Abstract
Airway smooth muscle cell (ASMC) remodeling contributes to the structural changes in the airways that are central to the clinical manifestations of asthma. Ca2+ signals play an important role in ASMC remodeling through control of ASMC migration and hypertrophy/proliferation. Upregulation of STIM1 and Orai1 proteins, the molecular components of the store-operated Ca2+ entry (SOCE) pathway, has recently emerged as an important mediator of vascular remodeling. However, the potential upregulation of STIM1 and Orai1 in asthmatic airways remains unknown. An important smooth muscle migratory agonist with major contributions to ASMC remodeling is the platelet-derived growth factor (PDGF). Nevertheless, the Ca2+ entry route activated by PDGF in ASMC remains elusive. Here, we show that STIM1 and Orai1 protein levels are greatly upregulated in ASMC isolated from ovalbumin-challenged asthmatic mice, compared to control mice. Furthermore, we show that PDGF activates a Ca2+ entry pathway in rat primary ASMC that is pharmacologically reminiscent of SOCE. Molecular knockdown of STIM1 and Orai1 proteins inhibited PDGF-activated Ca2+ entry in these cells. Whole-cell patch clamp recordings revealed the activation of Ca2+ release-activated Ca2+ (CRAC) current by PDGF in ASMC. These CRAC currents were abrogated upon either STIM1 or Orai1 knockdown. We show that either STIM1 or Orai1 knockdown significantly inhibited ASMC proliferation and chemotactic migration in response to PDGF. These results implicate STIM1 and Orai1 in PDGF-induced ASMC proliferation and migration and suggest the potential use of STIM1 and Orai1 as targets for ASMC remodeling during asthma.
Keywords: STIM1, Orai1, CRAC, SOCE, Asthma, Smooth muscle remodeling
Introduction
Asthma is a complex disease; it is clear that allergic, immunological, and inflammatory dysfunctions are major contributors to asthma [25, 37, 67]. It is also established that airway smooth muscle cell (ASMC) hyperresponsiveness is central to asthma manifestations [27, 50]. However, there is increasing evidence that structural changes or remodeling of the airways also contribute to asthma persistence, decline of pulmonary function, and clinical severity [2, 4, 19, 23]. It was shown, using a large database of airway cross sections, that ASMC remodeling is an early event that correlates with asthma severity [2, 4, 29, 62], suggesting that structural changes in asthmatic airways may precede clinical manifestations. Further, in animal models of asthma, airway remodeling persists even after resolution of inflammation [33, 34, 40]. ASMC remodeling represents an increased ASMC mass resulting from cell migration, proliferation, and/or hypertrophy [4, 24]. Incidentally, it was suggested that ASMC remodeling is likely to contribute to airway hyperresponsiveness in asthma, since ASMC constriction will depend not only on ASMC contractility but also on the number and the size of ASMC [50].
ASMC remodeling in asthma is believed to be the result of a phenotypic switch into a migratory proliferative or “synthetic” phenotype [24]. ASMC in asthmatic airways remodel inward towards the mucosal layer, which was suggested to result from ASMC migration in response to growth and chemokinetic factors secreted by epithelial cells [49]. One such potent smooth muscle migratory and proliferative factor is the platelet-derived growth factor (PDGF) [14, 28] which is secreted by epithelial cells and inflammatory cells from asthmatic airways [21, 45, 58] and colocalizes in the mucosal and submucosal layer of airways from asthmatic patients, and its production directly correlates with the severity of asthma [45].
Ca2+ signaling is essential for ASMC contractility, growth, and migration. One of the ubiquitous means of receptor-regulated Ca2+ entry in ASMC is through storeoperated Ca2+ (SOC) channels. A wide variety of growth factors that bind phospholipase C (PLC)-coupled receptors lead to PLC activation and subsequent production of the second messenger, inositol-1,4,5 trisphosphate (IP3)[7]. IP3-mediated depletion of internal Ca2+ stores then signals the activation of plasma membrane (PM) SOC channels [52, 53]. Pharmacological features of SOC channels include inhibition by 2-aminoethoxydiphenyl borate (2-APB; 50 μM) and low concentrations of gadolinium (5 μM Gd3+) [13, 63]. Stromal interaction molecule 1 (STIM1) is the Ca2+ sensor in the endoplasmic reticulum (ER) [35, 56] which oligomerizes in defined ER/PM junctional areas upon store depletion to activate the SOC channel at the PM formed by Orai1 [20, 66, 72]. The mechanisms of STIM1/Orai1 coupling involve direct binding of a ~100-amino acid C-terminal coiled-coil region of STIM1 (named SOAR for STIM/Orai activating region) to the C- and N-termini of Orai1 tetramers [42, 46, 71]. Molecular knockdown ascribed a role for STIM1/Orai1 in ASMC store-operated Ca2+ entry (SOCE) in response to passive store depletion by inhibitors of sarcoplasmic/endoplasmic reticulum Ca2+ ATPase such as thapsigargin or cyclopiazonic acid [47, 48].
ASMC remodeling in asthma is associated with abnormal Ca2+ handling and changes in expression of ion channels and pumps [38, 39, 50, 57, 59, 60]. Interestingly, similar disturbances occur in vascular occlusive diseases when vascular smooth muscle cells (VSMC) switch to a migratory proliferative phenotype [3, 15, 26, 36]. In the case of VSMC, the change to synthetic phenotype is accompanied by changes in ion channel expression, including upregulation by migratory proliferative VSMC of STIM1 and Orai1 proteins [5, 11, 51]. However, the potential upregulation of STIM1 and Orai1 in ASMC from asthmatic airways and their contribution to Ca2+ entry, membrane currents, and proliferative and migratory signaling in response to PDGF remain unknown, Here, we show that asthmatic mice upregulate protein levels of STIM1 and Orai1 in tracheobronchial ASMC. We also show that PDGF activates a Ca2+ entry pathway in synthetic ASMC that is pharmacologically reminiscent of SOCE. Indeed, molecular knockdown showed that PDGF-activated Ca2+ entry is mediated through STIM1 and Orai1. PDGF activates typical Ca2+ release-activated Ca2+ (CRAC) currents in ASMC that were STIM1- and Orai1-dependent. Furthermore, PDGF-mediated ASMC proliferation and migration was abrogated upon STIM1 and Orai1 knockdown.
Material and methods
Reagents
Gd3+ was purchased from Acros Organics, recombinant rat PDGF-BB from R & D Biosystems, 2-aminoethoxydiphenyl borate (2-APB) and Thapsigargin from Calbiochem; and BAPTA from Invitrogen. All siRNA sequences were obtained from Dharmacon. Specific primers for rat STIM1, Orai1 and Orai3, and siRNA sequences were reported previously [51]. Anti-STIM1 was purchased from BD Biosciences, anti-β-actin NH2-terminal domain from Sigma, GAPDH from Sigma, and anti-Orai1 (extracellular; catalog no. ACC-060) from Alomone. All other chemical products were obtained from Fisher Scientific unless specified otherwise.
ASMC dispersion and culture
Rat ASMCs were obtained from the tracheobronchial tissue of airways derived from 300- to 350-g male Sprague Dawley rats (Taconic Farms, Germantown, NY), Following the removal of the epithelial layer, ASMCs were enzymatically dispersed and cultured in DMEM–Ham’s F-12 medium with 10 % fetal bovine serum or 0,2 % where indicated, The rat ASMCs were maintained at 37 °C in a 5 % CO2-95 % atmospheric environment. Cells were split twice weekly, and media were changed every 3 days. Experiments were carried out in ASMCs from passages 3–9, Use of experimental animals was reviewed and approved by the Albany Medical College Institutional Care and Use Committee.
Cell transfections
Sets of four different siRNAs per target gene were initially assessed for their ability to reduce mRNA levels using quantitative PCR (qPCR) as described below. SiRNA sequences that induced significant decreases in their target mRNA without cross effects on other mRNAs were used in Western blotting to confirm protein knockdown as described below. All transfections in ASMCs were done using a Nucleofector device II (Amaxa Biosystems, Gaithersburg, MD) according to the manufacturer’s instructions, As a marker of cell transfection, 0,5 μg of green fluorescent protein was co-transfected with siRNA for identification of successfully transfected cells, For all siRNA experiments, the control siRNA is a scrambled siRNA sequence.
