Abstract
Background
In Germany at present, 64% of women and 59% of men who receive a diagnosis of cancer are still alive five years later. 45% of men and 57% of women with cancer are still of working age. Cancer can markedly harm their ability to work.
Methods
We analyzed data from selected publications to calculate the percentage of cancer patients in Germany who are now returning to work.
Results
The efficacy of oncological rehabilitation has not been demonstrated by a randomized controlled trial, nor is it clear whether the existing studies have accounted for potentially confounding variables. A combined assessment of reports from various countries reveals that 63% of cancer patients who are of working age go back to work after being unable to work for an average of five months. The situation varies markedly across countries: In Germany, the percentage of women with breast cancer who return to work is only 59%, compared to 80% in the USA and 82% in the United Kingdom. Younger and better educated patients are more likely to return to work, as are those who have received less invasive treatment with fewer complications.
Conclusion
Most cancer patients of working age go back to work, but the percentages vary widely from one country to another, perhaps reflecting differences in social systems.
Along with cardiovascular disease, malignant tumors are by a long way the most frequent cause of death in Germany. From 1980 to 2006, the number of new cases of cancer went up by 35% in women and more than 80% in men. Every other man and 43% of women must expect to develop cancer at some point in their lives (1). This trend is likely to continue in 2012. In 2007, a total of 459 000 men and women developed cancer; in 2012 the figure will be over 486 000 (1).
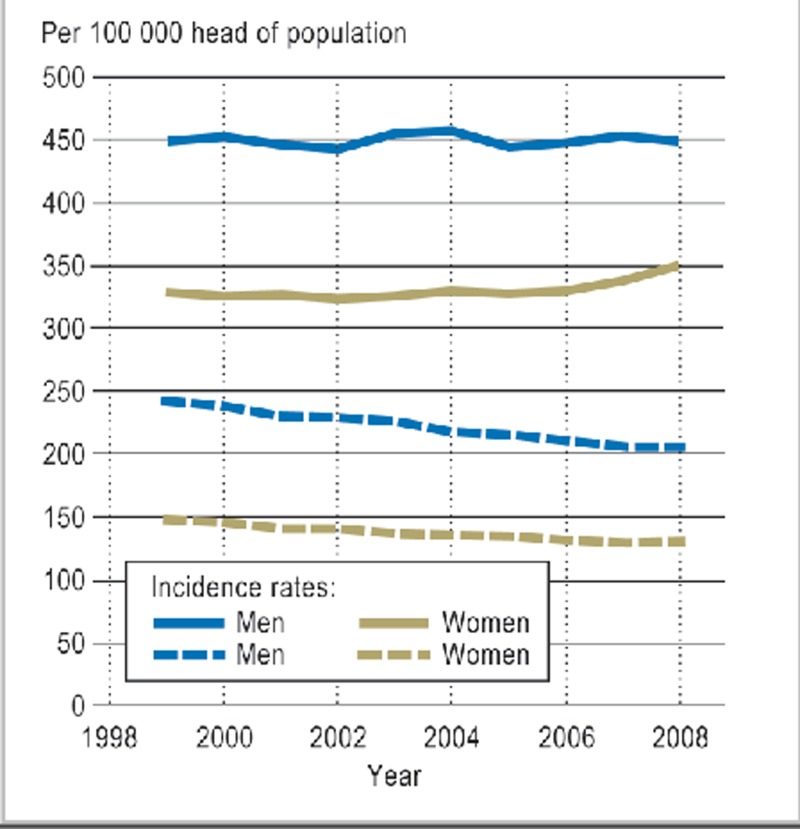
The mean age at diagnosis among both men and women is 69 years, but 45% of cancers in men and 57% of those in women occur before the age of 65 (1). Although the absolute rate of new cases of cancer has increased markedly in the past few decades, age-adjusted mortality in the past 30 years has gone down (Figure 1). The drop in mortality is not sex-specific, but shows the greatest effect among patients below the age of 65—those still of working age (1).
Figure 1.
Age-adjusted rates for incidence of cancer and mortality according to sex in Germany, 1999 to 2008 (1)
The corollary of falling cancer mortality is an increase in rates of cure or an increase in overall survival time in terms of all cancer cases. Among women, the 5-year survival rate across all tumor entities is currently 64%, and the rate among men is 59% (1). Assuming that this trend continues, the rates of cure will continue to improve, especially among people of working age, and thus measures to reintegrate them into the workplace will become increasingly important. In addition, the increase in number of lifetime working years, and the raising of the retirement age to 67, together with the shortage of skilled workers that is already becoming apparent, will exert further pressure for measures to retain cancer patients’ ability to work.
Methods
This review article is based on a selective literature search on PubMed that aimed to establish the percentage of cancer patients who return to work. Factors shown in the literature to either promote or impede returning to work were also analyzed.
The literature search included original articles in the form of retrospective analyses, meta-analyses, and reviews published in the past 10 years, which either related to several types of cancer or evaluated particular tumor entities. No information regarding correction for confounding variables was given in the publications studied.
