Abstract
Objective
To compare visual acuity (VA) scores after autorefraction versus research protocol manual refraction in eyes of patients with diabetes and a wide range of VA.
Methods
Electronic Early Treatment Diabetic Retinopathy Study (E-ETDRS) VA Test© letter score (EVA) was measured after autorefraction (AR-EVA) and after Diabetic Retinopathy Clinical Research Network (DRCR.net) protocol manual refraction (MR-EVA). Testing order was randomized, study participants and VA examiners were masked to refraction source, and a second EVA utilizing an identical manual refraction (MR-EVAsupl) was performed to determine test-retest variability.
Results
In 878 eyes of 456 study participants, median MR-EVA was 74 (Snellen equivalent approximately 20/32). Spherical equivalent was often similar for manual and autorefraction (median difference: 0.00, 5th and 95th percentiles −1.75 to +1.13 Diopters). However, on average, MR-EVA results were slightly better than AR-EVA results across the entire VA range. Furthermore, variability between AR-EVA and MR-EVA was substantially greater than the test-retest variability of MR-EVA (P<0.001). Variability of differences was highly dependent on autorefractor model.
Conclusions
Across a wide range of VA at multiple sites using a variety of autorefractors, VA measurements tend to be worse with autorefraction than manual refraction. Differences between individual autorefractor models were identified. However, even among autorefractor models comparing most favorably to manual refraction, VA variability between autorefraction and manual refraction is higher than the test-retest variability of manual refraction. The results suggest that with current instruments, autorefraction is not an acceptable substitute for manual refraction for most clinical trials with primary outcomes dependent on best-corrected VA.
Introduction
Visual acuity (VA) is a common outcome measure in clinical research for diabetic eye disease and, as a measure of visual function, is one of a small handful of well accepted primary endpoints for new drug registration with the United States Food and Drug Administration.1 For many years, clinical research studies have utilized the Early Treatment Diabetic Retinopathy Study (ETDRS) testing method for standardizing refraction and subsequent measurement of VA.2 However, this method requires substantial investment in training and certification of refractionists, and the procedure itself can be time consuming with substantial costs accrued over the course of a large phase 2 or 3 study due to associated personnel effort. Thus, an acceptable, less time intensive alternative to the rigorous ETDRS refraction procedure might result in substantial savings of cost and time for clinical trials in diabetic retinopathy. It might also improve clinical trial subject recruitment and retention because of shorter and less technically burdensome clinic visits.
One potential alternative to ETDRS manual refraction is autorefraction.3 This technique utilizes a computer-controlled device to provide an objective measure of an individual’s refractive error without the need for a skilled refractionist. Since first being described and validated against manual refraction in the early 1970’s,4-7 autorefractors have come into widespread clinical use due to the ease and speed of the semi-automated autorefraction procedure, the lack of need for a trained refractionist, and commercial availability.
In clinical trials, the role of autorefraction has been limited to providing starting information for subsequent manual refraction. However, results from a recent single site study sponsored by the Diabetic Retinopathy Clinical Research Network (DRCR.net) suggest that autorefraction using certain devices may be an acceptable substitute for the manual refraction in obtaining best corrected VA in eyes of patients with diabetes.8 The study was performed at a tertiary referral center for diabetes care and enrolled 216 eyes with varying degrees of diabetic retinopathy and visual acuity (20/16 to 20/800 measured with the Electronic Early Treatment Diabetic Retinopathy Study (E-ETDRS) VA Test©[EVA]) using a single autorefractor type. Refractive errors measured by autorefraction and manual refraction were relatively similar with a median vector dioptric difference (VDD) of 0.71 Diopters (D), and a VDD of < 1.00 D in 70% of eyes. On average, letter score (EVA) results after manual refraction (MR-EVA) were slightly better than EVA results after autorefraction (AR-EVA), with a median difference (AR-EVA – MR-EVA) of −1 letter (25th, 75th percentiles: −4, 2 letters). Furthermore, variability between AR-EVA and MR-EVA was similar to the test-retest variability of MR-EVA itself, and this similarity was present for all VA subgroups.
Although results from this pilot study suggested that autorefraction may be a feasible alternative to manual refraction, the study was relatively small, performed only at a single center with a single autorefractor type, and did not focus specifically on participants with diabetic macular edema which is a common inclusion criterion for trials in diabetic retinopathy. This paper reports results from a substantially larger DRCR.net sponsored multicenter study designed to compare VA scores obtained after autorefraction versus manual refraction in patients with and without center-involved DME across a diverse range of clinical sites, autorefractors, and certified refractionists with varying levels of experience.
Methods
The study was conducted at 26 sites participating in the DRCR.net. The study protocol was approved by the institutional review boards of each site, and each study participant gave verbal or written informed consent for participation in the study.
To be eligible for participation, a study participant was required to be at least 18 years old with type 1 or type 2 diabetes and have at least one eye with (1) OCT central subfield ≥250 microns, (2) Snellen equivalent VA 20/400 or better, and (3) diabetic macular edema (DME) as 2 the primary cause of any decreased vision.
A history was elicited from the study participant and extracted from available medical records. Data collected included age, gender, ethnicity and race, diabetes type (1 or 2), and concomitant ocular conditions that might contribute to decreased VA.
Testing Procedures
All study procedures were performed at the time of a single study visit by experienced 9 examiners certified by the DRCR.net for VA testing and/or refraction, and data were recorded on standardized forms. All study participants had 3 EVA tests on each eye, one EVA measurement performed using the refraction from an autorefractor, and two EVA measurements performed using the DRCR.net manual refraction. Each EVA test was performed first on the right eye and then the left eye. The VA technician was masked to refraction source in 92% and 93% of cases when the source was DRCR.net refraction and autorefraction, respectively (the technician was occasionally unmasked when only one technician was available to perform the testing). Tests were performed prior to dilation and prior to measurement of intraocular pressure. There was approximately a 5-minute rest period between each test.
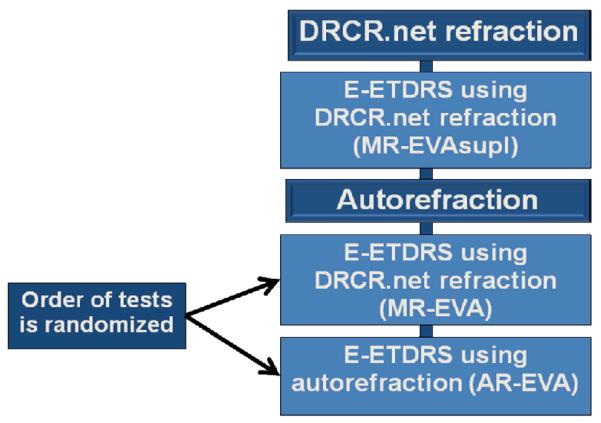
For 112 study participants participating in another DRCR.net protocol, DRCR.net manual refraction and EVA measurement using the manual refraction (MR-EVAsupl) were 1 performed according to the original protocol. A second refraction was then performed using an autorefractor. Two additional EVA measurements were then completed by a VA technician: 1) A repeat EVA measurement using the DRCR.net manual refraction (MR-EVA) and 2) an EVA measurement using the autorefraction (AR-EVA). The order of the two VA tests was randomized (Figure 1A) and study participants were masked during each VA test as to which refraction (autorefraction or manual refraction) was being used. The VA examiner was masked to the source of the refraction in 81% and 85% when the source was DRCR.net refraction and autorefraction, respectively.
Figure 1A.
Flowchart of Electronic Early Treatment Diabetic Retinopathy Study Visual Acuity Test Testing for Study Participants Participating in Another Diabetic Retinopathy Clinical Research Network Protocol.
