ESCC [1]. Studies have investigated other potential risk factors, such as obesity, gastroesophageal reflux and diet [1]; however, no previous analysis has considered how delivery rate impacts odds ratio trends (OR) with total exposure for smoking and for drinking.
Studies have typically estimated joint ORs by exposure rate (cigarettes/day [CPD] or drinks/day [DPD]) and exposure duration. Interpretation is however problematic, since ORs with increasing exposure rate for a fixed duration embed effects of increasing total exposure [2]. For example, for 30 years smoking, comparisons of ORs at 20 CPD and 30 CPD include different total exposures, i.e., 30 and 45 pack-years, respectively, where pack-years is the product of mean CPD and years of cigarette smoking. Thus, ORs for exposure rate and duration cannot be interpreted as separate effects. In contrast, we consider total exposure (pack-years or drink-years, defined as the product of mean DPD and years of alcohol consumption) and exposure rate (CPD or DPD), which reformulates analysis in terms of OR trends with total exposure and the modifying effects of delivery rate, where delivery rate represents the relative effects on the OR for an equal total exposure of a high exposure rate for a short duration compared with a low exposure rate for a long duration. For various environmental factors, e.g., cigarette smoking, alcohol consumption and inhaled arsenic, this approach enabled relatively simple characterizations of the joint ORs and generated novel etiologic insights [2–7]. Previous modeling of EAC, EGJA and ESCC data focused on a single factor, CPD or DPD, using splines or general additive models [8,9].
Using pooled data from the Barrett’s and Esophageal Adenocarcinoma Consortium (BEACON), we extend previous analyses of the associations of EAC and EGJA with smoking [10] and alcohol consumption [11], and include additional data on ESCC. Specifically, we consider: (i) trends in ORs by pack-years and drink-years and the influence of CPD and DPD; and (ii) effect modification of smoking patterns and drinking patterns by sex, body mass index (BMI=weight [kg]/height [m]2), occurrence of acid reflux and age, as well as the joint effects of smoking and drinking.
2. Material and methods
2.1 Study data
Data derived from 10 population-based case-control studies [12–21] and two case-control studies nested within cohorts [22–24] of EAC and EGJA (see Table 1 in Cook [10]). We additionally included data from seven BEACON studies which also enrolled ESCC cases [12–15,21–23]. Prior to the implementation of data restrictions (see below), there were 5,427 cases and 12,769 controls available for analysis. For additional study-specific information, see Supplement A.
The population-based studies ascertained amount and duration of cigarette use and smoking status at or within 2 years of diagnosis for cases or the index date for controls. For the two nested case-control studies, the baseline questionnaires administered at cohort enrollment provided exposure variables. The two nested studies ascertained CPD in categories [22,23], while one study ascertained duration of smoking in categories [23]. We assigned midpoint values for the categories. One study did not have duration information [22] which we estimated using either attained age or age at smoking cessation and 17 years as the age at smoking initiation. We minimized the influence of smoking cessation by restricting analysis to never, current and recent (<2 years) former cigarette smokers, thereby omitting 2,183 cases and 4,542 controls who were former smokers.
For alcohol consumption, all studies defined never-drinkers as lifelong abstainers, except four studies which ascertained drinking status one [22,23], five [20] or 20 years [15] before enrollment. We assigned midpoints for one study which collected DPD in categories [23]. Analyses included all subjects, since drinking cessation information was not available for all studies. We standardized DPD by equating one 12 ounce beer, 5 ounce glass of wine and 1.5 ounce of liquor.
Four studies (with 1,859 cases and 6,189 controls) lacked information on duration of alcohol consumption [15,19,20,22], and were not included in drinking analyses. However, we included these studies in smoking analyses by imputing drinking duration. For the eight studies with duration, we cross-classified control drinkers by sex, age (4 levels) and DPD (8 levels) and calculated cell-specific frequencies for duration using cell-specific decile cut-points. For each subject missing duration, we identified the appropriate sex, age and DPD category, randomly sampled a duration category using frequency weights and assigned mean duration. Inference for smoking analyses was similar using either single or multiple imputations, and we therefore present results for a single imputation. Analyses indicated that the inclusion of these four studies did not affect estimates of smoking-related parameters, as results were similar with these studies omitted.
BMI derived from self-reported height and weight, using usual adult weight [12,14,18,19], or, if unavailable, weight one year [13,17,21], five years [20] or 20 years [15] prior to the referent age. One study ascertained weight at age 20 and maximum adult weight (excluding pregnancies), for which we used the latter assuming it better reflected usual adult weight [16]. For the nested case-control studies, we used weight at cohort entry [22,23].
The Institutional Review Board or Research Ethics Committee for each study approved data collection and, if required, participation in the pooling.
2.2 Statistical models
We used binary logistic regression to estimate ORs for each category of smoking and alcohol consumption, as appropriate. For continuous pack-years, d, and CPD, x, the standard logistic model with exponentially increasing ORs across the full exposure range provided a poor fit to the data. We therefore fitted the model OR(z, d, x) = exp(α z) OR(d, x), where α and z were vectors of adjustment parameters and covariates, respectively, and
| (1) |
where β represented the excess odds ratio per pack-year (EOR/pack-year) and g(.) described variations of the EOR/pack-year with CPD, i.e., changes in strength with delivery rate [2,4]. After assessing alternatives, we set g(x)=exp{ϕ1 ln(x) + ϕ2 ln(x)2}, with g(0)=0. ORs by pack-years were approximately linear within a CPD category, i.e., OR(d) = 1 + γi d for the ith CPD category, where γi was the EOR/pack-year. We compared the fitted β g(x) with the γi estimates from the model:
| (2) |
where di equaled d within the ith CPD category and zero otherwise.
We also used model (1) for continuous drink-years and DPD after preliminary analysis revealed approximately linear relationships for ORs by drink-years within DPD categories. For ESCC, we used the same g(.). For EAC and EGJA, variations with DPD were complex and we modeled g(.) with restricted cubic splines (Supplement B), with the Akaike Information Criterion (AIC) advising on the number and placement of knots [25].
We considered effect modification by a categorical factor (f) with levels 1,…,S using
| (3) |
where distinct βs parameters and gs (.) functions replaced β and g(.), and where ds equaled d and xs equaled x within level s and zero otherwise. We used deviances to compare model fit and evaluate whether effect modification derived from total exposure (different β’s), exposure rate (different g(.)’s) or both. Starting with model (3), we constrained the β’s and/or g(.) functions to be equivalent across f and examined degradation in model fit. This approach, in contrast to starting with model (1) and enlarging the model, allowed the evaluation of the interaction of f and one factor (e.g., pack-years) while minimizing influence of the interaction of f and its closely related correlate (e.g., CPD).
Software for polytomous regression under model (1) to evaluate differences in the magnitude of associations between smoking and alcohol consumption across the three disease outcomes (EAC, EGJA, ESCC) was not available. As an alternative, we created one dataset with three “strata” consisting of EAC cases and controls, EGJA cases and controls and ESCC cases and controls and applied model (3) to test homogeneity across outcome. The approach was anti-conservative, since controls were replicated, but was generally comparable to use of categorical variables and standard polytomous logistic regression.
Analyses adjusted for the cross-classification of study (12 levels), age (<60, 60–64, 65–69, 70+ years) and sex, and for education (less than high school, high school graduate, more than high school, missing/not available) and BMI (<25, 25–29.9, 30.0–34.9, 35.0+ kg/m2). For smoking analyses, we further adjusted for drink-years and DPD categories, and for drinking analyses we adjusted for pack-years and CPD categories. Since results were similar, we did not adjust for race or occurrence of acid reflux, which was available in five studies only.
Cigarette smoking analyses included 927, 990 and 915 cases of EAC, EGJA and ESCC, respectively, 7,431 controls for EAC and EGJA and, due to fewer studies, 6,212 controls for ESCC. Alcohol consumption analyses included 1,103, 1,118 and 896 cases, respectively, and 5,719 controls for EAC and EGJA and 3,973 controls for ESCC.
Previous analyses of cigarette smoking suggested that <10 CPD smokers increased model instability due to a limited range for pack-years. We therefore repeated analyses in never and 10+ CPD smokers, which included 89.7% of cases and 89.1% of controls. For alcohol consumption, the relatively few heavy drinkers were highly influential. We repeated analyses in never and ≤10 DPD drinkers, which included 94.6% of cases and 98.4% of controls.
We fit the various models using the Epicure computer package[26].
