Abstract
Background
Fluorescein angiography (FA) has been performed as part of the management of diabetic macular edema (DME) for many years. Its current role relative to the role of optical coherence tomography (OCT) is not well defined.
Purpose
To evaluate the associations of FA features with visual acuity, and with OCT, and fundus photographic characteristics in eyes with DME.
Methods
In a clinical trial, conducted by the Diabetic Retinopathy Clinical Research Network to compare two methods of laser photocoagulation to treat DME, FA (film and digital), color photographs, OCT, and visual acuity measurements were obtained at baseline and at 1 year. Grading of morphologic features was performed at a reading center. Reproducibility of FAs was assessed and the correlations of FA features with visual acuity, OCT, and color photograph features were computed.
Results
From 79 clinical sites, data of 323 study eyes and 203 fellow non-study eyes were analyzed. Fluorescein leakage area at baseline was associated with reduced visual acuity, increased OCT measures of retinal thickness and volume, and color photographic measurements of retinal thickening (r = 0.33 – 0.58). No important associations were found with changes from baseline to 12 months in these parameters or with any of the other variables analyzed.
Conclusions
Fluorescein leakage is associated with visual acuity and some OCT and color photographic variables. We did not identify any unique FA variables that had a stronger association with visual acuity than OCT measures of retinal thickness. These data may be useful to investigators planning future DME clinical trials.
Keywords: Fluorescein angiography, diabetic macular edema, optical coherence tomography, fluorescein angiographic leakage, visual acuity, cystoid macular edema, digital fundus photography
Introduction
Re-evaluation of the utility of fluorescein angiography (FA) in clinical trials of diabetic macular edema (DME) is merited given the increasing shift from FA to optical coherence tomography (OCT) to document the presence and to quantify the severity of DME. Prior to the commercialization and widespread availability of OCT technology for ophthalmology, FA was employed commonly in clinical research and in patient care to evaluate, plan treatment for, and to monitor patients with DME.1, 2 Currently, many retina specialists use OCT to assist in the diagnosis and staging of macular disease. OCT has some advantages over FA in evaluation of DME, including shorter image acquisition time, lack of systemic risks, and reproducible quantitative reporting of retinal thickness measurements.3 Some interventional trials have employed OCT exclusively for morphologic characterization and monitoring of change in eyes with macular edema.4–6
We analyzed data from a Diabetic Retinopathy Clinical Research Network (DRCR.net) multicenter clinical trial in which two different methods of laser photocoagulation for treatment-naïve DME were compared.7 FA variables (at baseline and at 12 months) as graded by masked examiners at a reading center were compared with visual acuity and OCT, and color fundus photograph features in the same eyes at baseline and at 12 months. This analysis was undertaken to explore associations between FA and visual acuity, OCT, and color photographs to evaluate the extent to which FA characteristics correspond to changes in macular morphology and vision.
Methods
The design, methods, and results of the DRCR.net photocoagulation trial have been published.7 The trial adhered to the tenets of the Declaration of Helsinki. Best-corrected Electronic Early Treatment Diabetic Retinopathy Study method (primary) visual acuity measurements and OCT scanning were performed on both eyes at baseline and 3.5, 8, and 12 months after treatment. Fluorescein angiography images of both eyes were obtained at baseline and 12 months.
A total of 263 subjects (60 with two study eyes) from 79 sites were enrolled in the trial. Study eyes were randomized to receive laser photocoagulation using one of two protocols for treatment of DME at baseline. Photocoagulation was repeated during follow up according to specified study guidelines when DME was still present. To broaden the severity range of DME and to increase the dataset available for analysis, non-study eyes with no prior treatment for DME and a visual acuity letter score ≥19 were included in the analysis. Of the 462 eyes that were candidates for analysis in this report, 40 (9%) were excluded because of missing or ungradable images, leaving a total of 422 eyes (305 study eyes and 117 non-study eyes) of 252 participants. Study eyes had no prior treatment for DME; however, fellow eyes at baseline may have been treated for DME previously. There were no substantive differences in the baseline characteristics of the 40 excluded eyes compared with those of the 422 included eyes. These 422 eyes were included in all baseline analyses comparing OCT measurements and FA gradings. The baseline features of the 252 participants and 422 eyes are shown in Table 1. Only the 244 study eyes with gradable baseline and 12-month visit FAs, photographs, and OCT scans were eligible for analyses examining change between baseline and 12 months. Additional analyses of relationships between these morphologic measures and visual acuity excluded 24 eyes for baseline and 29 eyes for change between baseline and 12 month due to ocular abnormalities other than DME that were considered a likely cause of decreased visual acuity.
Table 1.
