Abstract
Three-week-old maize (Zea mays L.) plants were submitted to light/dark cycles and to prolonged darkness to investigate the occurrence of sugar-limitation effects in different parts of the whole plant. Soluble sugars fluctuated with light/dark cycles and dropped sharply during extended darkness. Significant decreases in protein level were observed after prolonged darkness in mature roots, root tips, and young leaves. Glutamine and asparagine (Asn) changed in opposite ways, with Asn increasing in the dark. After prolonged darkness the increase in Asn accounted for most of the nitrogen released by protein breakdown. Using polyclonal antibodies against a vacuolar root protease previously described (F. James, R. Brouquisse, C. Suire, A. Pradet, P. Raymond [1996] Biochem J 320: 283–292) or the 20S proteasome, we showed that the increase in proteolytic activities was related to an enrichment of roots in the vacuolar protease, with no change in the amount of 20S proteasome in either roots or leaves. Our results show that no significant net proteolysis is induced in any part of the plant during normal light/dark cycles, although changes in metabolism and growth appear soon after the beginning of the dark period, and starvation-related proteolysis probably appears in prolonged darkness earlier in sink than in mature tissues.
Carbohydrate deprivation is a fact of life for most higher plants. When the emergence of young seedlings and their transition to autotrophy is delayed (Elamrani et al., 1994b), when competition occurs between sink tissues such as roots, flowers, or fruits (Dejong and Grossman, 1995; Ho, 1996), or when under environmental constraints, the photosynthesis rate or the translocation of nutrients to sink tissues decreases (Amthor and McCree, 1990; Setter, 1990) and higher plants may experience carbon starvation. Similarly, in some types of senescence (Noodén, 1988) or in postharvest situations (King et al., 1990), the degradation of some tissue or cell structures is clearly related to the appearance of carbon-starvation symptoms, such as a decrease in sugar content, net lipid and protein breakdown, degradation of plastids, or loss of membrane selective permeability. The metabolic consequences of carbohydrate starvation have been studied in a number of plant models, and the situation may be summarized as follows: to survive, plant cells have to adapt to the lack of carbohydrates by substituting protein and lipid metabolism for sugar metabolism through autophagic processes (James, 1953; Thomas, 1978; Saglio and Pradet, 1980; Journet et al., 1986; Roby et al., 1987; Baysdorfer et al., 1988; Peoples and Dalling, 1988; King et al., 1990; Brouquisse et al., 1991; Tassi et al., 1992; Elamrani et al., 1994b).
There are many reports of degradation and synthesis of proteins during carbon deprivation. Thus, enzymic activities related to sugar metabolism and respiration (Journet et al., 1986; Brouquisse et al., 1991; Irving and Hurst, 1993), nitrogen reduction and assimilation (Thomas, 1978; Peeters and Van Laere, 1992; Brouquisse et al., 1992), or protein synthesis (Webster and Van't Hof, 1973; Tassi et al., 1992) decrease during starvation. In contrast, the activity of enzymes related to the catabolism of proteins (Thomas, 1978; Tassi et al., 1992; James et al., 1993, 1996; Moriyasu and Ohsumi, 1996), amino acids (Brouquisse et al., 1992), or fatty acids (Dieuaide et al., 1992, 1993; Ismail et al., 1997) increases. Some genes encoding enzymes involved in protein and lipid catabolism have been shown to be induced by sugar depletion (Koch, 1996), and it is clear that changes in selective synthesis or degradation of individual proteins could be important components in the coordinated response to carbon starvation (Graham, 1996; Koch, 1996).
In plant cells protein breakdown is mediated by different proteolytic systems: (a) vacuolar proteolysis, (b) selective nuclear and cytosolic proteolysis, and (c) organellar proteolysis (for review, see Vierstra, 1993). Because each cellular compartment is affected by sugar starvation, these different proteolytic systems might be involved in starvation-induced proteolysis. Vacuolar macroautophagy has been reported for tobacco and sycamore cells submitted to Suc deprivation (Aubert et al., 1996; Moriyasu and Ohsumi, 1996) and may occur in maize (Zea mays L.) root tips, where James et al. (1996) demonstrated the induction of a vacuolar Ser endopeptidase in response to carbon starvation. The ubiquitin-dependent pathway and 20S/26S proteasome, present in eukaryotic cells, mediate a selective cytosolic and nuclear proteolysis (Hershko and Ciechanover, 1992; Coux et al., 1996). Ubiquitin and components of the ubiquitin-dependent proteolysis have been shown to be induced in response to various stresses (Vierstra, 1993; Callis, 1995) but, to date, their involvment in response to carbon starvation has not been studied in plants. Very little is known about the changes in proteolytic activities in organelles under carbon deprivation. One study reports the induction of plastidial aminopeptidase and endopeptidase activities in cotyledons of sugar beet seedlings during prolonged dark growth (Elamrani et al., 1994a).
In all of these studies, relatively long-term carbohydrate starvation was investigated and, most of the time, the effects were analyzed in one type of organ (leaf, root, cotyledon, spear tip, etc.) or in model systems such as callus or cell suspensions. However, depending on the protocol used to induce carbon starvation (shading, darkening, leaf pruning, excision, girdling, etc.), the response to starvation might vary significantly (Feller and Fischer, 1994). Thus, in spite of the interest in such models and protocols, there are few data available on the changes in biochemical parameters at the level of the whole plant during carbon starvation.
In the present study we used 3-week-old maize plants submitted to light/dark cycles and to prolonged darkness to investigate the effects of moderate or strong sugar deprivation on protein breakdown in different parts of the plant. At various times during the experiment, the following plant parts were harvested: mature, senescent, and youngest leaves; mature roots; root tips; stems and sheaths; and green and yellow remainders (intermediate parts of the leaves). For each plant part, soluble sugar, protein, amino acid, and NH4+ content were analyzed, endopeptidase activities were measured, and the amount of the vacuolar RSIP, a protease previously shown to be induced in carbon-starved maize roots (James et al., 1996), and of the 20S proteasome (G. Basset, R. Brouquisse, L. Malek, and P. Raymond, unpublished data) were investigated by immunodetection. The growth rate of roots was also measured. The occurrence of carbon starvation during light/dark cycles and under prolonged darkness is discussed on the basis of changes in protein and free-amino acid content and proteolytic activities, used as carbon-starvation symptoms.
MATERIALS AND METHODS
Plant Culture and Harvest
Maize (Zea mays L. cv DEA, Pioneer France Maïs, Toulouse, France) seeds were germinated at 25°C in the dark between sheets of Whatman 3MM chromatography paper soaked in mineral nutrient solution (solution A) as described by Saglio and Pradet (1980). After 5 d the seedlings were transferred to a hydroponic culture system in a growth room for another 16 d. The culture medium consisted of solution A concentrated 2-fold and supplemented with 1 mm Fe-ethylenediamine-N,N-di(2-hydroxy-5-methylphenyl) acetic acid. Plants were grown under a 15-h photoperiod with a photosynthetic photon flux density of 200 to 250 μmol photons m−2 s−1. The light/dark temperature was 25/20°C and the RH was maintained close to 70%. Nitrate and phosphate concentrations were checked and readjusted daily.