Ca2+ measurements
Ca2+ was measured as described previously [1, 11, 41, 63, 65]. In brief, coverslips with attached cells were mounted in a Teflon chamber and incubated at 37 °C for 1 h in a culture medium containing 6 μM fura 2-AM (Molecular Probes, Eugene, OR). Cells were then washed and bathed in HEPES-buffered saline solution (in millimolars: 140 NaCl, 1,13 MgCl2, 4.7 KCl, 2 CaCl2, 10 d-glucose, and 10 HEPES, with pH adjusted to 7.4 with NaOH) for ~10 min before Ca2+ was measured. For Ca2+ measurements, fluorescence images of several cells were recorded and analyzed with a digital fluorescence imaging system (InCyt Im2, Intracellular Imaging, Cincinnati, OH). Fura 2 fluorescence at an emission wavelength of 510 nm was induced by excitation of Fura 2 alternately at 340 and 380 nm. The ratio of fluorescence at 340 nm to that at 380 nm was obtained on a pixel-by-pixel basis. All experiments were conducted at room temperature.
Whole-cell patch clamp electrophysiology
Whole-cell patch clamp recordings were carried out using an Axopatch 200B and Digidata 1440A (AxonInstruments, New York, NY) as previously published [64, 73]. Pipettes were pulled from borosilicate glass capillaries (World Precision Instruments, Inc., Sarasota, FL) with a P-97 flaming/brown micropipette puller (Sutter Instrument Company, Novatao, CA) and polished with DMF1000 (World Precision Instruments, Inc., Sarasota, FL). Resistances of filled glass pipettes were 2–3 MΩ. Series resistances were in the range of 2–8 MΩ. Only cells with tight seals (>16 GΩ) were selected to break in. Currents were low-pass filtered at 2 kHz and sampled at a rate of 10 kHz. All experiments were performed at room temperature (20–25 °C). Upon break-in, a pulse of divalent-free (DVF) bath solution is applied to the cells to determine the extent of background Na+ current. This background current is subsequently subtracted from the Na+ current obtained in DVF solutions once PDGF has been added for minutes and stores were presumably depleted. The nature of this background Na+ current is unknown; however, the size of these currents is not affected by STIM1 and Orai1 knockdown (See Fig. 4a-c).
Solutions employed for whole-cell patch clamp electrophysiology
Pipette solution Cs-Methanesulfonate (105 mM), 10 mM Cs-1,2-bis-(2-aminophenoxy) ethane-N,N,N’,N’-tetraacetic acid (Cs-BAPTA), 5 mMCaCl2, 8 mM MgCl2, and 10 mM HEPES (pH adjusted to 7.2 with CsOH), Based on the concentrations of Ca2+ and BAPTA in the pipette solution; free Ca2+ is 150 nM as calculated using Maxchelator software freely available at maxchelator,stanford,edu/
Bath solution Na-methanesulfonate (105 mM), 10 mM CsCl, 1.2 mM MgSO4, 10 mM HEPES, 20 mM CaCl2, and 5 mM glucose (pH was adjusted to 7.4 with NaOH). PDGF (200 ng/ml) was added.
Divalent-free bath solution Na-methanesulfonate (135 mM), 10 mM HEDTA, 1 mM EDTA, and 10 mM HEPES (pH 7,4, adjusted with NaOH)
For patch clamp experiments, 200 ng/ml PDGF was used to stimulate ASMC instead of 100 ng/ml used throughout the study. The reason for this increased concentration is to achieve ideal conditions for recordings by keeping the focal perfusion system as far away as possible from the cells to avoid disruption of the high GΩ seals while still eliciting significant currents.
Western blots on cell lysates
Cells were lysed using RIPA lysis buffer (50 mM Tris–HCl (pH 8), 150 mM NaCl, 1 % Triton X-100, 0.2 mM EDTA, 0.1 % SDS, 0.5 % sodium deoxycholate, 2 mM phenylmethylsulfonyl fluoride, 10 % protease inhibitor cocktail (Roche), 10 % phosphatase inhibitor cocktail (Roche)), protein concentrations were determined, and proteins (20–100 μg) in denaturing conditions were subjected to SDS-PAGE (8–14 %) and then electrotransferred onto polyvinylidene difluoride membranes (Bio-Rad), After the blots were blocked with 5 % nonfat dry milk (NFDM) dissolved in Tris-buffered saline containing 0,1 % Tween 20 (TTBS) either overnight or 2 h at room temperature, they were washed three times with TTBS for 5 min each and probed overnight at 4 °C with specific primary antibodies [anti-STIM1 (BD Biosciences, 1:250 dilution), anti-Orai1 (Alomone; 1:5,000 dilution), and β-actin NH2-terminal domain (1:25,000 dilution)] in TTBS containing 2 % NFDM. On the next day, membranes were washed (3 times for 5 min each) with TTBS and incubated for 1 h at room temperature with a horseradish peroxidase-conjugated secondary antibodies [anti-mouse antibody (1:10,000 dilution; Jackson) or antirabbit IgG (1:20,000 dilution; Jackson)] in TTBS containing 2 % NFDM. Bound antibodies were detected by enhanced chemiluminescence using Super Signal West Pico or Femto reagents (Pierce). Signal intensity was measured with a Fuji LAS4000 Imaging Station. Membranes were then stripped and reprobed with an antibody against beta actin to verify equal loading.
Ovalbumin sensitization and challenge protocol
Male and female mixed-background control mice (B6; FVB; 129) were sensitized to ovalbumin (OVA) as depicted in Fig. 1. Three- to four-week-old mice were intraperitoneally (i.p.) injected with 25 μg of OVA (Sigma) with 2 mg of the adjuvant aluminum hydroxide (Sigma) in 0.5 ml sterile physiological saline solution (PSS), once a week for 3 weeks, One week after final i.p. sensitization, mice were mildly anesthetized and challenged intranasally with 100 μg of OVA in 25 μl of PSS once daily for 10 successive days. Control animals were sensitized and challenged with sterile PSS. Twenty-four hours after final challenge, respiratory mechanics were performed on mice as described below. A group of mice were sacrificed with a lethal dose (100 μg/g) of i.p.-injected sodium pentobarbital and used for specimen collection including bronchoalveolar lavage (BAL) fluid and ASMC. All mice were housed in microisolator cages under pathogen-free conditions within the Albany Medical Center animal facility, Protocols were reviewed and approved by Albany Medical Center’s Institutional Animal Care and Use Committee.
Bronchoalveolar lavage IL-13 analysis
Twenty-four hours after final challenge, the airways from animals were lavaged using a cannulated microsyringe that was inserted into the trachea of the animal; 0.75 ml of sterile PSS was lavaged into the trachea and lungs of the experimental animals. Collected BAL fluid was centrifuged at 10,000 RPM for 1 min, and the supernatant was collected. Quantification of interleukin-13 (IL-13) concentration was carried out in duplicates by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN), as indicated by the manufacturer. The limit for detection of IL-13 concentration was 7.8 pg/ml.
Airway hyperreactivity
Twenty-four hours after final challenge, airway hyperresponsiveness was analyzed. Experimental animals were anesthetized with an i.p. injection of xylaxine (0.01 mg/g) and ketamine (0.1 mg/g). Animals were tracheotomized and connected to a computer-controlled small animal mechanical ventilator (flexivent, SCIREQ). The animals were then paralyzed with an i.p. injection of pancuronium bromide (0.8 mg/kg). Systemic airway resistance (Rrs) was determined using forced oscillation measurements during methacholine (Mch) (Sigma) dose response curves at concentrations of 0, 1.8, 3.8, 7.5, 15, 30, and 60 mg/ml. OVA-challenged animals were hyperresponsive to methacholine doses as compared to the saline-challenged animals. Rrs values were derived using the linear first-order single-compartment model accepting coefficient of determination values ≥0.9. The data are represented as mean ± SEM; n=6–7 mice per group.
Chemotactic cell migration assay
ASMC chemotactic migration assays were performed using the Boyden chamber method. Rat ASMC were transfected with either control siRNA or siRNA sequences targeting STIM1 and Orai1. Sixteen hours before initiating the assay, ASMC were serum-starved in HEPES-buffered 0.2 % FBS, DMEM/F-12, and penicillin and streptomyocin-supplemented medium. Cells were then harvested with trypsin, and 300,000 cells were seeded per six-well transwell cell culture chamber (BD Biosciences). Chamber membranes were polycarbonate with 8-μm pores. Cells were allowed to adhere to the membrane for 45 min without chemotactic stimulus, After 45 min, migration was induced by the addition of PDGF (100 ng/ml) in HEPES-buffered 0.2 % FBS, DMEM/F-12, and penicillin- and streptomyocin-supplemented medium, to the bottom of the lower compartment of the transwell chamber. After 6 h, non-migrated cells were removed from the upper chamber by cotton swabbing; migrated cells on the lower chamber were fixed in 4 % paraformaldehyde (USB Corporation) and stained with the nuclear stain DAPI (Vector). Cells were imaged on fluorescent Leica DMIRB scope and quantified using the ImageJ cell counter tool.