Baseline Characteristics (252 patients, 422 eyes)
| Age (yrs) - | |
| Median (25th, 75th percentile) | 60 (52, 66) |
| Range | [24, 88] |
| Gender: Women – n (%) | 103 (41%) |
| Race- n (%) | |
| White | 162 (64%) |
| African-American | 46 (18%) |
| Hispanic or Latino | 23 (9%) |
| Asian | 12 (5%) |
| Other | 9 (4%) |
| Diabetes Type - n (%) | |
| Type 1 | 17 (7%) |
| Type 2 | 235 (93%) |
| Duration of Diabetes (years) - | |
| Median (25th, 75th percentile) | 13 (8, 19) |
| Range | [0, 56] |
| HbA1c - | |
| Median (25th, 75th percentile) | 7.8 (6.8, 9.0) |
| Range | [4.6, 15.0] |
| E-ETDRS Visual Acuity letter score (Snellen equivalent) | |
| Median | 78 (20/32) |
| 25th, 75th percentile | 70, 85 (20/20, 20/40) |
| Range | [22, 95] |
| Central Subfield Thickness - | |
| Median (25th, 75th percentile) | 275 (233, 365) |
| Range | [127, 888] |
| DME Severity Scale (photographs) - n (%) | |
| Level 1 | 154 (36%) |
| Level 2 | 37 (9%) |
| Level 3 | 56 (13%) |
| Level 4 | 54 (13%) |
| Level 5a | 47 (11%) |
| Level 5b | 63 (15%) |
| Level 5c | 11 (3%) |
| Retinopathy Severity - n (%) | |
| DR Absent | 2 (<1%) |
| Mild NPDR | 63 (15%) |
| Moderate NPDR | 65 (15%) |
| Moderately severe NPDR | 198 (47%) |
| Severe NPDR | 51 (12%) |
| Mild PDR | 21 (5%) |
| Moderate PDR | 12 (3%) |
| High-risk PDR | 1 (<1%) |
| Cannot Grade | 9 (2%) |
| Lens - n (%) | |
| Phakic | 367 (87%) |
| Pseudophakic | 55 (13%) |
| Previous Panretinal Photocoagulation - n (%) | 12 (3%) |
ETDRS=Early Treatment Diabetic Retinopathy Study, DME=Diabetic Macular Edema, DD=Disc Diameter
Procedures
Fluorescein Angiograms
Early Treatment Diabetic Retinopathy Study (ETDRS) stereoscopic 30 or 35 degree single field FAs were obtained by certified photographers according to a standard protocol (available at http://drcr.net). The images were forwarded to the reading center for grading and consisted of either film negatives (Kodak TriX film) or digital images (from several approved manufacturers, after the camera system had been certified by the reading center). The images were digital for 252 (60%) eyes and film for 170 (40%) eyes. Grading was performed by University of Wisconsin Fundus Photograph Reading Center staff specifically trained and certified for the grading of FAs for DME. Film submissions were graded by viewing with stereoscopic 5X viewers and a calibrated light box. Distance and area measurements were estimated with the overlay of grids over the 35 mm slides. Digital images were displayed on calibrated 21-inch monitors in an application which allowed distance and area measurements and stereoscopic viewing. Each FA submission was by a single grader independent of other visits, with reference to the color photographs from the same visit (according to procedures from the ETDRS (8). Macular features were assessed using procedures slightly modified from the methodology employed in the ETDRS.8 Using the ETDRS macular grid, which the grader centered on what appeared to be the foveal center, features were assessed and measured for extent within each of the nine grid subfields and summed for a total area measurement for that feature within that eye. In digital display, areas were measured with planimetry in a similar manner, or areas were measured globally within the grid which yielded total area for specific characteristics but not subfield data. Areas of fluorescein leakage, cystoid abnormalities, capillary loss and leakage source within the ETDRS macular grid were the principle variables of interest for this analysis.
Fluorescein leakage was evaluated in the middle and late stages of the FA (2 minutes and 5 minutes) within the macular grid utilizing stereoscopic viewing and reference to earlier phase images. Any increased fluorescein intensity in the late phase images was considered leakage, which required distinguishing fluorescein leakage from background choroidal fluorescence, staining and window defect from retinal pigment epithelial lesions such as laser scars. Rather than categorizing leakage in each subfield as none, definite, moderate, and severe, according to the methodology for the ETDRS, the grading for this study employed area estimates for the grid as a whole in standard disc areas (DA).
Cystoid abnormalities and capillary loss were graded according to the methodology employed in the ETDRS without modification.8 The area of cystoid abnormalities was also included in the total leakage area assessment. Leakage source was graded using early and late FA images. The grader was asked to estimate the proportion total FA leakage that appeared to originate from microaneurysms (graded as none, < 33%, 33–67%, or >67%). This methodology was slightly modified from the procedures used in the ETDRS in that leakage intensity was not estimated, and leakage source was graded for the entire grid rather than by grid subfield.
For each variable, the grade of absent was employed when the grader assessed that the variable was not present or was suspected but with less than 50% certainty. The grade of questionable was used when the grader was 50 – 90% sure that the characteristic was present, but this answer did not allow area measurement of the characteristic. When images were of borderline or poor quality, such that identification and measurement of particular features was uncertain, the graders were advised to indicate the grade as “cannot grade” for individual features, because the feature could not be detected or measured reliably.
OCT
Zeiss Stratus OCT (Carl Zeiss Meditec; Dublin, CA) images (Humphrey OCT 2000 in 32 [8%] eyes, and Stratus OCT in 390 [92%] eyes at baseline) were obtained by a certified operator using 6 radial line scans of 6 mm length (Fast Macular scans with the Stratus OCT) and additional high resolution cross-hair 6 mm scans centered on the fovea (512 A-scan density). Data from both OCT scan models were pooled despite differences in axial resolution. Scans were submitted as hard copy paper prints to the reading center. Assessment included a quality evaluation to determine if the numeric report from the instrument software (the Fast MacularThickness map report) was reliable. Retinal thickness in the central subfield was analyzed in this report, because this measurement is commonly used clinically and was the principal OCT variable used in the trial. 9 When the value given by the OCT software for a given subfield was considered unreliable due to boundary line errors or decentration, that subfield was recorded as ungradable. If this included the center point thickness, the grader determined the center point of the macula by morphologic criteria, and the center point thickness was measured with digital calipers from the hard copy prints. From this value, the central subfield thickness was imputed, since the correlation between center point and central subfield thickness is extremely high (0.99).10, 11 Manual re-measurement of the center point thickness was required in 15% of OCT submissions. Reproducibility of central subfield thickness was analyzed in a previous DRCR.net report (in a different data set using the same methods) in which the half widths of the 95% confidence intervals for absolute and relative change between two measurements were 38 microns and 11%, respectively.3 We also evaluated the OCT total macular volume value, which is displayed in the Fast Macular Thickness Map report. In eyes with unreliable report numbers requiring manual remeasurement, the total macular volume was not analyzed further since it, too, was considered unreliable. Cystoid spaces were graded via a 5 step ordinal scale using a set of standard OCT images to delineate steps (Photograph 1A representing “mild” cystoid changes and 1B representing “moderate” changes).
Stereoscopic Color Fundus Photographs
Early Treatment Diabetic Retinopathy Study 30 – 35 degree 7-standard field stereoscopic fundus photographs were obtained using color film by certified photographers and sent to the DRCR.net reading center at the University of Wisconsin-Madison for grading. Grading methods for DME were the same as those used in the ETDRS, except that areas of retinal thickening and hard exudates were estimated as continuous variables rather than on ordinal scales.12
ETDRS DME Severity Scale
In the ETDRS, poorer baseline visual acuity and poorer visual outcomes were associated with larger areas of retinal thickening within 1 disc diameter (DD) of the center of the macula and with greater degree of thickening at the center assessed photographically at study entry.13 The ETDRS eyes were cross classified by baseline values of each of these measures, and mean baseline visual acuity was calculated for each cell in the table. Cells with similar visual acuity were combined using cluster analysis and clinical judgment to produce a 9-level DME severity scale. For use in this report, the scale was modified slightly. The scale, its modifications in this trial, and the reproducibility of the gradings have been reported (weighted kappa for the scale was 0.58).14
Statistical Methods
Reproducibility of FA gradings was assessed comparing replicate quality control and original area measurements for 4 FA measures: fluorescein leakage, cystoid abnormalities, capillary loss, and leakage source. Since both film and digital FAs were graded for this protocol, each medium was assessed separately for grading reproducibility to evaluate potential artifact or bias. Area measurements were expressed as DD after square root transformation. Expressing area measurements in DD as compared with DA partly adjusts for discrepancies which can develop in reporting changes in area that may be small in absolute value but large in relative change (i.e., a small area which doubles in size versus a large area doubling in size both have a relative change of area of 100%, but the absolute change for the larger area is substantially larger than the absolute change of the small area). Both the absolute difference between quality measurements and the relative absolute difference (a percentage calculated by dividing the absolute value of the difference by the mean of the two measurements and multiplying by 100) were computed and Bland-Altman plots constructed.