Returning to work after cancer
A meta-analysis of 36 studies—particularly from the USA and Europe, as well as some other countries—compared 20 366 long-term survivors of cancer with 157 603 healthy people in terms of occupation, without matching. While 15.2% of the healthy probands were unemployed, the unemployment rate among the long-term survivors was 33.8%, corresponding to a relative risk of 1.37 (95% confidence interval [95% CI]: 1.21 to 1.55) (2). This resulted in tumor patients’ having a 37% higher risk of unemployment than healthy probands.
In addition to this, another large meta-analysis recently showed that almost two-thirds of all tumor patients of working age (63.5%; range: 24% to 94%) can return to their previous occupation or another one (3). This meta-analysis included 64 studies published between January 2000 and November 2009. The mean duration of inability to work was 5 months. Between 26% and 53% of patients lost their job or gave up work in the 6 years after diagnosis. By comparison to healthy probands, many tumor patients changed jobs, worked a reduced number of hours, and experienced a drop in pay (3).
The percentages of patients who return to work varies in the different social systems. To allow comparisons, these are shown for breast cancer (Table 1) (4– 11). The figures show Germany (59%) at the tail end with South Korea (58%) (4, 9). There are no confirmed data relating to this fact. The most likely reason is that the German social system makes it possible to make special allowance for the health sequelae of cancer, with up to 78 weeks sick pay from the employer, sickness benefit provided by health insurance, and a partial or complete disability pension. These options do not exist for many cancer patients in other countries, and therefore financial pressures probably lead to their returning to work early.
Table 1. Percentage of breast cancer patients who return to work: an international comparison.
| Country | Patients (n) | Return to work (%)* | Reference |
| South Korea | 1594 | 58 | (4) |
| USA | 443 | 80 | (5) |
| Canada | 646 | 79 | (6) |
| Norway | n.d. | 74 | (7) |
| Netherlands | 514 | 71 | (8) |
| Germany | 446 | 59 | (9) |
| France | 586 | 79 | (10) |
| United Kingdom | 267, of whom 127 had breast cancer (40%) | 82 | (11) |
*Percentage of patients who have started working again; n.d., no data
Prognostic factors that promote or impede the return to working life are shown in Table 2. This makes clear that it is not only tumor type, cancer therapy, and the resulting physical and mental impairments that play a part—sociodemographic and work-related factors do too (4, 11– 22). In terms of the percentage of patients who return to work, there are no significant differences between patients with testicular tumors or malignant melanoma and non-tumor patients (7).
Table 2. Factors influencing the return to work.
| Promoting factors | Reference | Impeding factors | Reference |
| Younger age | (12) | Endocrine therapy | (16) |
| Higher educational level | (13) | Extensive surgery | (4) |
| No surgery | (11) | Advanced tumor stage | (4, 16, 17) |
| No absence from work due to illness | (11) | Chemo- and radiotherapy | (18, 23) |
| Few physical symptoms | (14) | Advanced age | (18– 20) |
| Male sex | (12) | Female sex | (19, 21) |
| Continuity of care | (15) | Lower educational level | (18, 21) |
| Urological tumors | (12) | Liver cancer, bronchial cancer, hematological cancer, CNS tumors, GI tumors, pancreatic cancer | (22) |
| Melanoma, Hodgkin lymphoma | (12) | Head and neck tumors, gynecological tumors | (12) |
CNS, central nervous system; GI, gastrointestinal tract
The immediate conditions in the workplace and the support provided for the return to work are a crucial promoting factor. The cancer survivor is more likely to be permanently able to work if he or she is integrated into the social network at the workplace, the employer ensures working conditions that are beneficial to his or her health, and professional help is offered in the return to working life, than if these factors are absent or even their opposite is true (5, 15, 24– 27).
Although more than 90% of patients report being able to compensate well for their limitations in respect of their work, in Norway 31% work with reduced physical and 23% with reduced mental function (28). It is noteworthy that, according to Short et al., around 73% of patients in the USA are back at work as early as the first year after completing their tumor therapy; by the fourth year after tumor diagnosis, the percentage has gone up to 84% (29).
For cancer survivors, the effects of unemployment are considerable. Loss of employment often leads to reduced quality of life, reduced self-respect, and financial problems (30).
Measures promoting a return to work
Medical cancer rehabilitation
In Germany, medical rehabilitation is an integral part of the social security system, and is laid down in law (31). This gives the patients concerned a legal right to an inpatient or outpatient rehabilitation program usually lasting 3 weeks (32). Medical rehabilitation in Germany is thus fundamentally different from the systems in other European countries (33).
Medical rehabilitation in Germany is largely paid for by the statutory pension insurance scheme (Deutsche Rentenversicherung, DRV) and by the health insurance companies. In 2010, 170 662 patients went through an inpatient or outpatient cancer rehabilitation program paid for by the DRV. This equates to 18% of all rehabilitation programs, putting cancer rehabilitation in third place among other programs such as rehabilitation due to psychological disorders and orthopedic conditions. Out of all cancer rehabilitation measures, 44% were carried out in people of working age, and 2% of these were in outpatient rehabilitation. In 2007, cancer rehabilitation measures were approved for 61 902 insurees with the objective of enabling them to return to work.