Cell proliferation assay
Rat ASMC were transfected with either control siRNA or siRNA sequences targeting either STIM1 or Orai1 (as described above), The next day, ASMC were growth arrested with 0,2 % FBS overnight, PDGF (100 ng/ml) was then added to cells for an additional 24 h and cell proliferation assayed 3 days post siRNA transfection, ASMC were trypsinized and counted in triplicates using a Beckman particle counter.
Western blots on tissue samples
Whole tissue extracts from mouse airways were harvested from experimental animals; the epithelial layer was mechanically removed, and the remaining tracheobronchial smooth muscle tissue was prepared in ice-cold RIPA lysis buffer (as described above) and used for immunoblotting. Tissue homogenate was centrifuged at 10,000 RPM for 10 min at 4 °C, and the supernatant was stored at −80 °C till analysis. Samples were separated in 10 % SDS polyacrylamide gel and transferred onto a nitrocellulose membrane, Monoclonal antibody specific for STIM1 (BD Laboratories) was used at a concentration of 1:250 in a blocking solution of 5% milk. Polyclonal antibody for Orai1 (Alomone) was used at a concentration of 1:2,000 in a blocking solution of 5 % milk. For Western blots on mice tissues, we used GAPDH as a loading control since β-actin levels change in remodeled smooth muscle cells.
Sections, hematoxylin/eosin staining
Morphological studies were carried out on tissue obtained from asthmatic and control animals 7 days after their final challenge, At this time point, animals were sacrificed and lungs were harvested (n=3, six airways from each animal each group) and fixed overnight in 10 % buffered formalin (Fisher Scientific), dehydrated, and embedded in paraffin blocks, The blocks were then sectioned in 5-μm sections and stained with hematoxylin and eosin (H&E) (Thermo Scientific). Lower airways (~200 μm in diameter) were examined for remodeling of the mucosal and submucosal layers using light microscopy at ×40 magnification.
RT-PCR and real-time PCR
Total RNA was extracted from cells using a Qiagen RNeasy Mini Kit following the manufacturer’s protocol. cDNA was made from 0.5 μg of RNA reverse transcribed using oligo(dT) primers (Invitrogen, Carlsbad, CA, USA) and SuperScript III reverse transcriptase (Invitrogen). PCR reactions were completed using Illustra PuReTaq Ready-To-Go PCR beads (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Real-time PCR analysis was performed using a Bio-Rad iCycler and iCycler iQ Optical System Software (Bio-Rad Laboratories). PCR reactions were performed using Bio-Rad iQ SYBR Green Supermix, The PCR protocol started with 5 min at 94 °C, followed by 45 cycles of 30 s at 94 °C, 30 s at 54.3 °C, and 45 s at 72 °C. Quantification was measured as sample fluorescence crossed a predetermined threshold value that was just above the background, Expressions of STIM1, Orai1, or Orai3 were compared to those of the housekeeping gene GAPDH and were measured using comparative threshold cycle values.
Statistical analysis
Data are expressed as means ± SE, and statistical analysis using either two sample Student’s t test; one-way ANOVA was done with Origin software (OriginLab, Northampton, MA) and repeated measures ANOVA with Tukey post hoc test (GraphPad Prism 4 for data in Fig. 1); *, **, and *** indicate p values <0,05, 0.01, and 0.001, respectively. Differences were considered significant when p<0.05.
Results
STIM1 and Orai1 are upregulated in ASMC from asthmatic mice
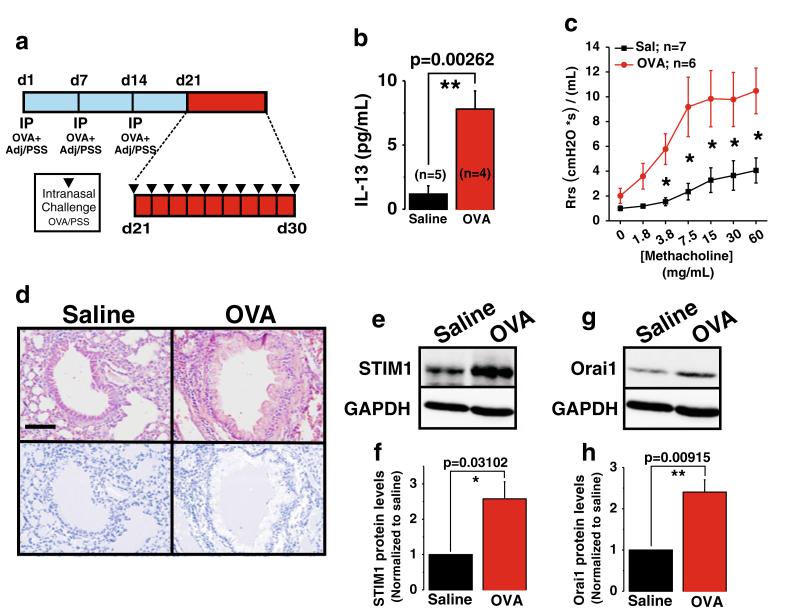
To determine whether STIM1 and Orai1 might contribute to ASMC remodeling during asthma, we sought to determine whether their protein levels are increased in tracheobronchial ASMC from asthmatic mice. A mouse model of allergen-induced asthma was established using the OVA challenge protocol described in “Material and methods” and represented in Fig. 1a, The model involves three intraperitoneal injections of OVA at days 1, 7, and 14 followed a week later by 10 daily intranasal challenges from day 21–30; control mice were subjected to the same protocol using saline instead of OVA (Fig. 1a). To confirm asthma development in OVA-challenged mice, we show that the concentration of the TH2 cytokine, IL-13, is increased in the airways (24 h post-final OVA challenge) in bronchoalveolar lavage fluid (BALF) from OVA-challenged mice vs control mice (Fig. 1b). Analysis of respiratory mechanics was conducted on mice 24 h after final OVA intranasal challenge and showed enhanced airway hyperresponsiveness during methacholine exposure in OVA-challenged mice vs control mice (Fig. 1c). The extent of airway remodeling in OVA-challenged and control mice was examined by H&E staining of lung tissue harvested 1 week after final challenge, showing extensive remodeling in the mucosa and submucosal layers of the lower airways in OVA-challenged mice but not in control mice (Fig. 1d). Western blotting on isolated tracheobronchial ASMC derived from OVA-treated and control mice (24 h post-final OVA challenge) shows increased protein expression of the SOCE components, STIM1 (Fig. 1e, f) and Orai1 (Fig. 1g, h), in OVA-challenged animals as compared to the control by approximately 2.5-fold.
Fig. 1.