After 3 weeks maize plants were submitted to two additional light/dark cycles and then to a 48-h dark period. Samples of two to three plants were harvested independently after 15 h of light, 9 h of darkness, 15 h of light, and 9, 17, 24, and 48 h of darkness. In each plant the following tissues were distinguished: root tips (the last 3 mm of primary and secondary roots), mature roots (whole roots minus the root tips), stems and sheaths, senescent leaves (the first 2 leaves), mature leaves (the apical 30 cm of the 4th, 5th, and 6th leaves), and the youngest leaves (i.e. the two most recently formed leaves; these are leaves 11 and 12 and are yellow). The remaining leaf tissues were pooled into two parts on the basis of their color and called the “green remainder” (the 3rd leaf and the remaining green part of the 4th, 5th, and 6th leaves) and “yellow remainder” (the remaining yellow part of the 4th, 5th, and 6th leaves, and the 7th–10th leaves). Immediately after harvest, the different tissues were weighed, rapidly frozen in liquid nitrogen, and either directly stored at −80°C (root tips, senescing leaves, and youngest leaves), or ground to a fine powder in a liquid nitrogen prechilled mortar and stored at −80°C. For sugar, protein, and amino acid analysis, tissue samples were freeze-dried for 24 h (Tray Drying Chamber, FTS Systems, Stone Ridge, NY) before metabolite extraction. Fresh tissues were used for enzymatic activity and NH4+ measurements and for immunoblotting experiments.
Extraction and Determination of Soluble Sugars and Amino Acids
Soluble sugars were extracted by boiling ethanol/water and analyzed according to the method of Moing et al. (1992). Free amino acids were extracted according to the method of Stitt and ap Rees (1978) and analyzed as described in Brouquisse et al. (1992).
Protein Analysis
Proteins were extracted with the following extraction buffer: 50 mm Hepes-Na, pH 7.3, 0.2% sodium deoxycholate, 0.1% Triton X-100, and 5 mm sodium ascorbate. They were measured according to the method of Bradford (1976) using BSA as a standard.
NH4+ Analysis
NH4+ was measured, after extraction from frozen powdered material with 0.1 m HCl, by the phenol-hypochlorite method, as described in King et al. (1990).
Proteolytic Activity Measurements
Forty to 500 mg of fresh tissue was crushed in a glass potter or in a mortar at 4°C with 200 μL to 1 mL of extraction medium (50 mm Tris-HCl, pH 7.5, 5 mm β-mercaptoethanol, and 0.5% [v/v] polyvinylpolypyrrolidone). Crushed extracts were then centrifuged (15,000g, 15 min) and the supernatants were used for endopeptidase and chymotrypsin-like activity measurements. Endopeptidase activities (against azocasein) were measured as described in James et al. (1993). The extinction coefficient E1% azocaseine in 1 m NaOH = 37 L cm−1 g−1 was used to calculate the azocaseinase activity. Chymotrypsin-like activities were measured using Succ-Leu-Leu-Val-Tyr-AMC as a substrate. Sixty microliters of enzymic extract, 20 μL of water, and 20 μL of substrate solution (2 mm stock in dimethyl formamide) were incubated for 1 h at 37°C. The reaction was stopped by the addition of 100 μL of 10% SDS (w/v) and 2 mL of 100 mm Tris-HCl, pH 9.0. The released 7-amino-4-methyl coumarin radicals were measured fluorometrically (excitation at 380 nm, emission at 460 nm) in an F-2000 spectrofluorimeter (Hitachi, Tokyo, Japan).
Electrophoresis and Immunoblots
Native- and SDS-PAGE were performed with 5% (w/v) and 12.5% (w/v) polyacrylamide gels, respectively, by the procedure of Laemmli (1970). Protein from native- and SDS-PAGE was transferred to a nitrocellulose membrane (BA 85, Schleicher & Schuell) for 1 h at 3 mA/cm2 in a Fast-Blot B33 semidry system (Biometra, Göttingen, Germany). Blots were blocked with TBS containing 0.2% Tween 20 and 5% (w/v) nonfat milk powder. RSIP and 20S proteasome were detected with polyclonal anti-RSIP (James et al., 1996) and anti-20S proteasome (G. Basset, R. Brouquisse, L. Malek, and P. Raymond, unpublished data) antibodies plus goat anti-rabbit IgG-alkaline phosphatase conjugate (Sigma).
Immunoprecipitation Method
One milliliter of each crude extract supernatant was desalted by centrifugation through a G-25 Sephadex column (Pharmacia) equilibrated with 50 mm Mes-Na, pH 6.1, and 20 mm NaCl. Equal azocaseinase activities (400 nmol azocasein min−1) were incubated for 2 h at 25°C with an increasing volume of immune or preimmune anti-RSIP serum. Immune complexes were incubated for 1 h at 6°C with a 2-fold (IgG-binding) excess of Protein A-Agarose (Bio-Rad) and then centrifuged for 10 min at 10,000g. The azocaseinase activity was then measured in each supernatant fraction.
Root-Growth Measurement
Attached maize roots were put on a sloping gutter in the growth chamber. Recycled nutrient solution was supplied continuously at the top of the gutter, creating a wet film around the bundle. A camera (Minolta, Ramsey, NJ) was fitted above the gutter to take pictures of the root tips every 3 h. The root system was kept in darkness except for short xenon flashes needed to take pictures. A grid was drawn at the bottom of the gutter and root length was measured manually on pictures with a 5-fold magnification of the actual root size.
RESULTS
To investigate changes in metabolite content and enzymic activities during light/dark cycles and during an extended dark period, 3-week-old maize plants were harvested at the end of the light (15 h) and the dark (9 h) periods of two successive light/dark cycles and then after 17, 24, and 48 h of darkness.