For 370 study participants not participating in another DRCR.net protocol, or study participants in another DRCR.net protocol without a protocol refraction or EVA scheduled as part of their current visit, a DRCR.net manual refraction was performed in each eye in addition to a refraction using an autorefractor. EVA was measured once in each eye using the DRCR.net manual refraction (MR-EVA) and once in each eye using the autorefraction (AR-EVA). The order of the two VA tests was randomized. The VA examiner was masked to the source of the refraction in 95% and 95% when the source was DRCR.net refraction and autorefraction, respectively. An unmasked, repeat EVA measurement using the DRCR.net7 manual refraction (MR-EVAsupl) was completed last (Figure 1B).
Figure 1B.
Flowchart of Electronic Early Treatment Diabetic Retinopathy Study Visual Acuity Testing for Study Participants not Participating in Another Diabetic Retinopathy Clinical Research Network Protocol
Twenty-two different autorefractor models were utilized in this study, based on availability at participating sites. The autorefractor models included in this study are listed in Table 1. The 3 most common types of autorefractors included those manufactured by Marco Nidek, Nidek and Topcon. Of the Topcon models, the 8000 series autorefractors differed from other autorefractor models included in this study in that they utilize a rotary prism technology that theoretically enables measurements of a wider retinal area through a smaller diameter pupil.
Table 1.
Autorefractor used (N=964) - N (%)
| Canon RK-2 2 | (<1%) |
| Canon RK-FI 8 | (<1%) |
| Grand Seiko WR-5100K | 42 (4%) |
| Humphrey 599 | 56 (6%) |
| Marco Epic 2100 | 14 (1%) |
| Marco Nidek ARK700A | 44 (5%) |
| Marco Nidek ARK730A | 18 (2%) |
| Marco Nidek ARK760A | 204 (21%) |
| Nidek AR 800 | 20 (2%) |
| Nidek AR3000 | 90 (9%) |
| Nidek ARK-900 | 12 (1%) |
| Nikon NRK-8000 | 28 (3%) |
| Nikon Retinomax 2 | 8 (<1%) |
| Nikon Speedy 1 | 2 (<1%) |
| Nikon Speedy K | 6 (<1%) |
| Topcon KR3000 | 38 (4%) |
| Topcon KR7000S | 2 (<1%) |
| Topcon KR8000 | 142 (15%) |
| Topcon KR8800 | 60 (6%) |
| Topcon KR8900 | 132 (14%) |
| Topcon RM 8000 | 14 (1%) |
| Topcon RM A7000 | 22 (2%) |
Statistical Methods
Eyes evaluated using portable autorefractors (e.g. Nikon Retinomax), outdated and not commercially available technology (e.g. Xinyuan fa-6000 and Topcon KR3000), or missing either of the MR-EVAs were not included in the analyses (59 of 964 eyes [6%]). The refractive error transformation method originally described by Long and later modified by Harris, and Thibos,9-11 was used for the comparison between auto and manual refraction. Refractive error data were transformed into a spherical equivalent and two Jackson-Cross cylinder powers with axis at 180° and 45° using the Thibos method and the vector dioptric difference (VDD) was calculated from these transformations with a modified formula that scales the unit vector to match that of the Harris method. In addition, autorefraction and manual refraction were compared and were considered the “Same” for spherical equivalent difference ≤ 0.25D and cylinder difference ≤ 0.25D; “Similar” for spherical equivalent difference > 0.25 and ≤ 0.5D, with cylinder difference ≤ 0.5D or the cylinder difference ≥ 0.5D and the axis difference ≤ 10 degrees; “Very Different” if the spherical equivalent difference ≥ 2D, the cylinder difference ≥ 2D, or the cylinder difference >1.5D and the axis difference ≥ 20 degrees; and “Moderately Different” if none of the above criteria were met.
Relationship of differences (MR-EVAsupl minus MR-EVA and AR-EVA minus MR-EVA) with VA was explored using Bland-Altman methods.12 Distributions of differences in VA and refractive error according to refraction method are described using percentiles, rather than limits of agreement, as the differences were not normally distributed. Computation of the Bland-Altman coefficient of repeatability (BACR) used the standard method, but differences greater than 3 times the initial BACR were truncated at this value and the BACR was re-calculated. A total of 5 MR-EVAsupl minus MR-EVA differences and 5 AR-EVA minus MR-EVA differences were truncated. As two measures of VA based on the (gold standard) manual refraction were available, estimation of underlying VA was based on averaging the two MR-EVAs, rather than basing it on an average of MR-EVA and AR-EVA. Comparisons of differences among subgroups were made using regression models with generalized estimating equations (GEE) to account for correlation of data from 2 eyes of the same participant, adjusting for underlying VA and autorefractor as potential confounders. Absolute differences between AR-EVA and MR-EVA were compared to absolute test-retest differences between MR-EVA and MR-EVAsupl, using a paired t-test with GEE to account for correlation between eyes. A rank-based transformation for normality (van der Waerden scores) was applied to the differences prior to these GEE analyses. Subgroup comparisons of MR-EVAsupl minus MR-EVA differences and AR-EVA minus MR-EVA differences used the t-test with GEE, and also adjusted for underlyingVA and autorefractor as potential confounders. All reported P values are 2-sided and unadjusted for multiple testing. In view of the large number of variables evaluated in the subgroup analyses, only associations with P values < 0.01 were considered to be unlikely due to chance. SAS version 9.1 (SAS Institute, Cary, NC) was used for all analyses.
Results
A total of 905 eligible eyes from 458 individuals with diabetes who enrolled in this study were included in the analyses. Of these eyes, 27 eyes (3%) had autorefraction that 8 resulted in “No Target” readings, leaving 878 eyes from 456 study participants included in the final analyses. The inability to obtain an autorefraction appeared associated with worse VA (0%, 1%, 4%, and 8%, respectively, when MR-EVA was: 20/20 or better, 20/25 to 20/32, 20/40 to 20/80, 20/100 or worse, P < 0.001). No associations were detected between age, pupil size, lens status, or autorefractor type and the ability to autorefract successfully.
Mean age of study participants (± standard deviation) was 63±11 years and 57% were men. Median MR-EVA Snellen equivalent was 20/32, being 20/20 or better in 18% (158), 20/25 to 20/32 in 32% (279), 20/40 to 20/80 in 35% (307), and ≤20/100 in 15% (134) of 878 eyes. Spherical equivalent of refractive error from the manual refraction ranged from −9.38 D to +6.88 D. Additional study participant and ocular characteristics are presented in Table 2.
Table 2.
Study Participant Characteristics and Ocular Characteristics
| Study Participant Characteristics | N=456 |
|---|---|
| Age (years) - Mean ± SD (Range) | 63 ± 11 (18, 90) |
| Gender - N (%) | |
| Women/Men | 198 (43%) / 258 (57%) |
| Race/Ethnicity - N (%) | |
| White | 307 (67%) |
| African-American | 73 (16%) |
| Hispanic | 65 (14%) |
| Asian | 2 (<1%) |
| Unknown/ not reported | 9 (2%) |
| Diabetes Type - N (%) | |
| Type 1 | 54 (12%) |
| Type 2 | 389 (85%) |
| Uncertain | 13 (3%) |
| Duration of Diabetes - Mean ± SD (Range) | 20 ± 11 (0, 69) |
|
| |
| Ocular Characteristics | N=878 |
|
| |
| Average MR-EVA Score (letters/Snellen equivalent) | 74 (20/32) |
| - Median (25th, 75th percentiles) | (63, 81) |
|
Average MR-EVA Letter Score (Snellen Equivalent) - N (%) |
|
| ≥84 (≥ 20/20) | 158 (18%) |
| 83-74 (20/25-20/32) | 279 (32%) |
| 73-57 (20/40-20/80) | 307 (35%) |
| ≤53 (≤20/100) | 134 (15%) |
| DME on Clinical Exam - N (%) | |
| None | 163 (18%) |
| Present, center not involved | 129 (15%) |
| Present, center involvement uncertain | 49 (6%) |
| Present, center involved | 529 (60%) |
| Cannot determine | 8 (<1%) |
| Primary Cause of Vision Loss - N (%) | |
| No vision loss | 188 (21%) |
| DME | 544 (62%) |
| Cornea | 2 (<1%) |
| Lens | 56 (6%) |
| Vitreous | 14 (2%) |
| Retina/choroid | 49 (6%) |
| Optic nerve (includes glaucoma) | 3 (<1%) |
| Other | 7 (<1%) |
| Undetermined | 15 (2%) |
|
Severity of DME (based on OCT CSF Thickness*) - N (%) |
|
| <250 | 269 (31%) |
| 250-<400 | 446 (52%) |
| 400-<500 | 89 (10%) |
| ≥500 | 52 (6%) |
| Manual Refraction Spherical Equivalent - N (%) | |
| < −3.00 | 64 (7%) |
| −3.00−<−1.00 | 133 (15%) |
| −1.00−<+1.00 | 467 (53%) |
| +1.00−<+3.00 | 176 (20%) |
| ≥ +3.00 | 38 (4%) |
| Pupil Size** - N (%) | |
| 1.0-3.0 | 397 (49%) |
| 3.5-6.0 | 415 (51%) |
| Lens status - N (%) | |
| Phakic | 585 (67%) |
| Pseudophakic | 293 (33%) |
Missing for 22 eyes; categories based on thickness on Zeiss Stratus; categories for Topcon 3D-OCT are <275, 275-<425, 425-<525, ≥525; categories for Zeiss Cirrus or Heidelberg Spectralis are <310, 310-<460, 460-<560, ≥560.