Orai1 and STIM1 proteins are upregulated in a mouse model of allergen-induced asthma. Schematic diagram of sensitization and challenge protocol for allergen-induced asthma (a). Three- to four-week-old C57BL/6J mice were sensitized to OVA by carrying out three weekly i.p. injections of 25 μg of OVA with adjuvant. One week after last sensitization, all mice were challenged intranasally with 100 μg of OVA for 10 consecutive days. A group of animals were sacrificed 24 h after final challenge, and BALF was collected to quantify airway IL-13 concentration as an indicator of airway inflammation (b; control n=5; OVA n=4). Twenty-four hours after final challenge, respiratory mechanics were performed to determine airway hyperresponsiveness by measuring systemic airway function (Rrs) with forced oscillations during challenges to increasing doses of methacholine (c; control n= 7; OVA n=6). Analysis of OVA-induced airway remodeling was examined by H&E staining of lung tissue harvested 1 week after final challenge and compared to saline control airways (d; for both experimental groups, n=3 mice with six airways each). Note in OVA-treated mice areas of extensive remodeling in the mucosa and submucosal layers of the lower airways. Images are taken at ×40; bar=40 μm. ImageJ plugin for color deconvolution was executed separating out the hematoxylin nuclear stain in order to visually distinguish the differences in cellular infiltrate surrounding control and OVA-treated airways (bottom images). Twenty-four hours after final challenge, Western blotting of ASM tissue derived from control saline-treated mice compared to OVA-treated animals shows increased protein expression of STIM1 (e, f) and Orai1 (g, h) in OVA-treated animals as compared to control animals. Quantification of band densitometry from n=3 independent mice/experiment is shown in f and h
PDGF-activated Ca2+ entry in ASMC is pharmacologically identical to SOCE activated by thapsigargin
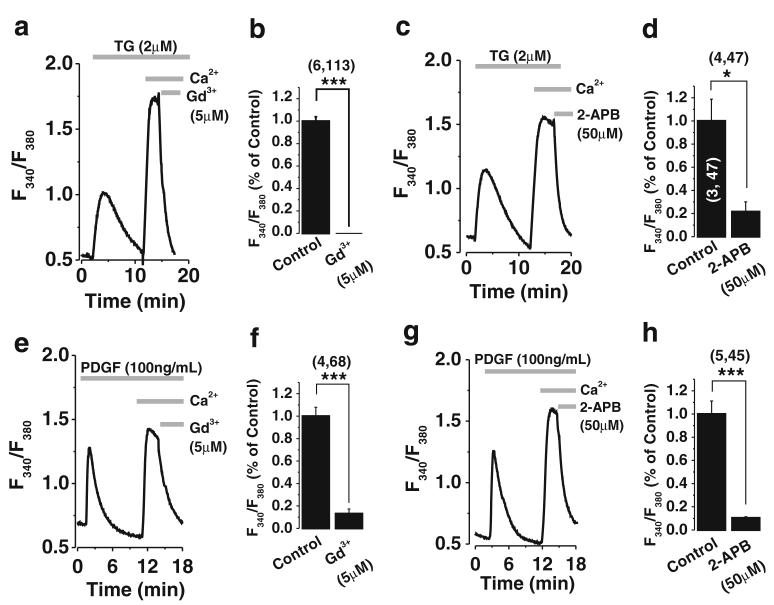
The increase in protein expression of the SOCE components, STIM1 and Orai1, in remodeled ASMC from asthmatic mice prompted us to undertake in vitro studies on migratory ASMC called “synthetic” (that are reminiscent of asthmatic airways) to determine whether PDGF, a potent remodeling, migratory, and proliferative smooth muscle agonist that is secreted by asthmatic airways, mediates Ca2+ entry and migratory signaling through STIM1 and Orai1. Before undertaking laborious knockdown studies, we sought to determine the pharmacological profile of PDGF-activated Ca2+ entry compared to classical SOCE activated by passive store depletion with thapsigargin (2 μM). Figure 2 shows that SOCE activated by thapsigargin in tracheobronchial ASMC (Fig. 2a-d) has an identical pharmacological profile to PDGF-activated Ca2+ entry in the same cells (Fig. 2e-h), namely inhibition by low concentrations of Gd3+ (5 μM; Fig. 2a, b, e, f) and 50 μM of the drug 2-APB (Fig. 2c, d, g, h).
Fig. 2.
PDGF-activated Ca2+ entry is pharmacologically identical to SOCE. Ca2+ signals were measured in rat ASMC using the ratiometric dye Fura2 in response to either 2 μM thapsigargin (TG; a, c) or 100 ng/ml PDGF (e, g) stimulation in nominally Ca2+-free bath solutions, followed by restoration of 2 mM Ca2+ to the bath, and subsequent addition of pharmacological inhibitors was indicated by the gray bars. The use of inhibitors known to inhibit SOCE 2-APB (50 μM) and Gd3+ (5 μM) inhibited thapsigargin-activated SOCE (a-d) as well as PDGF-activated Ca2+ entry (e-h). Traces represent averages from several cells assayed in a coverslip from a single experiment while bar graphs are averages of the total number of cells from several experiments. (x, y) next to bar graphs represent x=number of independent experiments and y=total number of cells
PDGF-activated Ca2+ entry in ASMC requires STIM1 and Orai1
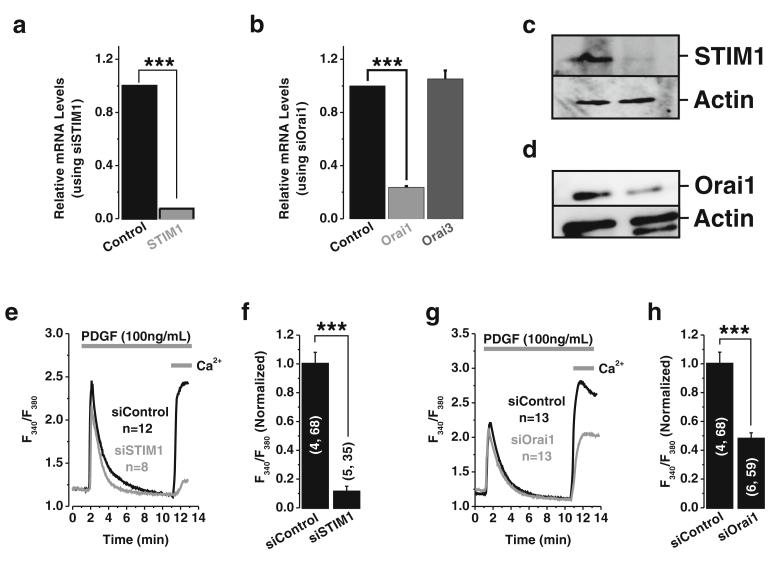
To firmly establish a role for STIM1 and Orai1 in PDGF-mediated Ca2+ entry in ASMC, we used siRNA to knockdown STIM1 and Orai1 proteins, Specific siRNA targeting STIM1 and Orai1 caused a substantial decrease in their respective mRNA (Fig. 3a, b). Furthermore, siRNA against Orai1 failed to affect the mRNA levels of Orai3, the Orai1 closest homologue (Fig. 3b). Western blotting on ASMC shows a substantial decrease in STIM1 and Orai1 protein levels upon transfection with their respective siRNA (Fig. 3c, d). However, similar to the data obtained when quantifying mRNA knockdown, the protein knockdown achieved with STIM1 siRNA was higher (~85 %) than that obtained with Orai1 siRNA (~55 %). To determine the effect of STIM1 and Orai1 knockdown on PDGF-activated Ca2+ entry, Ca2+ imaging was performed using a Fura2 protocol where Ca2+ release was assessed in nominally Ca2+-free buffer followed by restoration of 2 mM Ca2+ to the extracellular space to determine the extent of Ca2+ entry (Fig. 3e-h). STIM1 and Orai1 knockdown had no effect on Ca2+ release activated by PDGF but caused a significant reduction in Ca2+ entry (Fig. 3e-h). The effect of STIM1 knockdown on PDGF-activated Ca2+ entry is more evident (~89 % decrease in Ca2+ entry; Fig. 3f) compared to that of Orai1 knockdown (~53 % decrease in Ca2+ entry; Fig. 3h), consistent with levels of protein knockdown.
Fig. 3.
