Abstract
Reference strains of infectious pancreatic necrosis virus resembling the 10 recognized serotypes and local isolates of aquabirnaviruses isolated in northwestern Spain from reservoirs (mollusks) and from asymptomatic and carrier cultured fish were genotyped by restriction fragment length polymorphism (RFLP) and nucleic acid sequence analyses. The RFLP analysis yielded seven genogroups, each of which was clearly correlated with a serotype. Sequence analysis of the three open reading frames provided quite similar results in terms of genogrouping. Based on the results of this study and in order to unify the two types of assays, we propose placing aquabirnaviruses into six genogroups, four of which can be subdivided into two genotypes based on a two-step restriction analysis. The genotyping corresponds with serotyping as follows: genogroup I includes two genotypes corresponding to serotypes A9 (genotype I.1) and A1 (genotype I.2); genogroup II corresponds to serotype A3; genogroup III includes genotypes III.1 (serotype A2) and III.2 (serotype B1); genogroups IV and V include two genotypes, each corresponding to serotypes A5, A6, A7, and A8 (genotypes IV.1, IV.2, V.1, and V.2, respectively);and genogroup VI corresponds to serotype A4. As expected, most local isolates belonged to genotype III.1 and genogroup II. However, a few local isolates corresponded to the American types of genogroup I. Finally, based on the results of this study and due to its simplicity, the two-step restriction analysis assay is proposed as a method for typing new isolates of aquabirnaviruses, and the results correspond to the results of conventional serotyping.
Infectious pancreatic necrosis virus (IPNV), a member of the family Birnaviridae, is one of the mayor etiological agents found in fish farms, wild fish, and reservoirs in northwestern Spain (18, 22, 23). IPNV is an unenveloped icosaedrical virus with a bisegmented double-stranded RNA. Genome segment A contains two open reading frames (ORFs), a large ORF encoding a 106-kDa polyprotein (NH2-pVP2-NS-VP3-COOH) and a small ORF encoding VP5 and overlapping the amino end of the large ORF (7). Genome segment B encodes the minor internal polypeptide VP1 (94 kDa), which is the putative RNA-dependent RNA polymerase. Although some aquabirnaviruses cause lethal diseases and produce high mortalities in fry (1, 5, 20), the presence of the virus in the population does not always result in mortality or clinical disease, and the virus is commonly associated with apparently healthy asymptomatic fry stocks and breeders (24) or with environmental reservoirs (19, 23).
One of the main problems in epidemiological studies of aquabirnaviruses is the difficulty of typing new isolates due to the large range of serotypes that exist. Standard serotyping of strains of IPNV from aquatic organisms has been performed by neutralization with polyclonal antisera or by enzyme immunoassays with monoclonal antibodies (4, 14). Most aquabirnaviruses are antigenically related and belong to serogroup A, which includes nine serotypes, serotypes A1 (reference strain, West Buxton), A2 (Spajarup), A3 (Abild), A4 (Hecht), A5 (Tellina), A6 (Canada 1), A7 (Canada 2), A8 (Canada 3), and A9 (Jasper). Antigenically unrelated aquatic birnaviruses constitute serogroup B, which includes a single serotype, serotype B1 (reference strain, TV-1). However, this classification is controversial because serotyping may yield ambiguous or even nonrepeatable results due to the lack of standardized antisera (8, 14). Therefore, at present, an increasing number of researchers are employing molecular techniques for typing and classification of these viruses (3, 8).
Nucleotide sequencing is becoming the ultimate tool for characterization of aquabirnaviruses. Partial or complete genome sequences of an increasing number of aquabirnavirus strains provide important information on gene structure and have led to detection of group-specific and serotype-specific epitopes (4, 11, 17, 21, 25). Several authors have used genomic and/or deduced amino acid sequences for comparison of aquabirnavirus strains (3, 11, 13, 15) and have proposed genogroups which do not always correspond completely with previously established serogroups.
