Abstract
Metastatic and unresectable medullary thyroid carcinoma (MTC) is often difficult to treat as it is relatively unresponsive to radiation and conventional chemotherapy. This emphasizes the importance of the development of targeted therapies for advanced MTC. Vandetanib was approved by the US Food and Drug Administration for the treatment of symptomatic or progressive MTC in patients with advanced disease in April 2011. This therapy proved to be a breakthrough in the management of MTC. We review the efficacy and safety of this novel treatment and other treatments that are being evaluated in this disease.
Keywords: medullary thyroid cancer, pharmacotherapy, tyrosine kinase inhibitors, vandetanib
Introduction
There were an estimated 44,670 new cases of thyroid cancers in the United States in 2011; medullary thyroid cancer (MTC) accounts for about 4% of all new cases.1 MTC is considered a neuroendocrine tumor as it arises from the parafollicular C cells, which are derived from the neural crest. Approximately 80% are sporadic and 20% are familial, including Multiple Endocrine Neoplasia syndromes (MEN)—MEN 2A (60%), MEN 2B (5%) and Familial Medullary Thyroid Cancer (FMTC) (35%).2
While most of the familial cases have specifically rearranged during transfection mutations (RET, proto-oncogene RET protein), 30% to 50% of sporadic MTC cases may also harbor these mutations which may be germ-line or somatic.3 Somatic mutations, those detected only in tumor cells, are seen in approximately 50% of sporadic cases while germ line mutations, seen in most familial cases, are present in 6% to 7% of sporadic cases. In RET negative cases, a high amount of rat sarcoma gene (RAS) mutations (68%) have been identified.3
Management of MTC
MTC is preferably treated with surgical resection. Pharmacotherapy is indicated only for cases with metastatic or unresectable progressive tumors. Various drugs have been tried for the treatment on unresectable and metastatic MTC. Conventional chemotherapy has included bleomycin, anthracyclines (especially doxorubicin or Adriamycin) and cisplatin. However, chemotherapy has generally been used as a last resource in thyroid cancers, although MTC has been shown to be somewhat more responsive than differentiated or anaplastic thyroid cancers. The response rate for MTC has been greater with pulmonary metastases than with locally advanced or bony metastasis.6
Novel drugs such as tyrosine kinase inhibitors have shown more promise than traditional chemotherapy. Vandetanib (ZD6474) has been studied most extensively in clinical trials, and has proven to be a major breakthrough in the treatment of advanced metastatic and progressive MTC. It was approved by the United States Food and Drug Administration (FDA) in April 2011. However because of possible serious side effects, in order for a patient to be approved for treatment, a Risk Evaluation and Mitigation Strategy (REMS) has been developed to allow the drug to be dispensed by only pharmacies and providers that are certified through the REMS Vandetanib program.4
Other targeted therapy drugs that are currently in clinical trials include other tyrosine kinase inhibitors such as carbozantinib (XL184), motesanib, axitinib, sorafenib, and sunitinib, and capecitabine, a thymidylate synthetase inhibitor.5
Mechanism of Action of Newer Drugs
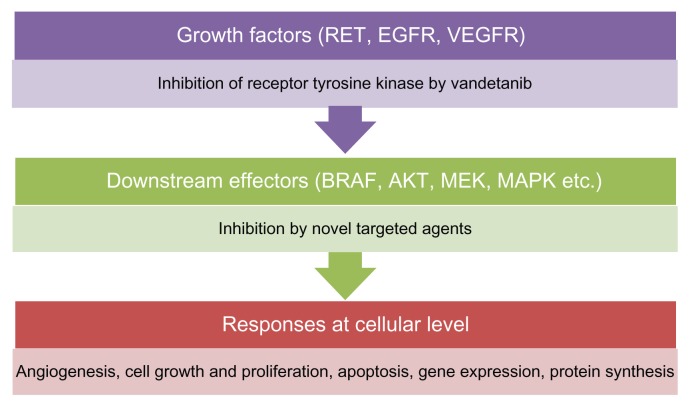
RET proto-oncogene protein is a trans-membrane receptor with an extracellular portion and an intracellular tyrosine kinase domain. Tyrosine kinase activates several signaling pathways, including the RAS (proto-oncogene RAS protein), PI3K (phosphoinositide-3-kinase), JNK (c-Jun N-terminal kinase), ERK5 (extracellular-signal-regulated kinase), and NFkB (nuclear factor κB) pathways. RAS in turn activates RAF (proto-oncogene RAF protein), MEK, and ERK through phosphorylation (Fig. 1). RAS is a signal transduction molecule that belongs to the low molecular weight G protein super family. RAS can bind to GTP (guanosine triphosphate) and GDP (guanosine diphosphate). Binding to GDP turns it off, while binding to GTP turns it on. RAS possesses GTPase activity that converts GTP to GDP and inactivates itself. Deregulation of RAS in cancers leads to its continuous activation.