Changes in Soluble Sugar and Protein Contents
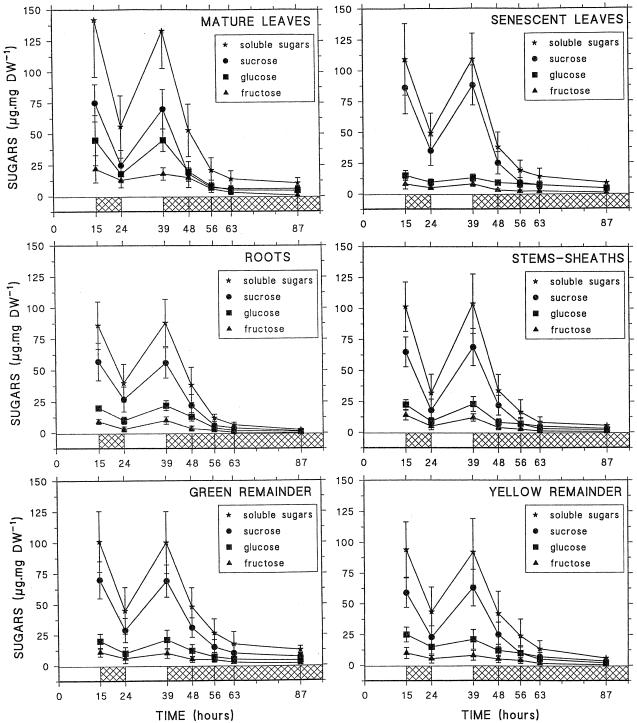
Soluble sugars were measured in whole roots (including root tips), stems and sheaths, senescent leaves, mature leaves, yellow remainder (including the youngest leaves), and green remainder (Fig. 1). Depending on the tissue, the total soluble sugar content varied from 85 to 140 μg mg−1 dry weight in whole roots and mature leaves, respectively, with Suc accounting for 50% to 80% of the soluble sugars. After measurement of the fresh and dry weights of the different tissues, dry weight was shown to represent about 15 ± 4% of the fresh weight (data not shown). Thus, the soluble sugar content was estimated to be between 70 and 120 μmol Glc equivalents g−1 fresh weight. In all of the tissues, soluble sugar content exhibited significant variations during light/dark cycles, showing a 50% drop at the end of the dark period. Under prolonged darkness soluble sugars continued to decrease and reached 10% of their initial value after 48 h of darkness. In roots soluble sugars decreased to 2.5 μg g−1 dry weight (around 2 μmol Glc equivalents g−1 fresh weight).
Figure 1.
Changes in soluble sugars, Suc, Glc, and Fru, in different maize plant parts (whole roots, mature leaves, senescent leaves, stems and sheaths, green remainder, and yellow remainder) during light/dark cycles and 48 h of darkness. Each point represents the mean (±sd) of four independent experiments. DW, Dry weight.
Such a decrease in soluble sugar content during more or less extended dark periods has been described for many plant species and may be related to cessation of photosynthetic activity during the dark period, whereas respiration and biosynthetic processes continue, although at a lower rate (Wittenbach, 1978; Frossard, 1985; Kerr et al., 1985). This situation, if naturally or artificially prolonged, may lead to a state of carbon starvation and to the beginning of controlled autophagic processes, particularly in sink organs with active metabolism (Thomas, 1978; Baysdorfer et al., 1988). The changes in proteins were thus investigated in the different tissues of the plant during light/dark cycles and extended darkness. As shown in Figure 2, the protein contents did not change significantly in any tissue during the light/dark cycle. However, the decrease in protein occurred after 48 h of darkness, particularly in growing tissues such as mature roots, root tips, and youngest leaves. In these tissues the protein decrease was 21% to 30% after 48 h of dark treatment. In the other tissues, the decrease in protein was significant only after 72 h of darkness (data not shown). Previous studies on sugar deprivation in excised maize root tips showed that protein degradation was linked to the release of the protein nitrogen as amino acids, particularly Asn, and NH4+ (Brouquisse et al., 1992) and to an increase in proteolytic activities (James et al., 1993, 1996). Thus, we investigated the fate of these various parameters in the different parts of the maize plant, and particularly in sink tissues (mature roots, root tips, and youngest leaves), in the course of the light/dark cycle and extended darkness.
Figure 2.
Changes in protein content in different maize plant tissues (root tips, mature roots, senescent leaves, mature leaves, youngest leaves, stems and sheaths, green remainder, and yellow remainder) during light/dark cycles and 48 h of extended darkness. Each point represents the mean (±sd) of five different experiments. DW, Dry weight.
Changes in Free Amino Acids and NH4+
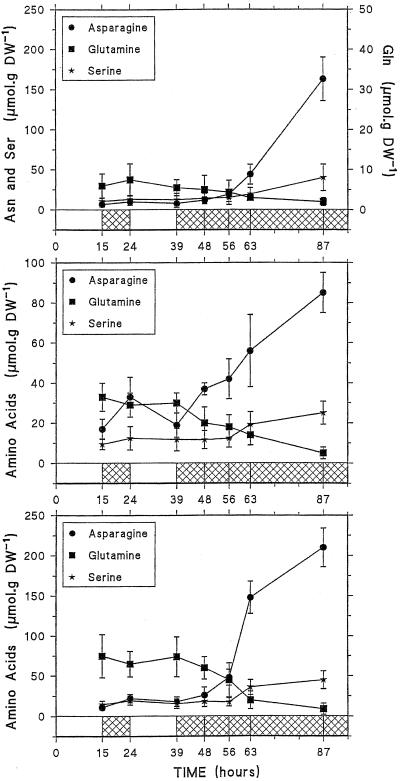
As shown in Figure 3, Gln and Asn changed in opposite ways under varying light conditions. Under a normal light/dark cycle, in mature roots and root tips, Gln content was higher than that of Asn, increasing during the day and decreasing during the night, whereas Asn content increased during the night and decreased during the day. Although smoothed by the variability between the samples of the four (root tips) or five (mature roots) experiment series, such changes between the end of the day and the end of the night were significant in a same series. These changes were not clearly observed in the youngest leaves. After 48 h of darkness, Gln dropped while Asn dramatically increased by a factor of 5, 16, and 18 in mature roots, root tips, and youngest leaves, respectively. It may be noted that Ser, which has been shown to accumulate in carbon-starved, excised root tips (Brouquisse et al., 1992), also increased in the different tissues with a pattern similar to that of Asn. In the other plant tissues Asn content (3–10 nmol g−1 fresh weight) increased by a factor of 1.2 to 9 after 48 h of darkness (data not shown). The increase in Asn has been related to the transient storage of the nitrogen released by the degradation of protein amino acids under carbohydrate starvation (James, 1953; Sieciechowicz et al., 1988; Genix et al., 1990; Brouquisse et al., 1992), since NH4+ is toxic for the cell and cannot be accumulated at a high concentration (Givan, 1979).
Figure 3.
Changes in free amino acids, Asn, Gln, and Ser, in youngest leaves (top), mature roots (middle), and root tips (bottom) of maize plants submitted to light/dark cycles and to 48 h of darkness. Each point represents the mean (±sd) of three (youngest leaves), four (root tips), or five (mature roots) independent experiments. DW, Dry weight.