Correlations of FA features with visual acuity, OCT, and color photograph features were tabulated and were computed in repeated measures models (to account for the correlation between eyes) based on the likelihood ratio as defined by Magee.15 Statistical analyses were performed using SAS software version 9.1.
Results
Reproducibility of FA Grading
A total of 106 FAs with both quality control and original gradings were available in this trial. For both digital and film images, intergrader agreement was high for detecting the presence or absence of FA leakage, capillary loss, and cystoid abnormalities. The relative error present when both gradings were >0.4 DD is shown in Table 2 and Figures 1, 2, and 3.
Table 2.
Comparative Reproducibility Within Film and Digital Images for Measurements of Fluorescein Leakage Area, Capillary Loss Area and Area of Cystoid Abnormalities*
|
|
||||||
|---|---|---|---|---|---|---|
| Film | Digital | |||||
|
| ||||||
| Fluorescien Leakage | Capillary Loss | Cystoid Abnormalities | Fluorescien Leakage | Capillary Loss | Cystoid Abnormalities | |
|
| ||||||
| N=40 | N=14 | N=40 | N=64 | N=28 | N=62 | |
|
|
||||||
| Agreement presence/absence** | 37 (93%) | 12 (86%) | 35 (88%) | 63 (98%) | 26 (93%) | 54 (87%) |
| both ≤0.4DD | 0 | 8 (57%) | 27 (68%) | 3 (5%) | 14 (50%) | 29 (47%) |
| both >0.4DD | 37 (93%) | 4 (29%) | 8 (20%) | 60 (94%) | 12 (43%) | 25 (40%) |
| Relative error when both >0.4DD*** | N=37 | N=4 | N=8 | N=60 | N=12 | N=25 |
| Maximum | 95% | 16% | 39% | 56% | 84% | 91% |
| 90th percentile | 59% | 16% | 39% | 38% | 52% | 71% |
| 75th percentile | 28% | 14% | 35% | 28% | 41% | 32% |
|
| ||||||
| Median | 16% | 11% | 21% | 10% | 29% | 20% |
|
| ||||||
| 25th percentile | 5% | 7% | 3% | 4% | 16% | 6% |
| 10th percentile | 2% | 2% | 1% | 2% | 6% | 4% |
| Minimum | 1% | 2% | 1% | 0 | 2% | 1% |
| within ± 10% | 38% | 25% | 38% | 50% | 17% | 40% |
| within ± 50% | 86% | 100% | 100% | 98% | 83% | 80% |
The relative absolute difference in area within the grid between two gradings after square root transformation (expressed as Disc Diameters (DD)).
Absence defined as both readings ≤0.4DD and presence defined as both readings >0.4DD.
Absolute value of the difference divided by the mean of the two readings, expressed as a percentage.
DD=Disc Diameter
Figure 1.
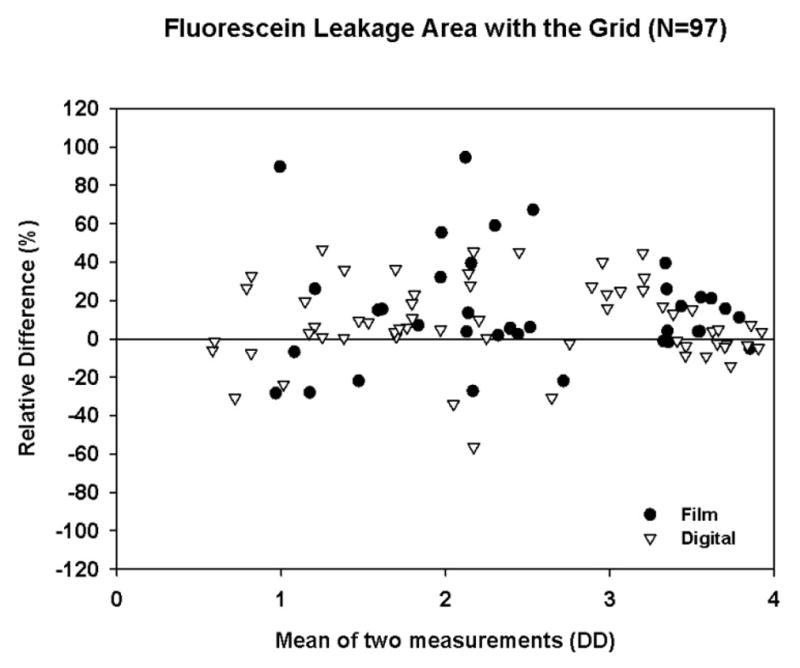
Bland-Altman Plot with Relative Difference for Fluorescein Leakage Area within the Grid (after Square Root Transformation, restricted to cases with both gradings >0.4DD).
Figure 2.