Missing for 66 eyes (pupil not done).
DME = Diabetic Macular Edema; CSF = central subfield; SD= standard deviation; OCT= Optical Coherence Tomography; MR-EVA= Manual refraction Electronic Early Treatment Diabetic Retinopathy Study Visual Acuity Test letter score.
Comparison of Refractive Error Determined with Autorefraction and with Manual Refraction
Refractive errors measured by autorefraction and manual refraction were similar (median VDD: 0.79 D, 5th and 95th percentiles 0.25 D to 3.55 D), with the VDD differing by ≥1.00 D in 337 (38%) eyes, and by ≥2.00 D in 122 (14%) eyes (Table 3). VDD was larger in eyes with worse VA (P < 0.001) or with larger refractive error (P < 0.001). Autorefraction spherical equivalent was similar compared with manual refraction spherical equivalent (median difference: 0.00, 5th and 95th percentiles −1.75 D to +1.13 D), although agreement between spherical equivalent from autorefraction versus manual refraction was worse in eyes with worse VA and in eyes with higher refractive error (Table 4 website [available at http://###], P<0.001 for both).
Table 3.
Autorefraction versus Manual Refraction - Vector Dioptric Difference
| N | Median (5th, 95th percentiles) |
≥0.25 N (%) |
≥ 0.50 N (%) |
≥ 1.00 N (%) |
|
|---|---|---|---|---|---|
| Overall | 878 | 0.79 (0.25, 3.55) | 837 (95%) | 669 (76%) | 337 (38%) |
|
| |||||
| Average MR-EVA | |||||
| ≥20/20 | 158 | 0.57 (0.00, 1.98) | 142 (90%) | 103 (65%) | 29 (18%) |
| 20/25-20/32 | 279 | 0.69 (0.25, 2.06) | 267 (96%) | 203 (73%) | 79 (28%) |
| 20/40-20/80 | 307 | 0.92 (0.25, 4.25) | 300 (98%) | 250 (81%) | 146 (48%) |
| ≤20/100 | 134 | 1.33 (0.25, 4.79) | 128 (96%) | 113 (84%) | 83 (62%) |
|
| |||||
|
Average Refraction
Spherical Equivalent* |
|||||
| <−3.00 | 73 | 0.86 (0.26, 7.10) | 71 (97%) | 61 (84%) | 33 (45%) |
| −3.00-<−1.00 | 152 | 0.79 (0.25, 3.55) | 145 (95%) | 112 (74%) | 66 (43%) |
| −1.00-<+1.00 | 449 | 0.77 (0.18, 2.45) | 426 (95%) | 341 (76%) | 152 (34%) |
| +1.00-<+3.00 | 163 | 0.71 (0.25, 3.08) | 155 (95%) | 118 (72%) | 59 (36%) |
| ≥+3.00 | 41 | 1.24 (0.40, 22.87) | 40 (98%) | 37 (90%) | 27 (66%) |
Average of manual refraction spherical equivalent and auto refraction spherical equivalent.
MR-EVA= Manual refraction Electronic Early Treatment Diabetic Retinopathy Study Visual Acuity Test letter score.
Table 4.
Autorefraction versus Manual Refraction - Spherical Equivalent
| Difference* | Absolute Difference** | |||||
|---|---|---|---|---|---|---|
| N | Median (5th, 95th percentiles) |
Median (5th, 95th percentiles) |
≥ 0.25 N (%) |
≥ 0.50 N (%) |
≥ 1.00 N (%) |
|
| Overall | 878 | 0.00 (−1.75, +1.13) | 0.38 (0.00, 2.25) | 645 (73%) | 404 (46%) | 178 (20%) |
|
| ||||||
| Average MR-EVA | ||||||
| ≥20/20 | 158 | 0.00 (−0.75, +0.63) | 0.25 (0.00, 1.13) | 101 (64%) | 41 (26%) | 10 (6%) |
| 20/25-20/32 | 279 | 0.00 (−1.13, +0.75) | 0.38 (0.00, 1.25) | 189 (68%) | 107 (38%) | 34 (12%) |
| 20/40-20/80 | 307 | −0.13 (−2.13, +1.25) | 0.50 (0.00, 2.38) | 244 (79%) | 172 (56%) | 79 (26%) |
| ≤20/100 | 134 | −0.25 (−2.88, +2.38) | 0.63 (0.00, 3.25) | 111 (83%) | 84 (63%) | 55 (41%) |
|
| ||||||
|
Average Refraction
Spherical Equivalent † |
||||||
| <−3.00 | 73 | 0.00 (−3.38, +1.25) | 0.50 (0.00, 3.38) | 58 (79%) | 38 (52%) | 25 (34%) |
| −3.00−<−1.00 | 152 | −0.19 (−2.25, +0.75) | 0.38 (0.00, 2.25) | 110 (72%) | 71 (47%) | 38 (25%) |
| −1.00−<+1.00 | 449 | −0.13 (−1.50, +0.75) | 0.38 (0.00, 1.63) | 334 (74%) | 201 (45%) | 66 (15%) |
| +1.00−<+3.00 | 163 | +0.13 (−1.13, +1.63) | 0.38 (0.00, 1.75) | 110(67%) | 69 (42%) | 32 (20%) |
| ≥+3.00 | 41 | +0.25 (−1.38, +15.88) | 0.50 (0.00, 15.88) | 33 (80%) | 25 (61%) | 17 (41%) |
Auto Refraction-Manual Refraction
Absolute Value of the Difference
Average of manual refraction spherical equivalent and auto refraction spherical equivalent
MR-EVA= Manual refraction Electronic Early Treatment Diabetic Retinopathy Study Visual Acuity Test letter score.
Of all the autorefractors, the Topcon 8000 series (Topcon RM 8000, KR8000, KR 8800, and KR 8900 models) generated autorefractions most similar to manual refraction. “Same” or “Similar” autorefractions were generated for 55% of eyes with a Topcon 8000 series machine as compared with 42%, 27% and 39% of eyes with Marco Nidek, Nidek, or other autorefractors (Table 5 website [available at http://###]).
Table 5.