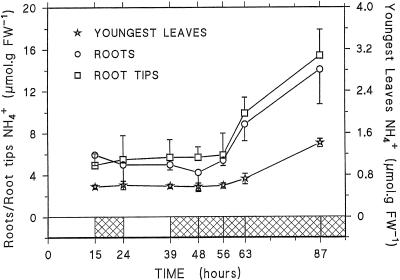
NH4+ content appeared to be quite different between the youngest leaves and the root tissues, at 0.5 and 5 μmol g−1 fresh weight, respectively (Fig. 4). In these tissues its content did not change during the light/dark cycle, but increased 2- to 3-fold between 17 and 48 h of darkness to reach 1.4, 14, and 15.4 μmol g−1 fresh weight in youngest leaves, mature roots, and root tips, respectively. In the other tissues the NH4+ content ranged between 0.6 and 2.3 μmol g−1 fresh weight and did not vary significantly during the experiment (data not shown). Considering that 85% of the fresh weight corresponds to cellular water and assuming an even distribution in cellular water, the [NH4+] in youngest leaves, roots, and root tips was estimated to be 0.7, 5.9, and 6.1 mm, respectively, at the end of the light period (as a comparison, the [NH4+] in the liquid medium was 0.75 mm) and 1.6, 16.5, and 18 mm after 48 h of darkness. However, it has been shown that most of the cell NH4+ is located in the vacuole (Lee and Ratcliffe, 1991; Roberts and Pang, 1992). In the present work, on the basis of certain physiological parameters (i.e. NH4+/NH3 pKa = 9.2, pH vacuole = 5.8, and pH cytoplasm = 7.3) and assuming that the cytoplasm and the vacuole each occupy 45% of the total tissue volume in the root tips (Patel et al., 1990), and 10% and 80%, respectively, in the mature roots, the cytoplasmic [NH4+] after 48 h of darkness was calculated to be 0.28 mm in the root tips and 0.37 mm in the mature roots.
Figure 4.
Changes in NH4+ content in youngest leaves, mature roots, and root tips of maize plants submitted to the light/dark cycle and to 48 h of darkness. Each point represents the mean of three independent experiments. For clarity, only one side of the sd is indicated for root-tip and mature root data. FW, Fresh weight.
Based on changes in proteins, amino acids, and NH4+, it was possible to calculate partial nitrogen balances before and after 48 h of darkness in the different tissues and in the whole plant (Table I, see ΔN values). In sink tissues (root tips, mature roots, youngest leaves, stems and sheaths, and yellow remainder), 38% to 94% of the nitrogen released by net protein breakdown was retrieved as Asn, for only 2% to 9% in mature or senescent tissues (mature and senescent leaves, green remainder). However, in these latter tissues, 54% (green remainder) to 82% (mature and senescent leaves) of the nitrogen released by proteins was lost after 48 h of darkness for only 30% to 49% in root tips, mature roots, and stems and sheaths. In contrast to this, the amount of nitrogen accumulated in NH4+, Asn, and other free amino acids in the youngest leaves and yellow remainder after 48 h of darkness was 19% and 53% higher than the amount of nitrogen released by protein breakdown (Table I). Thus, it appeared that the fate of nitrogen differed according to the tissue. Considered at the level of the whole plant, 53% of the nitrogen released by net protein breakdown was retrieved as free amino acids (two-thirds of which was Asn), 4% as NH4+, and the remaining 42% was lost, presumably as NH4+, in the hydroponic culture medium via the root system (which contained 76% of the ΔNH4+ accumulated in the whole plant, Table I).
Table I.
Distribution of nitrogen between protein, Asn, other free amino acids (other AA), and NH4+ in the different plant tissues and in the whole plant after 15-h light (15h L) and 48-h dark (48h D) periods
| Tissue | Fresh Wt | Nitrogen
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein
|
Asn
|
Other
AA
|
NH4+
|
Σ N
|
||||||||||||
| 15h L | 48h D | ΔN | 15h L | 48h D | ΔN | 15h L | 48h D | ΔN | 15h L | 48h D | ΔN | 15h L | 48h D | Δ (ΣN) | ||
| g plant−1 | μmol tissue−1 | |||||||||||||||
| Root tips | 0.03 ± 0.01 | 12 | 9.4 | −2.6 | 0.13 | 1.93 | 1.80 | 1.28 | 1.04 | −0.24 | 0.18 | 0.45 | 0.27 | 13.6 | 12.8 | −0.8 |
| Mature roots | 11.48 ± 2.03 | 2,239 | 1,619 | −620 | 58.5 | 293 | 234.5 | 227 | 245 | 18 | 57.4 | 177 | 119.6 | 2,582 | 2,334 | −248 |
| Youngest leaves | 0.80 ± 0.21 | 150 | 106 | −44 | 1.68 | 40.7 | 39.02 | 7.84 | 20.4 | 12.56 | 0.48 | 1.13 | 0.65 | 160 | 168.2 | 8.2 |
| Mature leaves | 15.80 ± 3.70 | 4,746 | 3,960 | −786 | 19 | 71 | 52 | 202 | 284 | 82 | 23.7 | 30 | 6.3 | 4,991 | 4,345 | −646 |
| Senescent leaves | 1.50 ± 0.47 | 388.5 | 322 | −66.5 | 0.9 | 6.75 | 5.85 | 7.95 | 14.7 | 6.75 | 1.5 | 1.2 | −0.3 | 399 | 345 | −54 |
| Stems and sheaths | 28.5 ± 5.90 | 7,638 | 6,384 | −1254 | 68.4 | 556 | 487.6 | 462 | 593 | 131 | 17 | 37 | 20 | 8,185 | 7,570 | −615 |
| Green remainder | 17.10 ± 5.31 | 4,891 | 4,429 | −462 | 51.3 | 60 | 8.7 | 357 | 559 | 202 | 27.4 | 28.2 | 0.8 | 5,326 | 5,076 | −250 |
| Yellow remainder | 12.80 ± 3.67 | 3,277 | 2,820 | −457 | 50 | 480 | 430 | 471 | 730 | 259 | 35.2 | 46.1 | 10.9 | 3,833 | 4,076 | 243 |
| Whole plant | 88.01 ± 21.09 | 23,342 | 19,649 | −3692 | 250 | 1509 | 1259 | 1736 | 2447 | 711 | 163 | 321 | 158 | 25,490 | 23,927 | −1563 |
The amount of nitrogen in protein was calculated assuming that the nitrogen content of protein is 16.7%. The conversion of protein, Asn, and other amino acid contents from micromoles per gram dry weight to micromoles per gram fresh weight was calculated on a basis that dry weight represents 15% of fresh weight, as mentioned in Results. Σ N represents the sum of (protein plus Asn plus other AA plus NH4+) nitrogen amounts present in each tissue. ΔN represents the difference in nitrogen amount present in either protein, Asn, other amino acids, NH4+, or Σ N between 48-h dark- and 15-h light-treated tissues.
Changes in Endoproteolytic Activities
In previous work we showed that endoproteolytic activities increase in excised maize root tips submitted to carbon starvation (James et al., 1993), and suggested that both the vacuolar and the specific nuclear and cytosolic proteolytic systems were potentially involved in the plant response to starvation (James et al., 1996). Thus, we followed first the changes in global endopeptidase activities in youngest leaves, roots, and root tips, and second, by western-blot experiments, the changes in the amounts of vacuolar RSIP and in the nuclear and cytosolic 20S proteasome.