STIM1 and Orai1 mediate PDGF-activated Ca2+ entry in ASMC. SiRNA sequences targeting STIM1 or Orai1 were transfected into ASMCs, and their efficiency of knockdown was assessed by quantification of relative mRNA levels by qPCR, 96 h post transfection. Employment of siRNA targeting STIM1 and Orai1 significantly reduced STIM1 and Orai1 mRNA levels by ~93 and ~76 %, respectively, as shown in a and b. However, Orai3 mRNA levels were not changed when transfecting ASMC with Orai1 siRNA (b). The efficiency of protein knockdown was documented by Western blot analysis and is shown in c and d for STIM1 and Orai1, respectively. Representative Ca2+ imaging traces (e) and statistical analysis (f) from cells transfected with either control siRNA or STIM1 siRNA and stimulated with PDGF (100 ng/ml) showing abrogation of the PDGF-activated Ca2+ entry (from 1±0.08069 to 0.1128±0.03757) in cells transfected with STIM1 siRNA as compared to siRNA non-targeting control. Similarly, Orai1 knockdown significantly inhibited PDGF-activated Ca2+ entry (from 1±0.08069 to 0.47926±0.04198) as shown in g; statistical analysis for this experiment is also shown h. (x, y) next to each data bar: x=number of independent runs, y=total number of cells
PDGF activates CRAC currents in ASMC that are encoded by STIM1 and Orai1
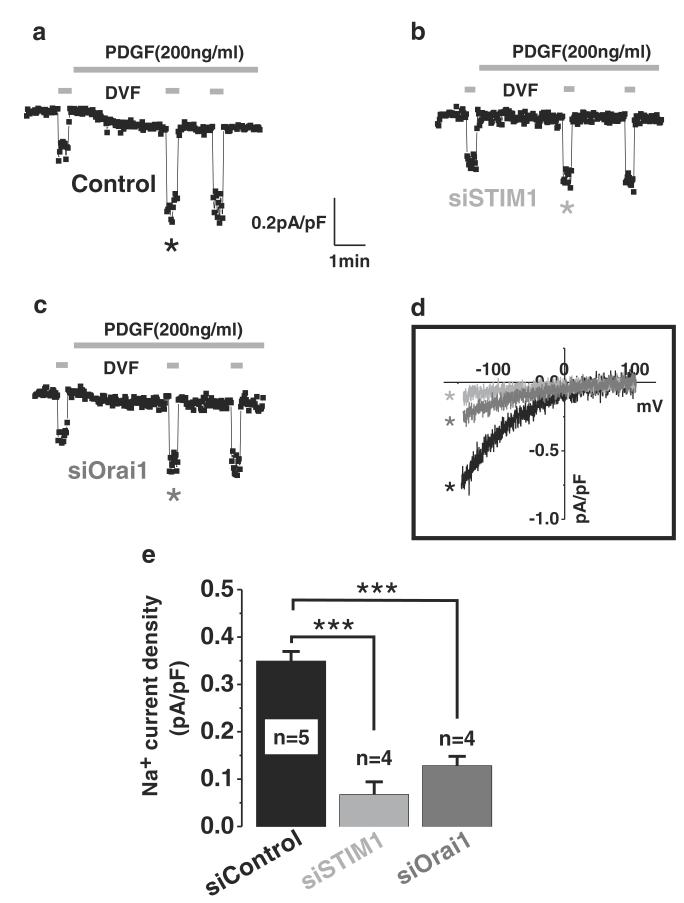
Patch clamp electrophysiology is the most direct and reliable means to assess ion channel function. Therefore, to confirm the Ca2+ imaging data, we performed whole-cell current recordings on ASMC stimulated with PDGF using a pipette solution where intracellular Ca2+ was buffered to 150 nM (see “Material and methods”). PDGF activates a small inwardly rectifying Ca2+ current that could be further amplified by the use of DVF bath solutions (Fig. 4a, d), Significantly, STIM1 or Orai1 knockdown using siRNA inhibited these PDGF-activated CRAC currents (Fig. 4b-e). Consistent with Ca2+ imaging and protein knockdown data, STIM1 siRNA caused a bigger decrease in CRAC currents (~82 % decrease) compared to Orai1 siRNA (~62 % decrease).
Fig. 4.
Whole-cell CRAC currents are activated by PDGF and encoded by STIM1 and Orai1 in ASMC. Whole-cell patch clamp electrophysiology in ASMCs transfected with either control siRNA or siRNA sequences targeting STIM1 or Orai1. STIM1 knockdown completely abrogated both Ca2+ and Na+ (assessed in DVF bath solutions) CRAC currents activated by PDGF (b) as compared to control (sicontrol 0.3494±0.02018 vs 0.06775± 0.02675 siSTIM1). Similarly, Orai1 knockdown (c) led to significant reduction of Ca2+ and Na+ CRAC currents activated by PDGF as compared to control (sicontrol 0.3494± 0.02018 vs 0.1285±0.01967 siOrai1). Na+ CRAC I/V relationships (d) show the requirement of STIM1 and Orai1 for PDGF-activated CRAC currents in ASMC. Statistical analyses are shown in e
STIM1 and Orai1 are required for PDGF-mediated ASMC chemotactic migration and proliferation
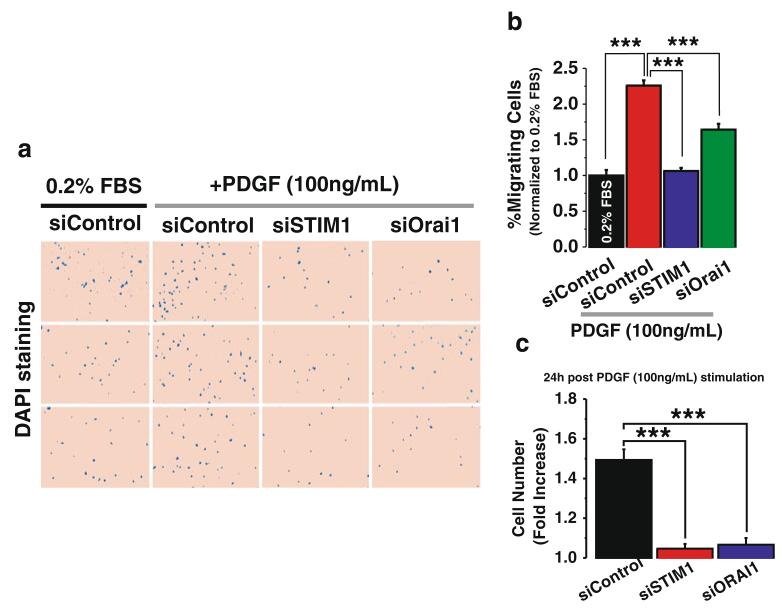
To determine the contribution of STIM1 and Orai1 to the migratory response of ASMC to PDGF, we performed Boyden chamber migration assays using PDGF as the chemotactic factor. Cells were maintained in a medium with low serum (0.2 %). Addition of PDGF to the lower chamber causes an increase in ASMC migration after 6 h, as visualized with staining of cell nuclei with DAPI (Fig. 5a; three random fields per condition are shown). Interestingly, either STIM1 or Orai1 knockdown caused a significant inhibition of the number of migrating ASMC (Fig. 5a, b). Data on the percentage of migrating cells (normalized to non-stimulated control) are shown in Fig. 5b; as noted for other assays described above, the effect of STIM1 siRNA on ASMC migration was more pronounced than that of Orai1 siRNA. ASMC proliferation was also assayed 3 days after transfection with STIM1 and Orai1 siRNA and 24 h post stimulation with 100 ng/ml PDGF (see “Material and methods”). Figure 5c shows fold induction of cell proliferation upon addition of PDGF relative to basal growth conditions (0.2 % FBS) for cells transfected with control siRNA (siControl), STIM1 siRNA (siSTIM1), and Orai1 siRNA (siOrai1).
Fig. 5.
STIM1 and Orai1 are required for chemotactic migration of ASMC in response to PDGF. Rat ASMC were transfected with either control siRNA or siRNA sequences targeting STIM1 and Orai1. Sixteen hours before migration experiments, cells were serum-starved in media containing 0.2 % FBS. Cells were then harvested with trypsin, and the role of STIM1 and Orai1 in PDGF-induced chemotactic cell migration was determined in a Boyden chamber. Migrating cells were fixed and stained with the nuclear stain DAPI. Representative fields from membranes assayed in triplicates are depicted (a). ASMC chemotactic cell migration was significantly increased in control cells stimulated with 100 ng/ml of PDGF (a). This increase in PDGF-induced cell migration was attenuated in ASMC transfected with either STIM1 siRNA or Orai1 siRNA (a). Quantification of these differences are depicted in b where total number of migrating cells per condition was quantified by analyzing 10 fields per membrane, in triplicates from two independent experiments. c Proliferation assay was performed as described in the “Material and methods.” Rat ASMC were transfected with either siSTIM1, siOrai1, or siControl. Twenty-four hours after PDGF stimulation, ASMC were detached and counted. Cell numbers (after 24 h with 100 ng/ml PDGF) were normalized to control cells under basal conditions (24 h in 0.2%FBS). Values represent fold induction per condition normalized to basal growth conditions (0.2 % FBS)
Discussion
Numerous studies have documented increased ASMC mass in asthma [2, 4, 16, 17, 22, 24, 30, 39, 50]. This pathophysiological remodeling of ASMC is a phenotypic change from a quiescent to a migratory and proliferative/hypertrophic phenotype [24, 50] and is the major cause of decreased pulmonary function in asthmatic patients [2, 4, 23, 43]. ASMC phenotypic modulation is of great clinical importance but still remains poorly understood. ASMC remodeling in asthma shares similarities with cardiomyocyte and VSMC remodeling that occurs in cardiac hypertrophy, atherosclerosis, restenosis, and hypertension, including changes in expression and/or activation of Ca2+ handling proteins, channels, and pumps [6, 12, 15, 18, 26, 31, 32, 36, 38, 39, 44, 55, 57, 59-61, 68-70]. However, the potential upregulation of STIM1 and Orai1 in ASMC during remodeling that occurs in asthma remained unknown.