Autorefraction versus Manual Refraction – Refraction Difference* (website)
| Autorefractor Type | |||||
|---|---|---|---|---|---|
| Overall | Marco Nidek |
Nidek | Topcon 8000 Series |
Other | |
| N | 878 | 251 | 114 | 335 | 178 |
| Same | 221 (25%) | 47 (19%) | 19 (17%) | 111 (33%) | 44 (25%) |
| Similar | 168 (19%) | 57 (23%) | 11 (10%) | 75 (22%) | 25 (14%) |
| Mod different | 399 (45%) | 118 (47%) | 56 (49%) | 134 (40%) | 91 (51%) |
| Very different | 90 (10%) | 29 (12%) | 28 (25%) | 15 (4%) | 18 (10%) |
The definition is as lollOws: Same if (1) difference in spherical equivalent ≤ 0.25D and (2) difference in cylinder ≤ 0.25D, otherwise Similar if (1) difference in spherical equivalent ≤ 0.5D and (2) difference in cylinder ≤ 0.5D and (3) difference in cylinder either ≤ 0.25D or difference in cylinder ≥0.5D and difference in axis ≤ 10 degrees; and criteria for ‘same’ not met; otherwise Very Different if (1) difference in spherical equivalent ≥ 2D or (2) difference in cylinder ≥ 2D or (3) difference in cylinder >1.5D and difference in axis ≥ 20 degrees; otherwise Moderately Different if none of the criteria above are met.
Test-retest Electronic Early Treatment Diabetic Retinopathy Study (E-ETDRS) VA Test Score Reproducibility
The median difference between the 2 MR-EVAs (MR-EVAsupl minus MR-EVA) for all 878 eyes was 0 letters (5th and 95th percentiles −5 to +7 letters) and the median absolute difference was 2 letters (5th and 95th percentiles 0 to 9 letters) (Figure 2 and Table 6a). Thirteen percent of test-retest scores differed by ≥5 but <10 letters, 2% by ≥10 but <15 letters, and 2% by ≥15 letters (Table 6a). The overall Bland-Altman coefficient of repeatability (the half-width of the interval containing 95% of test-retest differences) was 9 letters, and was larger in eyes with worse VA (5 to 13 letters, Table 6a). Greater absolute differences in test-retest scores were associated with worse VA (P<0.001), although the median letter difference between MR-EVAs was not statistically different among VA subgroups (P=0.18).
Figure 2.
Bland-Altman Plot of Difference between Electronic Early Treatment Diabetic Retinopathy Study Visual Acuity Tests Measured Using Manual Refraction
Solid reference line indicates median; dashed lines indicate 5th and 95th percentiles
MR-EVA= Manual refraction Electronic Early Treatment Diabetic Retinopathy Study Visual Acuity Test letter score; MR-EVAsupl= Supplemental Manual refraction Electronic Early Treatment Diabetic Retinopathy Study Visual Acuity Test letter score
Table 6a.
Comparison of Manual Refraction with Auto Refraction and Supplemental Manual Refraction Visual Acuity Letter Score
| MR-EVAsupl - MR-EVA | AR-EVA - MR-EVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Average MR-EVA | Overall | Average MR-EVA | |||||||
| ≥20/20 (≥84) |
20/25-20/32 (83-74) |
20/40-20/80 (73-54) |
≤20/100 (≤53) |
≥20/20 (≥84) |
20/25-20/32 (83-74) |
20/40-20/80 (73-54) |
≤20/100 (≤53) |
|||
| N | 878 | 158 | 279 | 307 | 134 | 878 | 158 | 279 | 307 | 134 |
| Letter Difference*- Median (5th, 95th percentiles) | 0 (−5, +7) | 0 (−3, +4) | 0 (−4, +5) | +1 (−7, +9) | 0 (−7, +9) | −1 (−17, +7) | −2 (−15, +5) | −2 (−15, +6) | −1 (−22, +10) | 0 (−12, +9) |
|
Letter Difference Group - N (%) |
||||||||||
| ≤ −15 | 5 (1%) | 0 | 0 | 4 (1%) | 1 (1%) | 56 (6%) | 8 (5%) | 14 (5%) | 28 (9%) | 6 (4%) |
| −14 to −10 | 9 (1%) | 0 | 1 (<1%) | 4 (1%) | 4 (3%) | 49 (6%) | 8 (5%) | 12 (4%) | 21 (7%) | 8 (6%) |
| −9 to −5 | 38 (4%) | 2 (1%) | 7 (3%) | 17 (6%) | 12 (9%) | 142 (16%) | 29 (18%) | 41 (15%) | 46 (15%) | 26 (19%) |
| −4 to +4 | 728 (83%) | 151 (96%) | 249 (89%) | 230 (75%) | 98 (73%) | 545 (62%) | 105 (66%) | 194 (70%) | 168 (55%) | 78 (58%) |
| +5 to +9 | 77 (9%) | 5 (3%) | 20 (7%) | 38 (12%) | 14 (10%) | 63 (7%) | 8 (5%) | 16 (6%) | 28 (9%) | 11 (8%) |
| +10 to +14 | 12 (1%) | 0 | 0 | 9 (3%) | 3 (2%) | 10 (1%) | 0 | 1 (<1%) | 6 (2%) | 3 (2%) |
| ≥+15 | 9 (1%) | 0 | 2 (1%) | 5 (2%) | 2 (1%) | 13 (1%) | 0 | 1 (<1%) | 10 (3%) | 2 (1%) |
|
Absolute Value of the Letter Difference * - Median (5th, 95th percentiles) |
2 (0, 9) | 1 (0, 4) | 2 (0, 7) | 2 (0, 11) | 2(0, 11) | 4 (0, 20) | 3 (0, 15) | 3 (0, 16) | 4 (0, 24) | 4 (0, 16) |
| < 5 - N(%) | 728 (83%) | 151 (96%) | 249 (89%) | 230 (75%) | 98 (73%) | 545 (62%) | 105 (66%) | 194 (70%) | 168 (55%) | 78 (58%) |
| <10 - N(%) | 843 (96%) | 158 (100%) | 276 (99%) | 285 (93%) | 124 (93%) | 750 (85%) | 142 (90%) | 251 (90%) | 242 (79%) | 115 (86%) |
| <15 - N(%) | 864 (98%) | 158 (100%) | 277 (99%) | 298 (97%) | 131 (98%) | 809 (92%) | 150 (95%) | 264 (95%) | 269 (88%) | 126 (94%) |
|
Coefficient of
Repeatability |
9 | 5 | 7 | 13 | 11 | 18 | 16 | 14 | 22 | 15 |
Supplemental MR EVA - MR EVA and Auto Refraction EVA - Manual Refraction EVA, respectively
Absolute Value of the Difference
MR-EVA= Manual refraction Electronic Early Treatment Diabetic Retinopathy Study Visual Acuity Test letter score; AR-EVA= Autorefraction Electronic Early Treatment Diabetic Retinopathy Study Visual Acuity Test letter score; MR-EVAsupl= Supplemental Manual refraction Electronic Early Treatment Diabetic Retinopathy Study Visual Acuity Test letter score
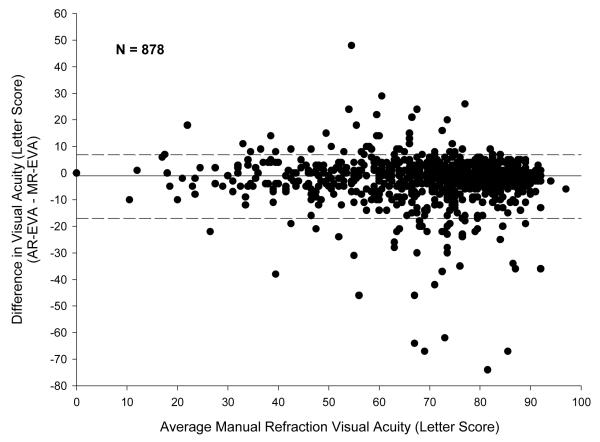
Comparison of VA Obtained with Manual Refraction versus Autorefraction
Overall, EVA obtained with manual refraction was slightly better than that obtained with autorefraction. The median difference between the EVA measurements (AR-EVA – MR-EVA) was −1 letters (5th and 95th percentiles −17 to +7 letters) (Figure 3 and Table 6a). Of the 878 eyes included in the analysis, 545 (62%) were within −4 to +4 letters, 247 (28%) had an EVA better by 5 or more letters after manual refraction, 86 (10%) had an EVA better by 5 or more letters after autorefraction. The median absolute difference between AR-EVA and MR-EVA was 4 letters (5th and 95th percentiles 0 to 20 letters), with 23% of measurements differing by ≥5 but <10 letters, 7% differing by ≥10 but <15 letters, and 8% differing by ≥15 letters (Table 6a). Greater differences and greater absolute differences between the two EVA measurements were associated with worse VAs (P<0.001 and P=0.02, respectively).