3.0 Results
3.1 Marginal and joint odds ratios by pack-years and cigarettes/day
ORs by pack-years increased significantly for all outcomes, with ESCC exhibiting the strongest association (Table 1). Adjusted for pack-years, ORs by CPD increased, leveled, then even decreased, suggesting variations with delivery rate. The test of no linear trend with CPD rejected only for ESCC, although a test of no linear-quadratic variation rejected for EAC (P=0.02) and EGJA (P=0.05).
Table 1.
Odds Ratios (OR) with 95% Confidence Intervals (CI) By Cigarette Smoking And Alcohol Consumption By Disease Type. Data From The Barrett’s Esophagus And Esophageal Adenocarcinoma Consortium (BEACON).
| Variable | Cases a | Controls | OR | 95% CI | OR | 95% CI | OR | 95% CI | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EAC | EGJA | ESCC | EAC/EGJA | ESCC | EAC | EGJA | ESCC | ||||
| Pack-years of smokingb | |||||||||||
| 0 | 411 | 393 | 203 | 4,587 | 3,720 | 1.00 c | 1.00 c | 1.00 c | |||
| 1–29 | 61 | 47 | 67 | 596 | 527 | 1.66 | 1.1–2.4 | 1.24 | 0.8–1.8 | 2.63 | 1.8–4.0 |
| 30–39 | 73 | 108 | 121 | 608 | 529 | 1.45 | 0.8–2.5 | 1.87 | 1.1–3.1 | 2.69 | 1.6–4.6 |
| 40–49 | 117 | 135 | 166 | 485 | 407 | 2.22 | 1.2–4.0 | 2.01 | 1.1–3.5 | 3.93 | 2.2–7.1 |
| 50–59 | 104 | 144 | 178 | 684 | 630 | 1.92 | 1.0–3.6 | 1.93 | 1.1–3.5 | 4.62 | 2.5–8.5 |
| 60+ | 161 | 163 | 180 | 471 | 399 | 2.77 | 1.4–5.6 | 1.86 | 1.0–3.6 | 5.63 | 2.7–11.7 |
| P for no linear trend d | <0.01 | <0.01 | <0.01 | ||||||||
| Cigarettes/day e | |||||||||||
| 1–9 | 46 | 35 | 43 | 315 | 254 | 1.00 c | 1.00 c | 1.00 c | |||
| 10–19 | 140 | 141 | 198 | 955 | 825 | 1.30 | 0.8–2.2 | 1.29 | 0.8–2.1 | 1.42 | 0.9–2.3 |
| 20–29 | 178 | 245 | 281 | 1,067 | 978 | 1.33 | 0.7–2.4 | 1.82 | 1.0–3.2 | 0.89 | 0.5–1.6 |
| 30–39 | 73 | 100 | 91 | 206 | 170 | 1.65 | 0.8–3.4 | 2.59 | 1.5–5.0 | 1.03 | 0.5–2.1 |
| 40+ | 79 | 76 | 99 | 301 | 265 | 1.34 | 0.6–2.9 | 2.12 | 1.0–4.3 | 0.71 | 0.3–1.5 |
| P for no linear trend | 0.40 | 0.35 | (<0.01) | ||||||||
| Drink-years f | |||||||||||
| 0 | 146 | 143 | 44 | 1,054 | 579 | 1.00 c | 1.00 c | 1.00 c | |||
| 1–49 | 468 | 480 | 181 | 2,697 | 1,847 | 0.74 | 0.6–0.9 | 0.86 | 0.7–1.1 | 1.31 | 0.9–1.9 |
| 50–99 | 198 | 209 | 131 | 882 | 666 | 0.77 | 0.5–1.1 | 0.93 | 0.6–1.3 | 2.18 | 1.3–3.8 |
| 100–149 | 96 | 103 | 114 | 436 | 347 | 0.60 | 0.4–0.9 | 0.68 | 0.4–1.0 | 2.96 | 1.6–5.3 |
| 150–199 | 60 | 70 | 107 | 231 | 191 | 0.62 | 0.4–1.1 | 0.62 | 0.4–1.0 | 3.52 | 1.8–6.9 |
| 200+ | 135 | 113 | 319 | 419 | 343 | 0.67 | 0.4–1.2 | 0.49 | 0.3–0.9 | 3.82 | 1.9–7.8 |
| P for no linear trend | (0.22) | (0.34) | <0.01 | ||||||||
| Drinks/day g | |||||||||||
| 0.1–1.0 | 376 | 386 | 140 | 2,149 | 1,491 | 1.00 c | 1.00 c | 1.00 c | |||
| 1.0–2.9 | 309 | 314 | 197 | 1,520 | 1,112 | 1.09 | 0.8–1.4 | 0.88 | 0.7–1.1 | 1.32 | 0.9–1.9 |
| 3.0–4.9 | 130 | 142 | 188 | 547 | 439 | 1.27 | 0.8–1.9 | 1.24 | 0.8–1.8 | 2.15 | 1.3–3.6 |
| 5.0–9.9 | 104 | 105 | 199 | 332 | 269 | 1.56 | 0.9–2.7 | 1.90 | 1.1–3.2 | 2.74 | 1.5–5.2 |
| 10+ | 38 | 28 | 128 | 117 | 83 | 1.87 | 0.9–3.7 | 1.74 | 0.9–3.4 | 4.12 | 2.0–8.4 |
| P for no linear trend | 0.90 | 0.60 | 0.39 | ||||||||
Includes esophageal adenocarcinoma (EAC), esophagogastric junctional adenocarcinoma, (EGJA), and esophageal squamous cell carcinoma (ESCC) cases. There were fewer controls for ESCC cases, since cases enrolled in seven studies only. ORs obtained from standard binary logistic regression adjusted for study/center, education, age, sex.
Never and current cigarette smokers. ORs additionally adjusted for BMI, drink-years, DPD and CPD. ORs by pack-years at the referent category of <10 CPD smokers, based on a logistic main effects model for the two factors.
Referent category.
P-value for one degree of freedom chi-square test of no trend. Parentheses denote negative trend.
Never and current cigarette smokers. ORs additionally adjusted for BMI, drink-years, DPD and pack-years.
All data, omitting subjects with missing drink-years. ORs additionally adjusted for BMI, pack-years, CPD and DPD. ORs by drink-years at the referent category of <1.0 DPD drinkers, based on a multiplicative logistic model for main effects.
All data, omitting subjects with missing drink-years. ORs additionally adjusted for BMI, pack-years, CPD and drink-years.
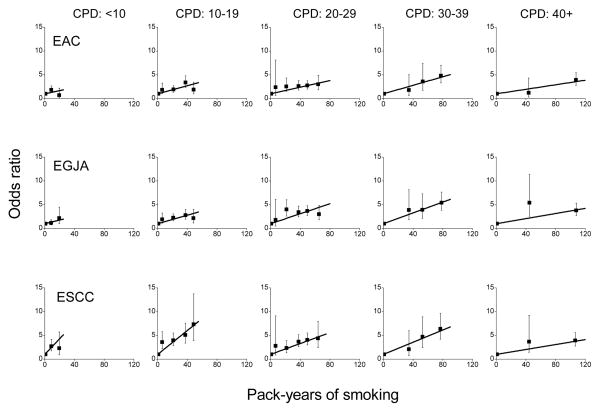
For joint categories of pack-years and CPD, ORs relative to never-smokers increased approximately linearly with pack-years within CPD categories (Figure 1). Four of 15 tests rejected linearity at the 0.05-level (for EAC <10 CPD, for EGJA 20–29 and 40+ CPD, and for ESCC 40+ CPD). A sensitivity analysis revealed that after omitting two studies [17,21] only one of 15 tests rejected linearity (for EGJA 20–29 CPD), consistent with expectation.
Figure 1.
Odds ratios for esophageal adenocarcinoma (EAC), esophagogastric junctional adenocarcinoma (EGJA) and esophageal squamous cell carcinoma (ESCC) by categories of pack-years of cigarette smoking and number of cigarettes smoked per day (CPD) (solid symbol) and fitted linear models in pack-years (see text). Bars represent 95% confidence intervals. Data for never and current cigarette smokers from the Barrett’s Esophagus and Esophageal Adenocarcinoma Consortium (BEACON).