Because of the relatively high cost of this technology and since it is not available in all laboratories, some researchers have developed a different approach for molecular typing of aquabirnavirus. Restriction fragment length polymorphism (RFLP) patterns have been used to compare reference type strains with local isolates from different areas (2, 12, 16). In these studies, the amplified fragments have not always been the same. Heppell at al. (12) employed a 359-bp fragment corresponding to the NS variable region, while Lee et al. (16) and Biering et al. (2) applied RFLP analysis to a larger region representing most of the VP2 coding region. On the other hand, Heppell at al. (12) employed reference strains of all the serotypes of serogroup A, and they did not find that there was a complete correlation with RFLP genotyping results. Using the complete VP2 region yielded better results for a correlation between serotyping and genotyping, although in these studies (2, 16) the authors did not employ reference strains for all of the existing serotypes.
In the present study, we sequenced the complete viral genome and used RFLP analysis of the VP2 coding region to genotype a number of aquabirnaviruses isolated from our area. The reference strains of the 10 serotypes of serogroups A and B were also evaluated.
MATERIALS AND METHODS
Cells and viruses.
Reference strains of serotypes A1 (West Buxton), A2 (Spajurup), A3 (Abild), A4 (Hecht), A5 (Tellina), A6 (Canada 1), A7 (Canada 2), A8 (Canada 3), A9 (Jasper), and B1 (TV-1) were purchased from the American Type Culture Collection. CV-HB1, a reservoir strain from clams, was kindly provided by C.-F. Lo (Academia Sinica, Taipei, Taiwan). Local strains from Galicia (northwestern Spain) were isolated in our laboratory and included isolates from fish farms (isolates 2290 and 2310 from salmon [Salmo salar]; isolates 24FO, 405, 533, 534, 1146, 2284, and 2464 from trout [Oncorhynchus mykiss]; isolates 152 and 578 from turbot [Scophthalmus maximus]) and environmental isolates from several kinds of mollusks acting as reservoirs (23) near those fish farms, including isolates 24R and 55R from mussels and isolate 88R from oysters. Based on the electrophoretic mobilities of their genomes, these Galician strains were previously classified into electropherogroups by Cutrin et al. (6). All the viruses were propagated in Chinook salmon embryo cells (CHSE-214) at 15°C by using Eagle's minimum essential medium supplemented with 100 IU of penicillin per ml, 100 μg of streptomycin per ml, and 10% fetal calf serum.
RNA extraction.
The supernatant from an infected cell culture monolayer was collected when extensive cytopathic effects were observed. Cell debris was removed by centrifugation at 2,500 × g for 10 min. The pellets were resuspended in 1× SSC buffer (0.15 M NaCl plus 0.015 M sodium citrate, pH 7.0) and sonicated (30 s at 20 kHz). The virus was concentrated by centrifugation at 78,000 × g for 90 min at 4°C, and each pellet was resuspended again in 1× SSC buffer. After treatment with 2 mg of proteinase K (Sigma) per ml and 0.5% sodium dodecyl sulfate for 2 h at 56°C, the RNA was extracted with phenol-chloroform-isoamyl alcohol (25:24:1) and precipitated with 2.5 volumes of 100% ethanol and 0.1 volume of 3 M sodium acetate at −20°C overnight. The RNA was centrifuged at 14,000 × g for 30 min and washed with 70% ethanol. The pellets were then vacuum dried with a Speedvac (Savant) for about 10 min and resuspended in TE buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA). Viral RNA was quantified by spectrophotometry at 260 nm and stored at −20°C.
RT-PCR amplification.