Figure 1.
Targets of MTC treatments.
Abbreviations: AKT, protein kinase B; MAPK, mitogen-activated protein kinase; MEK, MAPK or ERK kinase; NF κB, nuclear factor κB; p38 MAPK, p38 mitogen-activated protein kinase; PI3K, phosphoinositide-3-kinase; RAF, proto-oncogene RAF protein; RET, proto-oncogene RET protein.7
Tyrosine kinase activation in MTC leads to down-stream activation of signal transducers, leading to increased cell growth and proliferation and inhibition of apoptosis (Fig. 1). VEGF (vascular endothelial growth factor) activation in VHL (von Hipple Lindau syndrome) has also been associated with MTC.7
Vandetanib in MTC
Vandetanib acts by RET, VEGF and EGFR inhibition. VEGF acts by binding of the VEGF receptor on the extracellular surface and promoting angiogenesis around the tumor. VEGF inhibition by vandetanib, particularly of VEGFR2, which is known to cause neovascularization, can thwart tumor growth.
Pharmacokinetics
Vandetanib is an oral tablet taken once daily with a half-life of 10 days. Its absorption is not significantly affected by food.8 Dose adjustments may be needed in subjects with renal impairment. Exposure to the drug was not changed in subjects with hepatic impairment, and thus does not necessitate dose adjustments for patients with liver disease.9
Vandetanib is partly metabolized by cytochrome P450 (CYP) 3A4. Studies have shown decrease in blood levels by about 40% during co-administration with potent inducers of CYP3A4 (eg, rifampin). The increase in exposure of co-administration with inhibitors like itroconazole was found to be low at 9%. It is prudent to avoid CYP3A4 inducers with Vandetanib.10 It should also be avoided with drugs that prolong QT interval. Metabolites such as the N-desmethyl and N-oxide metabolites and even unchanged Vandetanib can be detected in the urine, feces and plasma of patients.8
Side effects of vandetanib
Phase 1 trials were first conducted on 77 patients in the United States and Australia. These patients received different doses of the drug ranging, from 50 mg to 600 mg. The dosages administered (and respective number of patients) were 50 mg (n = 9), 100 mg (n = 19), 200 mg (n = 8), 300 mg (n = 25), 500 mg (n = 8) and 600 mg (n = 8).5
Dose limiting toxicities were seen in 6 of the 16 patients receiving 500 mg and 600 mg doses (3 in each). Major side effects included hypertension in 5% (n = 4), diarrhea in 5% (n = 4) and rash in 4% (n = 3) of patients.
Asymptomatic QTc prolongation occurred at 100 mg, 300 mg, 600 mg (1 patient each) and 200 mg and 500 mg (2 patients each), thus making it less subject to the amount of dose received.5
In a phase 1 study conducted in Japan, eighteen patients were given different doses of the drug, ranging from 100 mg to 400 mg. The dosages administered (and respective number of patients) were 100 mg (n = 3), 200 mg (n = 6), 300 mg (n = 6), and 400 mg (n = 3). On completion of two cycles, the dose limiting toxicities were hypertension in 17% (n = 3), diarrhea in 5% (n = 1), headache in 5% (n = 1), rash in 5% (n = 1), and alanine aminotransferase increase in 5% (n = 1) of patients. In another study in Japan, one person with 400 mg dose had grade 3 ALT elevation and grade 3 hypertension, whereas one person treated with 600 mg dose had thrombocytopenia.11 A dose of 400 mg per day was considered to exceed the maximum tolerated dose (MTD).12
In a study conducted in 36 Chinese subjects,13 42% (n = 15) had rash, and 39% (n = 14) had diarrhea. No QTc prolongation was seen, whereas ALT elevation was seen in 3% (n = 1) of patients.