Figure 3.
Bland-Altman Plot of Difference between Electronic Early Treatment Diabetic Retinopathy Study Visual Acuity Tests Measured Using Manual Refraction and Auto Refraction
Solid reference line indicates median; dashed lines indicate 5th and 95th percentiles
MR-EVA=Manual refraction Electronic Early Treatment Diabetic Retinopathy Study Visual Acuity Test letter score; AR-EVA= Autorefraction Electronic Early Treatment Diabetic Retinopathy Study Visual Acuity Test letter score.
The absolute differences between AR-EVA and MR-EVA (Table 5a) were slightly larger than the differences between MR-EVA and MR-EVAsupl (P < 0.001), with the median absolute difference between AR-EVA and MR-EVA ranging from 3 to 4 letters as compared with the median absolute difference between MR-EVAs ranging from 1 to 2 letters according to VA level (Figure 3 and Table 6a). A greater absolute difference between AR-EVA and MR-EVA compared with the absolute difference between the 2 MR-EVAs was present for all VA subgroups.
In the 122 eyes that had markedly different refractions, 76 (62%) had MR-EVA of 5 or more letters better than AR-EVA and 13 (11%) had AR-EVA of 5 or more letters better than MR-EVA while 58 (48%) had MR-EVA 10 or more letters better than AR-EVA and 6 (5%) had AR-EVA of 10 or more letters better than MR-EVA.
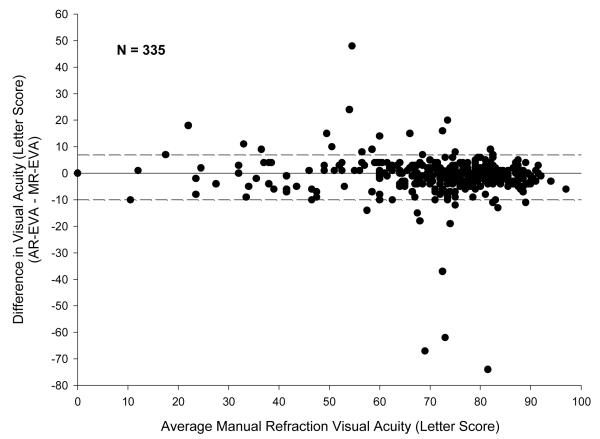
Differences and absolute differences between AR-EVA and MR-EVA were not associated with study participant age, gender, race, DME severity, primary cause of vision loss, pupil size, lens status, or refractive error (Table 7 website [available at http://###]). However, both differences and absolute differences between AR-EVA and MR-EVA scores were highly associated with the type of autorefractor used (P < 0.001) (Table 6b). Of all the autorefractor models included in this study, autorefractors from the Topcon 8000 series generated refractions that yielded VA results most similar to manual refraction. In general, although results with the Topcon 8000 series models were better than results with other autorefractor models, there was still substantially more variability between Topcon 8000 AR-EVA and MR-EVA than between MR-EVAsupl and MR-EVA. The median difference in AR-EVA and MR-EVA in the Topcon 8000 series group was 0 letters (5th and 95th percentiles −10 to +7 letters) but the median absolute difference between AR-EVA and MR-EVA in this group was 3 letters (5th and 95th percentiles 0 to 13 letters) (Figure 4). Test-retest absolute differences for MR-EVA of 2 lines or more were present in 4% of eyes, whereas in the Topcon group, absolute differences of 2 lines or more between AR-EVA and MR-EVA were present for 9% of eyes, as compared with 18% of eyes tested with other autorefractor models. Three eyes (1%) tested with Topcon 8000 series machines had a greater than 60 letter difference between AR-EVA and MR-EVA. Results obtained with the Topcon 8000 series machines were generally consistent between the 8 clinical sites that employed these models, although one of these sites appeared to have a greater variability than the others between AR and MR-EVAs, accounting for 4 of the 5 observations with AR-EVA and MR-EVA absolute difference ≥ 30 letters.
Table 7.
Comparison of Manual Refraction with Auto Refraction and Supplemental Manual Refraction Visual Acuity Letter Score by Subgroups (website)
| MR-EVAsupl – MR-EVA | AR-EVA – MR-EVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Difference* | Absolute Difference** | N | Difference* | Absolute Difference** | |||||
| Median (5th, 95th percentiles) |
P value | Median (5th, 95th percentiles) |
P value | Median (5th, 95th percentiles) |
P value | Median (5th, 95th percentiles) |
P value | |||
| VA Tester Masked | 0.05 | 0.006 | 0.02 | 0.71 | ||||||
| Yes | 35 | 0 (−7, +2) | 1 (0, 7) | 800 | −2 (−19, +7) | 3(0, 21) | ||||
| No | 843 | 0 (−5, +7) | 2 (0, 9) | 78 | −1 (−13, +8) | 4 (0, 14) | ||||
| Age | 0.91 | 0.87 | 0.01 | 0.14 | ||||||
| <60 | 311 | 0 (−5, +7) | 2 (0, 9) | 311 | −1 (−14, +7) | 3 (0, 18) | ||||
| 60−<70 | 337 | 0 (−4, +8) | 2 (0, 8) | 337 | −1 (−19, +9) | 4 (0, 23) | ||||
| ≥70 | 230 | 0 (−6, +6) | 2 (0, 9) | 230 | −2 (−20, 5) | 4 (0, 21) | ||||
| Gender | 0.55 | 0.05 | 0.35 | 0.50 | ||||||
| Women | 380 | 0 (−5, +7) | 2 (0, 8) | 380 | −1 (−17, +8) | 3 (0, 20) | ||||
| Men | 498 | 0 (−6, +7) | 2 (0, 9) | 498 | −1 (−17, +7) | 4 (0, 20) | ||||
| Race/Ethnicity | 0.75 | 0.33 | 0.58 | 0.99 | ||||||
| White | 591 | 0 (−5, +6) | 2 (0, 8) | 591 | −2 (−17, +7) | 4 (0, 20) | ||||
| Non-White | 287 | 0 (−6, +8) | 2 (0, 10) | 287 | −1 (−16, +7) | 3 (0, 22) | ||||
| DME on clinical exam a | 0.36 | 0.12 | 0.18 | 0.07 | ||||||
| None | 163 | 0 (−6, +8) | 2 (0, 9) | 163 | −2 (−20, +7) | 4 (0, 22) | ||||
| Present, center involvement none/uncertain |
178 | 0 (−5, +7) | 2(0, 11) | 178 | −1 (−23, +8) | 4 (0, 23) | ||||
| Present, center involved | 529 | +1 (−5, +7) | 2 (0, 8) | 529 | −1 (−16, +7) | 3 (0, 16) | ||||
|
Primary cause of vision
loss b |
0.53 | 0.36 | 0.31 | 0.47 | ||||||
| DME | 544 | +1 (−5, +7) | 2 (0, 9) | 544 | −1 (−17, +8) | 4 (0, 20) | ||||
| Other | 146 | +1 (−5, +9) | 2 (0, 9) | 146 | 0 (−20, +9) | 4 (0, 22) | ||||
| Pupil Size c | 0.75 | 0.98 | 0.30 | 0.02 | ||||||
| 1.0-3.0 | 397 | 0 (−6, +9) | 2 (0, 10) | 397 | −2 (−23, +8) | 4 (0, 24) | ||||
| 3.5-6.0 | 415 | 0 (−5, +6) | 2 (0, 7) | 415 | −1 (−11, +6) | 3 (0, 12) | ||||
| Lens status | 0.59 | 0.55 | 0.35 | 0.54 | ||||||
| Phakic | 585 | 0 (−5, +7) | 2 (0, 9) | 585 | −1 (−16, +7) | 3 (0, 19) | ||||
| Pseudophakic | 293 | 0 (−6, +6) | 2 (0, 8) | 293 | −2 (−21, +8) | 4 (0, 21) | ||||
|
Severity of DME (based
on OCT CSF thicknessd) |
0.75 | <.001 | 0.73 | 0.002 | ||||||
| <250 | 269 | 0 (−6, +8) | 2 (0, 10) | 269 | −2 (−23, +8) | 4 (0, 26) | ||||
| 250-<400 | 446 | 0 (−5, +6) | 2 (0, 8) | 446 | −2 (−15, +7) | 3 (0, 16) | ||||
| 400-<500 | 89 | 0 (−4, +6) | 2 (0, 8) | 89 | −1 (−15, +6) | 3 (0, 18) | ||||
| ≥500 | 52 | +1 (−5, +6) | 2 (0, 9) | 52 | −3 (−14, +5) | 4 (0, 14) | ||||
|
Manual Refraction
Spherical Equivalent |
0.36 | 0.49 | 0.82 | 0.35 | ||||||
| < −3.00 | 64 | +1 (−5, +6) | 2 (0, 8) | 64 | −1 (−7, +9) | 3 (0, 11) | ||||
| −3.00−<−1.00 | 133 | 0 (−4, +8) | 2 (0, 9) | 133 | 0 (−14, +7) | 4 (0, 18) | ||||
| −1.00−<+1.00 | 467 | 0 (−5, +7) | 2 (0, 8) | 467 | −1 (−16, +7) | 3 (0, 20) | ||||
| +1.00-<+3.00 | 176 | 0 (−7, +8) | 2 (0, 9) | 176 | -3 (−22, +6) | 4 (0, 24) | ||||
| ≥ +3.00 | 38 | +1 (−4, +5) | 2 (0, 9) | 38 | -2 (−20, +5) | 4 (0, 20) | ||||
MR EVAsupl minus MR EVA and AR- EVA minus MR- EVA, respectively.