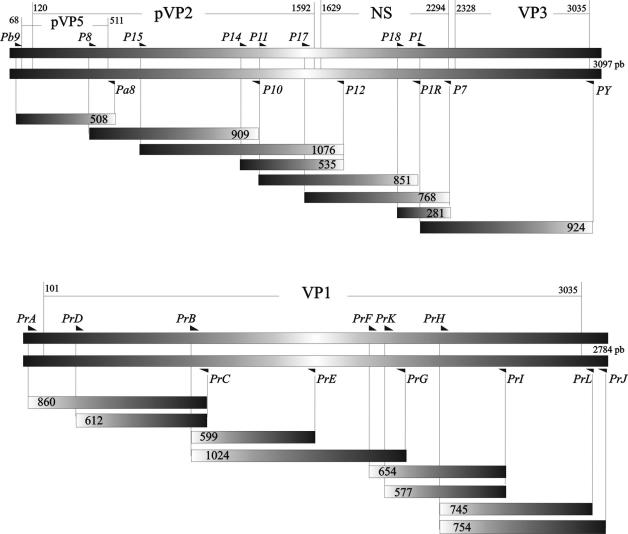
Random hexamers (Promega) at a final concentration of 1.25 mM were annealed to 100 ng of purified viral RNA per μl in nuclease-free water by heating the preparation at 80°C for 10 min and incubating it at 37°C for another 10 min. Samples were cooled on ice and briefly centrifuged at 10,000 × g. For cDNA synthesis, a reverse transcription (RT) mixture containing 200 U of Superscript II RNase H RT− (Gibco BRL), deoxyribonucleoside triphosphates (1 mM each; Promega), 40 U of RNase inhibitor (Promega), and 5 mM MgCl2 in Mg-free PCR buffer (Gibco BRL) was added and incubated for 1 h at 37°C. Prior to PCR amplification, the samples were denatured for 10 min at 99°C and briefly centrifuged at 10,000 × g. Ten microliters of the denatured RT solution was transferred to a PCR mixture (final volume, 50 μl) containing 2 mM MgCl2, both specific primers at a concentration of 0.5 μM, and 2.5 U of Taq polymerase (Perkin-Elmer). Following an initial 2-min denaturation step at 94°C, the mixture was subjected to 35 cycles of amplification (denaturation for 45 s at 94°C, annealing for 45 s at 59°C, and extension for 90 s at 72°C) with a thermal cycler (MJ Research, Inc.). A final extension step consisting of 7 min at 72°C was performed before storage of the reaction product at 4°C. TE buffer was used as a negative control. The sequences and positions of primers P9 (forward) and P10 (reverse) employed for amplification of the specific 1,179-bp fragment used for RFLP analysis are shown in Table 1. Table 1 also shows the sequences of all primer pairs employed for sequencing both genomic segments, and the positions and distributions of the primers are shown in Fig. 1.
TABLE 1.
RT-PCR primer sets employed for sequencing and RFLP analysis
| Primer | Sequence (5′-3′) | Fragment length (bp)a | Map positionb | Coding region |
|---|---|---|---|---|
| Pb9 | GAG ACG TCT TAC GGA GGA G | 508 | 39-547 | Noncoding-VP2 |
| Pa8 | GAC ATC AGG CTG TTG TAG G | |||
| P9 | TGA GAT CCA TTA TGC TTC CC | 1,179 | 151-1330 | VP2 |
| P10 | GAC AGG ATC ATC TTG GCA TAG T | |||
| P8 | ACG GAA ATA CGA CAT CCA GAG C | 909 | 421-1330 | pVP2 |
| P10 | GAC AGG ATC ATC TTG GCA TAG T | |||
| P15 | GAA CGG AGC AAG GAT GAG GTG | 1,076 | 683-1759 | VP2-NS |
| P12 | TGC ACC ACA GGA AAG ATG ACT C | |||
| P14 | GTG TCC AAC TAC GAG CTG ATC | 535 | 1224-1759 | VP2-NS |
| P12 | TGC ACC ACA GGA AAG ATG ACT C | |||
| P11 | ACT ATG CCA AGA TGA TCC TGT C | 851 | 1309-2160 | VP2-NS |
| P1R | GTT CAT GGG CGG CTA TGG CTT T | |||
| P17 | CCA GTT CAT CGG AGA TCT CAC | 768 | 1544-2312 | NS |
| P7 | TCT CAT CAG CTG GCC CAG GTA C | |||
| P18 | CTG TCC CTG CTC GTC AAC GAC | 281 | 2031-2312 | NS |
| P7 | TCT CAT CAG CTG GCC CAG GTA C | |||