Other side effects include congestive heart failure, nausea, vomiting, cough, nasopharyngitis, back pain, fatigue, headache, asthenia, anorexia and teratogenicity.
QTc prolongation is considered a major side effect due to its association with congestive heart failure and arrhythmias. In a meta-analysis of 2188 patients who received 300 mg Vandetanib as single dose, 16.4% [95% CI 8.1%–30.4%] had all grade and 3.7% high grade QTc prolongation. These numbers were reported for patients treated for all kinds of cancer. However, MTC patients were reported to have 18% [95% CI 10.7%–28.6%] all grade and 12% [95% CI 4.5%–28%] high grade (greater than 500 ms or symptomatic) QTc prolongation. The higher incidence of these events was due to the fact that MTC patients took the drug for longer duration. The RR of high grade QTc prolongation was found to be 3.24 (1.57–6.71) compared to the subjects on Vandetanib for cancers other than MTC.14
In a meta-analysis from studies in Pubmed published between 1996 and 2011, data from 2961 patients who received vandetanib 300 mg as a single agent were culled. Out of 2961 patients, 46.1% [95% confidence interval (CI) 40.6% to 51.8%] developed rash (RR.2.43 compared to controls). Of the 2961 patients, 261 had MTC, 54.3% [95% CI 33.4% to 73.8%] of those 261 developed low grade rash, and 3.4% [95% CI 1.8%–6.5%] developed high grade rash. The endpoints for the study were rash, rash/desquamation and acneiform dermatitis.
The percentage of patients developing rash was higher in other target therapy drugs: 75.2% [95% CI 71.3%–78.7%] patients on erlotinib developed low grade rash, whereas 9.1% [95% CI 7%–11.7%] developed a high grade rash. The highest incidence was seen with cetuximab, 88.2% [95% CI 84.8%–91%] developing a low grade rash and 11.3% [95% CI 8.8%–14.3%] developing a high grade rash.15 Steven-Johnson Syndrome resulting in death was also observed. Due to its long half-life, sunscreens and protective clothing should be used for four months after discontinuation of treatment.16
The prolonged treatment can result in an unusual adverse neurological event, reversible cerebral vaso-constriction syndrome (RCVS), which was reported in a patient in his fifties. Thunder-clap headaches were first experienced at a time when he was starting to urinate. Upon investigation, he was found to have multifocal segmental arterial constrictions and a dilation of cerebral arteries on brain angiography consistent with a diagnosis of RCVS. He was successfully treated with oral nimodipine.17
Vandetanib was found to be fetotoxic, embryotoxic and teratogenic to rats, and while no human data are currently available, it is recommended that it should be avoided in pregnancy.16
Efficacy of vandetanib in MTC
In general, vandetanib has been found to be more useful in patients in whom metastases progress rapidly (in less than 12 months) and diffusely.17 The risk of presenting with lymph node and distant metastases increases in patients with sporadic medullary thyroid cancer with three or four risk allele mutations of RET, stressing the importance of target therapy in such patients (OR = 5.84).18 The RET S836S variant in particular is associated with early onset and increased risk of metastatic disease in patients with hereditary, as well as sporadic, MTC.19
Two separate studies with different doses of vandetanib were conducted to evaluate its efficacy in MTC. Out of the 30 patients who received 300 mg vandetanib as a single agent, 30% (n = 6) showed partial response, whereas, in 53% (n = 16), the disease was found to be stable at 24 weeks. The median duration of response at data cut off was 10.2 months and the disease control rate was thus 73% (22 of 30).20
Vandetanib given in a dose of 100 mg showed that 16% (n = 3) [95% confidence interval 3.4–39.6] of the total 19 patients (13 males) showed a partial response. In 53% (n = 10) patients, the disease remained stable over a period of 24 weeks. The overall disease control rate was noted to be 68% [95% confidence interval 43.4–87.4]. Calcitonin and CEA sustained 50% or greater decrease in 16% (n = 3) and 5% (n = 1) of patients respectively.21
In a phase III registration trial, the estimated progression-free survival for the patients treated with vandetanib was 30.5 months, compared with 19.3 months for patients who received placebo. In this double-blind randomized control trial of patients with locally advanced or metastatic disease, 331 patients were randomly assigned to receive treatment with either vandetanib or placebo. Patients in the placebo arm found to have disease progression were allowed to opt for vandetanib. There were 52 such patients, making the true survival improvement rate throughout the trial difficult to assess. Vandetanib not only improved progression-free survival, but the objective response rate was also higher, and several other disease-related measures were better in the vandetanib arm than in the placebo arm. Since most patients in the trial had RET mutations, the effect of vandetanib on patients without RET mutations was difficult to ascertain.22
Cost of vandetanib
Vandetanib is sold under the brand name Caprelsa, developed by AstraZeneca (London, UK). A 30 day supply of the drug, 300 mg taken once daily, costs approximately $5000 to 10,000 dollars.23
The cost effectiveness of this medication is yet to be established. Many studies have used a threshold of cost effective ratios of below $50,000 to $100,000 dollars per quality adjusted life year to demonstrate a cost effective treatment.24 Studies on other chronic disease conditions may be better comparisons than those of aggressive cancer treatments, given that MTC can often have an indolent course.