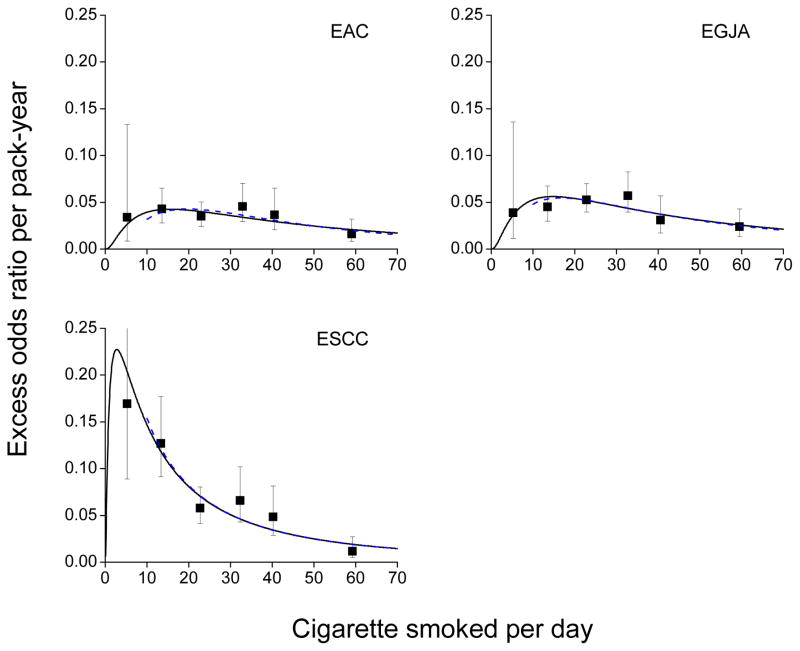
The EOR/pack-year estimates from model (2), i.e., slopes, were generally larger for ESCC than for EAC and EGJA, which were themselves similar (Table 2). EOR/pack-year estimates (square symbol) and the fitted model (1) (solid line) declined at higher CPDs, indicating a decreasing strength of association (Figure 2). For each outcome, the variation of the pack-years association with CPD, g(.), was significant (Supplement Table C1).
Table 2.
Estimated Excess Odds Ratio (EOR) Per Pack-Year By Categories Of Cigarettes/Day (CPD), Overall And By Levels Of Potential Effect Modifiers a. Data For Never And Current Cigarette Smokers From The Barrett’s Esophagus And Esophageal Adenocarcinoma Consortium (BEACON).
| Modifier | Level | Cigarettes/day | P b | P b,c | |||||
|---|---|---|---|---|---|---|---|---|---|
| <10 | 10–19 | 20–29 | 30–39 | 40–49 | 50+ | ||||
| Esophageal adenocarcinoma carcinoma cases and controls | |||||||||
| None | 0.034 | 0.043 | 0.035 | 0.045 | 0.036 | 0.016 | |||
| Sex | Females | 0.066 | 0.062 | 0.102 | 0.084 | 0.052 | 0.000 | 0.17 | 0.21 |
| Males | 0.025 | 0.039 | 0.029 | 0.042 | 0.034 | 0.017 | |||
| BMI d | <25 | 0.028 | 0.034 | 0.036 | 0.060 | 0.022 | 0.035 | 0.51 | 0.36 |
| 25–29 | 0.020 | 0.062 | 0.039 | 0.037 | 0.055 | 0.012 | |||
| 30+ | 0.085 | 0.027 | 0.025 | 0.039 | 0.043 | 0.006 | |||
| Age | <55 | 0.021 | 0.055 | 0.040 | 0.069 | 0.027 | 0.023 | 0.64 | 0.80 |
| 55–64 | 0.028 | 0.054 | 0.031 | 0.047 | 0.058 | 0.005 | |||
| 65+ | 0.042 | 0.028 | 0.033 | 0.028 | 0.023 | 0.023 | |||
| Acid reflux | No | 0.000 | 0.047 | 0.026 | 0.050 | 0.046 | 0.017 | 0.57 | 0.69 |
| Yes | 0.031 | 0.066 | 0.025 | 0.034 | 0.050 | 0.035 | |||
| Drink-yrs | Never | 0.127 | 0.057 | 0.022 | 0.145 | 0.195 | 0.005 | 0.14 | 0.79 |
| <50 | 0.016 | 0.027 | 0.046 | 0.035 | 0.038 | 0.031 | |||
| 50–99 | 0.000 | 0.051 | 0.035 | 0.028 | 0.015 | 0.013 | |||
| 100+ | 0.150 | 0.068 | 0.025 | 0.050 | 0.033 | 0.009 | |||
| Esophagogastric junctional adenocarcinoma cases and controls | |||||||||
| None | 0.039 | 0.045 | 0.053 | 0.057 | 0.031 | 0.024 | |||
| Sex | Females | 0.083 | 0.060 | 0.101 | 0.077 | 0.109 | 0.069 | 0.09 | 0.08 |
| Males | 0.027 | 0.041 | 0.045 | 0.053 | 0.026 | 0.019 | |||
| BMI | <25 | 0.015 | 0.057 | 0.056 | 0.067 | 0.024 | 0.038 | 0.03 | 0.28 |
| 25–29 | 0.127 | 0.043 | 0.053 | 0.047 | 0.042 | 0.017 | |||
| 30+ | 0.000 | 0.023 | 0.050 | 0.065 | 0.034 | 0.018 | |||
| Age | <55 | 0.000 | 0.045 | 0.062 | 0.079 | 0.031 | 0.020 | 0.32 | 0.36 |
| 55–64 | 0.053 | 0.054 | 0.077 | 0.045 | 0.061 | 0.039 | |||
| 65+ | 0.049 | 0.041 | 0.033 | 0.054 | 0.013 | 0.011 | |||
| Acid reflux | No | 0.051 | 0.070 | 0.062 | 0.058 | 0.041 | 0.023 | 0.29 | 0.36 |
| Yes | 0.027 | 0.043 | 0.082 | 0.088 | 0.032 | 0.115 | |||
| Drink-yrs | Never | 0.000 | 0.095 | 0.088 | 0.141 | 0.083 | 0.031 | 0.06 | 0.09 |
| <50 | 0.061 | 0.037 | 0.047 | 0.050 | 0.022 | 0.029 | |||
| 50–99 | 0.000 | 0.028 | 0.080 | 0.041 | 0.018 | 0.014 | |||
| 100+ | 0.030 | 0.042 | 0.029 | 0.049 | 0.032 | 0.019 | |||
| Esophageal squamous cell carcinoma case and controls | |||||||||
| None | 0.169 | 0.127 | 0.058 | 0.066 | 0.048 | 0.012 | |||
| Sex | Females | 0.168 | 0.170 | 0.056 | 0.037 | 0.199 | 0.008 | 0.65 | 0.79 |
| Males | 0.168 | 0.111 | 0.056 | 0.070 | 0.042 | 0.012 | |||
| BMI | <25 | 0.192 | 0.154 | 0.066 | 0.083 | 0.045 | 0.012 | 0.08 | 0.06 |
| 25–29 | 0.188 | 0.119 | 0.057 | 0.042 | 0.062 | 0.012 | |||
| 30+ | 0.000 | 0.031 | 0.025 | 0.077 | 0.054 | 0.012 | |||
| Age | <55 | 0.314 | 0.319 | 0.106 | 0.091 | 0.078 | 0.007 | 0.01 | <0.01 |
| 55–64 | 0.135 | 0.131 | 0.051 | 0.060 | 0.077 | 0.028 | |||
| 65+ | 0.151 | 0.071 | 0.048 | 0.067 | 0.024 | 0.012 | |||
| Acid reflux | No | 0.205 | 0.186 | 0.094 | 0.063 | 0.060 | 0.012 | 0.45 | 0.39 |
| Yes | 0.194 | 0.142 | 0.073 | 0.053 | 0.202 | 0.086 | |||
| Drink-yrs | Never | 0.012 | 0.168 | 0.000 | 0.065 | 0.003 | 0.011 | 0.19 | 0.12 |
| <50 | 0.155 | 0.156 | 0.073 | 0.044 | 0.081 | 0.015 | |||
| 50–99 | 0.000 | 0.200 | 0.156 | 0.176 | 0.049 | 0.042 | |||
| 100+ | 0.141 | 0.083 | 0.036 | 0.049 | 0.041 | 0.006 | |||
Estimates of EOR/pack-year based on linear odds ratios by pack-years relative to never-smokers within CPD categories.
P-values for chi-square tests of homogeneity of model (3) for continuous pack-years and CPD across levels of modifying factor, based on 3 (2-level modifier ) or 6 (3-level modifier) degrees of freedom. Additional test results given in Supplement Table C2.
P-value for data restricted to never and 10+ CPD smokers.
Body mass index.
Figure 2.
Estimated excess odds ratios per pack-year for esophageal adenocarcinoma (EAC), esophagogastric junctional adenocarcinoma (EGJA) and esophageal squamous cell carcinoma (ESCC) within categories of cigarettes per day (CPD) (square symbol), plotted at the category-specific mean CPD, and model (1) fitted to all data (solid line) and to never and 10+ CPD smokers (dash line). Bars represent 95% confidence intervals. Data for never and current cigarette smokers from the Barrett’s Esophagus and Esophageal Adenocarcinoma Consortium (BEACON).