| P1 | AAA GCC ATA GCC GCC CAT GAA C | 924 | 2139-3063 | VP3 |
| PY | GAT CAG TCA GGA AAG AGA GTA G | |||
| PrA | GGC ACC CGA CAT ACC ACG AC | 860 | 23-883 | Noncoding-VP1 |
| PrC | TGC CGT CGT GTC TCC TTT GGT | |||
| PrD | CGG ATA CTT AGG CCG CGA GC | 612 | 271-883 | VP1 |
| PrC | TGC CGT CGT GTC TCC TTT GGT | |||
| PrB | GAC AAG TCA TAT CTG CCA CAT GT | 599 | 799-1398 | VP1 |
| PrE | GAG CCG TCC TCG TTC GTC CA | |||
| PrB | GAC AAG TCA TAT CTG CCA CAT GT | 1,024 | 799-1823 | VP1 |
| PrG | CGA ACA TCT CCA TTT GGC GGC | |||
| PrF | GCG CGA GCT GAA AAA CCT TAG | 654 | 1653-2307 | VP1 |
| PrI | CGG AGT TTG GTC CTC TGG TC | |||
| PrK | GCT CCG ACC TAC CAC CGC ACA G | 577 | 1730-2307 | VP1 |
| PrI | CGG AGT TTG GTC CTC TGG TC | |||
| PrH | CAC ATG TCC CTC GAC AAG AGG | 745 | 1996-2741 | VP1 |
| PrL | TGG CAC GTT GTC GGG TCA CTA CTT | |||
| PrH | CAC ATG TCC CTC GAC AAG AGG | 754 | 1996-2750 | VP1 |
| PrJ | TCA TGT TGG TGG CAC GTT GTC G |
FIG. 1.
Map positions of primer pairs employed for sequencing both genomic segments of aquabirnaviruses, based on the previously published sequences of the Jasper strain (9, 10). The designations of primers are shown in italics. pb, base pairs.
RFLPs.
The amplification products were extracted with phenol-chlorophorm-isoamyl alcohol (25:24:1) and ethanol precipitated as described above, and each pellet was resuspended in 25 μl of TE buffer. Purified PCR products were subjected to restriction endonuclease digestion with XhoI, BamHI, EcoRI, Aat II, PvuII, BstEII, MboI, AvaII, DdeI, or HaeIII at 37°C overnight in the digestion buffer supplied and under the conditions indicated by the manufacturer. DNA fragments from restriction endonuclease digestion were separated by electrophoresis in 2% agarose gels (1% Nusieve GTG agarose and 1% SeaPlaque low-gelling-temperature agarose; FMC Bioproducts) in TAE buffer (40 mM Tris, 20 mM acetic acid, 2 mM EDTA) at 100 V for 4 h (16). The gels were stained with ethidium bromide (0.5 mg/ml) for 30 min and destained in water for 30 min, and the bands were visualized under UV light. The sizes of the fragments were determined by comparison with molecular weight markers (φX174 DNA digested with HaeIII). The expected number of restriction sites in a PCR product was determined based on the previously published sequence of the Jasper strain (9) by using the PCgene (Lasergene, Inc.) computer program. Restriction profiles were analyzed, and the data were subjected to hierarchical cluster analysis by using the Statistical Package for Social Sciences (SPSS 6.1). Cluster analysis by the unweighted pair group method with arithmetic averages was performed by taking into account only the presence or absence of restriction cleavage sites.
cDNA sequencing.