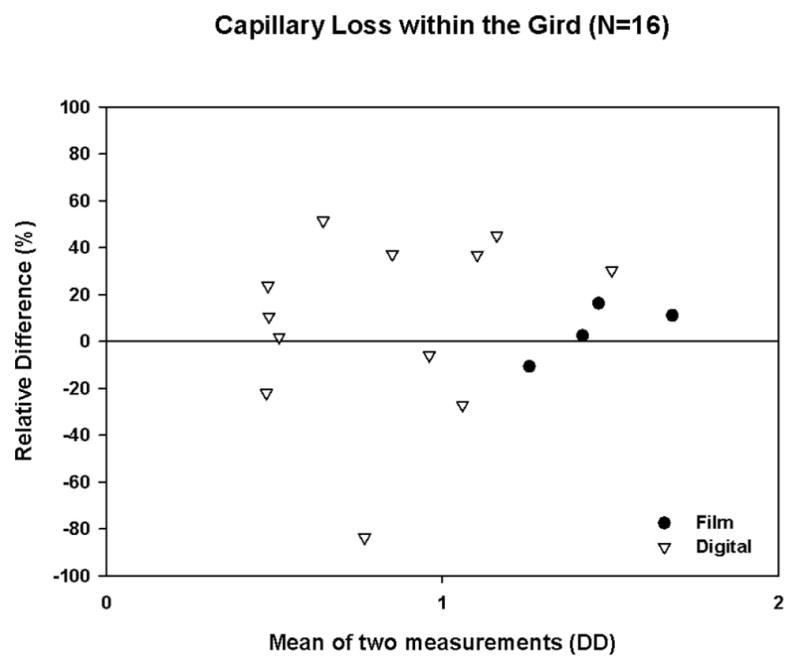
Bland-Altman Plot with Relative Difference for Capillary Loss within the Grid (after Square Root Transformation, restricted to cases with both gradings >0.4DD)
Figure 3.
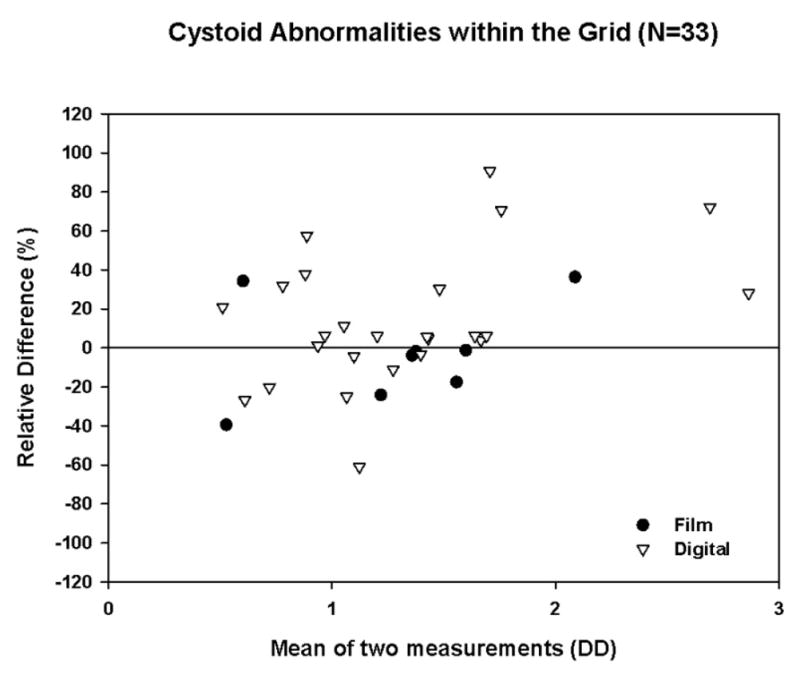
Bland-Altman Plot with Relative Difference for Cystoid Abnormalities withing the Grid (after Square Root Transformation, restricted to cases with both gradings >0.4DD)
Leakage Associations
At baseline, the associations of FA leakage area with: visual acuity, central subfield thickness, total macular volume, photographic DME area, the ETDRS DME Severity Scale, and photographic macular thickening at the center ranged from r = 0.33 to 0.58, with the strongest being OCT total macular volume, DME area, and ETDRS DME Severity Scale. (Table 3). There were no notable associations between baseline fluorescein leakage area and change in these variables from baseline (data not shown). The associations between change in fluorescein leakage from baseline to 12 months and change in: central subfield thickness, total macular volume, photographic DME area, and the DME Severity Scale ranged from r = 0.30 – 0.44, with the strongest being change in OCT total macular volume and change in photographic DME area.
Table 3.
Baseline Fluorescein Leakage Area within the Grid (N=422 eyes)
| Fluorescein Leakage within Grida | |||
|---|---|---|---|
| 0 – <3 DA | ≥ 3 DA | r | |
| Baseline Visual Acuity letter score (Snellen Equivalent)b | 0.33 | ||
| ≥ 84 (≥20/20) | 92 (43%) | 36 (19%) | |
| 83–69 (<20/20 to 20/40) | 96 (45%) | 98 (53%) | |
| 68–19 (<20/40 to 20/400) | 24 (11%) | 51 (28%) | |
| Total | 212(100%) | 185 (100%) | |
| Baseline Central Subfield Thickness | 0.38 | ||
| < 300 | 154 (69%) | 101 (51%) | |
| 300 – < 425 | 54 (24%) | 50 (25%) | |
| ≥ 425 | 14 (6%) | 48 (24%) | |
| Total | 222 (100%) | 199 (100%) | |
| Baseline OCT Volumec | 0.58 | ||
| < 7 | 45 (24%) | 2 (1%) | |
| 7 – < 8 | 94 (50%) | 40 (25%) | |
| 8 – < 9 | 34 (18%) | 55 (35%) | |
| ≥ 9 | 16 (8%) | 60 (38%) | |
| Total | 189 (100%) | 157 (100%) | |
| Baseline DME area | 0.56 | ||
| < 4 DA | 180 (81%) | 82 (41%) | |
| ≥ 4 DA | 42 (19%) | 117 (59%) | |
| Total | 222 (100%) | 199 (100%) | |
| Baseline Retinal thickening at center of macula | 0.37 | ||
| None | 115 (52%) | 47 (24%) | |
| Questionable | 22 (10%) | 19 (10%) | |
| Definite, < 1X reference | 23 (10%) | 25 (13%) | |
| Definite, < 2X reference | 60 (27%) | 97 (49%) | |
| Definite, ≥ 2X reference | 2 (<1%) | 11 (6%) | |
| Total | 222 (100%) | 199 (100%) | |
| Baseline DME Severity Scale | 0.45 | ||
| Level 1 | 111 (50%) | 43(22%) | |
| Level 2 | 21 (9%) | 16 (8%) | |
| Level 3 | 29 (13%) | 27 (14%) | |
| Level 4 | 29 (13%) | 25 (13%) | |
| Level 5a | 21 (9%) | 26 (13%) | |
| Level 5b | 9 (4%) | 53 (27%) | |
| Level 5c | 2 (<1%) | 9 (5%) | |
| Total | 222 (100%) | 199 (100)% | |
Missing for 1 eye
Excluded 24 eyes with ocular abnormalities other than DME that were considered a likely cause of decreased visual acuity.