Cigarette smoking patterns for EAC, EGJA and ESCC differed significantly (P<0.01) (Table C1). Relative to model (3) with outcome-specific βs and gs(.), there was less degradation in fit with common β (P=0.09 and P=0.17 in the full and restricted data, respectively) than with common g(.) (P=0.04 and P=0.07), suggesting the greater EORs for ESCC derived from differential smoking rate effects, g(.). A similar evaluation indicated homogeneity of smoking-related parameters for EAC and EGJA (P=0.63) (not shown).
3.2 Marginal and joint odds ratios by drink-years and drinks/day
Using binary logistic regression ORs for EAC and EGJA by drink-years were <1.0, while ORs by DPD adjusted for drink-years were about 1.0 for <5 DPD and >1.0 for 5+ DPD (Table 1). These patterns, based on marginal effects, suggested a protective association with drink-years in low DPD consumers but a deleterious association in high DPD consumers. For ESCC, ORs by drink-years increased significantly. After adjustment for drink-years, ORs by DPD increased. Although the test of no linear trend did not reject (P=0.39), the test of no linear-quadratic trend did reject (P<0.01).
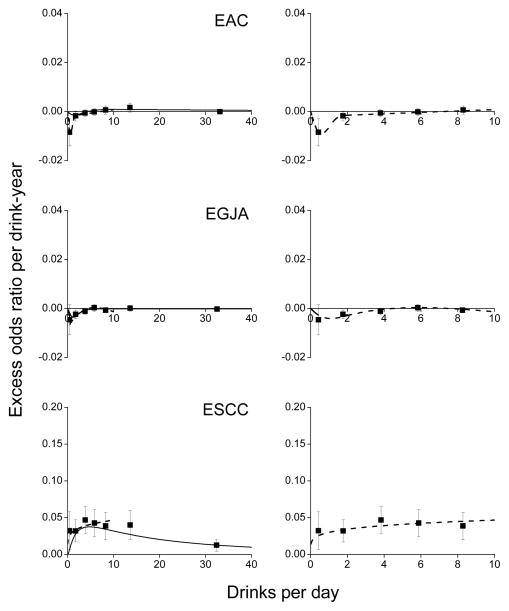
For joint categories of drink-years and DPD, ORs relative to never-drinkers increased approximately linearly with drink-years within DPD categories (Figure 3). Two of 15 tests (for EGJA 5.0–9.9 and ESCC 10+ DPD) significantly rejected linearity. For EAC and EGJA, ORs with drink-years declined in <5 DPD categories. For the five categories, p-values for a test of no trend with drink-years were <0.01, 0.06, 0.33, 0.85 and 0.30 for EAC, respectively, and 0.10, <0.01, 0.04, 0.62 and <0.01 for EGJA.
Figure 3.
Odds ratios for esophageal adenocarcinoma (EAC), esophagogastric junctional adenocarcinoma (EGJA) and esophageal squamous cell carcinoma (ESCC) by categories of drink-years and number of drinks per day (DPD) (solid symbol) and fitted linear models in drink-years (see text). Bars represent 95% confidence intervals. Data from the Barrett’s Esophagus and Esophageal Adenocarcinoma Consortium (BEACON).
Expanding DPD categories and fitting model (2), there were inverse associations with drink-years for <5 DPD drinkers (Table 3 and Figure 4), with EOR/drink-year estimates (×10) increasing monotonically from −0.085 to 0.016 for <20 DPD for EAC and from −0.046 to 0.002 for <7 DPD for EGJA. Applying model (1), there were significant associations with alcohol consumption for EAC (P=0.06) and for EGJA (P=0.03), with DPD significantly modifying the EOR/drink-year estimates (P=0.04 and P=0.02) (Table C3).
Table 3.
Estimated Excess Odds Ratio (EOR) Per 10-Drink-Years By Categories Of Drinks/Day (DPD), Overall And By Levels Of Potential Effect Modifiers a. Data From The Barrett’s Esophagus And Esophageal Adenocarcinoma Consortium (BEACON).
| Modifier | Level | Drinks/day | P b | P b,c | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <1 | 1–2.9 | 3–4.9 | 5–6.9 | 7–9.9 | 10–19.9 | 20+ | ||||
| Esophageal adenocarcinoma carcinoma cases and controls | ||||||||||
| None | −0.085 | −0.018 | −0.007 | −0.002 | 0.006 | 0.016 | −0.001 | |||
| Sex | Female | −0.066 | 0.018 | 0.069 | −0.032 | 0.699 | −0.053 | 0.000 | 0.31 | 0.21 |
| Males | −0.088 | −0.021 | −0.008 | −0.002 | 0.003 | 0.016 | −0.001 | |||
| BMI d | <25.0 | −0.090 | −0.003 | 0.022 | −0.005 | −0.003 | 0.024 | 0.001 | 0.97 | 0.79 |
| 25–29 | −0.086 | −0.026 | −0.009 | −0.008 | 0.030 | 0.008 | −0.003 | |||
| 30+ | −0.082 | −0.031 | −0.032 | 0.023 | −0.011 | 0.025 | −0.030 | |||
| Age | <55 | −0.116 | 0.022 | 0.006 | 0.043 | 0.035 | −0.005 | −0.003 | 0.47 | 0.18 |
| 55–64 | −0.122 | −0.042 | −0.014 | −0.009 | 0.031 | 0.022 | −0.002 | |||
| 65+ | −0.068 | −0.013 | −0.005 | −0.005 | −0.009 | 0.012 | 0.003 | |||
| Acid reflux | No | −0.072 | −0.048 | −0.024 | −0.018 | 0.000 | 0.018 | 0.000 | 0.72 | 0.03 |
| Yes | −0.124 | −0.015 | −0.004 | 0.007 | −0.008 | 0.008 | −0.005 | |||
| Pack-yrs | 0 | 0.024 | 0.052 | 0.011 | 0.056 | 0.071 | 0.029 | −0.005 | 0.50 | 0.10 |
| <30 | −0.050 | 0.005 | 0.010 | 0.001 | 0.019 | 0.042 | 0.009 | |||
| 30–44 | 0.009 | −0.003 | −0.003 | 0.019 | 0.002 | 0.032 | 0.003 | |||
| 45+ | −0.113 | −0.025 | −0.003 | −0.005 | 0.003 | 0.008 | 0.000 | |||
| Esophagogastric junctional adenocarcinoma cases and controls | ||||||||||
| None | −0.046 | −0.024 | −0.012 | 0.002 | −0.007 | 0.001 | −0.003 | |||
| Sex | Female | −0.020 | −0.015 | −0.004 | 0.054 | −0.035 | 0.071 | 0.000 | 0.92 | 0.98 |
| Males | −0.049 | −0.025 | −0.012 | 0.002 | −0.007 | 0.000 | −0.003 | |||
| BMI | <25.0 | −0.023 | −0.027 | −0.001 | 0.022 | −0.003 | 0.001 | −0.005 | 0.04 | 0.30 |
| 25–29 | 0.038 | 0.016 | 0.003 | −0.002 | 0.014 | −0.005 | 0.000 | |||
| 30+ | −0.089 | −0.025 | −0.009 | 0.021 | −0.009 | 0.068 | 0.060 | |||
| Age | <55 | −0.123 | −0.041 | −0.011 | 0.002 | −0.027 | −0.013 | −0.003 | 0.92 | 0.45 |
| 55–64 | −0.067 | −0.027 | −0.004 | 0.016 | 0.022 | −0.003 | −0.001 | |||
| 65+ | −0.028 | −0.021 | −0.015 | −0.005 | −0.012 | 0.010 | −0.004 | |||
| Acid reflux | No | −0.036 | −0.039 | −0.020 | −0.005 | −0.002 | −0.008 | −0.003 | 0.09 | 0.14 |
| Yes | −0.011 | −0.010 | 0.001 | 0.015 | −0.003 | 0.009 | −0.005 | |||
| Pack-yrs | 0 | 0.008 | 0.009 | 0.030 | 0.033 | −0.015 | 0.009 | 0.023 | 0.89 | 0.72 |
| <30 | 0.069 | 0.005 | 0.005 | 0.006 | 0.015 | −0.003 | −0.005 | |||
| 30–44 | −0.050 | −0.007 | −0.015 | −0.001 | 0.006 | 0.005 | −0.003 | |||
| 45+ | −0.052 | −0.025 | −0.004 | 0.012 | −0.002 | 0.006 | −0.001 | |||
| Esophageal squamous cell carcinoma case and controls | ||||||||||
| None | 0.323 | 0.320 | 0.468 | 0.427 | 0.386 | 0.400 | 0.124 | |||
| Sex | Female | 0.023 | 0.252 | 0.287 | 4.019 | 0.561 e | 0.38 | 0.05 | ||
| Males | 0.849 | 0.498 | 0.694 | 0.594 | 0.551 | 0.559 | 0.175 | |||
| BMI | <25 | 0.414 | 0.327 | 0.476 | 0.357 | 0.351 | 0.372 | 0.106 | 0.04 | 0.06 |
| 25–29 | 0.359 | 0.462 | 0.575 | 0.608 | 0.462 | 0.440 | 0.143 | |||
| 30+ | −0.054 | 0.095 | 0.307 | 0.445 | 0.534 | 0.618 | 668.7 | |||
| Age | <55 | 0.072 | 0.881 | 1.282 | 1.224 | 1.291 | 1.346 | 0.286 | <0.01 | <0.01 |
| 55–64 | 0.323 | 0.404 | 0.624 | 0.449 | 0.481 | 0.334 | 0.090 | |||
| 65+ | 0.203 | 0.176 | 0.236 | 0.270 | 0.198 | 0.261 | 0.121 | |||
| Acid reflux | No | 0.134 | 0.140 | 0.216 | 0.203 | 0.261 | 0.278 | 0.087 | 0.18 | 0.30 |
| Yes | 0.043 | 0.019 | 0.190 | 0.272 | 0.111 | 0.186 | 0.024 | |||
| Pack-yrs | 0 | 0.189 | 0.195 | 0.597 | 0.762 | 0.416 | 0.850 | 0.570 | <0.01 | 0.26 |
| <30 | 0.509 | 0.243 | 0.409 | 0.516 | 0.534 | 0.531 | 0.090 | |||
| 30–44 | 0.193 | 0.231 | 0.490 | 0.293 | 0.457 | 0.256 | 0.016 | |||
| 45+ | 0.447 | 0.623 | 0.548 | 0.472 | 0.347 | 0.422 | 0.346 | |||
Estimates of EOR/10 drink-years based on linear odds ratios by drink-years relative to never-drinkers within DPD categories.