Mechanism of resistance to vandetanib and other TKIs
Since these drugs work on various intracellular pathways, different molecular mechanisms may account for their resistance.
Primary resistance, also known as intrinsic resistance, is host-related, and may be due to pharmacokinetic causes. The drug may not be absorbed in sufficient quantities, or may be rapidly metabolized to inactive compounds. It may also be due to defects in the immune system of the body. It is not related to the duration of treatment.25
Secondary, or acquired resistance, tends to occur after prolonged treatment. The cancer cells may become resistant with continued exposure to drugs due to the generation of new clone cells that contain genetic alterations. Due to this instability, they can find an alternative pathway which helps them survive and multiply even in the presence of the drug. For example, a previous study demonstrated that mutation of tyrosine 806 to cysteine (Y806C) induced RET kinase resistance to ZD 6474.26
Other drugs for treatment of MTC
Tyrosine kinase inhibitors sorafenib and sunitnib are currently available for treatment of other solid cancers. They have been tried for patients with MTC and have shown some efficacy. However, they are not curative and have not been shown to improve survival rates. Motesanib, cabozantinib, a multi-kinase inhibitor, and axitinib have also been tried for metastatic MTC, resulting in a partial response or stable disease state. Clinical trials are yet to be done for bortezomib (proteasome inhibitor), valproic acid (histone deacetylase inhibitor), capecitabine (5-FU prodrug), indomethacin (a well-known non-steroidal anti-inflammatory), cardiac natriuretic hormones, and an extract of the plant cautleya gracilis. Various other drugs are in clinical trials and are being evaluated for their efficacy and safety in the treatment of MTC. These include phase III clinical trials in subjects with locally advanced unresectable or metastatic MTC. The trials were designed to evaluate the progression-free survival in patients receiving cabozantinib compared to placebo.28 This study evaluated 330 patients with progressive MTC who were randomized to receive cabozantinib or placebo. Although the progression free survival favored the cabozantinib group (11.2 vs. 4 months P < 0.0001), there was no statistical difference in overall survival.29 Phase II clinical trials are being undertaken for lithium carbonate, SOM 230 with or without RAD001, imatinib with dacarbazine and capecitabine and Vandetanib plus bortezomib (Table 3).30
Table 3.
Current studies of new agents in the treatment of MTC.
| Title | Status | Phase |
|---|---|---|
| SOM230 alone or in combination with RAD001 in patients with medullary thyroid CANCER | Recruiting | 2 |
| An initial study of Lithium in patients with medullary thyroid cancer | Recruiting | 2 |
| Imatinib in combination with dacarbazine and capecitabine in medullary thyroid cancer | Active not recruiting | 1 |
| A targeted phase I/II study of ZD6474 (Vandetanib) plus the proteosome inhibitor bortezomib (Velcade) in adults with solid tumors with a focus on hereditary or sporadic, locally advanced or metastatic medullary thyroid cancer | Active not recruiting | 1/2 |
Conclusion
Tyrosine kinase inhibitors have shown promise in the treatment of metastatic MTC, which has rein-vigorated research interest in this area. While RET mutations are well described in MTC, new discoveries at the molecular level are providing novel targets for unconventional compounds. In the authors’ institution, patients with metastatic medullary thyroid cancer are divided into different categories including those who have a poor performance status, those with a good performance status but stable disease, and those with a good performance status and progressive or symptomatic disease. It is only the latter group that is offered treatment with vandetanib and care is made to look for side effects. Performance status can be measured using the Eastern Co-operative Oncology Group (ECOG) scale (Table 4). The management of these side effects is discussed below.