Total endopeptidase activities, measured with azocasein as a substrate, were found to be higher in mature roots than in the youngest leaves (25–30 and 5–7 μg azocasein min−1 g−1 fresh weight, respectively), and to remain essentially unchanged during light/dark cycles (Fig. 5A). However, after 48 h of darkness a significant and reproducible 1.5-fold increase in endopeptidase activity was observed in mature roots, and to a lesser extent in the youngest leaves. Western-blot data showed that in both tissues the amount of 20S proteasome remained unchanged throughout the experiment (Fig. 5B). RSIP was found to be present in the mature roots and to slightly increase under permanent darkness, but it was not detectable in the youngest leaves (Fig. 5B). These last results are in agreement with a previous study (James et al., 1996) that showed that RSIP is absent in leaves and only present in roots of young maize seedlings. The increase in endopeptidase activity in the whole roots was linked to the increase in RSIP amount, since in both 15-h light and 48-h dark tissue extracts, RSIP was found to represent about 70% to 75% of the total activity after immunoprecipitation experiments (Table II).
Figure 5.
Changes in total endopeptidase (EP) activity and immunodetection by western-blot analysis of RSIP (SDS-PAGE) and 20S proteasome (native-PAGE) in mature roots (Root) and youngest leaves (Y.L.) of maize plants submitted to light/dark cycles and 48 h of darkness. A, Azocasein was used as a substrate. Each point represents the mean (±sd) of three (youngest leaves) or six (mature roots) independent experiments. B, The equivalent to 0.5 mg dry weight of mature roots (bottom, 100–70 μg of protein) and youngest leaves (top, 90–60 μg of protein) was loaded onto each lane. FW, Fresh weight.
Table II.
Percentage of endopeptidase activity brought about by RSIP in mature roots and root tips after 15-h light and 48-h dark periods
| Tissue | 15 h of Light | 48 h of Dark |
|---|---|---|
| % | ||
| Mature roots | 74 –79 | 67 –78 |
| Root tips | 35 –39 | 72 –83 |
Equal amounts of azocaseinolytic activity (400 nmol azocasein min−1) were analyzed by an immunoprecipitation test in the presence of immune or preimmune anti-RSIP serum, as described in Methods. The data are the results of two independent experiments.
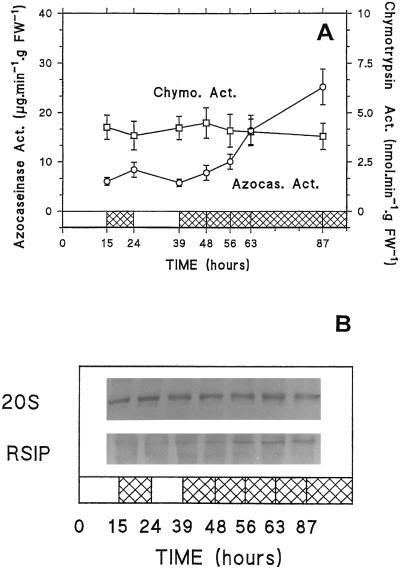
In root tips global endopeptidase activities (6.1 μg azocasein min−1 g−1 fresh weight) were found to be lower than in mature roots (Fig. 6A). However, these activities showed significant and reproducible variations during light/dark cycles, increasing and decreasing during the dark and the light periods, respectively, and then increased 5-fold after 48 h of darkness (Fig. 6A). This increase in endopeptidase activities was linked to a significant enrichment of the root tips in RSIP, as shown after immunodetection on western-blot (Fig. 6B) and immunoprecipitation experiments (Table II). Using a synthetic substrate, Succ-Leu-Leu-Val-Tyr-AMC, we also measured the chymotrypsinlike activity, which was found to be a good marker for the activity of the 20S proteasome in maize root tips (G. Basset, R. Brouquisse, L. Malek, and P. Raymond, unpublished data). Neither the chymotrypsin-like activity nor the amount of 20S proteasome protein showed any change during light/dark cycles or prolonged darkness (Fig. 6) in spite of the decrease in the bulk of cell proteins.
Figure 6.
Changes in total endopeptidase and chymotrypsin-like activities and immunodetection by western-blot analysis of RSIP (SDS-PAGE) and 20S proteasome (native-PAGE) in root tips of maize plants submitted to light/dark cycles and 48 h of darkness. A, Azocasein was used as a substrate for total endopeptidase activity measurement, and Succ-Leu-Leu-Val-Tyr-MCA was used for chymotrypsin-like activity measurement. Each point represents the mean (±sd) of four (azocasein) or two (chymotrypsin-like) independent experiments. B, The equivalent to 0.25 mg dry weight of root tips (100–75 μg of protein) was loaded onto each lane. FW, Fresh weight; Chymo. Act., chymotrypisin activity; Azoca. Act., azocaseinase activity.
In senescent leaves, mature leaves, and stems and sheaths, endoproteolytic activities ranged from 3 to 8 μg azocasein min−1 g−1 fresh weight and did not change significantly during the experiment (data not shown).
Root-Growth Measurement
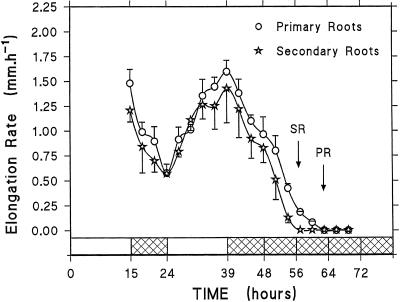
To assess the effect of carbon deprivation on root growth, we followed root elongation during the light/dark cycle and under extended darkness. As reported in Figure 7, primary and secondary roots exhibited changing elongation rates during light/dark cycles, speeding up in the light and slowing down in the dark. Such an effect has been reported in various studies and was attributed to the decrease of carbohydrate supply to the root system (Williams and Farrar, 1990; Bingham and Stevenson, 1994; Merlo et al., 1994). In the present experiment the pattern of the root elongation rate (Fig. 7) closely resembled that of root sugar content (Fig. 1). It is striking that the mean elongation rate dropped within the first 3 h of the dark period. Under prolonged darkness the roots ceased to elongate after 15 to 24 h, when the decrease in protein and the increase in Asn, NH4+, and in endopeptidase activities became significant in the root tips (Figs. 2, 3, 4, and 6). In addition, secondary root elongation stopped after 15 to 18 h of darkness, whereas primary roots continued to grow up to 21 to 24 h (Fig. 7, arrows). This suggests that sugar limitation was effective in the secondary roots first and only 6 h later in the primary roots. On the basis of root elongation, it was found that roots survived at least to 96 h in darkness, since they were able to reinitiate their growth after return to initial light/dark conditions (data not shown).
Figure 7.
Changes in the elongation rate of primary and secondary roots of maize plants during light/dark cycles and extended darkness. These data are from a representative experiment that has been repeated two times. The first and second points of each curve represent the mean growth rates over the last 3 h of the light period and the first 3 h of the dark period, respectively. Each point represents the mean (±sd) of two (primary roots) or four (secondary roots) measurements. For clarity, only one side of the sd was indicated. Arrows indicate the moment when secondary (SR) or primary (PR) roots stopped growing.