Growth, humoral, and contractile factors that signal through receptor tyrosine kinase or G protein-coupled receptors play an important role in ASMC function through initiation of Ca2+ signaling [50]. However, in certain conditions, altered signaling through these receptors can contribute to disease. These agonists impact on Ca2+ signaling through activation of isoforms of PLC to cause Ca2+ release from the internal stores and Ca2+ entry via numerous pathways across the PM [8]. Agonist-regulated Ca2+ entry channels can be either store-dependent (SOC) activated as a consequence of the fall in ER Ca2+ content [54] or store-independent (non-SOC) activated by a wide range of second messengers [10]. PDGF is a potent migratory and proliferative growth factor [9] that acts via a receptor tyrosine kinase and couples to PLCγ activation and subsequent Ca2+ signaling. PDGF is a major contributor to smooth muscle remodeling; its production increases in asthmatic airways and directly correlates with the severity of asthma [21, 45, 58]. Nevertheless, the molecular components of PDGF-activated Ca2+ entry pathway in ASMC and the contribution of this Ca2+ entry pathway to ASMC migration remained unknown.
In this study, we show that tracheobronchial ASMC from asthmatic mice show increased protein expression of STIM1 and Orai1. To our knowledge, this is the first report to show upregulation of STIM1 and Orai1 in ASMC from asthmatic mice. We further show that cultured tracheobronchial ASMC mediates Ca2+ entry in response to PDGF via a pathway that pharmacologically resembles classical SOCE. This pathway is inhibited by low concentrations of lanthanides (5 μM Gd3+) and 2-APB (50 μM). PDGF activated inwardly rectifying highly Ca2+-selective CRAC currents in ASMC. Furthermore, we show that PDGF-activated Ca2+ entry and CRAC currents were significantly inhibited by knockdown of either STIM1 or Orai1.
The primary cultured tracheobronchial ASMC used in this study are migratory and proliferative cells compared with the quiescent freshly isolated ASMC. These cells that are referred to as synthetic ASMC are reminiscent of in vivo remodeling of ASMC during asthma. We further show that knockdown of STIM1 or Orai1 inhibits synthetic ASMC migration in vitro using Boyden chamber assays with PDGF as the chemotactic factor. These results suggest that STIM1 and Orai1 are likely contributing to in vivo PDGF-mediated migratory signaling during ASMC remodeling in asthma. Future studies are required to determine the contributions of STIM1 and Orai1 and their homologues (STIM2, Orai2, Orai3) to Ca2+ entry pathways activated by other growth factors/agonists with importance in ASMC remodeling and asthma. Additional studies on STIM1 and Orai1 smooth muscle-specific knockout mice will likely determine whether these molecules could represent good targets for the treatment of ASMC remodeling during asthma.
Acknowledgments
This work was mainly supported by grant HL097111 from NIH to MT and in part by NIH grants R01HL49426 to HAS and HL095566 to KM.
Footnotes
Amy Spinelli, José C. González-Cobos, Xuexin Zhang, and Rajender K. Motiani contributed equally to this work.
Contributor Information
Amy M. Spinelli, Center for Cardiovascular Sciences, Albany Medical College, Mail Code 8, 47 New Scotland Ave, Albany, NY 12208, USA
José C. González-Cobos, Center for Cardiovascular Sciences, Albany Medical College, Mail Code 8, 47 New Scotland Ave, Albany, NY 12208, USA
Xuexin Zhang, Center for Cardiovascular Sciences, Albany Medical College, Mail Code 8, 47 New Scotland Ave, Albany, NY 12208, USA.
Rajender K. Motiani, Center for Cardiovascular Sciences, Albany Medical College, Mail Code 8, 47 New Scotland Ave, Albany, NY 12208, USA
Sarah Rowan, Center for Cardiovascular Sciences, Albany Medical College, Mail Code 8, 47 New Scotland Ave, Albany, NY 12208, USA.
Wei Zhang, Center for Cardiovascular Sciences, Albany Medical College, Mail Code 8, 47 New Scotland Ave, Albany, NY 12208, USA.
Joshua Garrett, Center for Cardiovascular Sciences, Albany Medical College, Mail Code 8, 47 New Scotland Ave, Albany, NY 12208, USA.
Peter A. Vincent, Center for Cardiovascular Sciences, Albany Medical College, Mail Code 8, 47 New Scotland Ave, Albany, NY 12208, USA
Khalid Matrougui, Department of Physiology, Hypertension and Renal Center of Excellence, Tulane University, New Orleans, LA 70112, USA.
Harold A. Singer, Center for Cardiovascular Sciences, Albany Medical College, Mail Code 8, 47 New Scotland Ave, Albany, NY 12208, USA
Mohamed Trebak, Center for Cardiovascular Sciences, Albany Medical College, Mail Code 8, 47 New Scotland Ave, Albany, NY 12208, USA.
References
- 1.Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res. 2008;103(11):1289–1299. doi: 10.1161/01.RES.0000338496.95579.56. doi:10.1161/01.RES.0000338496.95579.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai TR. Evidence for airway remodeling in chronic asthma. Curr Opin Allergy Clin Immunol. 2010;10(1):82–86. doi: 10.1097/ACI.0b013e32833363b2. [DOI] [PubMed] [Google Scholar]
- 3.Beech DJ. Ion channel switching and activation in smoothmuscle cells of occlusive vascular diseases. Biochem Soc Trans. 2007;35(Pt 5):890–894. doi: 10.1042/BST0350890. doi:10.1042/BST0350890. [DOI] [PubMed] [Google Scholar]
- 4.Bentley JK, Hershenson MB. Airway smooth muscle growth in asthma: proliferation, hypertrophy, and migration. Proc Am Thorac Soc. 2008;5(1):89–96. doi: 10.1513/pats.200705-063VS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol Cell Physiol. 2008;295(3):C779–C790. doi: 10.1152/ajpcell.00173.2008. doi:10.1152/ajpcell.00173.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berridge MJ. Remodelling Ca2+ signalling systems and cardiac hypertrophy. Biochem Soc Trans. 2006;34(Pt 2):228–231. doi: 10.1042/BST20060228. [DOI] [PubMed] [Google Scholar]
- 7.Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta. 2009;1793:933–940. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Berridge MJ. Smooth muscle cell calcium activation mechanisms. J Physiol. 2008;586(Pt 21):5047–5061. doi: 10.1113/jphysiol.2008.160440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004;15(4):215–228. doi: 10.1016/j.cytogfr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Bird GS, Aziz O, Lievremont JP, Wedel BJ, Trebak M, Vazquez G, Putney JW., Jr Mechanisms of phospholipase C-regulated calcium entry. Curr Mol Med. 2004;4(3):291–301. doi: 10.2174/1566524043360681. [DOI] [PubMed] [Google Scholar]
- 11.Bisaillon JM, Motiani RK, Gonzalez-Cobos JC, Potier M, Halligan KE, Alzawahra WF, Barroso M, Singer HA, Jourd’heuil D, Trebak M. Essential role for STIM1/Orai1-mediated calcium influx in PDGF-induced smooth muscle migration. Am J Physiol Cell Physiol. 2010;298(5):C993–C1005. doi: 10.1152/ajpcell.00325.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bobe R, Hadri L, Lopez JJ, Sassi Y, Atassi F, Karakikes I, Liang L, Limon I, Lompre AM, Hatem SN, Hajjar RJ, Lipskaia L. SERCA2a controls the mode of agonist-induced intracellular Ca(2 +) signal, transcription factor NFAT and proliferation in human vascular smooth muscle cells. J Mol Cell Cardiol. 2011;50:621–33. doi: 10.1016/j.yjmcc.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broad LM, Cannon TR, Taylor CW. A non-capacitative pathway activated by arachidonic acid is the major Ca2+ entry mechanism in rat A7r5 smooth muscle cells stimulated with low concentrations of vasopressin. J Physiol. 1999;517(Pt 1):121–134. doi: 10.1111/j.1469-7793.1999.0121z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlin SM, Roth M, Black JL. Urokinase potentiates PDGF-induced chemotaxis of human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2003;284(6):L1020–L1026. doi: 10.1152/ajplung.00092.2002. [DOI] [PubMed] [Google Scholar]