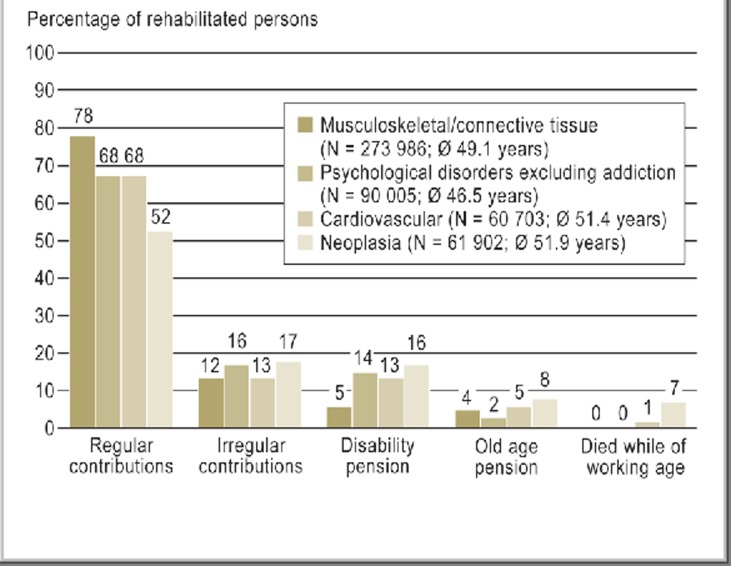
Out of this group, 52% were once again able to pay their statutory pension contributions regularly because they were in work, and 17% of patients made irregular contributions. Sixteen percent of the patient received disability pension (Figure 2) (34). The most frequent users of cancer rehabilitation were breast cancer patients (26%). In second place were those with malignancies of the digestive organs (19%), followed by malignant tumors of the male genital organs (18%). Most of the cases in the last group were men with prostate cancer (34).
Figure 2.
Two-year statutory pension insurance data on patients in various diagnostic groups who underwent medical rehabilitation in 2007 (34)
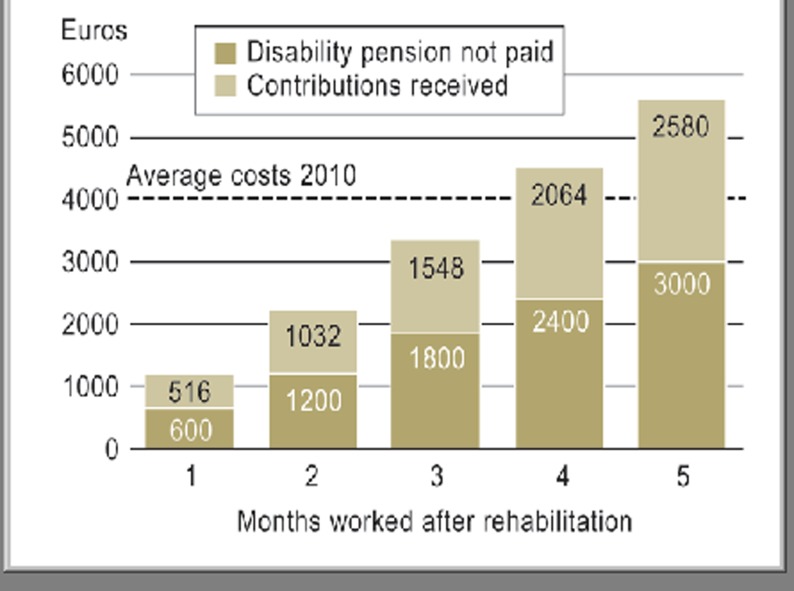
For the DRV, successful rehabilitation into working life is the main criterion of success (the slogan is “Reha vor Rente,” rehab before pension) (35). Financially, the DRV comes out ahead if successful rehabilitation can postpone early retirement on grounds of disability by at least 4 months (Figure 3) (34). At present there are no independent studies outside the DRV’s own dataset on this subject.
Figure 3.
Amortization model of medical rehabilitation (34)
Just 4 months longer at work, or 4 months’ delay in early retirement on grounds of disability, are enough to balance out the costs of rehabilitation with the savings in disability pensions and the contributions received. Fictitious model calculation based on data on rehabilitated persons in 2010
Before starting a cancer rehabilitation program, it must be tested whether a need for rehabilitation exists. Examples of the criteria by which this is decided are shown in Table 3. According to § 8 of the Rehabilitation Guideline of the Joint Federal Committee (Gemeinsamer Bundesausschuss), a need for rehabilitation exists “when, for reasons of physical, mental, or psychological impairment, restrictions of everyday activity exist that are probably not temporary, which may be expected to restrict the ability to work in the foreseeable future, or already restrict it, and which require multidimensional and interdisciplinary medical rehabilitation in addition to curative care. Such restrictions include the need for nursing care.”