P-values for chi-square test of homogeneity of model (3) for continuous drink-years and DPD across levels of modifying factor, based on 3 (2-level modifier ), 6 (3-level modifier) or 8 (4-level modifier) degrees of freedom. Additional test results given in Supplement Table C4.
P-value using data restricted to never and ≤ 10 DPD drinkers.
Body mass index.
Includes 7+ DPD.
Figure 4.
Estimated excess odds ratios per drink-year for esophageal adenocarcinoma (EAC), esophagogastric junctional adenocarcinoma (EGJA) and esophageal squamous cell carcinoma (ESCC) within categories of drinks per day (DPD) (square symbol), plotted at the category-specific mean DPD, for all data (left panels) and for #10 DPD (right panels). Bars represent 95% confidence intervals. For EAC and EGJA, model (1) included DPD effects estimated by restricted cubic splines with four interior knots at 0.2, 0.5, 3.0 and 10.5 DPD for EAC and at 0.1, 1.0, 2.0 and 7.0 for EGJA (solid line), and at 0.1, 0.3, 1.3 and 2.0 DPD for EAC and at 0.1, 0.2, 2.0 and 9.8 for EGJA for never and #10 DPD drinkers (dash line). For ESCC, model (1) included DPD effects defined by an exponential function (see text) fitted to all data (solid line) and to restricted data (dash line). Note the aspect ratio for EAC and EGJA panels was 2-times the aspect ratio for the ESCC panels. Data from the Barrett’s Esophagus and Esophageal Adenocarcinoma Consortium (BEACON).
For ESCC, EOR/drink-year estimates tended to increase with DPD then decrease (Table 3 and Figure 4). We fitted model (1) with g(x)=exp{ϕ1 ln(x) + ϕ2 ln(x)2} for all data and with g(x)=exp{ϕ1 ln(x)}for ≤10 DPD since inclusion of ln(x)2 did not improve fit (P=0.94). For all data, the 3-parameter model (β, ϕ1 and ϕ2) underestimated the EOR/drink-year at lower DPD (solid line), while the 2-parameter model (β and ϕ1) provided a good fit for ≤10 DPD (dash line).
3.3 Effect modification of cigarette smoking and alcohol consumption excess odds ratios
Using model (3), we evaluated effect modification of cigarette smoking ORs by sex, BMI, age, acid reflux and drink-years. For EAC and EGJA, there was no significant effect modification (Tables 2 and C2). For ESCC, there was significant modification by attained age, with smoking patterns enhanced at younger ages (P=0.01) (Figure C1). The enhancement at younger ages derived primarily from the interaction of CPD and age (P=0.03) and not pack-years and age (P=0.57) (Table C2). There was a suggestion of enhanced smoking effects at lower BMIs (Table 2, P=0.08, with P=0.06 in the restricted data), but relationships were not consistent across CPD categories.
We evaluated effect modification of ORs for alcohol consumption (Tables 3 and C4). For EAC and EGJA, we found no significant effect modification by sex, BMI, age or pack-years, but did observe a suggested modification by acid reflux (P=0.03 for EAC and P=0.14 for EGJA). EOR/drink-year estimates for <7 DPD categories were greater for those reporting acid reflux, except in the <1 DPD category for EAC, suggesting acid reflux mitigated the inverse drink-years association (Table 3). Combining EAC and EGJA cases, acid reflux significantly modified alcohol consumption patterns (P=0.02) (not shown). For ESCC, there was significant modification only by attained age, with drinking patterns enhanced at younger attained ages (P<0.01) (Figure C2). The modification of ORs by sex in the restricted data (P=0.05), which was suggested in Table 3, was due to <1 DPD drinkers and omission of those subjects resulted in P=0.37 for the test of homogeneity. Finally, BMI significantly modified drinking patterns (P=0.04), but results were not consistent, with reduced EOR/drink-year estimates for 30+ BMI subjects but only in <5 DPD categories.
3.4 Consistency of smoking and drinking results by study
Using model (3) within the restricted data, tests of homogeneity of smoking effects across studies did not reject for EAC (p=0.08) or for EGJA (p=0.13), but did reject for ESCC (p=0.02). The latter test was influenced by one study (23), which, when omitted, resulted in no rejection of homogeneity (p=0.16) (not shown). ORs for ESCC by pack-years for the Kaiser-Permanente Study (23) were smaller than the other studies, and may have been influenced by the few ESCC smokers (70 of 92 total ESCC cases) and the limited number of distinct values for pack-years due to the use of category mid-points for duration and CPD.
Using model (3) with a restricted cubic spline for g(.), tests for homogeneity of drinking effects for EAC and for EGJA across studies did not reject (p=0.99 and p=0.98, respectively). Seven BEACON studies contributed cases for ESCC analyses. With the additional requirement of information for duration of drinking, ESCC analyses were limited to four studies (12–14, 21). The test of homogeneity of drinking effects was rejected (p<0.01), although it was not rejected (p=0.24) after omitting the Australian study (21). The Australian study had a similar pattern of increasing EOR/drink-year estimates for ESCC; however, the EOR/drink-year parameter (β) was lower.
4.0 Discussion
Our analysis of BEACON data revealed distinct associations for EAC, EGJA and ESCC with cigarette smoking and alcohol consumption and differential effect modification. For cigarette smoking, each outcome exhibited similar inverse delivery rate patterns above 10–15 CPD, whereby for equal pack-years smoking more CPD for shorter duration was less deleterious than fewer CPD for longer duration. This pattern has occurred consistently with smoking-related cancers, including lung, oral cavity, pharynx, larynx, bladder, kidney, liver and pancreas [2,3,5,7,27], and may reflect saturation of activation pathways [28–30], increased detoxification [31] or enhanced DNA repair [32,33]. These patterns may also have reflected CPD-dependent inhalation, whereby heavier smokers inhaled less vigorously and thereby ingested fewer carcinogens per cigarette. However, a sensitivity analysis based on the association between urinary cotinine and CPD for a smoking and lung cancer study concluded that CPD-dependent inhalation did not explain the inverse delivery rate pattern [34].
Previous studies have reported greater smoking-related ORs for ESCC than for EAC and EGJA [1]; however, our analysis went further and suggested that these differences derived from delivery rate effects, i.e., different g(.) functions, and not pack-years, i.e., similar β’s. This implied that factors which stochastically influence pathways that predispose towards a specific histology were not related to the carcinogenic impact of lifetime cigarette consumption but to the relative modulating influence of delivery rate. In particular, the greater smoking-related ORs for ESCC derived from heightened responsiveness to differing delivery rates.