Table 4.
ECOG performance status.
| ECOG performance status | Definition | Ability to take systemic treatment |
|---|---|---|
| 0 | No symptoms | Yes |
| 1 | Symptomatic but able to maintain activities of daily living | Yes |
| 2 | Symptoms keep subject in bed less than 50% of the day | Yes |
| 3 | Symptoms keep subject in bed more than 50% of the day | No |
| 4 | Symptoms keep subject in bed 100% of the day | No |
Skin toxicities are managed with the assistance of an experienced dermato-oncologist with topical antibiotic and if needed, steroid creams or systemic tetracyclines or macrolide antibiotics. If these are not effective then the medication is withheld until toxicities resolve and the medication is restarted at a lower dose.
The authors’ management of QTc prolongation involves avoiding the use of vandetanib in patients who have a history of QTc prolongation or Torsades de Pointes, or who have a baseline QTc interval of greater than 450 ms. ECG’s should be obtained at 2–4 weeks and 8–12 weeks and then every 3 months while taking the medication. Medications that prolong the QTc interval should be avoided. If the QTc interval is prolonged to greater than 500 ms while taking the medication, vandetanib should be held until the QTc interval falls to less than 450 ms and vandetanib can then be restarted at a lower dose. If more than 2 dose reductions are needed then the medication should not be continued.
Hypertension is a common side effect of many tyrosine kinase inhibitors and occurs in 32% of patients with MTC taking vandetanib. 8.8% of patients develop high grade hypertension.31
In the authors’ institution, hypertension is managed using an angiotensin converting enzyme inhibitor (ACE) or beta blocker for non-black patients and a calcium channel blocker for black patients. If the blood pressures remain elevated then combinations of anti-hypertensives are used together with consultation with hypertensive specialists. If this along with dose reductions do not control blood pressure levels, then vandetanib should be discontinued.
Table 1.
Side effects associated with vandetanib.
| Side effect | US and Australia phase 129 | Japan phase 112 | China phase 113 | Phase III ZETA study22 | Meta-analysis with 300 mg dose14,15 |
|---|---|---|---|---|---|
| Hypertension | 5% | 17% | 9% | ||
| Diarrhea | 5% | 5% | 39% (all grades) | 11% | |
| Rash | 4% | 5% | 42% (all grades) | 4% | 54.3% |
| QTc prolongation | 4% | 8% | |||
| Alanine aminotransferase (ALT) increase | 5% | ||||
| Grade 3 ALT elevation | 5% | 3% | |||
| Thrombocytopenia | 5% | ||||
| QTc prolongation all grades | 18% | ||||
| QTc prolongation grades 3 and 4 | 12% |
Table 2.
Efficacy of vandetanib.
| Effect | Vandetanib22 | Placebo22 | Vandetanib 300 mg20 | Vandetanib 100 mg21 |
|---|---|---|---|---|
| Progression free survival | 30.5 months | 19.3 months | 10.2 months | Not determined |
| Partial response | 45% | 13% | 30% | 16% |
| Stable disease ≥ 24 weeks | 87% | 71% | 53% | 53% |
| Maintained calcitonin decrease | 69% | 3% | 80% | 16% |
| Maintained carcinoembryonic antigen reduction | 52% | 2% | 53% | 5% |
Footnotes
Author Contributions
Wrote the first draft of the manuscript: HD, KS, JS, SR. Contributed to the writing of the manuscript: HD, KS, JS, SR. Agree with manuscript results and conclusions: HD, KS, JS, SR. Jointly developed the structure and arguments for the paper: HD, KS, JS, SR. Made critical revisions and approved final version: JS, SR. All authors reviewed and approved of the final manuscript.
Competing Interests
JAS has received consulting fees from Exelixis and speaking fees from Veracyte. Other authors disclose no competing interests.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest. Provenance: the authors were invited to submit this paper.
Funding
Author(s) disclose no funding sources.