Absolute Value of the Difference
Exclude 8 eyes (Cannot determine)
Exclude 188 eyes (no vision loss)
Missing for 66 eyes (pupil not done)
Missing for 22 eyes; categories based on thickness on Zeiss Stratus; categories for Topcon 3D-OCT are <275, 275-<425, 425-<525, ≥525; categories for Zeiss Cirrus or Heidelberg Spectralis are <310, 310-<460, 460-<560, ≥560.
MR-EVA= Manual refraction Electronic Diabetic Retinopathy Study Visual Acuity Test letter score; AR-EVA= Autorefraction Electronic Early Treatment Diabetic Retinopathy Study Visual Acuity Test letter score; MR-EVAsupl= Supplemental Manual refraction Electronic Early Treatment Diabetic Retinopathy Study Visual Acuity Test letter score; OCT= Optical Coherence Tomography; CSF= Central Subfied
Table 6b.
Comparison of Manual Refraction with Auto Refraction Visual Acuity Letter Score by Autorefractor Type
| Autorefractor Type† |
|||||
|---|---|---|---|---|---|
| Overall | Marco Nidek | Nidek | Topcon 8000 Series |
Other | |
| N | 878 | 251 | 114 | 335 | 178 |
|
| |||||
|
Letter Difference* - Median (5th, 95thpercentiles) |
−1 (−17, +7) | −2 (−19, +8) | −3 (−35, +7) | 0 (−10, +7) | −3 (−18, +6) |
|
Letter Difference Group - n (%) |
|||||
| ≤-15 | 56 (6%) | 18 (7%) | 19 (17%) | 7 (2%) | 12 (7%) |
| −14 to −10 | 49 (6%) | 16 (6%) | 12 (11%) | 12 (4%) | 9 (5%) |
| −9 to −5 | 142 (16%) | 47 (19%) | 17 (15%) | 41 (12%) | 37 (21%) |
| −4 to +4 | 545 (62%) | 141 (56%) | 55 (48%) | 245 (73%) | 104 (58%) |
| +5 to +9 | 63 (7%) | 22 (9%) | 9 (8%) | 20 (6%) | 12 (7%) |
| +10 to +14 | 10 (1%) | 3 (1%) | 1 (1%) | 3 (1%) | 3 (2%) |
| ≥+15 | 13 (1%) | 4 (2%) | 1 (1%) | 7 (2%) | 1 (1%) |
|
| |||||
|
Absolute Value of the Letter Difference** -Median (5th, 95th percentiles) |
4 (0, 20) | 4 (0, 21) | 5 (0, 35) | 3 (0, 13) | 4 (0, 21) |
| < 5 - N(%) | 545 (62%) | 141 (56%) | 55 (48%) | 245 (73%) | 104 (58%) |
| <10 - N (%) | 750 (85%) | 210 (84%) | 81 (71%) | 306 (91%) | 153 (86%) |
| <15- N (%) | 809 (92%) | 229 (91%) | 94 (82%) | 321 (96%) | 165 (93%) |
Auto Refraction EVA - Manual Refraction EVA
Absolute Value of the Difference
The difference and absolute difference were significantly less in the Topcon 8000 series group compared with Marco Nidek, Nidek, and Other groups (P value is 0.01, <.001, and .001 respectively for the difference and P value is <0.001, <.001, and 0.009 respectively for the absolute difference)
Figure 4.
Bland-Altman Plot of Difference between Early Electronic Treatment Diabetic Retinopathy Study Visual Acuity Tests Measured Using Manual Refraction and Auto Refraction by Topcon 8000 Series Machines
Solid reference line indicates median; dashed lines indicate 5th and 95th percentiles
MR-EVA= Manual refraction Electronic Early Treatment Diabetic Retinopathy Study Visual Acuity Test letter score; AR-EVA= Autorefraction Electronic Early Treatment Diabetic Retinopathy Study Visual Acuity Test letter score.
Discussion
The training and certification of examiners to accurately refract study participants for the determination of best corrected VA is both time-consuming and expensive. The rigorous manual refraction protocol currently in use also is time consuming to perform and lengthens visits for study participants. Thus, the ability to substitute an automated refraction for manual refraction could streamline study visits and result in substantial savings of time and cost for clinical staff. However, our results, obtained across a broad range of sites with a large variety of autorefractors, do not support the general substitution of autorefraction for a standardized manual refraction for most clinical trial protocols at this time.
Although differences in performance were noted between autorefractor models, with Topcon 8000 series autorefractors performing on average most similarly to manual refraction, there still was wide variability among results generated by certified VA examiners employing the Topcon machines. There was an over 2 fold increase in the percentage of eyes with a 2 or more line difference using Topcon 8000 AR-EVA as compared with repeat MR-EVA. Thus, even though the variability from MR-EVA would be decreased if all sites were to use this same consistent autorefractor model, the discrepancies of MR-EVA to AR-EVA likely do not support using AR-EVA in lieu of MR-EVA routinely for clinical trial purposes. Topcon 8000 series autorefractors differ from other autorefractor models included in this study in that they utilize a rotary prism technology enabling evaluation of a wider retinal area through a smaller diameter pupil. It is conceivable that the differences in autorefractor performance seen in this study are tied to this difference in autorefractor hardware and that further improvements in autorefraction are possible. It is unlikely that differences between autorefractors were due to differences in performance across clinical sites because overall there was no substantial difference in variability of VA measurements among the individual sites that used the Topcon 8000 series machines.