Table 3. Treatment-related functional impairments that substantiate a need for rehabilitation (selected examples).
| Acute medical therapy | Impairment of function |
| General | |
|
|
| Surgery | |
| Breast cancer |
|
| Gastrointestinal tract |
|
| Urological tumors |
|
| Bronchial carcinoma |
|
| Radiotherapy | |
|
|
| Chemotherapy | |
|
|
In terms of the therapeutic principles of cancer rehabilitation, identifying and treating the sequelae of neoplastic disease and/or its treatment are to the fore. Only when a need for rehabilitation has been determined can rehabilitation goals be formulated and rehabilitation therapy be initiated. The rehabilitation program attempts to train and motivate patients until they are able to continue what they have learned (e.g., movement therapy, nutritional behavior, etc.) in their home environment (sustainable rehabilitation). The aim is to re-establish social integration and thus the ability to return to working and social life (36– 38).
So far, the effectiveness of cancer rehabilitation has not been proven in either national or international randomized studies. This is largely due to the fact that the rehabilitation of cancer patients differs according to public health systems around the world, and is carried out in different ways (33). While in Germany a 3-week inpatient rehabilitation program is usually the norm, in Scandinavia and the Netherlands, rehabilitation is usually on an outpatient basis in the form of long-term care provided by a multiprofessional rehabilitation team (33, 39). In the USA, likewise, outpatient rehabilitation facilities with a small inpatient capacity have developed, mostly at the national tumor centers (40). In a North American analysis of 427 patients given rehabilitation care at the M. D. Anderson Cancer Center, the mean inpatient stay was 11 days, and 76% of patients were discharged to outpatient rehabilitation (e1). In one North American and three German uncontrolled studies, positive effects of inpatient cancer rehabilitation were shown in terms of general quality of life (e2– e5). In addition, a self-evaluation questionnaire survey of 883 tumor patients showed a significant improvement in physical and psychological status, whereas functional status remained unchanged (39).
One area in which there is good evidence strongly relevant to cancer rehabilitation is movement therapy. A systematic review of randomized studies showed that controlled physical activities adapted to patients’ abilities can significantly improve patients’ cardiovascular fitness and physical performance after chemotherapy (e6). In addition, for patients with colorectal, prostate, or breast cancer, it was shown that a program of movement therapy reduced tumor-specific mortality (e7– e9).
Because of the positive correlation between nutritional behavior and risk of recurrence, this area is particularly important in the educative component of cancer rehabilitation. For both patients with colon cancer and those with breast cancer, cohort studies have shown that a diet rich in animal fats significantly increases the risk of recurrence (e10, e11).
The use of physiotherapy in cancer rehabilitation is another area for which there are no published randomized studies, so the evidence for it is low. However, uncontrolled studies have been cited that show an improvement in respiratory function after lung surgery or optimization of shoulder mobility after breast surgery or neck dissection (e12– e15).
In the same way there is only limited evidence to support “psycho-oncologic” therapy. In two observation studies a reduction in anxiety and depression was shown, both immediately after cancer rehabilitation and six months later (e16, e17). An important reason for psycho-oncologic treatment for depression during rehabilitation is that a meta-analysis found a statistically significant correlation between depression, tumor progression, and mortality (e18).
In view of the data situation described above, the components of cancer rehabilitation in Germany today are a program of training in dealing with the sequelae of tumors and/or treatment, and in changing prognostically unfavorable lifestyles (e.g., nicotine and alcohol consumption, little physical activity, a fat-rich diet, psychological stress). These are an adjunct to the active therapy elements in the sequential rehabilitation of physical function (e19). To these, in medical therapy for work-oriented rehabilitation for patients of working age, are added counseling using, for example, the diagnostic instrument AVEM (Arbeitsbezogenes Verhaltens- und Erlebensmuster, Work-Related Behavior and Perception Patterns), and a structured work-oriented training program (e20).
Instruments of occupational rehabilitation
In cases where cancer rehabilitation cannot entirely achieve the desired results, other measures promoting the return to work come into play. These measures may be listed as:
Financial support for the employer
Help in adapting a vehicle (e.g., aids to getting in and out, automatic gearbox, gear shift on the steering wheel)
Support for vocational training and preparation
Measures to help the cancer survivor keep or get employment.
In 2010, 40% of all measures promoting a return to work were for the purpose of helping cancer survivors keep or get a job, while support for vocational training made up 22%. Six years earlier, the ratio was 24% to 30%, which shows that the DRV is increasingly following the goal of maintaining existing jobs using the instruments of occupational rehabilitation (e21).
Gradual reintegration
One long-established, successful instrument is gradual reintegration (stufenweise Wiedereingliederung, STW) (e22). The purpose of this is to take patients after long, serious illness and guide them gradually back to full-time work at their previous job and reintegrate them there.
In cancer rehabilitation, it is important to tell patients who still have a job, and who will on medical grounds be able to return to it within the foreseeable future, about the gradual reintegration program. Taking only these patients into account, a survey of insurees showed that a total of 86% of insurees opt for gradual reintegration when it is recommended to them. Careful selection of candidates for gradual reintegration usually reaches those patients who themselves express a desire for gradual reintegration.