Missing for 75 eyes.
OCT=Optical Coherence Tomography, DME=Diabetic Macular Edema, DA=Disc Area
Cystoid Abnormality Associations
Cystoid abnormalities were present on FA in 91 (22%) of 417 evaluable eyes at baseline compared with 128 (31%) on OCT (Table 4). There were notable discrepancies, such as 24 eyes with no cysts by OCT but a measurable cystoid area on FA. These cases were retrospectively reviewed by one of us (RPD): all 24 FAs were confirmed to have definite cystoid abnormalities on FA, but no cystoid abnormalities were observed in 20 of the 24 OCTs, with questionable cystoid abnormalities in 2 and subtle cystoid abnormalities in 2 (with knowledge of the FA findings at the time of review). A majority of the 20 cases where cystoid abnormalities were not graded on OCT had poor image quality: in 6 the scans came from the OCT 2000 and 5 Stratus OCT images had very low signal strength. In the remaining 9 cases, the radial lines of the OCT failed to sample the cystoid abnormalities, which were not foveal in location. Conversely, there were 6 eyes with severe cysts on OCT (Grade ≥1B), but cystoid abnormalities were not observed on FA. When the FAs were retrospectively reviewed with knowledge of the OCT grading, subtle cystoid abnormalities could be discerned in 4. Two cases had no cystoid abnormalities by FA upon review.
Table 4.
Cystoid Abnormalities Within the Grid at Baseline (N=422 eyes)
| Baseline Cystoid Changes Within Grida on FA | |||
|---|---|---|---|
| N | 0 | >0 | |
| OCT Cystoid Abnormalities | |||
| No evidence | 248 | 224 (54%) | 24 (6%) |
| Questionable | 41 | 30 (7%) | 11 (3%) |
| Definite, <1A | 75 | 49 (12%) | 26 (6%) |
| ≥ 1A, <1B | 33 | 16 (4%) | 17 (4%) |
| ≥ 1B | 20 | 7 (2%) | 13 (3%) |
Missing for 5 eyes.
OCT=Optical Coherence Tomography
Capillary Loss Associations
Definite capillary loss was present in 83 (37%) of the 224 evaluable FAs, generally of minimal area (median 0.12 DA, interquartile range 0.05–0.26). A cross-tabulation of the presence or absence of capillary loss with features from OCT, color photography, FA and visual acuity showed no meaningful associations at baseline (r = 0.02 – 0.30) (Table 5). There were no important correlations between baseline capillary loss and change from baseline in these other variables.
Table 5.
Baseline Capillary Loss Area within the ETDRS Macular Grid on FA (N=422 eyes for baseline analysis and N=244 eyes for change from baseline to 12 months analysis)
| Baseline Capillary Loss Within Grida | |||
|---|---|---|---|
| 0 | >0 | r | |
| Baseline VA letter score (Snellen equivalent)b | 0.06 | ||
| ≥84 (≥20/20) | 38 (28%) | 17 (22%) | |
| 83–69 (<20/20 to 20/40) | 63 (47%) | 46 (60%) | |
| 68–19 (<20/40 to 20/400) | 34 (25%) | 14 (18%) | |
| Total | 135 (100%) | 77 (100%) | |
| Baseline Central Subfield Thickness | 0.02 | ||
| < 300 | 61 (43%) | 42 (51%) | |
| 300 – < 425 | 55 (39%) | 23 (28%) | |
| ≥ 425 | 25 (18%) | 18 (22%) | |
| Total | 141 (100%) | 83 (100%) | |
| Baseline Total Macular Volumec | 0.09 | ||
| < 7 | 8 (7%) | 3 (4%) | |
| 7 – < 8 | 47 (44%) | 23 (32%) | |
| 8 – < 9 | 30 (28%) | 19 (27%) | |
| ≥ 9 | 23 (21%) | 26 (37%) | |
| Total | 108 (100%) | 71 (100%) | |
| Baseline DME Area Within the Grid | 0.24 | ||
| < 4 DA | 87 (62%) | 33 (40%) | |
| ≥4 DA | 54 (38%) | 50 (60%) | |
| Total | 141 (100%) | 83 (100%) | |
| Baseline Retinal Thickening at Center of Macula | 0.13 | ||
| None | 39 (28%) | 12 (14%) | |
| Questionable | 17 (12%) | 7 (8%) | |
| Definite, < 1X reference | 21 (15%) | 15 (18%) | |
| Definite, < 2X reference | 61 (43%) | 44 (53%) | |
| Definite, ≥ 2X reference | 3 (2%) | 5 (6%) | |
| Total | 141 (100%) | 83 (100%) | |
| Baseline Fluorescein Leakage Within Gridd | 0.30 | ||
| 0 – < 3DA | 85 (61%) | 20 (24%) | |
| ≥ 3DA | 55 (39%) | 63 (76%) | |
| Total | 140 (100%) | 83 (100%) | |
| Change in VAe, f | 0.01 | ||
| ≥ 10 letters better | 13 (14%) | 6 (9%) | |
| within ± 9 letters | 73 (77%) | 51 (77%) | |
| ≥ 10 letters worse | 9 (9%) | 9 (14%) | |
| Total | 95 (100%) | 66 (100%) | |
| Change in Fluorescein Leakage Within Gride | 0.29 | ||
| > 0.5 DA better | 58 (52%) | 47 (66%) | |
| within ± 0.5 DA | 40 (36%) | 11 (15%) | |
| > 0.5 DA worse | 13 (12%) | 13 (18%) | |
| Total | 111 (100%) | 71 (100%) | |
| Change in Central Subfield Thicknesse | 0.06 | ||
| > 40 microns better | 49 (44%) | 34 (48%) | |
| within ± 40 microns | 46 (41%) | 25 (35%) | |
| > 40 microns worse | 16 (14%) | 12 (17%) | |
| Total | 111 (100%) | 71 (100%) | |
| Change in DME Severity Scalee | 0.02 | ||
| ≥ 2 levels better | 32 (29%) | 22 (31%) | |
| within ± 1 levels | 56 (50%) | 41 (58%) | |
| ≥ 2 levels worse | 23 (21%) | 8 (11%) | |
| Total | 111 (100%) | 71 (100%) | |
Missing for 198 eyes (24 nongradable and 174 not assessed).
Excluded 12 eyes with ocular abnormalities other than DME that were considered a likely cause of decreased visual acuity.
Missing for 45 eyes.
Missing for 1 eye.