If substitution of autorefraction for manual refraction is desired, these results suggest that some types of clinical trials with specific outcomes or treatment algorithms may lend themselves more readily than others to this approach. To some extent, increased variability of VA measurements associated with autorefraction could be accounted for in study design by increasing sample size (Table 8). However, the wide range of differences seen between vision tested after autorefraction versus manual refraction would make autorefraction a poor substitute for manual refraction in clinical trials in which treatment decisions for individual patients are driven by relatively small changes in VA (e.g. 5 letters). Autorefraction might be a more feasible substitute for manual refraction in studies in which VA is evaluated solely for determination of outcomes across a large study population or was a secondary or tertiary outcome variable. However, in these studies careful consideration of the value of VA for assessing adverse events would also have to be considered.
Table 8.
Distribution of Manual Refraction, Auto Refraction and Supplemental Manual Refraction Visual Acuity Approximate Snellen Equivalent (Letter Score) (website)
| MR-EVA | AR-EVA | MR-EVAsupl | |
|---|---|---|---|
| N | 878 | 878 | 878 |
| ≥ 20/20 (≥84) | 168 (19%) | 128 (15%) | 171 (19%) |
| 20/25-20/32 (83-74) | 267 (30%) | 255 (29%) | 284 (32%) |
| 20/40-20/80 (73-54) | 300 (34%) | 314 (36%) | 293 (33%) |
| ≤20/100 (≤53) | 143 (16%) | 181 (21%) | 130 (15%) |
| Mean | 69 | 67 | 70 |
| Standard Deviation | 17 | 18 | 17 |
| Variance | 287 | 340 | 283 |
MR-EVA= Manual refraction Electronic Early Treatment Diabetic Retinopathy Study Visual Acuity Test letter score; AR-EVA= Autorefraction Electronic Early Treatment Diabetic Retinopathy Study Visual Acuity Test letter score; MR-EVAsupl= Supplemental Manual refraction Electronic Early Treatment Diabetic Retinopathy Study Visual Acuity Test letter score
Results from this study also confirm that there is high test retest reliability of EVA measurements in eyes with DME. EVA was performed twice with the same manual refraction in this study to serve as the benchmark for comparison of the AR and MR-EVAs. The test-retest variability of MR-EVA for eyes with DME in this study was similar to that from eyes without DME. Furthermore, in this study, results from the 81% of eyes with DME (half-width of two sided confidence intervals [CI]: 9 letters, 83% and 96% within 5 and 10 letters, respectively) were comparable to a previous study in which only a small proportion (10%) of participants had DME (half-width of two-sided CI: 8 letters, 89% and 98% within 5 and 10 letters).13 Limitations of this study include the fact that a small percentage of VA examiners were not masked to refraction source. However, use of the E-ETDRS testing protocol to measure VA minimizes the potential bias that could be attributed to an examiner and no statistically significant difference was seen in absolute differences between VA based on autorefraction versus manual refraction between results from the over 90% of VA examinations that were performed in a masked fashion versus those that were unmasked. In addition, although a relatively low percentage of eyes in the study had decreased VA, we were still able to determine a significant difference between AR-EVA and MR-EVA as measured in different VA subgroups. We were able to enroll substantial numbers of eyes to assess three most commonly available autorefraction models (Marco Nidek, Nidek, and Topcon) but, for other, generally older autorefractor models, we were limited by relatively small numbers. Finally, although this study evaluated the test-retest reliability of MR-EVA, it did not assess the test-retest reliability of AR-EVA. It is still possible that autorefraction, if highly repeatable, might be useful for following changes in VA over time.
In summary, these results demonstrate that VA after autorefraction tends to be slightly worse than that after manual refraction. Variability between VA after autorefraction and VA after manual refraction is substantially higher than the test-retest variability of manual refraction. We also observed important differences between autorefractor models. Although in general autorefraction may not be an acceptable substitute for manual refraction, specific elements of study design including increased sample size and nonreliance of treatment algorithm on small differences in VA may allow limited substitution of autorefraction for manual refraction in some studies.
Supplementary Material
Acknowledgments
Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services EY14231, EY14229, EY018817
Diabetic Retinopathy Clinical Research Network Clinical Sites that participated on this protocol:
Sites are listed in order by number of subjects enrolled into the study. The number of subjects enrolled is noted in parenthesis preceded by the site location and the site name. Personnel are listed as (I) for Study Investigator, (C) for Coordinator, (V) for Visual Acuity Tester, and (P) for Photographer.
Boston, MA Joslin Diabetes Center (163): Jennifer K. Sun(I); Sabera T. Shah(I); Timothy J. Murtha(I); Paul G. Arrigg(I); Lloyd Paul Aiello(I); George S. Sharuk(I); Deborah K. Schlossman(I); Christopher M. Andreoli(I); Margaret E Stockman (C,V); Troy Kieser (C,V); Julie A. Barenholtz (C,V); Sharon M. Eagan(V); Dorothy Tolls(V); John C. BuAbbud(V); Jamy Borbidge(V); Jerry D. Cavallerano(V); William Carli(V); Mary Ann Robertson(V); Mathew M. Coppola(V); Anna Fagan(V); Leslie L. Barresi(P) Lakeland, FL Florida Retina Consultants (70): Oren Z. Plous(I); Scott M. Friedman(I); Kelly A. Blackmer(C); Karen Sjoblom (C,V); Jolleen S. Key (C,V); Damanda A. Fagan(V); Allen McKinney(P); Kimberly A Williamson(P)0 Lancaster, PA Family Eye Group (50): Michael R Pavlica(I); Noelle S Matta (C,V); Sara Weit(V); Cristina M. Brubaker(P) Baltimore, MD Elman Retina Group, P.A. (24): Michael J. 2 Elman(I); JoAnn Starr(C); Theresa M. Butcher(C); Dena Y. Salfer-Firestone(V); Pamela V. Singletary(V); Nancy Gore(V); Teresa Coffey(V) Detroit, MI Henry Ford Health System, Dept of Ophthalmology and Eye Care Services (24): Paul Andrew Edwards(I); Uday Desai(I); Janet Murphy (C,V); Alexa M. Lipman (C,V); Julianne Hall (C,V); Melanie A Gutkowski (C,V); Dorena F. Wilson(V) Indianapolis, IN Raj K. Maturi, M.D., P.C. (22): Raj K. Maturi(I); Laura A. Bleau (C,V); Carolee K. Novak (C,V) Loma Linda, CA Loma Linda University Health Care, Department of Ophthalmology (20): Joseph T. Fan(I); Mukesh Bhogilal Suthar(I); Michael E. Rauser(I); Cara L Davidson (C,V); Kara E. Rollins (C,V); Blen D. Eshete (C,V); Gisela Santiago(V); William H. Kiernan(V) Charlotte, NC Charlotte Eye, Ear, Nose and Throat Assoc., PA (12): David Browning(I); Andrew Nicholas Antoszyk(I); Danielle R. Brooks (C,V); Angela K. Price (C,V); Sarah A. Ennis(V); Angella S. Karow(V) Houston, TX Retina and Vitreous of Texas (12): H. Michael Lambert(I); Pam S. Miller(C); Valerie N. Lazarte(V); Debbie Fredrickson(V) Hershey, PA Penn State College of Medicine (11): Ingrid U. Scott(I); Susan M. Chobanoff (C,V) New York, NY The New York Eye and Ear Infirmary/Faculty Eye Practice (10): Ronald C. Gentile(I); Estuardo Alfonso Ponce(I); Anita Ou (C,V); Catiria Guerrero(V); Julie A. Paa(V); Violete