In addition, the attitude of the employer plays an important part in the return to work. If the employer supports it, the probability of a successful return to work increases markedly. The main reasons given by patients for not taking up the offer of gradual reintegration include:
Health reasons
Lack of (personal) motivation
Logistic obstacles relating to work (e.g., a long commute)
Lack of support from the employer for gradual reintegration (e22, e23).
However, there are no data from controlled randomized studies.
Summary
On the basis of the current literature, it can be postulated that more than half of all patients with breast cancer in Germany return to work after their illness. This percentage is quite low in comparison to other countries. The most likely reason is that in Germany there is good financial support from the state for those who are unable to work.
Whether and to what extent a patient is able to return to work, and in what form, depend on many factors relating to both the disease and its treatment. Commitment on the part of the employer in making use of measures supporting the return to work, and the personal motivation of the patient as regards continuing to work, are also important.
Supporting measures to assist rehabilitation after cancer can be significant for the return to work. This assumption, however, is still unsupported by high-quality studies. Measures supporting a return to work, which should be initiated before the end of a cancer rehabilitation program, can also be useful elements promoting reintegration into working life. This is another point on which the evidence base is poor. In our opinion, therefore, the current rehabilitation measures available to promote the return to social and working life need to be more intensively evaluated, their effectiveness needs to be underpinned by prospective randomized studies, and further educative and psychosocial care leading on from cancer rehabilitation should be established.
Key Messages.
More than half of cancer patients in Germany return to work after their illness.
The percentage of patients who return to work is much lower in Germany than in other countries.
Returning to work is conditioned by individual factors as well as those related to disease and treatment, and could be influenced by medical and vocational rehabilitation measures.
Cancer rehabilitation could lead to an improvement in the numbers returning to social and working life.
Scientific evaluation of cancer rehabilitation needs to be intensified, as do social security measures promoting the return to work.
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
Footnotes
Conflict of interest statement
Dr. Korsukewitz was and Dr. Seifart is an employee of the German statutory pension insurance scheme (Deutsche Rentenversicherung, DRV), which pays for most of the cancer rehabilitation in Germany.
Both authors are employees of rehabilitation facilities.
References
- 1.Robert-Koch-Institut, Deutschland GEKID Krebs in Deutschland 2007-2008. Häufigkeiten und Trends. Gesundheitsberichterstattung des Bundes. 8th revised edition 2012. [Google Scholar]
- 2.de Boer AG, Taskila T, Ojajärvi A, van Dijk FJ, Verbeek JH. Cancer survivors and unemployment: a meta-analysis and meta-regression. JAMA. 2009;301:753–762. doi: 10.1001/jama.2009.187. [DOI] [PubMed] [Google Scholar]
- 3.Mehnert A. Employment and work-related issues in cancer survivors. Crit Rev Oncol Hematol. 2011;77:109–130. doi: 10.1016/j.critrevonc.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Ahn E, Cho J, Shin DW, Park BW, Ahn SH, Noh DY, Nam SJ, Lee ES, Yun YH. Impact of breast cancer diagnosis and treatment on work-related life and factors affecting them. Breast Cancer Res Treat. 2009;116:609–616. doi: 10.1007/s10549-008-0209-9. [DOI] [PubMed] [Google Scholar]
- 5.Bouknight RR, Bradley CJ, Luo Z. Correlates of return to work for breast cancer survivors. J Clin Oncol. 2006;24:345–353. doi: 10.1200/JCO.2004.00.4929. [DOI] [PubMed] [Google Scholar]
- 6.Drolet M, Maunsell E, Brisson J, Brisson C, Mâsse B, Deschênes L. Not working 3 years after breast cancer: predictors in a population-based study. J Clin Oncol. 2005;23:8305–8312. doi: 10.1200/JCO.2005.09.500. [DOI] [PubMed] [Google Scholar]
- 7.Syse A, Tretli S, Kravdal Ø. Cancer’s impact on employment and earnings-a population-based study from Norway. J Cancer Surviv. 2008;2:149–158. doi: 10.1007/s11764-008-0053-2. [DOI] [PubMed] [Google Scholar]