DISCUSSION
In 3-week-old maize plants grown under medium light intensity, the soluble sugar content strongly changed during the light/dark cycle in all of the plant tissues, and dropped dramatically during extended darkness (Fig. 1). Numerous studies have shown that following carbohydrate depletion, the growth and respiration of plant tissues decrease, and net protein and lipid breakdown starts (James, 1953; Thomas, 1978; Wittenbach, 1978; Baysdorfer et al., 1988; King et al., 1990; Williams and Farrar, 1990; Brouquisse et al., 1991; Bingham and Stevenson, 1994). In the present experiments no net protein breakdown was observed during the light/dark cycle, but when the dark period was extended, proteins significantly decreased in growing tissues such as roots or youngest leaves, whereas they moderately decreased in mature tissues (Fig. 2). Actively metabolizing and growing tissues are known to be very sensitive to carbohydrate depletion, and the early decrease in protein may be related to the cessation of growth and to the beginning of degradative processes (Journet et al., 1986; Baysdorfer et al., 1988; Brouquisse et al., 1991; Moriyasu and Ohsumi, 1996). Carbon deprivation-related proteolysis is generally accompanied by associated symptoms such as increased Asn and NH4+ contents and increased endopeptidase activities (Thomas, 1978; King et al., 1990; Brouquisse et al., 1992; Peeters and van Laere, 1992; James et al., 1993).
During light/dark cycles Gln and Asn exhibited opposite trends in root tissues (Fig. 3). Both amino acids play a crucial role in plant growth and development, since they are the primary nitrogen-transport compounds within most plants (Urquhart and Joy, 1981) and their synthesis is regulated by sugar supply and other regulatory signals (for review, see Lam et al., 1996). Thus, whereas in light-grown plants Gln is preferentially synthesized, in dark-grown plants in which photosynthetic carbon is limiting, Asn is synthesized preferentially to transport nitrogen (Lam et al., 1996). In the present experiment, as neither NH4+ nor proteins changed significantly during the light/dark cycle (Figs. 2 and 4), the increase in root Asn during the dark period cannot be ascribed to an increase in net protein breakdown, but may rather be explained by the regulation of the nitrogen assimilation pathways.
Under prolonged darkness the situation is different: the dramatic increase in Asn is related to the breakdown of proteins and to an increase in Ser and NH4+contents (Figs. 2, 3 and 4). As already suggested (Brouquisse et al., 1992), in carbon-starved tissues Asn plays the role of a nitrogen storage compound and avoids a toxic accumulation of NH4+ inside the cell (Givan, 1979; Siechiechowicz et al., 1988). Considering the compartmentation of NH4+ between cytoplasm (<0.4 mm) and vacuole (19–36 mm) in the root tissues, NH4+ should not affect cytoplasmic metabolism. As reported in Table I, the nitrogen released by protein degradation is differently metabolized according to the plant tissue. In actively growing sink tissues (root tips, youngest leaves, and yellow remainder), it is significantly retrieved as Asn (more than 70%) and other free amino acids, whereas it is essentially lost (more than 80%) in mature or senescent source tissues (mature and senescent leaves), the other tissues (mature roots, stems and sheaths, and green remainder) showing intermediate behavior. Taken together, these observations suggest that in whole maize plants submitted to transient starvation, the nitrogen issued from net proteolysis is metabolized or lost. In aerial source tissues (mature and senescent leaves, and to some extent, green remainder), it is massively exported to be either released in the incubation medium via the root system or partially reallocated to aerial sink tissues (youngest leaves and yellow remainder). In contrast, in the sink organs (root tips, youngest leaves, yellow remainder, and to a lesser extent mature roots and stems and sheaths), the nitrogen issued from proteolysis is primarily stored as Asn or free amino acids and, to a lesser extent, partly lost into the incubation medium. Thus, in these latter tissues, dividing and elongating cells will be able to use a “cheap” (in terms of energy cost) and readily available source of nitrogen as soon as the carbon supply to growing tissues increases again (Brouquisse et al., 1992). The synthesis of Asn is probably catalyzed by Asn synthetase, the main route for Asn synthesis in plants (Siechiechowicz et al., 1988; Lam et al., 1996), because its activity and mRNA level were found to increase in maize root tips submitted to carbon starvation (Brouquisse et al., 1992; Chevalier et al., 1996).
No net protein degradation and no increase in endopeptidase activities were observed during light/dark cycles. However, after 48 h of darkness total endopeptidase activities increased in root tips and mature roots (Figs. 2, 5, and 6). In the roots the increase in proteolytic activity was associated with the induction of RSIP, a vacuolar Ser endopeptidase shown to account for up to 80% of the endopeptidase activity in roots of whole plants (Table II) and sugar-starved, excised maize roots (James et al., 1996). As in yeast (Baba et al., 1994) and animal cells (Seglen and Boley, 1992), autophagy has been shown to occur in plant cells submitted to sugar starvation (Aubert et al., 1996; Moriyasu and Ohsumi, 1996). In tobacco cells deprived of Suc, the appearance of autophagic vacuoles has been associated with an increase in endopeptidase activities (Moriyasu and Ohsumi, 1996), and in Suc-starved sycamore cells, the development of autophagic vacuoles was correlated with an increase in P-choline, a marker of phospholipid degradation (Aubert et al., 1996). The presence of an increasing amount of RSIP in maize roots during extended darkness (Fig. 6B; Table II) suggests that vacuolar autophagy may be involved in the degradative processes (e.g. proteolysis) that take place in the root system. Although endopeptidase activities did not increase significantly in the youngest leaves, net protein breakdown and Asn increase clearly occurred after 48 h of darkness. Preexisting proteolytic systems may be active enough to account for the net degradation of the proteins in the youngest leaves or the delay in endopeptidase induction in youngest leaves compared with root tissues. Alternatively, azocasein could be a poor substrate for the newly induced proteases in leaves, and the azocaseinolytic measurement would not account for all of the endopeptidase induction during the dark period.
Although vacuolar proteolysis and autophagy are known to be nonselective processes (Marty, 1978; Seglen and Bohley, 1992), the maintenance or increase of some enzymes, in a general context of degradation, suggests that some degradative processes should be selective. In addition, the observation that changes in metabolic state (as shown by the change in Gln/Asn, Fig. 3) and in growth rate (Fig. 7) occur before any significant decrease in the amount of total protein (Fig. 2) suggests that the protein equipment is changed through increased protein turnover, in which selective proteolysis may have a major role. The proteasome-mediated, ubiquitin-dependent proteolysis, which is present in the cytosol and the nucleus of eukaryotic cells, is highly selective (Coux et al., 1996). Furthermore, ubiquitin and proteasome have been shown to increase in skeletal rat muscle during starvation (Medina et al., 1995). As we showed that 20S proteasome is present in maize plants, and particularly in growing tissues (G. Basset, R. Brouquisse, L. Malek, and P. Raymond, unpublished data), it was tempting to investigate its fate during the light/dark cycle and extended darkness. On the basis of its immunosignal on western blots and, in the case of the root tips, on its chymotrypsin-like activity (Figs. 5 and 6), it can be seen that the proteasome remained steady for up to 48 h of darkness, whereas the total protein decreased. This suggests that in the cytosol and the nucleus of carbon-deprived cells, proteasome-mediated proteolysis could selectively hydrolyze some proteins, thus allowing the synthesis of enzymes or proteic factors necessary for the acclimation to starvation.