Studies have linked increased alcohol consumption with ESCC, as well as cancers of the oral cavity, pharynx, larynx, liver, colon/rectum and breast [35]. This association may derive from the ethanol metabolite acetaldehyde, a possible human carcinogen (Group 2B) [36], which may increase reactive oxygen species [37] or enhance cell permeability to environmental carcinogens, e.g., tobacco smoke [35,38]. Our analysis observed a direct delivery rate pattern for ≤10 DPD, whereby the drink-years association strengthened with increasing DPD, suggesting that alcohol-related causal mechanisms were not rate limited. Above 10 DPD, the drink-years association weakened, a pattern which also occurred for oral cavity, pharyngeal and laryngeal cancers [4,5]. However, interpretation of results for heavy drinkers may be problematic, since data at extreme levels of daily consumption were limited and potentially subjected to increased misclassification. Nevertheless, the decreasing strength of the drink-years association above 10 DPD was consistent, suggesting that this “reduced potency” or “wasted exposure” pattern may not be an artifact.
In contrast to ESCC, studies of EAC and EGJA and alcohol consumption have been less definitive, with reports of a decreasing association[14,16,39], no association[9,14,15,17,22,40–43] and an increasing association[13,39,44,45]. A recent BEACON analysis of EAC and EGJA reported suggestive evidence of an inverse association in modest drinkers[11]. Our modeling found a significant inverse association with drink-years, but limited mainly to <5 DPD consumers, with no association in heavier drinkers. We think it unlikely that increasing misclassification of DPD greatly influenced the diminution of the inverse drink-years association. Assuming duration was accurate, increasing DPD misclassification should have induced progressively greater curvilinearity of the drink-years association, a pattern we did not observe (Figure 3).
Healthy lifestyle factors may have confounded results for EAC and EGJA through a link to moderate drinking and reduced consequences of insulin resistance or elevated serum lipids and lipoproteins or increased antioxidants [11]. However, alcohol-related ORs were similar in never and current smokers and within BMI categories (not shown), which argues against lifestyle confounding.
We evaluated effect modification of smoking ORs by sex, BMI, age, acid reflux and drink-years and drinking ORs by sex, BMI, age, acid reflux and pack-years. For EAC, only the occurrence of acid reflux significantly modified ORs for alcohol consumption. For <7 DPD, inverse associations with drink-years were greater in those without reflux (P=0.01 for the test of no association) than with reflux (P=0.29), suggesting acid reflux may dissipate any health benefits from lower DPD. A similar pattern occurred for EGJA, with a significant inverse drink-years association among those without (P=0.05), but not with (P=0.77), acid reflux, although homogeneity was not rejected. This result needs corroboration, since information on acid reflux in the pooled data were limited [19,46–48].
For ESCC, we found evidence of effect modification by BMI, with ORs for smoking and drinking enhanced for those under 30 BMI. Confounding by reverse causation (disease-related weight loss) may have influenced results[49]; however, such effects are thought weak for most cancers[50] and moreover reverse causation would had to have been outcome specific. In BEACON, the OR for ESCC by BMI <25 relative to 25+ was OR=1.27 (95% confidence interval [CI] 0.98, 1.65) and OR=1.44 (95% CI 1.22, 1.69) in never and ever smokers, respectively, an association consistent with other ESCC studies [13,23,42,51,52], as well as studies of lung[53–56], oral cavity, pharyngeal and laryngeal cancers[5,57]. In addition, the enhancement of ORs for smoking and drinking at lower BMIs was also observed for oral cavity and pharyngeal cancers[5]. In contrast, ORs for EAC and EGJA increased with greater BMI (not shown), and BMI did not modify smoking and drinking ORs. Mechanisms that link lower BMIs with increased ORs for ESCC, lung, oral cavity and pharynx cancers and with enhancement of smoking-related and drinking-related ORs are unknown[57], but may involve altered caloric absorption and utilization, greater oxidative stress or altered DNA repair[57–60].
We found no effect modification of smoking ORs by drinking or drinking ORs by smoking, indicating consistency with a multiplicative joint association. This agreed with most previous studies [12,14,43,45,61], although not all [9].
Our analysis had several strengths. We pooled original data from 12 studies conducted in diverse settings, while similarities of study instruments allowed substantial harmonization of variables for smoking, alcohol consumption and other important risk factors. The large numbers of case patients increased our power to assess main effects, as well as more subtle patterns, such as delivery rate effects and effect modifiers, and variations by histology and site. The use of pooled data also enabled the direct assessment of the consistency of the observed associations across independent studies.
Limitations in our results included potential recall bias from the retrospective collection of information for the 10 case-control studies, although this was balanced by data from two case-control studies which were nested in cohorts and which used data ascertained at enrollment and prior to disease incidence. Consistency of results across studies and differential patterns by outcome types suggested that recall bias was not substantial.
In summary, smoking-related ORs exhibited an inverse delivery rate pattern, whereby for equal pack-years smoking more CPD for shorter duration was less deleterious than smoking fewer CPD for longer duration. For EAC and EGJA, there was a significant inverse association with drink-years in <5 DPD drinkers, primarily in those reporting no acid reflux, and no association in heavier drinkers. For ESCC, there was an increasing OR trend with drink-years, which strengthened with greater DPD in light and moderate drinkers. Although consistent across studies, our results require further confirmation, but provide important guidance for the development of more directed hypotheses.
Supplementary Material
Acknowledgments
Drs. Abnet, Chow, Cook, Freedman, Kamangar, Lubin and Ward were supported by the Intramural Program of the National Institutes of Health. The Population Health Study was funded by the Intramural Program of the National Institutes of Health. The Larynx, Esophagus, and Oral Cavity (LEO) Study was funded by grants R01-CA30022 and R37-CA41530 (both awarded to TLV, David Thomas, Scott Davis, Bonnie Worthington Roberts, Ruth Little, and Mary Rogers). The US Multi-Center Study was funded by grants U01-CA57949 (awarded to TLV), U01-CA57983 (awarded to MDG), and U01-CA57923 (awarded to HAR). The Swedish Esophageal Cancer Study was funded by grant number R01 CA57947–03 (awarded to ON and Hans-Olov Adami). The United Kingdom Study of Oesophageal Cancer in Women was funded by Chief Scientist Office (Scotland) (awarded to Patricia McKinney), the LORS (East Anglia) (awarded to Nick E. Day), Special Trustees of the Nottingham University Hospitals (awarded to Clair Chilvers), and the Medical Research Council (awarded to Paula Cook Mozaffari). The Los Angeles County Multi-ethnic Case-control Study was funded by grants 3RT-0122 (‘Smoking and Risk of Proximal Vs. Distal Gastric Cancer’, awarded to AHW) and 10RT-0251 (‘Smoking, microsatellite instability & gastric cancers’, awarded to AHW) from the California Tobacco Related Research Program and grant CA59636 (awarded to LB) from the National Cancer Institute. The Nebraska Health Study was funded by the Intramural Program of the National Institutes of Health. The Nova Scotia Barrett Esophagus Study was supported by the Nova Scotia Health Research Foundation (‘Molecular mechanisms and lifestyle risk factor interactions in the pathogenesis of human esophageal adenocarcinoma’, N419, awarded to AGC). The Factors Influencing the Barrett’s Adenocarcinoma Relationship (FINBAR) study was funded by an Ireland-Northern Ireland Co-operation Research Project Grant sponsored by the Northern Ireland Research & Development Office, and the Health Research Board, Ireland (All-Ireland case-control study of Oesophageal Adenocarcinoma and Barrett’s Oesophagus, awarded to LJM and Harry Comber). The Australian Cancer Study was supported by the Queensland Cancer Fund and the National Health and Medical Research Council (NHMRC) of Australia (Program no. 199600, awarded to David C. Whiteman, Adele C. Green, Nicholas K. Hayward, Peter G. Parsons, David M. Purdie, and Penelope M. Webb). Dr. Whiteman is funded by a Future Fellowship from the Australian Research Council and Drs Webb and Pandeya are funded by NHMRC Research Fellowships. NIH-AARP was funded by the Intramural Program of the National Institutes of Health. Reported analyses with the Kaiser-Permanente Multiphasic Health Checkup Study were funded by NIH grant number R01 DK063616 (Epidemiology and Incidence of Barrett’s Esophagus, Kaiser Permanente, awarded to DAC) and NIH grant R21DKO77742 (Barrett’s Esophagus: Risk Factors in Women, awarded to DAC and Nicholas J. Shaheen).