- 8.Roelen CA, Koopmans PC, Groothoff JW, van der Klink JJ, Bültmann U. Return to work after cancer diagnosed in 2002, 2005 and 2008. J Occup Rehabil. 2011;21:335–341. doi: 10.1007/s10926-011-9319-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehnert A, Koch U. Predictors of employment among cancer survivors after medical rehabilitation—a prospective study. Scand J Work Environ Health. 2012 doi: 10.5271/sjweh.3291. online first article; doi: 10.5271/sjweh.3291. [DOI] [PubMed] [Google Scholar]
- 10.Peugniez C, Fantoni S, Leroyer A, Skrzypczak J, Duprey M, Bonneterre J. Return to work after treatment for breast cancer: single center experience in a cohort of 273 patients. Ann Oncol. 2010;21:2124–2125. doi: 10.1093/annonc/mdq556. [DOI] [PubMed] [Google Scholar]
- 11.Amir Z, Moran T, Walsh L, Iddenden R, Luker K. Return to paid work after cancer: A British experience. Cancer Surviv. 2007;1:129–136. doi: 10.1007/s11764-007-0021-2. [DOI] [PubMed] [Google Scholar]
- 12.Schultz PN, Beck ML, Stava C, Sellin RV. Cancer survivors work related issues. AAOHN J. 2002;50:220–226. [PubMed] [Google Scholar]
- 13.Taskila-Brandt T, Martikainen R, Virtanen SV, Pukkala E, Hietanen P, Lindbohm ML. The impact of education and occupation on the employment status of cancer survivors. Eur J Cancer. 2004;40:2488–2493. doi: 10.1016/j.ejca.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 14.Pryce J, Munir F, Haslam C. Cancer survivorship and work: symptoms, supervisor response, co-worker disclosure and work adjustment. J Occup Rehabil. 2007;17:83–92. doi: 10.1007/s10926-006-9040-5. [DOI] [PubMed] [Google Scholar]
- 15.Verbeek J, Spelten E, Kammeijer M, Sprangers M. Return to work of cancer survivors: a prospective cohort study into the quality of rehabilitation by occupational physicians. Occup Environ Med. 2003;60:352–357. doi: 10.1136/oem.60.5.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnsson A, Fornander T, Olsson M, Nystedt M, Johansson H, Rutqvist LE. Factors associated with return to work after breast cancer treatment. Acta Oncol. 2007;46:90–96. doi: 10.1080/02841860600857318. [DOI] [PubMed] [Google Scholar]
- 17.Bouknight RR, Bradley CJ, Luo Z. Correlates of return to work for breast cancer survivors. J Clin Oncol. 2006;24:345–353. doi: 10.1200/JCO.2004.00.4929. [DOI] [PubMed] [Google Scholar]
- 18.Fantoni SQ, Peugniez C, Duhamel A, Skrzypczak J, Frimat P, Leroyer A. Factors related to return to work by women with breast cancer in northern France. J Occup Rehabil. 2010;20:49–58. doi: 10.1007/s10926-009-9215-y. [DOI] [PubMed] [Google Scholar]
- 19.Lee MK, Lee KM, Bae JM, Kim S, Kim YW, Ryu KW, Lee JH, Noh JH, Sohn TS, Hong SK, Yun YH. Employment status and work-related difficulties in stomach cancer survivors compared with the general population. Br J Cancer. 2008;98:708–715. doi: 10.1038/sj.bjc.6604236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlsen K, Dalton SO, Diderichsen F, Johansen C. Danish Cohort Study. Risk for unemployment of cancer survivors: a Danish cohort study. Eur J Cancer. 2008;44:1866–1874. doi: 10.1016/j.ejca.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Chan F, Strauser D, da Silva Cardoso E, Xi Zheng L, Chan JY, Feuerstein M. State vocational services and employment in cancer survivors. J Cancer Surviv. 2008;2:169–178. doi: 10.1007/s11764-008-0057-y. [DOI] [PubMed] [Google Scholar]
- 22.Park JH, Park EC, Park JH, Kim SG, Lee SY. Job loss and re-employment of cancer patients in Korean employees: a nationwide retrospective cohort study. J Clin Oncol. 2008;26:1302–1309. doi: 10.1200/JCO.2007.14.2984. [DOI] [PubMed] [Google Scholar]
- 23.de Boer AG, Verbeek JH, Spelten ER, Uitterhoeve AL, Ansink AC, de Reijke TM, et al. Work ability and return-to-work in cancer patients. Br J Cancer. 2008;98:1342–1347. doi: 10.1038/sj.bjc.6604302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taskila T, Martikainen R, Hietanen P, Lindbohm ML. Comparative study of work ability between cancer survivors and their referents. Eur J Cancer. 2007;43:914–920. doi: 10.1016/j.ejca.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Taskila T, Lindbohm ML, Martikainen R, Lehto US, Hakanen J, Hietanen P. Cancer survivors’ received and needed social support from their work place and the occupational health services. Support Care Cancer. 2006;14:427–435. doi: 10.1007/s00520-005-0005-6. [DOI] [PubMed] [Google Scholar]