References
- 1.Thyroid cancer. [Accessed Mar 18, 2012]. Available at: http://www.cancer.org/Cancer/ThyroidCancer/DetailedGuide/thyroid-cancer?docSelected=thyroid-cancer-what-is-thyroid-cancer.
- 2.Brandi ML, Gagel RF, Angeli A, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001 Dec;86(12):5658–71. doi: 10.1210/jcem.86.12.8070. [DOI] [PubMed] [Google Scholar]
- 3.Moura MM, Cavaco BM, Pinto AE, Leite V. High prevalence of RAS mutations in RET-negative sporadic medullary thyroid carcinomas. J Clin Endocrinol Metab. 2011 May;96(5):E863–8. doi: 10.1210/jc.2010-1921. Epub Feb 16, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Lauri Scudder D. Game changers in pharmacy practice 2011: Game changers in oncology. [Accessed Mar 13, 2012]. Available at: http://www.medscape.com/viewarticle/753823_3.
- 5.Deshpande H, Marler V, Sosa JA. Clinical utility of vandetanib in the treatment of patients with advanced medullary thyroid cancer. Onco Targets Ther. 2011;4:209–15. doi: 10.2147/OTT.S17422. Epub Dec 9, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahuja S, Ernst H. Chemotherapy of thyroid carcinoma. J Endocrinol Invest. 1987 Jun;10(3):303–10. doi: 10.1007/BF03348135. [DOI] [PubMed] [Google Scholar]
- 7.Schlumberger M, Carlomagno F, Baudin E, Bidart JM, Santoro M. New therapeutic approaches to treat medullary thyroid carcinoma: Molecular therapeutic targets in medullary thyroid carcinoma. Nat Clin Pract Endocrinol Metab. 2008 Jan;4(1):22–32. doi: 10.1038/ncpendmet0717. [DOI] [PubMed] [Google Scholar]
- 8.Martin P, Oliver S, Kennedy SJ, et al. Pharmacokinetics of vandetanib: Three phase I studies in healthy subjects. Clin Ther. 2012 Jan;34(1):221–37. doi: 10.1016/j.clinthera.2011.11.011. Epub Dec 28, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Weil A, Martin P, Smith R, et al. Pharmacokinetics of vandetanib in subjects with renal or hepatic impairment. Clin Pharmacokinet. 2010 Sep;49(9):607–618. doi: 10.2165/11534330-000000000-00000.. [DOI] [PubMed] [Google Scholar]
- 10.Martin P, Oliver S, Robertson J, Kennedy SJ, Read J, Duvauchelle T. Pharmacokinetic drug interactions with vandetanib during coadministration with rifampicin or itraconazole. Drugs R D. 2011;11(1):37–51. doi: 10.2165/11586980-000000000-00000.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heymach JV. ZD6474—clinical experience to date. Br J Cancer. 2005 Jun;92( Suppl 1):S14–20. doi: 10.1038/sj.bjc.6602604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamura T, Minami H, Yamada Y, et al. A phase I dose-escalation study of ZD6474 in japanese patients with solid, malignant tumors. J Thorac Oncol. 2006 Nov;1(9):1002–9. [PubMed] [Google Scholar]
- 13.Zhang L, Li S, Zhang Y, et al. Pharmacokinetics and tolerability of vandetanib in chinese patients with solid, malignant tumors: An open-label, phase I, rising multiple-dose study. Clin Ther. 2011 Mar;33(3):315–27. doi: 10.1016/j.clinthera.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Zang J, Wu S, Tang L, et al. Incidence and risk of QTc interval prolongation among cancer patients treated with vandetanib: A systematic review and meta-analysis. PLoS One. 2012;7(2):e30353. doi: 10.1371/journal.pone.0030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen AC, Wu S, Damse A, Sherman E, Lacouture ME. Risk of rash in cancer patients treated with vandetanib: Systematic review and meta-analysis. J Clin Endocrinol Metab. 2012 Apr;97(4):1125–33. doi: 10.1210/jc.2011-2677. Epub Feb 29, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Caprelsa (vandetanib) tablets. [Accessed Mar 18, 2012]. Available at: http://www1.astrazeneca-us.com/pi/vandetanib.pdf.