Finally, the time course of the appearance of starvation symptoms varied according to the tissue; proteolysis first appears in sink tissues and later in mature tissues. These observations raise the problem of the regulation of proteolysis by sugar supply. The expression of numerous plant genes is known to be regulated by carbohydrate status (for review, see Koch, 1996) and it is clear that global protein breakdown is also regulated by sugars (James et al., 1993). The potential involvement of different proteolytic systems (i.e. vacuolar- and ubiquitin-dependent proteolysis) raises questions concerning their regulation and their respective roles in the response to starvation. Whereas the involvement of the lysosomal/vacuolar pathway in autophagy associated with nutrient deprivation is well established, ubiquitin- and proteasome-dependent proteolysis has been associated with actively growing tissues well supplied with nutrients (Ichihara and Tanaka, 1995; Coux et al., 1996). The identification of the natural substrates of the proteases and of the signals (and their transduction pathways) that regulate the expression of the gene encoding these proteases are two major objectives of our ongoing research on proteolysis.
ACKNOWLEDGMENTS
We wish to warmly thank Soazig Ledeleter, Emmanuelle Constant, Frédéric Madec, and Mikaël Laizet, the undergraduate students who helped us to prepare and analyze the 2716 extracts necessary for this study.
Abbreviations:
- RSIP
root starvation-induced protease
- Succ-Leu-Leu-Val-Tyr-AMC
N-succinyl-Leu-Leu-Val-Tyr-7-amino-4-methyl coumarin
Footnotes
This work was supported by the French Institut National de la Recherche Agronomique.
LITERATURE CITED
- Amthor JS, McCree KJ (1990) Carbon balance of stressed plants: a conceptual model for integrating research results. In RG Alscher, JC Cumming, eds, Stress Responses in Plants: Adaptation and Acclimation Mechanisms. Wiley-Liss & Sons, Inc., New York, pp 1–15
- Aubert S, Gout E, Bligny R, Marty-Mazars D, Barrieu F, Alabouvette J, Marty F, Douce R. Ultrastructural and biochemical characterization of autophagy in higher plant cells subjected to carbon deprivation: control by the supply of mitochondria with respiratory substrates. J Cell Biol. 1996;133:1251–1263. doi: 10.1083/jcb.133.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M, Takeshige K, Baba N, Ohsumi Y. Ultrastructural analysis of the autophagic process in yeast: detection of autophagosomes and their characterization. J Cell Biol. 1994;124:903–913. doi: 10.1083/jcb.124.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baysdorfer C, Warmbrodt RD, Van Der Woude WJ. Mechanisms of starvation tolerance in pearl millet. Plant Physiol. 1988;88:1381–1387. doi: 10.1104/pp.88.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham IJ, Stevenson EA. Control of root growth: effects of carbohydrates on the extension, branching and rate of respiration of different fractions of wheat roots. Physiol Plant. 1994;88:149–158. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brouquisse R, James F, Pradet A, Raymond P. Asparagine metabolism and nitrogen distribution during protein degradation in sugar-starved maize root tips. Planta. 1992;188:384–395. doi: 10.1007/BF00192806. [DOI] [PubMed] [Google Scholar]
- Brouquisse R, James F, Raymond P, Pradet A. Study of glucose starvation in excised maize root tips. Plant Physiol. 1991;96:619–626. doi: 10.1104/pp.96.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callis J. Regulation of protein degradation. Plant Cell. 1995;7:845–857. doi: 10.1105/tpc.7.7.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier C, Bourgeois E, Just D, Raymond P. Metabolic regulation of asparagine synthetase gene expression in maize (Zea mays L.) root tips. Plant J. 1996;9:1–11. doi: 10.1046/j.1365-313x.1996.09010001.x. [DOI] [PubMed] [Google Scholar]
- Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- Dejong TM, Grossman YL. Quantifying sink and source limitations on dry matter partitioning to fruit growth in peach trees. Physiol Plant. 1995;95:437–443. [Google Scholar]
- Dieuaide M, Brouquisse R, Pradet A, Raymond P. Increased fatty acid β-oxidation after glucose starvation in maize root tips. Plant Physiol. 1992;99:595–600. doi: 10.1104/pp.99.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieuaide M, Couée I, Pradet A, Raymond P. Effects of glucose starvation on the oxidation of fatty acids by maize root tip mitochondria and peroxisomes: evidence for fatty acid β-oxidation and acyl-coA dehydrogenase activity in higher plants. Biochem J. 1993;296:199–207. doi: 10.1042/bj2960199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elamrani A, Couée I, Carde J-P, Gaudillère J-P, Raymond P. Modification of etioplasts in cotyledons during prolonged dark growth of sugar beet seedlings. Plant Physiol. 1994a;106:1555–1565. doi: 10.1104/pp.106.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elamrani A, Gaudillère J-P, Raymond P. Carbohydrate starvation is a major determinant of the loss of greening capacity in cotyledons of dark-grown sugar beet seedlings. Physiol Plant. 1994b;91:56–64. [Google Scholar]
- Feller U, Fischer A. Nitrogen metabolism in senescing leaves. Crit Rev Plant Sci. 1994;13:241–273. [Google Scholar]
- Frossard JS. L'éclairement du feuillage, facteur de régulation du rythme nycthéméral de la respiration des racines. Physiol Vég. 1985;23:163–173. [Google Scholar]
- Genix P, Bligny R, Martin JB, Douce R. Transient accumulation of asparagine in sycamore cells after a long period of sucrose starvation. Plant Physiol. 1990;94:717–722. doi: 10.1104/pp.94.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givan CV. Metabolic detoxification of ammonia in tissues of higher plants. Phytochemistry. 1979;18:375–382. [Google Scholar]
- Graham IA. Carbohydrate control of gene expression in higher plants. Res Microbiol. 1996;147:572–580. doi: 10.1016/0923-2508(96)84014-9. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system for protein degradation. Annu Rev Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- Ho LC. Tomato. In: Zamski E, Schaffer AA, editors. Photoassimilate Distribution in Plants and Crops. Source-Sink Relationships. Inc., New York: Marcel Dekker; 1996. pp. 709–728. [Google Scholar]
- Ichihara A, Tanaka K. Role of proteasomes in cell growth. Mol Biol Rep. 1995;21:49–52. doi: 10.1007/BF00990970. [DOI] [PubMed] [Google Scholar]