Missing for 62 eyes (17 nongradable and 45 not assessed).
Excluded 21 eyes with ocular abnormalities other than DME that were considered a likely cause of decreased visual acuity.
OCT=Optical Coherence Tomography, DME=Diabetic Macular Edema, DA=Disc Area, VA=Visual Acuity
Leakage Source Associations
Only 19 eyes were graded to have leakage source < 33% from microaneurysms, which limits the conclusions that can be drawn from this assessment. No associations were found between baseline leakage source and baseline or 12-month features or with change (r ≤ 0.13) in these features. Reproducibility of grading this feature was poor (kappa -0.04).
Discussion
This report is the first multicenter clinical trial to investigate the associations between FA, fundus photograph, and OCT features in DME. The strongest associations between baseline FA variables and other measures were found between baseline fluorescein leakage and baseline total macular volume (by OCT), DME area and DME severity scale (by color photographs) (Table 3). Change in fluorescein leakage from baseline to 12 months also had associations with change from baseline to 12 months in total macular volume and DME area (Table 6). Cystoid abnormalities seen on OCT were frequently absent on FA (Table 4) although FA did demonstrate some cystoid abnormalities not evident on OCT. Relatively few eyes had capillary loss by FA (a measure not assessable with OCT), and those that had this feature had minimal involvement. No notable associations were seen between capillary loss and any baseline variables or change in these variables (Table 5).
Table 6.
Change in Fluorescein Leakage Area (Square Root Transformation) Within Grid (N=244 eyes)
| Change in Fluorescein Leakage | |||||
|---|---|---|---|---|---|
| N | > 0.3 DD better* | Within Grid within ± 0.3 DD | > 0.3 DD worse | r | |
| Change in Visual Acuitya | 0.22 | ||||
| ≥10 letters better | 19 (9%) | 6 (3%) | 1 (<1%) | ||
| within ± 9 letters | 83 (39%) | 65 (30%) | 14 (7%) | ||
| ≥10 letters worse | 10 (5%) | 9 (4%) | 8 (4%) | ||
| Total | 215 | ||||
| Change in Central Subfield Thickness | 0.30 | ||||
| > 40 microns better | 66 (27%) | 31 (13%) | 12 (5%) | ||
| within ± 40 microns | 47 (19%) | 46 (19%) | 4 (2%) | ||
| > 40 microns worse | 13 (5%) | 15 (6%) | 10 (4%) | ||
| Total | 244 | ||||
| Change in OCT Volumeb | 0.44 | ||||
| >0.5 better** | 57 (33%) | 22 (13%) | 3 (2%) | ||
| within ±0.5 | 32 (18%) | 40 (23%) | 5 (3%) | ||
| >0.5 worse | 3 (2%) | 5 (3%) | 7 (4%) | ||
| Total | 174 | ||||
| Change in DME Area | 0.39 | ||||
| >0.3 DD better | 68 (28%) | 33 (14%) | 6 (2%) | ||
| within ±0.3 DD | 31 (13%) | 27 (11%) | 4 (2%) | ||
| >0.3 DD worse | 27 (11%) | 32 (13%) | 16 (7%) | ||
| Total | 244 | ||||
| Change in DME Severity Scale | 0.30 | ||||
| ≥2 levels better | 47 (19%) | 23 (9%) | 2 (<1%) | ||
| within ± 1 level | 59 (24%) | 50 (20%) | 16 (7%) | ||
| ≥2 levels worse | 20 (8%) | 19 (8%) | 8 (3%) | ||
| Total | 244 | ||||
less leakage
reduction in OCT retinal volume
Excluded 29 eyes with ocular abnormalities other than DME that were considered a likely cause of decreased visual acuity.
Missing for 36 eyes at baseline, 21 eyes at 12 months, and 13 eyes at both baseline and 12 months.
OCT=Optical Coherence Tomography, DME=Diabetic Macular Edema, DD=Disc Diameter
This multicenter trial employed both film and digital FA, in contrast to the ETDRS which analyzed film images only. There were not large differences in the reproducibility of grading for images obtained with each method. However, this finding does not imply equal validity of the gradings with each method, which would require a study in which each eye was imaged by both methods.
The comparison of OCT and FA variables should be presented with the caveat that the precise area of the macula measured differs considerably between modalities, which presumably weakens any correlations. The ETDRS grid employed in the standard grading of photographic and FAs has a diameter of 4 DD, which equals 7.2 mm (by the modern convention of 1 DD equals 1.8 mm on the retina). In contrast, the Stratus OCT uses a grid with a diameter of 6.0 mm. The area of the ETDRS grid, and each of the 9 photographic subfields, is 44% larger than the corresponding OCT subfield, which adds imprecision to the comparison of characteristics measured by these modalities. A small number of scans in this study (8%) were performed with the OCT 2000 which has a lower scan density than the Stratus OCT (100 versus 128 A scans per B scan in the fast macular map scans). This may have increased variability of measurements as well as decreased the sensitivity of detection of morphology such as cysts.
The Early Treatment Diabetic Retinopathy Study and others previously noted the substantial correlation between fluorescein leakage and extent of retinal thickening as appreciated on color fundus photography in DME, 2, 16, 17 but not all have confirmed this association holds in macular edema from other causes.18 In a series of 30 eyes with DME, Neubauer et al noted that there was a significant correlation between fluorescein leakage severity and specific OCT parameters,19 including central subfield thickness (r=0.46) and mean thickness in the outer subfields (r=0.54) but not the inner subfields. Our study confirms that there are associations between fluorescein leakage and some, but not all, measures of retinal thickening by OCT and photography (Table 3). Because FA and color photographs were graded concurrently by the same grader in this study, the conclusions regarding relationship between these two modalities are to be interpreted cautiously because of the potential for bias. Prior publications have qualitatively described good topographic co-localization of FA leakage and the extent of retinal thickening by OCT in DME.20–25 Conversely, the ETDRS and others have noted that fluorescein leakage can occur in macular regions that do not appear to have retinal thickening.1, 18 Others have reported some eyes with DME with increased retinal thickening by OCT in the absence of fluorescein leakage.26, 27 This may be a factor which weakens the correlations between OCT measured thickness and fluorescein leakage in our study.