- 17.Duplomb S, Benoit A, Mechtouff-Cimarelli L, et al. Unusual adverse event with vandetanib in metastatic medullary thyroid cancer. J Clin Oncol. 2012 Jan 10;30(2):e21–3. doi: 10.1200/JCO.2011.38.2796. Epub Dec 12, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Ceolin L, Siqueira DR, Ferreira CV, et al. Additive effect of ret polymorphisms on sporadic medullary thyroid carcinoma susceptibility and tumor aggressiveness. Eur J Endocrinol. 2012 May;166(5):847–54. doi: 10.1530/EJE-11-1060. Epub Feb 17, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Siqueira DR, Romitti M, da Rocha AP, et al. The RET polymorphic allele S836S is associated with early metastatic disease in patients with hereditary or sporadic medullary thyroid carcinoma. Endocr Relat Cancer. 2010 Oct 5;17(4):953–63. doi: 10.1677/ERC-09-0312. Print Dec 2010. [DOI] [PubMed] [Google Scholar]
- 20.Wells SA, Jr, Gosnell JE, Gagel RF, et al. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol. 2010 Feb 10;28(5):767–772. doi: 10.1200/JCO.2009.23.6604. Epub Jan 11, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson BG, Paz-Ares L, Krebs A, Vasselli J, Haddad R. Vandetanib (100 mg) in patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Endocrinol Metab. 2010 Jun;95(6):2664–71. doi: 10.1210/jc.2009-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells SA, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: A randomized double blind Phase III trial. J Clin Oncol. 2012 Jan 10;30(2):134–41. doi: 10.1200/JCO.2011.35.5040. Epub Oct 24, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vandetanib (caprelsa) for medullary thyroid cancer. Med Lett Drugs Ther. 2012 Jan 9;54(1381):3–4. [PubMed] [Google Scholar]
- 24.Benucci M, Saviola G, Manfredi M, Sarzi-Puttini P, Atenzi F. Cost Effectiveness Analysis of Disease-Modifying Antirheumatic Drugs in Rheumatoid Arthritis. A Systematic review Literature. Int J Rheumatol. 2011;2011:845496. doi: 10.1155/2011/845496. Epub Nov 22, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgillo F, Bareschino MA, Bianco R, Tortora G, Ciardiello F. Primary and acquired resistance to anti-EGFR targeted drugs in cancer therapy. Differentiation. 2007 Nov;75(9):788–99. doi: 10.1111/j.1432-0436.2007.00200.x. Epub Jul 2, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Carlomagno F, Guida T, Anaganti S, et al. Identification of tyrosine 806 as a molecular determinant of RET kinase sensitivity to ZD6474. Endocr Relat Cancer. 2009 Mar;16(1):233–41. doi: 10.1677/ERC-08-0213. Epub Nov 24, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Morgillo F, Martinelli E, Troiani T, Orditura M, De Vita F, Ciardiello F. Antitumor activity of sorafenib in human cancer cell lines with acquired resistance to EGFR and VEGFR tyrosine kinase inhibitors. PLoS One. 2011;6(12):e28841. doi: 10.1371/journal.pone.0028841. Epub Dec 9, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Sugawara M, Geffner DL, Martinez D, Hershman JM. Novel treatment of medullary thyroid cancer. Curr Opin Endocrinol Diabetes Obes. 2009 Oct;16(5):367–72. doi: 10.1097/MED.0b013e3283304f0c. [DOI] [PubMed] [Google Scholar]
- 29.Schoffski P, Elisei R, Muller S, et al. An international, double-blind, randomized, placebo-controlled phase III trial (EXAM) of cabozantinib (XL-184) in medullary thyroid carcinoma (MTC) patients (pts) with documented RECIST progression at baseline. J Clin Oncol. 2012;30 Suppl; abstr 5508. [Google Scholar]
- 30.Thyroid gland-medullary carcinoma. [Mar 13, 2012]. Available at: http://www.cancer.gov/clinicaltrials/search/results?protocolsearchid=10227324.
- 31.Qi WX, Shen Z, Lin F, et al. Incidence and Risk of Hypertension with Vandetanib in Cancer Patients: A Systematic review and Meta-analysis of clinical trials. Br J Clin Pharmacol. 2012 Aug 10; doi: 10.1111/j.1365-2125.201204417.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]