- 15.Cheong A, Bingham AJ, Li J, Kumar B, Sukumar P, Munsch C, Buckley NJ, Neylon CB, Porter KE, Beech DJ, Wood IC. Downregulated REST transcription factor is a switch enablingcritical potassium channel expression and cell proliferation. Mol Cell. 2005;20(1):45–52. doi: 10.1016/j.molcel.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 16.Ebina M, Takahashi T, Chiba T, Motomiya M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma. A 3-D morphometric study. Am Rev Respir Dis. 1993;148(3):720–726. doi: 10.1164/ajrccm/148.3.720. [DOI] [PubMed] [Google Scholar]
- 17.Ebina M, Yaegashi H, Chiba R, Takahashi T, Motomiya M, Tanemura M. Hyperreactive site in the airway tree of asthmatic patients revealed by thickening of bronchial muscles. A morphometric study. Am Rev Respir Dis. 1990;141(5 Pt 1):1327–1332. doi: 10.1164/ajrccm/141.5_Pt_1.1327. [DOI] [PubMed] [Google Scholar]
- 18.Eder P, Molkentin JD. TRPC channels as effectors of cardiac hypertrophy. Circ Res. 2011;108(2):265–272. doi: 10.1161/CIRCRESAHA.110.225888. [DOI] [PubMed] [Google Scholar]
- 19.Edwards JN, Friedrich O, Cully TR, von Wegner F, Murphy RM, Launikonis BS. Upregulation of store-operated Ca2+ entry in dystrophic mdx mouse muscle. Am J Physiol Cell Physiol. 2010;299(1):C42–C50. doi: 10.1152/ajpcell.00524.2009. [DOI] [PubMed] [Google Scholar]
- 20.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441(7090):179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 21.Gabazza EC, Taguchi O, Tamaki S, Takeya H, Kobayashi H, Yasui H, Kobayashi T, Hataji O, Urano H, Zhou H, Suzuki K, Adachi Y. Thrombin in the airways of asthmatic patients. Lung. 1999;177(4):253–262. doi: 10.1007/pl00007645. [DOI] [PubMed] [Google Scholar]
- 22.Graham SJ, Black MJ, Soboloff J, Gill DL, Dziadek MA, Johnstone LS. Stim1, an endoplasmic reticulum Ca2+ sensor, negatively regulates 3 T3-L1 pre-adipocyte differentiation. Differentiation: Res Biol Divers. 2009;77(3):239–247. doi: 10.1016/j.diff.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halwani R, Al-Muhsen S, Hamid Q. Airway remodeling in asthma. Curr Opin Pharmacol. 2010;10(3):236–245. doi: 10.1016/j.coph.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Hirst SJ, Martin JG, Bonacci JV, Chan V, Fixman ED, Hamid QA, Herszberg B, Lavoie JP, McVicker CG, Moir LM, Nguyen TT, Peng Q, Ramos-Barbon D, Stewart AG. Proliferative aspects of airway smooth muscle. J Allergy Clin Immunol. 2004;114(2 Suppl):S2–S17. doi: 10.1016/j.jaci.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 25.Holtzman MJ, Byers DE, Benoit LA, Battaile JT, You Y, Agapov E, Park C, Grayson MH, Kim EY, Patel AC. Immune pathways for translating viral infection into chronic airway disease. Adv Immunol. 2009;102:245–276. doi: 10.1016/S0065-2776(09)01205-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.House SJ, Potier M, Bisaillon J, Singer HA, Trebak M. The non-excitable smooth muscle: calcium signaling and phenotypic switching during vascular disease. Pflugers Arch. 2008;456(5):769–785. doi: 10.1007/s00424-008-0491-8. doi:10.1007/s00424-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu Q, Zheng G, Zweier JL, Deshpande S, Irani K, Ziegelstein RC. NADPH oxidase activation increases the sensitivity of intracellular Ca2+ stores to inositol 1,4,5-trisphosphate in human endothelial cells. J Biol Chem. 2000;275(21):15749–15757. doi: 10.1074/jbc.M000381200. [DOI] [PubMed] [Google Scholar]
- 28.Ito I, Fixman ED, Asai K, Yoshida M, Gounni AS, Martin JG, Hamid Q. Platelet-derived growth factor and transforming growth factor-beta modulate the expression of matrix metalloproteinases and migratory function of human airway smooth muscle cells. Clin Exp Allergy. 2009;39(9):1370–1380. doi: 10.1111/j.1365-2222.2009.03293.x. [DOI] [PubMed] [Google Scholar]
- 29.James AL, Bai TR, Mauad T, Abramson MJ, Dolhnikoff M, McKay KO, Maxwell PS, Elliot JG, Green FH. Airway smooth muscle thickness in asthma is related to severity but not duration of asthma. Eur Respir J. 2009;34(5):1040–1045. doi: 10.1183/09031936.00181608. [DOI] [PubMed] [Google Scholar]
- 30.James AL, Pare PD, Hogg JC. The mechanics of airway narrowing in asthma. Am Rev Respir Dis. 1989;139(1):242–246. doi: 10.1164/ajrccm/139.1.242. [DOI] [PubMed] [Google Scholar]
- 31.Ju H, Scammel La, Fleur T, Dixon IM. Altered mRNA abundance of calcium transport genes in cardiac myocytes induced by angiotensin II. J Mol Cell Cardiol. 1996;28(5):1119–1128. doi: 10.1006/jmcc.1996.0103. [DOI] [PubMed] [Google Scholar]
- 32.Kumar B, Dreja K, Shah SS, Cheong A, Xu SZ, Sukumar P, Naylor J, Forte A, Cipollaro M, McHugh D, Kingston PA, Heagerty AM, Munsch CM, Bergdahl A, Hultgardh-Nilsson A, Gomez MF, Porter KE, Hellstrand P, Beech DJ. Upregulated TRPC1 channel in vascular injury in vivo and its role in human neointimal hyperplasia. Circ Res. 2006;98(4):557–563. doi: 10.1161/01.RES.0000204724.29685.db. doi:10.1161/01.RES.0000204724.29685.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leigh R, Ellis R, Wattie J, Southam DS, De Hoogh M, Gauldie J, O’Byrne PM, Inman MD. Dysfunction and remodeling of the mouse airway persist after resolution of acute allergeninduced airway inflammation. Am J Respir Cell Mol Biol. 2002;27(5):526–535. doi: 10.1165/rcmb.2002-0048OC. [DOI] [PubMed] [Google Scholar]
- 34.Leung SY, Eynott P, Noble A, Nath P, Chung KF. Resolution of allergic airways inflammation but persistence of airway smooth muscle proliferation after repeated allergen exposures. Clin Exp Allergy. 2004;34(2):213–220. doi: 10.1111/j.1365-2222.2004.01870.x. [DOI] [PubMed] [Google Scholar]
- 35.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+ -store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15(13):1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipskaia L, del Monte F, Capiod T, Yacoubi S, Hadri L, Hours M, Hajjar RJ, Lompre AM. Sarco/endoplasmic reticulum Ca2+ -ATPase gene transfer reduces vascular smooth muscle cell proliferation and neointima formation in the rat. Circ Res. 2005;97(5):488–495. doi: 10.1161/01.RES.0000180663.42594.aa. [DOI] [PubMed] [Google Scholar]
- 37.Locksley RM. Asthma and allergic inflammation. Cell. 2010;140(6):777–783. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahn K, Hirst SJ, Ying S, Holt MR, Lavender P, Ojo OO, Siew L, Simcock DE, McVicker CG, Kanabar V, Snetkov VA, O’Connor BJ, Karner C, Cousins DJ, Macedo P, Chung KF, Corrigan CJ, Ward JP, Lee TH. Diminished sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) expression contributes to airway remodelling in bronchial asthma. Proc Natl Acad Sci U S A. 2009;106(26):10775–10780. doi: 10.1073/pnas.0902295106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahn K, Ojo OO, Chadwick G, Aaronson PI, Ward JP, Lee TH. Ca(2+) homeostasis and structural and functional remodelling of airway smooth muscle in asthma. Thorax. 2010;65(6):547–552. doi: 10.1136/thx.2009.129296. [DOI] [PubMed] [Google Scholar]