Some association between change in fluorescein leakage and change in OCT measures of retinal thickness was found (r=0.30 to 0.44). Other studies have reported that qualitative change in fluorescein leakage severity over time correlates with change in retinal thickening by OCT after various interventions.28, 29, 30 Bandello et al evaluated “light” versus “heavy” laser photocoagulation treatment of previously untreated eyes with DME with FA and OCT.31 At 12 months, 19 of 29 eyes had decreased fluorescein leakage and 13 of 29 had a decrease in central subfield thickness by at least 10% (from Table 2 of their report). Ten of the 13 eyes with decreased central subfield thickness also had decreased fluorescein leakage. The current study also demonstrates that change in fluorescein leakage from baseline to 12 months has associations with some measures of change by OCT and photographically determined retinal thickening (central subfield thickness, total macular volume, DME area, DME severity scale) (Table 6).
The association between baseline visual acuity and fluorescein leakage has been noted previously in the ETDRS;2 there was some association in this cohort (r = 0.33, Table 3). In a prior DRCR.net publication analyzing this same data set baseline fluorescein leakage in a multivariate regression model demonstrated a small incremental increase in the prediction of the variance of baseline visual acuity over OCT central subfield thickness in univariate analysis.9 Univariate analysis calculated the association between OCT central subfield thickness and VA to have an r 2 value 23%, and association between FA leakage and VA to be slightly worse with r 2 14%.32 Because the methodology for measuring fluorescein leakage has changed from an ordinal scale in the ETDRS to a continuous scale in the DRCR.net,8 the reproducibility of grading for FA leakage area and intensity from the ETDRS (kappa 0.43, weighted kappa 0.72) cannot be directly compared to this one (Figures 1, 2, and 3). However, the inter grader reproducibility of fluorescein leakage evaluation appears roughly comparable between this study and the ETDRS, being only “fair” overall. The grading of lesion characteristics from FA inherently has some measurement variability due to variable image quality and the subjective nature of the assessments which may obscure underlying correlations. In addition, FA interpretation of leakage is made more difficult due to background staining of laser scars, which may mask vascular leakage (8). This potentially affected grading of follow up examinations as well as some fellow eyes which had laser scars at baseline.
The association between cystoid abnormalities by OCT and FA has been noted by several groups which have considered DME with cystoid abnormalities to be more advanced than DME without cystoid abnormalities.20, 23, 33 By histopathology and by OCT the larger petaloid cystoid abnormalities on FA correspond to collections of fluid in the outer plexiform layer, while the smaller “pebbly” cystoid abnormalities correspond to fluid collections in the inner nuclear layer in the extrafoveal regions of the macula.24, 34 In the current study, the association between area of cystoid abnormalities graded on FA and a 5-step scale by OCT was unimpressive (Table 5). There was a greater likelihood that cystoid abnormalities are detected by OCT than FA, indicating greater sensitivity of the former. Cases of cystoid abnormalities in DME and in other forms of macular edema where no leakage was seen on FA, but there were cystoid abnormalities by clinical examination and OCT are well documented.20, 26, 27, 34 In addition, cystoid abnormalities observed on FA but not on OCT have been detailed.24 Some cases of cystoid abnormalities without fluorescein leakage may be the result of longstanding cystoid abnormalities with structural alterations in the neural retina.34, 35
A unique attribute of FA assessment is visualization of the small caliber retinal vessels that allows detection and quantification of capillary ischemia (or capillary loss). However, the grading of capillary loss is nearly at the limits of resolution by optical camera systems, and a relatively small degradation of image quality (e.g., poor focus or cataract) can make this variable very difficult to grade. Only 37% of eyes gradable for this feature had capillary loss (likely an underestimate of the true incidence for the reasons mentioned). An association between capillary loss and FA leakage has been noted previously.8 In this study, the association was weak and there were no other notable associations with this feature.
Fluorescein angiography indirectly reflects an important pathophysiologic process (vascular leakage and ischemia) which is not measured by OCT and does not always correlate with retinal thickening. FA might provide evidence of a treatment effect in clinical studies of diseases which feature retinal vascular leakage. However, we did not identify any unique FA variables which correlated with visual acuity outcome better than OCT measures of retinal thickness. This trial did not have an untreated control group or large differences in outcomes between treatment arms, which limits the extent to which these results can be extrapolated to other trials with different designs. Because of the relatively invasive nature of FA and its cost, its implementation in large studies should be balanced against the expected value of fluorescein leakage in support of other endpoints.
Conclusions
In this multicenter study of laser-treated DME, some characteristics measured from FA correlate with OCT-measured retinal thickness and visual acuity, and others do not. Retinal thickening and FA leakage do not always correlate, and the importance of identifying FA leakage is unclear. Fluorescein angiography may be useful in studies exploring the biological effects of treatment and may provide supportive evidence for a treatment effect in early development trials. However, it may not be very useful for large clinical trials when other validated endpoints are available, particularly for parameters for which grading reproducibility is low.
Acknowledgments
Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services EY14231, EY14269, EY14229.
Footnotes
The most recently published list of the Diabetic Retinopathy Clinical Research Network can be found in Ophthalmology 2008 Sep;115(9):1447–1459.e10 with a current list available at www.drcr.net
DRCRnet investigator financial disclosures are posted at www.drcr.net
There are no conflicts of interest.