- 26.Nieuwenhuijsen K, Bos-Ransdorp B, Uitterhoeve LL, Sprangers MA, Verbeek JH. Enhanced provider communication and patient education regarding return to work in cancer survivors following curative treatment: a pilot study. J Occup Rehabil. 2006;16:647–657. doi: 10.1007/s10926-006-9057-9. [DOI] [PubMed] [Google Scholar]
- 27.Böttcher HM, Steimann M, Koch U, Bergelt C. Rückkehr zur Arbeit - Erfahrungen und Erwartungen von Patientinnen und Patienten in der stationären onkologischen Rehabilitation. Die Rehabilitation. 2012;51:31–38. doi: 10.1055/s-0031-1285888. [DOI] [PubMed] [Google Scholar]
- 28.Torp S, Nielsen RA, Gudbergsson SB, Dahl AA. Worksite adjustments and work ability among employed cancer survivors. Support Care Cancer. 2012;20:2149–2156. doi: 10.1007/s00520-011-1325-3. [DOI] [PubMed] [Google Scholar]
- 29.Short PF, Vasey JJ, Tuncelli K. Employment pathways in a large cohort of adult cancer survivors. Cancer. 2005;103:1292–1301. doi: 10.1002/cncr.20912. [DOI] [PubMed] [Google Scholar]
- 30.Lauzier S, Maunsell E, Drolet M, Coyle D, Hébert-Croteau N, Brisson J, et al. Wage losses in the year after breast cancer: extent and determinants among Canadian women. J Natl Cancer Inst. 2008;100:321–332. doi: 10.1093/jnci/djn028. [DOI] [PubMed] [Google Scholar]
- 31.Gerdes N, Zwingmann C, Jäckel WH Research in rehabilitation. Results from a research network in southwest Germany. Stuttgart: Schattauer Verlag; 2006. The system of rehabilitation in Germany; pp. 3–19. [Google Scholar]
- 32.Bengel J, Herwig JE, Koch U Research in rehabilitation. Results from a research network in southwest Germany. Stuttgart: Schattauer Verlag; 2006. Research in rehabilitation in Germany; pp. 20–7. [Google Scholar]
- 33.Hellbom M, Bergelt C, Bergenmar M, Gijsen B, Loge JH, Rautalahti M, Smaradottir A, Johansen C. Cancer rehabilitation: A Nordic and European perspective. Acta Oncol. 2011;50:179–186. doi: 10.3109/0284186X.2010.533194. [DOI] [PubMed] [Google Scholar]
- 34.DRV Bund (eds.) Reha-Bericht 2012. Die medizinische und berufliche Rehabilitation der Rentenversicherung im Licht der Statistik. 2012 [Google Scholar]
- 35.Widera T. Workshop „Ergebnisqualität in der medizinischen Rehabilitation der Rentenversicherung“ held on November 25th at Deutsche Rentenversicherung Bayern Süd in Munich. RVaktuell. 2009;56:98–99. [Google Scholar]
- 36.Müller D, Mehnert A, Gärtner U, et al. Identifikation von Angst, Depression und Progredienzangst bei onkologischen Rehabilitanden. Psychother Psych Med. 2005;55 [Google Scholar]
- 37.Teichmann J Bundesarbeitsgemeinschaft für Rehabilitation. Rehabilitation und Teilhabe. Köln: Deutscher Ärzte-Verlag; 2005. Hämatologische Krankheitsbilder und onkologische Grundprinzipien bei Malignomen; pp. 410–432. [Google Scholar]
- 38.Thies S, Leibbrand B, Barth J, et al. Individuelle Rehabilitationsziele und Rehabilitationsmotivation in der onkologischen Rehabilitation. Phys Rehab Kur Med. 2008;18:318–323. [Google Scholar]
- 39.Teichmann JV. Onkologische Rehabilitation: Evaluation der Effektivität stationärer onkologischer Rehabilitationsmaßnahmen. Rehabilitation. 2002;41:53–63. doi: 10.1055/s-2002-19952. [DOI] [PubMed] [Google Scholar]
- 40.Yadav R. Rehabilitation of surgical cancer patient at university of texas M D. Anderson Cancer Center. J Surg Oncol. 2007;95:361–369. doi: 10.1002/jso.20775. [DOI] [PubMed] [Google Scholar]
- e1.Shin KY, Guo Y, Konzen B, Fu J, Yadav R, Bruera E. Inpatient cancer rehabilitation: the experience of a national comprehensive cancer center. J Phys Med Rehabil. 2011;90:63–68. doi: 10.1097/PHM.0b013e31820be1a4. [DOI] [PubMed] [Google Scholar]
- e2.Hartmann U, Ring C, Reuss-Brost MA. Verbesserung der gesundheitsbezogenen Lebensqualität bei Brustkrebspatientinnen durch stationäre Rehabilitation. Med Klin. 2004;99:422–429. doi: 10.1007/s00063-004-1050-5. [DOI] [PubMed] [Google Scholar]
- e3.Hartmann U, Muche R, Reuss-Borst M. Effects of a step-by-step inpatient rehabilitation programme on quality of life in breast cancer patients. A prospective randomized study. S Karger. 2007;30:177–182. doi: 10.1159/000099989. [DOI] [PubMed] [Google Scholar]
- e4.Singer S, Schulte T. Lebensqualität von älteren Tumorpatienten - Bedarf an und Nutzung von Anschlussheilbehandlungen. Dtsch Med Wochenschr. 2009;134:121–126. doi: 10.1055/s-0028-1123968. [DOI] [PubMed] [Google Scholar]
- e5.Strauss-Blasche G, Gnad E, Ekmekcioglu C, Hladschik B, Marktl W. Combined inpatient rehabilitation and spa therapy for breast cancer patients: effects on quality of life and CA 15-3. Cancer Nurs. 2005;28:390–398. doi: 10.1097/00002820-200509000-00009. [DOI] [PubMed] [Google Scholar]