Abbreviations
- BEACON
Barrett’s Esophagus and Esophageal Adenocarcinoma Consortium
- CDP
cigarettes per day
- CI
confidence interval
- DPD
drinks per day
- EAC
esophageal adenocarcinoma
- EGJA
esophagogastric junctional adenocarcinoma
- EOR
excess odds ratio
- ESCC
esophageal squamous cell carcinoma
- OR
odds ratio
Footnotes
Conflict of interest: None declared.
References
- 1.Blot WJ, Mclaughlin JK, Fraumeni JF., Jr . Esophageal cancer. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. New York: Oxford University Press, Inc; 2006. pp. 697–706. [Google Scholar]
- 2.Lubin JH, Caporaso N. Cigarette smoking and lung cancer: modeling total exposure and intensity. Cancer Epidemiol Biomarkers Prev. 2006;15:517–23. doi: 10.1158/1055-9965.EPI-05-0863. [DOI] [PubMed] [Google Scholar]
- 3.Lubin JH, Virtamo J, Weinstein SJ, Albanes D. Cigarette smoking and cancer: intensity patterns in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study in Finnish men. Am J Epidemiol. 2008;167:970–5. doi: 10.1093/aje/kwm392. [DOI] [PubMed] [Google Scholar]
- 4.Lubin JH, Purdue M, Kelsey KT, Zhang ZF, Winn DM, Wei QY, et al. Total exposure and exposure rate effects for alcohol and smoking and risk of head and neck cancer: a pooled analysis of case-control studies. Am J Epidemiol. 2009;170:937–47. doi: 10.1093/aje/kwp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lubin JH, Gaudet MM, Olshan AF, Kelsey K, Boffeta P, Brennan P, et al. Body mass index, cigarette smoking and alcohol consumption and cancers of the oral cavity, pharynx and larynx: modeling odds ratios in pooled case-control data. Am J Epidemiol. 2010;171:1250–61. doi: 10.1093/aje/kwq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lubin JH, Moore LE, Fraumeni JF, Cantor KA. Respiratory cancer and inhaled inorganic arsenic in copper smelters workers: a linear relationship with cumulative exposure that increases with concentration. Environ Health Perspect. 2008;116:1661–5. doi: 10.1289/ehp.11515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lubin JH, Alavanja MCR, Caporaso N, Brown LM, Brownson RC, Field RW, et al. Cigarette smoking and cancer: modeling total exposure and intensity. Am J Epidemiol. 2007;166:479–89. doi: 10.1093/aje/kwm089. [DOI] [PubMed] [Google Scholar]
- 8.Pandeya N, Williams GM, Sadhegi S, Green AC, Webb PM, Whiteman DC. Associations of duration, intensity, and quantity of smoking with adenocarcinoma and squamous cell carcinoma of the esophagus. Am J Epidemiol. 2008;168:105–14. doi: 10.1093/aje/kwn091. [DOI] [PubMed] [Google Scholar]
- 9.Pandeya N, Williams G, Green AC, Webb PM, Whiteman DC. Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology. 2009;136:1215–24. doi: 10.1053/j.gastro.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 10.Cook MB, Kamangar F, Whiteman DC, Freedman ND, Gammon MD, Bernstein L, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the International BEACON Consortium. J Natl Cancer Inst. 2010;102:1344–53. doi: 10.1093/jnci/djq289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freedman ND, Murray LJ, Kamangar F, Abnet CC, Cook MB, Nyren O, et al. Alcohol intake and risk of oesophageal adenocarcinoma: a pooled analysis from the BEACON Consortium. Gut. 2011;60:1029–37. doi: 10.1136/gut.2010.233866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown LM, Silverman DT, Pottern LM, Schoenberg JB, Greenberg RS, Swanson GM, et al. Adenocarcinoma of the esophagus and esophagogastric junction in White men in the United-States - alcohol, tobacco, and socioeconomic-factors. Cancer Causes Control. 1994;5:333–40. doi: 10.1007/BF01804984. [DOI] [PubMed] [Google Scholar]
- 13.Vaughan TL, Davis S, Kristal A, Thomas DB. Obesity, alcohol, and tobacco as risk-factors for cancers of the esophagus and gastric cardia - adenocarcinoma versus squamous-cell carcinoma. Cancer Epidemiol Biomarkers Prev. 1995;4:85–92. [PubMed] [Google Scholar]
- 14.Gammon MD, Schoenberg JB, Ahsan H, Risch HA, Vaughan TL, Chow WH, et al. Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1997;89:1277–84. doi: 10.1093/jnci/89.17.1277. [DOI] [PubMed] [Google Scholar]
- 15.Lagergren J, Bergstrom R, Lindgren A, Nyren O. The role of tobacco, snuff and alcohol use in the aetiology of cancer of the oesophagus and gastric cardia. Int J Cancer. 2000;85:340–6. [PubMed] [Google Scholar]
- 16.Cheng KK, Sharp L, McKinney PA, Logan RFA, Chilvers CED, Cook-Mozaffari P, et al. A case-control study of oesophageal adenocarcinoma in women: a preventable disease. Br J Cancer. 2000;83:127–32. doi: 10.1054/bjoc.2000.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu AH, Wan P, Bernstein L. A multiethnic population-based study of smoking, alcohol and body size and risk of adenocarcinomas of the stomach and esophagus (United States) Cancer Causes Control. 2001;12:721–32. doi: 10.1023/a:1011290704728. [DOI] [PubMed] [Google Scholar]
- 18.Chen HL, Ward MH, Graubard BI, Heineman EF, Markin RM, Potischman NA, et al. Dietary patterns and adenocarcinoma of the esophagus and distal stomach. Am J Clin Nutr. 2002;75:137–44. doi: 10.1093/ajcn/75.1.137. [DOI] [PubMed] [Google Scholar]
- 19.Veugelers PJ, Porter GA, Guernsey DL, Casson AG. Obesity and lifestyle risk factors for gastroesophageal reflux disease, Barrett esophagus and esophageal adenocarcinoma. Dis Esophagus. 2006;19:321–8. doi: 10.1111/j.1442-2050.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 20.Anderson LA, Watson RGP, Murphy SJ, Johnston BT, Comber H, Mc Guigan J, et al. Risk factors for Barrett’s oesophagus and oesophageal adenocarcinoma: results from the FINBAR study. World J Gastroenterol. 2007;13:1585–94. doi: 10.3748/wjg.v13.i10.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whiteman DC, Sadeghi S, Pandeya N, Smithers BM, Gotley DC, Bain CJ, et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57:173–80. doi: 10.1136/gut.2007.131375. [DOI] [PubMed] [Google Scholar]
- 22.Freedman ND, Abnet CC, Leitzmann MF, Mouw T, Subar AF, Hollenbeck AR, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol. 2007;165:1424–33. doi: 10.1093/aje/kwm051. [DOI] [PubMed] [Google Scholar]
- 23.Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev. 2008;17:352–8. doi: 10.1158/1055-9965.EPI-07-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abnet CC, Freedman ND, Hollenbeck AR, Fraumeni JF, Leitzmann M, Schatzkin A. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer. 2008;44:465–71. doi: 10.1016/j.ejca.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akaike H. Information theory and an extension of the maximum likelihood principal. In: Petrov EB, Csaki F, editors. 2nd Annual Symposium on Information Theory and Control. Budapest, Hungary: Akademia Kiado; 1973. pp. 267–81. [Google Scholar]
- 26.Preston DL, Lubin JH, Pierce DA, McConney ME. Epicure User’s Guide. Seattle, Washington, USA: HiroSoft International Corporation; 2006. [Google Scholar]
- 27.Lynch SM, Vrieling A, Lubin JH, Kraft P, Mendelsohn JB, Hartge P, et al. Cigarette smoking and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Am J Epidemiol. 2009;170:403–13. doi: 10.1093/aje/kwp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewtas J, Walsh D, Williams R, Dobias L. Air pollution exposure DNA adduct dosimetry in humans and rodents: evidence for non-linearity at high doses. Mutat Res-Fundam Mol Mech Mutagen. 1997;378:51–63. doi: 10.1016/s0027-5107(97)00097-3. [DOI] [PubMed] [Google Scholar]
- 29.Lutz WK. Dose-response relationships in chemical carcinogenesis: superposition of different mechanisms of action, resulting in linear-nonlinear curves, practical thresholds, J-shapes. Mut Res-Fundam Mol Mech Mutagen. 1998;405:117–24. doi: 10.1016/s0027-5107(98)00128-6. [DOI] [PubMed] [Google Scholar]