- 40.McMillan SJ, Lloyd CM. Prolonged allergen challenge in mice leads to persistent airway remodelling. Clin Exp Allergy. 2004;34(3):497–507. doi: 10.1111/j.1365-2222.2004.01895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Motiani RK, Abdullaev IF, Trebak M. A novel native storeoperated calcium channel encoded by Orai3: selective requirement of Orai3 versus Orai1 in estrogen receptor-positive versus estrogen receptor-negative breast cancer cells. J Biol Chem. 2010;285(25):19173–19183. doi: 10.1074/jbc.M110.102582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muik M, Fahrner M, Derler I, Schindl R, Bergsmann J, Frischauf I, Groschner K, Romanin C. A cytosolic homomerization and a modulatory domain within STIM1 C-terminus determine coupling to ORAI1 channels. J Biol Chem. 2009;284:8421–8426. doi: 10.1074/jbc.C800229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy DM, O’Byrne PM. Recent advances in the patho-physiology of asthma. Chest. 2010;137(6):1417–1426. doi: 10.1378/chest.09-1895. [DOI] [PubMed] [Google Scholar]
- 44.Neylon CB, Lang RJ, Fu Y, Bobik A, Reinhart PH. Molecular cloning and characterization of the intermediate-conductance Ca(2+)-activated K(+) channel in vascular smooth muscle: relationship between K(Ca) channel diversity and smooth muscle cell function. Circ Res. 1999;85(9):e33–e43. doi: 10.1161/01.res.85.9.e33. [DOI] [PubMed] [Google Scholar]
- 45.Ohno I, Nitta Y, Yamauchi K, Hoshi H, Honma M, Woolley K, O’Byrne P, Dolovich J, Jordana M, Tamura G, et al. Eosinophils as a potential source of platelet-derived growth factor B-chain (PDGF-B) in nasal polyposis and bronchial asthma. Am J Respir Cell Mol Biol. 1995;13(6):639–647. doi: 10.1165/ajrcmb.13.6.7576701. [DOI] [PubMed] [Google Scholar]
- 46.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136(5):876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peel SE, Liu B, Hall IP. A key role for STIM1 in store operated calcium channel activation in airway smooth muscle. Respir Res. 2006;7:119. doi: 10.1186/1465-9921-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peel SE, Liu B, Hall IP. ORAI and store-operated calcium influx in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2008;38(6):744–749. doi: 10.1165/rcmb.2007-0395OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pepe C, Foley S, Shannon J, Lemiere C, Olivenstein R, Ernst P, Ludwig MS, Martin JG, Hamid Q. Differences in airwayremodeling between subjects with severe and moderate asthma. J Allergy Clin Immunol. 2005;116(3):544–549. doi: 10.1016/j.jaci.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 50.Perez-Zoghbi JF, Karner C, Ito S, Shepherd M, Alrashdan Y, Sanderson MJ. Ion channel regulation of intracellular calcium and airway smooth muscle function. Pulm Pharmacol Ther. 2009;22(5):388–397. doi: 10.1016/j.pupt.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, Trebak M. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J. 2009;23(8):2425–2437. doi: 10.1096/fj.09-131128. doi:10.1096/fj.09-131128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 53.Putney JW., Jr Capacitative calcium entry revisited. Cell Calcium. 1990;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- 54.Putney JW. Capacitative calcium entry: from concept to molecules. Immunol Rev. 2009;231(1):10–22. doi: 10.1111/j.1600-065X.2009.00810.x. [DOI] [PubMed] [Google Scholar]
- 55.Qi M, Puglisi JL, Byron KL, Ojamaa K, Klein I, Bers DM, Samarel AM. Myosin heavy chain gene expression in neonatal rat heart cells: effects of [Ca2+]i and contractile activity. Am J Physiol. 1997;273(2 Pt 1):C394–C403. doi: 10.1152/ajpcell.1997.273.2.C394. [DOI] [PubMed] [Google Scholar]
- 56.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169(3):435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shepherd MC, Duffy SM, Harris T, Cruse G, Schuliga M, Brightling CE, Neylon CB, Bradding P, Stewart AG. KCa3,1 Ca2+ activated K+channels regulate human airway smooth muscle proliferation. Am J Respir Cell Mol Biol. 2007;37(5):525–531. doi: 10.1165/rcmb.2006-0358OC. [DOI] [PubMed] [Google Scholar]
- 58.Shimizu S, Gabazza EC, Hayashi T, Ido M, Adachi Y, Suzuki K. Thrombin stimulates the expression of PDGF in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;279(3):L503–L510. doi: 10.1152/ajplung.2000.279.3.L503. [DOI] [PubMed] [Google Scholar]
- 59.Snetkov VA, Hirst SJ, Twort CH, Ward JP. Potassium currents in human freshly isolated bronchial smooth muscle cells. Br J Pharmacol. 1995;115(6):1117–1125. doi: 10.1111/j.1476-5381.1995.tb15926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Snetkov VA, Hirst SJ, Ward JP. Ion channels in freshly isolated and cultured human bronchial smooth muscle cells. Exp Physiol. 1996;81(5):791–804. doi: 10.1113/expphysiol.1996.sp003977. [DOI] [PubMed] [Google Scholar]
- 61.Tharp DL, Wamhoff BR, Turk JR, Bowles DK. Upregulation of intermediate-conductance Ca2+ -activated K + channel (IKCa1) mediates phenotypic modulation of coronary smooth muscle. Am J Physiol Heart Circ Physiol. 2006;291(5):H2493–H2503. doi: 10.1152/ajpheart.01254.2005. [DOI] [PubMed] [Google Scholar]
- 62.Tillie-Leblond I, de Blic J, Jaubert F, Wallaert B, Scheinmann P, Gosset P. Airway remodeling is correlated with obstruction in children with severe asthma. Allergy. 2008;63(5):533–541. doi: 10.1111/j.1398-9995.2008.01656.x. [DOI] [PubMed] [Google Scholar]
- 63.Trebak M, Bird GS, McKay RR, Putney JW., Jr Comparison of human TRPC3 channels in receptor-activated and storeoperated modes. Differential sensitivity to channel blockers suggests fundamental differences in channel composition. J Biol Chem. 2002;277(24):21617–21623. doi: 10.1074/jbc.M202549200. doi:10.1074/jbc.M202549200. [DOI] [PubMed] [Google Scholar]
- 64.Trebak M, Lemonnier L, Dehaven WI, Wedel BJ, Bird GS, Putney JW., Jr Complex functions of phosphatidylinositol 4,5-bisphosphate in regulation of TRPC5 cation channels. Pflugers Arch. 2009;457(4):757–769. doi: 10.1007/s00424-008-0550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trebak M, St JBG, McKay RR, Birnbaumer L, Putney JW., Jr Signaling mechanism for receptor-activated canonical transient receptor potential 3 (TRPC3) channels. J Biol Chem. 2003;278(18):16244–16252. doi: 10.1074/jbc.M300544200. doi:10.1074/jbc.M300544200. [DOI] [PubMed] [Google Scholar]
- 66.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science (New York, NY) 2006;312(5777):1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walter MJ, Holtzman MJ. A centennial history of research on asthma pathogenesis. Am J Respir Cell Mol Biol. 2005;32(6):483–489. doi: 10.1165/rcmb.F300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu X, Eder P, Chang B, Molkentin JD. TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc Natl Acad Sci USA. 2010;107(15):7000–7005. doi: 10.1073/pnas.1001825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiao JH, Zheng YM, Liao B, Wang YX. Functional role of TRPC1 and TRPC3 in normal and asthmatic airway smooth muscle. Am J Respir Cell Mol Biol. 2010;43:17–25. doi: 10.1165/rcmb.2009-0091OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA. 2004;101(38):13861–13866. doi: 10.1073/pnas.0405908101. doi:10.1073/pnas.0405908101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11(3):337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc Natl Acad Sci USA. 2006;103(24):9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang W, Halligan KE, Zhang X, Bisaillon JM, Gonzalez-Cobos JC, Motiani RK, Hu G, Vincent PA, Zhou J, Barroso M, Singer HA, Matrougui K, Trebak M. Orai1-mediated I (CRAC) is essential for neointima formation after vascular injury. Circ Res. 2011;109(5):534–542. doi: 10.1161/CIRCRESAHA.111.246777. [DOI] [PMC free article] [PubMed] [Google Scholar]