- e6.Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. J Clin Oncol. 2005;23:3830–3842. doi: 10.1200/JCO.2005.02.148. [DOI] [PubMed] [Google Scholar]
- e7.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- e8.Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical Activity and survival after prostate cancer diagnosis in the health professionals follows-up study. J Clin Oncol. 2011;29:726–732. doi: 10.1200/JCO.2010.31.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e9.Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Thomas J, Nelson H, Whittom R, Hantel A, Schilsky RL, Fuchs CS. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer. J Clin Oncol. 2006;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- e10.Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Hu FB, Mayer RJ, Nelson H, Whittom R, Hantel A, Thomas J, Fuchs CS. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298:754–764. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- e11.Gold EB, Pierce JP, Natarajan L, Stefanick ML, Laughlin GA, Caan BJ, Flatt SW, Emond JA, Saquib N, Madlensky L, Kealey S, Wasserman L, Thomson CA, Rock CL, Parker BA, Karanja N, Jones V, Hajek RA, Pu M, Mortimer JE. Dietary pattern influences breast cancer prognosis in women without hot flashes: the women’s healthy eating and living trial. J Clin Oncol. 2009;27:352–359. doi: 10.1200/JCO.2008.16.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Lauchlan DT, McCaul JA, McCarron T. Neck dissection and the clinical appearance of post-operative shoulder disability: the post-operative role of physiotherapy. Eur J Cancer Care. 2008;17:542–548. doi: 10.1111/j.1365-2354.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- e13.Scaffidi M, Vulpiani MC, Vetrano M, Conforti F, Marchetti MR, Bonifacion A, Marchetti P, Saraceni VM, Ferretti A. Early rehabilitation reduces the onset of complications in the upper limb following breast cancer surgery. Eur J Phys Rehabil Med. 2012 Epub ahead of print. [PubMed] [Google Scholar]
- e14.Cesario A, Ferri L, Geletta D, Bonassi S, Clini E, Biscione G, Cardaci V, di Toro S, Zarzana A, Margaritora S, Piraino A, Russo P, Sterzi S, Granone P. Post-operative respiratory rehabilitation after lung resection for non-small cell lung cancer. Lung Cancer. 2007;57:175–180. doi: 10.1016/j.lungcan.2007.02.017. [DOI] [PubMed] [Google Scholar]
- e15.Schultz K, Bergmann KC, Kenn K, Petro W, Heitmann RH, Fischer R, Lang SM. Effektivitäten der pneumologischen Anschluss-Rehabilitation (AHB) - Ergebnisse einer multizentrischen prospektiven Beobachtungsstudie. Dtsch Med Wochenschr. 2006;131:1793–1798. doi: 10.1055/s-2006-949155. [DOI] [PubMed] [Google Scholar]
- e16.Kramer R, Meißner B, Schultze-Berndt A, Franz IW. Verlaufsstudie psychologischer Effekte in der stationären Rehabilitation (VESPER-Studie) Dtsch Med Wochenschr. 2003;128:1470–1474. doi: 10.1055/s-2003-40290. [DOI] [PubMed] [Google Scholar]
- e17.Hartmann U, Kluge A, Ring C, Reuss-Borst M. Verbesserung der Angst und Depression bei Brustkrebspatientinnen während stationärer onkologischer Rehabilitation - Ergebnisse einer prospektiven Studie. Rehabilitation. 2006;45:88–94. doi: 10.1055/s-2005-915336. [DOI] [PubMed] [Google Scholar]
- e18.Satin JR, Linden W, Philips MJ. Depression as a predictor of disease progression and mortality in cancer patients. Cancer. 2009;115:5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- e19.Rau J, Teichmann J, Petermann F. Motivation zu sportlicher Aktivität bei onkologischen Patienten nach der Rehabilitationsmaßnahme - Ergebnisse einer randomisiert-kontrollierten Wirksamkeitsstudie. Psychother Psych Med. 2009;59:300–306. doi: 10.1055/s-2008-1067485. [DOI] [PubMed] [Google Scholar]
- e20.Schaarschmidt U, Fischer AW. Frankfurt a M.: Verlag Swets und Zeitlinger; 2003. [Google Scholar]
- e21.Erbstößer S, Verhorst H, Lindow B, Klosterhuis H. Leistungen zur Teilhabe am Arbeitsleben durch die Deutsche Rentenversicherung - ein Überblick. RVaktuell. 2008;11:343–350. [Google Scholar]
- e22.Bürger W, Glaser-Möller N, Kulick B, Pallenberg C, Stapel M. Stepwise occupational reintegration under the German pension insurance scheme-results of comprehensive routine data analyses and participants surveys. Rehabilitation. 2011;50:74–85. doi: 10.1055/s-0030-1261900. [DOI] [PubMed] [Google Scholar]
- e23.Bundesarbeitsgemeinschaft für Rehabilitation (eds.) Würzburg: Böhler Verlag GmbH; 2004. Arbeitshilfe für die stufenweise Wiedereingliederung in den Arbeitsprozess. [Google Scholar]