- Irving DE, Hurst PL. Respiration, soluble carbohydrates and enzymes of carbohydrate metabolism in tips of harvested asparagus spears. Plant Sci. 1993;94:89–97. [Google Scholar]
- Ismail I, De Bellis L, Alpi A, Smith SM. Expression of glyoxylate cycle genes in cucumber roots responds to sugar supply and can be activated by shading or defoliation of the shoots. Plant Mol Biol. 1997;35:633–640. doi: 10.1023/a:1005840522049. [DOI] [PubMed] [Google Scholar]
- James F, Brouquisse R, Pradet A, Raymond P. Changes in proteolytic activities in glucose-starved maize root tips: regulation by sugars. Plant Physiol Biochem. 1993;31:845–856. [Google Scholar]
- James F, Brouquisse R, Suire C, Pradet A, Raymond P. Purification and biochemical characterization of a vacuolar serine endopeptidase induced by glucose starvation in maize roots. Biochem J. 1996;320:283–292. doi: 10.1042/bj3200283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James WO. Plant Respiration. The. Oxford, UK: Clarendon Press; 1953. [Google Scholar]
- Journet EP, Bligny R, Douce R. Biochemical changes during sucrose deprivation in higher plant cells. J Biol Chem. 1986;261:3193–3199. [PubMed] [Google Scholar]
- Rufty PSJR, Kerr TW, Huber SC. Changes in nonstructural carbohydrates in different parts of soybean (Glycine max [L.] Merr.) plants during a light/dark cycle and in extended darkness. Plant Physiol. 1985;78:576–581. doi: 10.1104/pp.78.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GA, Woollard DC, Irving DE, Borst WM. Physiological changes in asparagus spear tips after harvest. Physiol Plant. 1990;80:393–400. [Google Scholar]
- Koch K. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam HM, Coschigano KT, Oliveira IC, Melo-Oliveira R, Coruzzi GM. The molecular-genetics of nitrogen assimilation into amino acids in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:569–593. doi: 10.1146/annurev.arplant.47.1.569. [DOI] [PubMed] [Google Scholar]
- Lee RB, Ratcliffe RG. Observation of the subcellular distribution of the ammonium ion in maize root tissue using in-vivo 14N-nuclear magnetic resonance spectroscopy. Planta. 1991;183:359–367. doi: 10.1007/BF00197734. [DOI] [PubMed] [Google Scholar]
- Marty F. Cytochemical studies on GERL, provacuoles, and vacuoles in root meristematic cell of Euphorbia. Proc Natl Acad Sci USA. 1978;75:852–856. doi: 10.1073/pnas.75.2.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina R, Wing SS, Goldberg AL. Increase in levels of polyubiquitin and proteasome mRNA in skeletal muscle during starvation and denervation atrophy. Biochem J. 1995;307:631–637. doi: 10.1042/bj3070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo L, Ferretti M, Passera C, Ghisi R. Effect of decreased irradiance on N and C metabolism in leaves and roots of maize. Physiol Plant. 1994;91:72–80. [Google Scholar]
- Moing A, Carbonne F, Rashad MH, Gaudillère J-P. Carbon fluxes in mature peach leaves. Plant Physiol. 1992;100:1878–1884. doi: 10.1104/pp.100.4.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyasu Y, Ohsumi Y. Autophagy in tobacco suspension-cultured cells in response to sucrose starvation. Plant Physiol. 1996;111:1233–1241. doi: 10.1104/pp.111.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noodén LD (1988) Postlude and prospects. In LD Noodén, AC Leopold, eds, Senescence and Aging in Plants. Academic Press, Inc., San Diego, CA, pp 499–517
- Patel DD, Barlow PW, Lee RB. Development of vacuolar volume in the root tips of pea. Ann Bot. 1990;65:159–169. [Google Scholar]
- Peeters KMU, Van Laere AJ. Ammonium and amino acid metabolism in excised leaves of wheat (Triticum aestivum) senescing in the dark. Physiol Plant. 1992;84:243–249. [Google Scholar]
- Peoples MB, Dalling MJ (1988) The interplay between proteolysis and amino acid metabolism during senescence and nitrogen reallocation. In LD Noodén, AC Leopold, eds, Senescence and Aging in Plants. Academic Press, Inc., San Diego, CA, pp 181–217
- Roberts JKM, Pang MKL. Estimation of ammonium ion distribution between cytoplasm and vacuole using nuclear magnetic resonance spectroscopy. Plant Physiol. 1992;100:1571–1574. doi: 10.1104/pp.100.3.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby C, Martin JB, Bligny R, Douce R. Biochemical changes during sucrose deprivation in higher plant cells: 31P-nuclear magnetic resonance studies. J Biol Chem. 1987;262:5000–5007. [PubMed] [Google Scholar]
- Saglio PH, Pradet A. Soluble sugars, respiration and energy charge during aging of excised maize root tips. Plant Physiol. 1980;66:516–519. doi: 10.1104/pp.66.3.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen PO, Boley P. Autophagy and other vacuolar protein degradation mechanisms. Experientia. 1992;48:158–172. doi: 10.1007/BF01923509. [DOI] [PubMed] [Google Scholar]
- Setter TL (1990) Transport/harvest index: photosynthate partitionning in stressed plants. In RG Alscher, JC Cumming, eds, Stress Responses in Plants: Adaptation and Acclimation Mechanisms. Wiley-Liss & Sons, Inc., New York, pp 17–36
- Sieciechowicz KA, Joy KW, Ireland RJ. The metabolism of asparagine in plants. Phytochemistry. 1988;27:663–671. [Google Scholar]
- Stitt M, ap Rees T, (1978) Pathways of carbohydrate oxidation in leaves of Pisum sativum and Triticum aestivum. Phytochemistry 17: 1251–1256
- Tassi F, Maestri E, Restivo FM, Marminoli N. The effects of carbon starvation on cellular metabolism and protein and RNA synthesis in Gerbera callus cultures. Plant Sci. 1992;83:127–136. [Google Scholar]
- Thomas H. Enzymes of nitrogen mobilization in detached leaves of Folium temulentum during senescence. Planta. 1978;142:161–169. doi: 10.1007/BF00388207. [DOI] [PubMed] [Google Scholar]
- Urquhart AA, Joy KW. Use of phloem exudate technique in the study of amino acid transport in pea plant. Plant Physiol. 1981;68:750–754. doi: 10.1104/pp.68.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra RD. Protein degradation in plants. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:385–410. [Google Scholar]
- Webster PL, Van't Hof J. Polyribosomes in proliferating and non-proliferating root meristem cells. Am J Bot. 1973;60:117–121. [Google Scholar]
- Williams JHH, Farrar JF. Control of barley root respiration. Physiol Plant. 1990;79:259–266. [Google Scholar]
- Wittenbach VA. Breakdown of ribulose carboxylase and change in proteolytic activity during dark-induced senescence of wheat seedlings. Plant Physiol. 1978;62:604–608. doi: 10.1104/pp.62.4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]