Perez(V) Baltimore, MD Wilmer Eye Institute at Johns Hopkins (8): Sharon D. Solomon(I); Susan Bressler(I); Diana V. Do(I); Adrienne Williams Scott(I); Mary Frey (C,V); Sandra West (C,V); Deborah Donohue(V) McAllen, TX Valley Retina Institute (8): Victor Hugo Gonzalez(I); Nehal R. Patel(I); Marcos Silva(C); Melody Cruz(C); Monica R. Cantu(V); Marlene Lopez(V); Rachel Rodriguez(V) New Albany, IN John-Kenyon American Eye Institute (7): Howard S. Lazarus(I); Debra Paige Bunch (C,V); Angela D. Ridge(C); Kelly Booth(V) Chapel Hill, NC University of North Carolina, Dept of Ophthalmology (6): Seema Garg(I); Travis A. Meredith(I); Odette M. Houghton(I); Cassandra J. Barnhart (C,V); Nabeel Barakat (C,V); Harpreet Kaur (C,V) Winston-Salem, NC Wake Forest University Eye Center (6): Craig Michael Greven(I); M. Madison Slusher(I); Joan Fish (C,V); Lori N. Cooke (C,V); Cara Everhart (C,V) Chicago, IL University of Illinois at Chicago Medical Center (5): Jennifer I. Lim(I); Michael P. Blair(I); Marcia Niec(C); Yesenia Ovando(V); Tametha Johnson(V) Paducah, KY Paducah Retinal Center (5): Carl W. Baker(I); Tracey M. Caldwell(C); Lynnette F. Lambert (C,V); Tracey R. Martin(V); Mary J. Palmer(V) Milwaukee, WI Medical College of Wisconsin (4): Judy E. Kim(I); Kimberly E. Stepien(I); Dennis P. Han(I); Vesper V. Williams(C); Vicki Barwick(V); Judy Flanders(V) Rochester, NY University of Rochester (4): David Allen DiLoreto(I); George Ogara(C,V); Malinda M. Goole(V); Terrance Schaefer(V) Dubuque, IA Medical Associates Clinic, P.C. (3): Michael H. Scott(I); Philomina M. Wiegman(C); Thomas R. Dvorak(V); Marcia J. Moyle(P); Brenda L. Tebon(P) Kingsport, TN Southeastern Retina Associates, PC (2): Howard L. Cummings(I); Deanna Jo Long(C); Stacy Carpenter(V) Pittsburgh, PA Retina Vitreous Consultants (2): Karl R. Olsen(I); Tara L. Wilson(C); Kim Whale(V); Pamela Rath(I); Christina Schultz(V); David Steinberg(P); Heather Shultz(P) San Antonio, TX Retinal Consultants of San Antonio (2): Calvin E. Mein(I); Moises A. Chica(I); Lita Kirschbaum (C,V); Christopher Sean Weineke(P) Boston, MA Ophthalmic Consultants of Boston (1): Trexler M Topping(I); Lesley-Anne Freese(C); Jennifer L. Stone(V) St. Louis, MO Barnes Retina Institute (1): Rajendra S. Apte(I); Kevin J. Blinder(I); Carolyn L. Walters (C,V); Lynda K. Boyd(V)
DRCR.net Coordinating Center: Jaeb Center for Health Research, Tampa, FL (staff as of 6/8/2011): Adam R. Glassman (Director and Principal Investigator), Roy W. Beck (Executive Director) Talat Almukhtar, Bambi J. Arnold, Brian B. Dale, Alyssa Baptista, Sharon R. Constantine, Simone S. Dupre, Allison R. Edwards, Meagan L. Huggins, Paula A. Johnson, Lee Anne Lester, Brenda L. Loggins, Emily B. Malka, Shannon L. McClellan, Michele Melia, Kellee M. Miller, Pamela S. Moke, Haijing Qin, Rosa Pritchard, Eureca Scott, Cynthia R. Stockdale.
Fundus Photograph Reading Center: University of Wisconsin-Madison, Madison, WI (staff as of 6/8/11): Matthew D. Davis (Director Emeritus), Sapna Gangaputra (Co-Director), Ronald P. Danis (Director and Principal Investigator), Larry Hubbard (Associate Director), James Reimers (Lead Color Photography Evaluator), Pamela Vargo (Lead Photographer), Ericka Moeller (Digital Imaging Specialist), Dawn Myers (Lead OCT Evaluator), Kristjan Burmeister (Project Manager), Vonnie Gamma (Data Management)
DRCR.net Operations Center: Johns Hopkins University School of Medicine, Baltimore, MD (staff as of 6/8/2011): Neil M. Bressler (Network Chair and Principal Investigator), Connie Lawson, Peggy R. Orr, Beth Wellman.
DRCR.net Vice Chairs: Susan B. Bressler (2009-current), Scott Friedman (2009-current), Carl W. Baker (2011-current), Ingrid U. Scott (2009-2010).
National Eye Institute: Eleanor Schron (2009-current), Donald F. Everett (2003-2006, 2007-2009), Päivi H. Miskala (2006-2007)
Executive Committee: Raj K. Maturi (2009-present; Chair 2010) Neil M. Bressler (2006-present; Chair 2006 - 2008), Lloyd Paul Aiello (2002-present; Chair 2002 – 2005), Carl W. Baker (2009-present), Roy W. Beck (2002-present), Susan B. Bressler (2009-Present), Alexander J. Brucker (2009-present), Kakarla V. Chalam (2009-present) Ronald P. Danis (2004-present), Matthew D. Davis (2002-present), Michael J. Elman (2006-present; Chair 2009), Frederick L. Ferris III (2002-present), Scott Friedman (2007 –present), Adam R. Glassman (2005-present), Joseph Googe, Jr. (2009-present), Eleanor Schron (2009-present), JoAnn Starr (2009-present), Jennifer K. Sun (2009-present). Prior Members: Andrew N. Antoszyk (2009), Abdhish Bhavsar (2007 –2008), David M. Brown (2006-2007), David J. Browning (2005-2006), Donald F. Everett (2002-2009), Joan Fish (2008 - 2009), Andreas Lauer (2007-2008), Kim McLeod (2002-2006), Päivi H. Miskala (2005-2007), Cynthia J. Grinnell (2006-2007), Ingrid U. Scott (2009-2010).
Footnotes
Financial Disclosures:
An Address for reprints will not be provided A complete list of all DRCR.net investigator financial disclosures can be found at www.drcr.net
References
- 1.Ferris FL, 3rd, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–6. [PubMed] [Google Scholar]
- 2.Early Treatment Diabetic Retinopathy Study Research Group Early treatment diabetic retinopathy study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology. 1991;98:741–56. doi: 10.1016/s0161-6420(13)38009-9. [DOI] [PubMed] [Google Scholar]
- 3.Pesudovs K, Weisinger HS. A comparison of autorefractor performance. Optom Vis Sci. 2004;81(7):554–8. doi: 10.1097/00006324-200407000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Cornsweet TN, Crane HD. Servo-controlled infrared optometer. J Opt Soc Am. 1970;60(4):548–54. doi: 10.1364/josa.60.000548. [DOI] [PubMed] [Google Scholar]
- 5.Knoll HA, Mohrman R. The ophthalmetron, principles and operation. Am J Optom Arch Am Acad Optom. 1972;49(2):122–8. doi: 10.1097/00006324-197202000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Knoll HA, Mohrman R, Maier WL. Automatic objective refraction in an office practice. Am J Optom Arch Am Acad Optom. 1970;47(8):644–9. doi: 10.1097/00006324-197008000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Safir A, Knoll H, Mohrman R. Automatic objective refraction. Report of a clinical trial. Trans Am Acad Ophthalmol Otolaryngol. 1970;74(6):1266–75. [PubMed] [Google Scholar]
- 8.Sun JK, Aiello LP, Cavallerano JD, et al. Visual acuity testing using autorefraction or pinhole occluder compared with a manual protocol refraction in individuals with diabetes. Ophthalmology. 2011;118(3):537–42. doi: 10.1016/j.ophtha.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thibos LN, Wheeler W, Horner D. Power vectors: an application of Fourier analysis to the description and statistical analysis of refractive error. Optom Vis Sci. 1997;74(6):367–75. doi: 10.1097/00006324-199706000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Harris W. The mean and variance of samples of dioptric powers: the basic calculations. Clinical and Experimental Optometry. 1990;73:89–92. [Google Scholar]
- 11.Long WF. A matrix formalism for decentration problems. Am J Optom Physiol Opt. 1976;53(1):27–33. doi: 10.1097/00006324-197601000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–60. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 13.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135(2):194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.