- 30.Phillips DH. Smoking-related DNA and protein adducts in human tissues. Carcinogenesis. 2002;23:1979–2004. doi: 10.1093/carcin/23.12.1979. [DOI] [PubMed] [Google Scholar]
- 31.Gu J, Liang D, Wang YF, Lu C, Wu XF. Effects of N-acetyl transferase 1 and 2 polymorphisms on bladder cancer risk in Caucasians. Mut Res-Genetic Toxicol Environ Mutagen. 2005;581:97–104. doi: 10.1016/j.mrgentox.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Shen HB, Spitz MR, Qiao YW, Guo ZZ, Wang LE, Bosken CH, et al. Smoking, DNA repair capacity and risk of nonsmall cell lung cancer. Int J Cancer. 2003;107:84–8. doi: 10.1002/ijc.11346. [DOI] [PubMed] [Google Scholar]
- 33.Wei QY, Cheng L, Amos CI, Wang LE, Guo ZZ, Hong WK, et al. Repair of tobacco carcinogen-induced DNA adducts and lung cancer risk: a molecular epidemiologic study. J Natl Cancer Inst. 2000;92:1764–72. doi: 10.1093/jnci/92.21.1764. [DOI] [PubMed] [Google Scholar]
- 34.Lubin JH, Caporaso N, Wichmann HE, Schaffrath-Rosario A, ALAVANJA MCR. Cigarette smoking and lung cancer: modeling effect modification of total exposure and intensity. Epidemiology. 2007;18:639–48. doi: 10.1097/EDE.0b013e31812717fe. [DOI] [PubMed] [Google Scholar]
- 35.Marshall JR, Freudenheim J. Alcohol. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. New York: Oxford University Press, Inc; 2006. pp. 243–58. [Google Scholar]
- 36.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Re-Evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide. Lyon, France: 1999. [PMC free article] [PubMed] [Google Scholar]
- 37.Seitz HK, Maurer B, Stickel F. Alcohol consumption and cancer of the gastrointestinal tract. Dig Dis. 2005;23:297–303. doi: 10.1159/000090177. [DOI] [PubMed] [Google Scholar]
- 38.Seitz HK, Becker P. Alcohol metabolism and cancer risk. Alcohol Res Health. 2007;30:38–47. [PMC free article] [PubMed] [Google Scholar]
- 39.Brown LM, Hoover R, Gridley G, Schoenberg JB, Greenberg RS, Silverman DT, et al. Drinking practices and risk of squamous-cell esophageal cancer among Black and White men in the United States. Cancer Causes Control. 1997;8:605–9. doi: 10.1023/a:1018446430228. [DOI] [PubMed] [Google Scholar]
- 40.Anderson LA, Cantwell MM, Watson RGP, Johnston BT, Murphy SJ, Ferguson HR, et al. The association between alcohol and reflux esophagitis, Barrett’s esophagus, and esophageal adenocarcinoma. Gastroenterology. 2009;136:799–805. doi: 10.1053/j.gastro.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Kubo A, Levin TR, Block G, Rumore GJ, Quesenberry CP, Buffler P, et al. Alcohol types and sociodemographic characteristics as risk factors for Barrett’s esophagus. Gastroenterology. 2009;136:806–15. doi: 10.1053/j.gastro.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindblad M, Rodriguez LAG, Lagergren J. Body mass, tobacco and alcohol and risk of esophageal, gastric cardia, and gastric non-cardia adenocarcinoma among men and women in a nested case-control study. Cancer Causes Control. 2005;16:285–94. doi: 10.1007/s10552-004-3485-7. [DOI] [PubMed] [Google Scholar]
- 43.Hashibe M, Boffetta P, Janout V, Zaridze D, Shangina O, Mates D, et al. Esophageal cancer in Central and Eastern Europe: tobacco and alcohol. Int J Cancer. 2007;120:1518–22. doi: 10.1002/ijc.22507. [DOI] [PubMed] [Google Scholar]
- 44.Zhang ZF, Kurtz RC, Sun M, Karpeh M, Yu GP, Gargon N, et al. Adenocarcinomas of the esophagus and gastric cardia: medical conditions, tobacco, alcohol, and socioeconomic factors. Cancer Epidemiol Biomarkers Prev. 1996;5:761–8. [PubMed] [Google Scholar]
- 45.Kabat GC, Ng SKC, Wynder EL. Tobacco, alcohol intake, and diet in relation to adenocarcinoma of the esophagus and gastric cardia. Cancer Causes Control. 1993;4:123–32. doi: 10.1007/BF00053153. [DOI] [PubMed] [Google Scholar]
- 46.Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med. 2006;166:965–71. doi: 10.1001/archinte.166.9.965. [DOI] [PubMed] [Google Scholar]
- 47.Locke GR, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ. Risk factors associated with symptoms of gastroesophageal reflux. Am J Med. 1999;106:642–9. doi: 10.1016/s0002-9343(99)00121-7. [DOI] [PubMed] [Google Scholar]
- 48.Nocon M, Labenz J, Willich SN. Lifestyle factors and symptoms of gastro-oesophageal reflux - a population-based study. Aliment Pharmacol Ther. 2006;23:169–74. doi: 10.1111/j.1365-2036.2006.02727.x. [DOI] [PubMed] [Google Scholar]
- 49.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 50.Flegal KM, Graubard BI, Williamson DF, Cooper RS. Reverse causation and illness-related weight loss in observational studies of body weight and mortality. Am J Epidemiol. 2010;173:1–9. doi: 10.1093/aje/kwq341. [DOI] [PubMed] [Google Scholar]
- 51.Chow WH, Blot WJ, Vaughn TL, Risch HA, Gammon MD, Stanford JL, et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1998;90:150–5. doi: 10.1093/jnci/90.2.150. [DOI] [PubMed] [Google Scholar]
- 52.Pandeya N, Williams GM, Green AC, Webb PM, Whiteman DC. Do low control response rates always affect the findings? Assessments of smoking and obesity in two Australian case-control studies of cancer. Aust N Z J Public Health. 2009;33:312–9. doi: 10.1111/j.1753-6405.2009.00401.x. [DOI] [PubMed] [Google Scholar]
- 53.Kabat GC, Miller AB, Rohan TE. Body mass index and lung cancer risk in women. Epidemiology. 2007;18:607–12. doi: 10.1097/ede.0b013e31812713d1. [DOI] [PubMed] [Google Scholar]
- 54.Kabat GC, Kim M, Hunt JR, Chlebowski RT, Rohan TE. Body mass index and waist circumference in relation to lung cancer risk in the Women’s Health Initiative. Am J Epidemiol. 2008;168:158–69. doi: 10.1093/aje/kwn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang L, Yang GH, Zhou MG, Smith M, Ge H, Boreham J, et al. Body mass index and mortality from lung cancer in smokers and nonsmokers: a nationally representative prospective study of 220,000 men in China. Int J Cancer. 2009;125:2136–43. doi: 10.1002/ijc.24527. [DOI] [PubMed] [Google Scholar]
- 56.Nonemaker JM, Garrett-Mayer E, Carpenter MJ, Ford ME, Silvestri G, Lackland DT, et al. The risk of dying from lung cancer by race: a prospective cohort study in a biracial cohort in Charleston, South Carolina. Ann Epidemiol. 2009;19:304–10. doi: 10.1016/j.annepidem.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 57.Gaudet MM, Olshan AF, Berthiller J, Chuang SC, Zhang ZF, Lissowska J, et al. Body mass index and risk of head and neck cancer in a pooled analysis of case-control studies in the International Head and Neck Cancer Epidemiology (INHANCE) Consortium. Int J Epidemiol. 2010;39:1091–102. doi: 10.1093/ije/dyp380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Godschalk RWL, Feldker DEM, Borm PJA, Wouters EFM, Van Schooten FJ. Body mass index modulates aromatic DNA adduct levels and their persistence in smokers. Cancer Epidemiol Biomarkers Prev. 2002;11:790–3. [PubMed] [Google Scholar]
- 59.Palli D, Vineis P, Russo A, Berrino F, Krogh V, Masala G, et al. Diet, metabolic polymorphisms and DNA adducts: The EPIC-Italy cross-sectional study. Int J Cancer. 2000;87:444–51. doi: 10.1002/1097-0215(20000801)87:3<444::aid-ijc21>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 60.Mizoue T, Tokunaga S, Kasai H, Kawai K, Sato M, Kubo T. Body mass index and oxidative DNA damage: a longitudinal study. Cancer Sci. 2007;98:1254–8. doi: 10.1111/j.1349-7006.2007.00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA. Alcohol consumption, cigarette smoking and risk of subtypes of oesophageal and gastric cancer: a prospective cohort study. Gut. 2010;59:39–48. doi: 10.1136/gut.2009.191080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.