An address for reprints will not be provided
References
- 1.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study report no. 4. Int Ophthalmol Clin. 1987;27(4):265–72. doi: 10.1097/00004397-198702740-00006. [DOI] [PubMed] [Google Scholar]
- 2.Early Treatment Diabetic Retinopathy Study Research Group. Fluorescein angiographic risk factors for progression of diabetic retinopathy. EDTRS report number 13. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:834–40. [PubMed] [Google Scholar]
- 3.Diabetic Retinopathy Clinical Research Network. Reproducibility of macular thickness and volume using Zeiss optical coherence tomography in patients with diabetic macular edema. Ophthalmology. 2007;114(8):1520–5. doi: 10.1016/j.ophtha.2006.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, et al. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology. 2007;114(4):743–50. doi: 10.1016/j.ophtha.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 5.Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143(4):566–83. doi: 10.1016/j.ajo.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld PJ, Fung AE, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005;36:336–9. [PubMed] [Google Scholar]
- 7.Fong DS, Strauber SF, et al. Writing Committee for the Diabetic Retinopathy Clinical Research Network. Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol. 2007;125(4):469–80. doi: 10.1001/archopht.125.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Early Treatment Diabetic Retinopathy Study Research Group. Classification of diabetic retinopathy from fluorescein angiograms. ETDRS report number 11. Ophthalmology. 1991;98:807–22. [PubMed] [Google Scholar]
- 9.Diabetic Retinopathy Clinical Research Network. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114(3):525–36. doi: 10.1016/j.ophtha.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diabetic Retinopathy Clinical Research Network. Randomized trial of peribulbar triamcinolone acetonide with and without focal photocoagulation for mild diabetic macular edema: a pilot study. Ophthalmology. 2007;114(6):1190–6. doi: 10.1016/j.ophtha.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott IU, Edwards A, et al. Diabetic Retinopathy Clinical Research Network. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmal. 2007;114(10):1860–7. doi: 10.1016/j.ophtha.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs - an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 13.Early Treatment Diabetic Retinopathy Study Research Group. Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline: ETDRS report no. 19. Arch Ophthalmol. 1995;113:1144–55. [PubMed] [Google Scholar]
- 14.Gangnon R, Hubbard LD, Aiello LM, et al. A Severity Scale for Diabetic Macular Edema (DME) Developed from ETDRS Data. Invest Ophthalmol Vis Sci. 2008 doi: 10.1167/iovs.08-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magee L. R2 measures based on Wald and likelihood ratio joint significance tests. Amer Stat. 1990;44(3):250–3. [Google Scholar]
- 16.Bresnick GH. Diabetic maculopathy. A critical review highlighting diffuse macular edema. Ophthalmology. 1983;90(11):1301–17. doi: 10.1016/s0161-6420(83)34388-8. [DOI] [PubMed] [Google Scholar]
- 17.Bresnick GH. Diabetic macular edema. A review. Ophthalmology. 1986;93(7):989–97. doi: 10.1016/s0161-6420(86)33650-9. [DOI] [PubMed] [Google Scholar]
- 18.Nussenblatt RB, Kaufman SC, Palestine AG, et al. Macular thickening and visual acuity. Measurement in patients with cystoid macular edema. Ophthalmology. 1987;94(9):1134–9. doi: 10.1016/s0161-6420(87)33314-7. [DOI] [PubMed] [Google Scholar]
- 19.Neubauer AS, Chryssafis C, Priglinger SG, et al. Topography of diabetic macular oedema compared with fluorescein angiography. Acta Ophthalmol Scand. 2007;85(1):32–9. doi: 10.1111/j.1600-0420.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 20.Catier A, Tadayoni R, Paques M, et al. Characterization of macular edema from various etiologies by optical coherence tomography. Am J Ophthalmol. 2005;140(2):200–6. doi: 10.1016/j.ajo.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 21.Goebel W, Kretzchmar-Gross T. Retinal thickness in diabetic retinopathy. A study using optical coherence tomography (OCT) Retina. 2002;22(6):759–67. doi: 10.1097/00006982-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Hee MR, Puliafito C, Duker JS, et al. Topography of diabetic macular edema with optical coherence tomography. Ophthalmology. 1998;105(2):360–70. doi: 10.1016/s0161-6420(98)93601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang SW, Park CY, Ham DI. The correlation between fluorescein angiographic and optical coherence tomographic features in clinically significant diabetic macular edema. Am J Ophthalmol. 2004;137(2):313–22. doi: 10.1016/j.ajo.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Otani T, Kishi S. Correlation between optical coherence tomography and fluorescein angiography findings in diabetic macular edema. Ophthalmology. 2007;114(1):104–7. doi: 10.1016/j.ophtha.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 25.Kisilevsky M, Hudson C, Flanagan JG, et al. Agreement of the Heidelberg Retina Tomograph II macula edema module with fundus biomicroscopy in diabetic maculopathy. Arch Ophthalmol. 2006;124(3):337–42. doi: 10.1001/archopht.124.3.337. [DOI] [PubMed] [Google Scholar]
- 26.Ghazi NG, Ciralsky JB, Shah SM, et al. Optical coherence tomography findings in persistent diabetic macular edema: the vitreomacular interface. Am J Ophthalmol. 2007;144(5):747–54. doi: 10.1016/j.ajo.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Ozdek SC, Erdinc MA, Gurelik G, et al. Optical coherence tomographic assessment of diabetic macular edema: comparison with fluorescein angiographic and clinical findings. Ophthalmologica. 2005;219(2):86–92. doi: 10.1159/000083266. [DOI] [PubMed] [Google Scholar]
- 28.Bakri SJ, Beer PM. Intravitreal triamcinolone injection for diabetic macular edema: a clinical and fluorescein angiographic case series. Can J Ophthalmol. 2004;39(7):755–60. doi: 10.1016/s0008-4182(04)80069-3. [DOI] [PubMed] [Google Scholar]
- 29.Chieh JJ, Roth DB, Liu M, et al. Intravitreal triamcinolone acetonide for diabetic macular edema. Retina. 2005;25(7):828–34. doi: 10.1097/00006982-200510000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Johnson MW. Tractional cystoid macular edema: a subtle variant of the vitreomacular traction syndrome. Am J Ophthalmol. 2005;140(2):184–92. doi: 10.1016/j.ajo.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 31.Bandello F, Polito A, Del Borrello M, et al. “Light” versus “classic” laser treatment for clinically significant diabetic macular edema. Br J Ophthalmol. 2005;89(7):864–70. doi: 10.1136/bjo.2004.051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Browning DJ, Apte RS, Bressler SB, et al. Association of the extent of diabetic macular edema as assessed by optical coherence tomography with visual acuity and retinal outcome variables. Retina. 2009;29(3):300–5. doi: 10.1097/IAE.0b013e318194995d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soliman W, Sander B, Jorgensen TM. Enhanced optical coherence patterns of diabetic macular oedema and their correlation with the pathophysiology. Acta Ophthalmol Scand. 2007;85(6):613–7. doi: 10.1111/j.1600-0420.2007.00917.x. [DOI] [PubMed] [Google Scholar]
- 34.Gass JD, Norton EW. Cystoid macular edema and papilledema following cataract extraction. A fluorescein fundoscopic and angiographic study. Arch Ophthalmol. 1966;76(5):646–61. doi: 10.1001/archopht.1966.03850010648005. [DOI] [PubMed] [Google Scholar]
- 35.Van de Moere A, Sandhu SS, Talks SJ. Correlation of optical coherence tomography and fundus fluorescein angiography following photodynamic therapy for choroidal neovascular membranes. Br J Ophthalmol. 2006;90(3):304–6. doi: 10.1136/bjo.2005.079947. [DOI] [PMC free article] [PubMed] [Google Scholar]