The RNA was extracted and subjected to RT-PCR as described above. Different primers sets were employed for sequencing the complete length of the viral genome segments (Table 1); the locations of these primer sets are indicated in Fig. 1. The RT-PCR product was subjected to electrophoresis through a 2% SeaPlaque agarose gel (PMC Bioproducts) containing ethidium bromide (0.5 mg/ml) in TAE buffer. A specific band was visualized under UV light, cut out from the gel, and transferred to a microcentrifuge tube. The agarose was melted by heating the preparation at 65°C for 5 min, and the preparation was treated with 5 U of β-agarase (Sigma). The samples were then incubated overnight at 37°C. The specific band was cycle sequenced by using an ABI PRISM dye terminator Ready Reaction kit (Perkin-Elmer). Eight microliters of PCR product (corresponding to 1 μg of DNA) was mixed with 1 μl of primer (final concentration, 10 μM), 10 μl of Terminator Premix, and enough nuclease-free water to obtain a final volume of 20 μl. The reaction was performed with a thermal cycler (Perkin-Elmer model 480) by using the following program: 25 cycles of 96°C for 30 s, 50°C for 15 s, and 60°C for 4 min. Unincorporated nucleotides were removed from the solution by using a prewashed Spin column (Sepharose 100; Centricon). The sample was vacuum dried and resuspended with 4 μl of loading buffer (deionized formamide and 25 mM EDTA [pH 8.0] at a ratio of 5:1 containing 50 mg of blue dextran per ml). The sample was denatured at 90°C for 2 min, immediately transferred into an ice bath, and loaded onto a 7% polyacrylamide gel in an ABI model 373A DNA sequencer. Sequences were edited by using the Sequence Navigator program (Applied Biosystems, Inc.). Deduced amino acid sequences were determined with the DNASTAR EditSeq computer program (Lasergene, Inc.). Multiple-sequence alignment was performed by using the DNASTAR MegAlign program (Lasergene, Inc.) with the following suggested parameters: unweighted gap penalty, 10; and gap length penalty, 10. Phylogenetic trees were constructed with the MegAlign program by using a Clustal algorithm. For comparative purposes, nucleotide sequences of other aquabirnaviruses deposited in GenBank were used; the accession numbers of these sequences were AF342727 (West Buxton), AF343571 (Dry Mills), AF343572 (VR299), AF343573 (Buhl), AF342728 (Spajarup), U48225 (Sp-Mason), AY026482 (Bonnamy), AY026483 (d'Honnincthun), AY026484 (OV2 gene for polyprotein), AF342729 (Abild), AY026489 (CV-HB1), AF342730 (Hecht), AF342731 (Tellina), AF342732 (Canada 1), AF342733 (Canada 2), AF342734 (Canada 3), AF342735 (Ja-ATCC), M18049 (Ja-Dobos), D00701 (N1), and D26526 (DRT) for the polyprotein gene; D00701 (N1), AF160258 (E1S), L40580 (Abild), L40581 (Canada 2), L40582 (d'Honnincthun), L40584 (VR299), and M18049 (Ja-Dobos) for VP5; and M58756 (Ja-Dobos), M58757 (Sp-Dobos), and D26527 (DRT) for the VP1 gene.
Nucleotide sequence accession numbers.
The nucleotide sequences of local isolates obtained in the present study have been deposited in the European Molecular Biology Laboratory nucleotide sequence database of the European Bioinformatics Institute. The accession numbers for isolates 1146, 2284, 2290, 2310, 2464, 24R, 578, and 88R are AJ489222 to AJ489229, respectively, for the polyprotein ORF; AJ489230 to AJ489237, respectively, for the VP5 ORF; and AJ489238 to AJ489245, respectively, for the VP1 ORF.
RESULTS
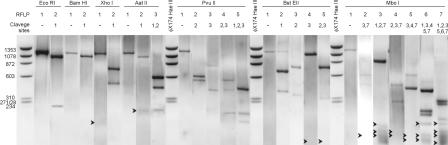
RFLP profiles obtained for each enzyme are shown in Fig. 2. Only one restriction site was present in the amplified cDNA fragments for endonucleases EcoRI, BamHI and XhoI. These endonucleases generated two possible RFLPs, one corresponding to the uncleaved fragment (absence of restriction sites) and one corresponding to presence of the restriction site, which resulted in a two-fragment profile that was unique for each enzyme. Assays with Aat II resulted in three different RFLPs; RFLP 1 corresponded to the uncleaved fragment, RFLP 2 was generated by the presence of one restriction site, and RFLP 3 included three fragments from two specific sites. Five RFLPs, from three possible restriction targets, were obtained with enzymes PvuII and BstEII. A total of seven possible targets specific for MboI generated seven RFLPs, and the highest numbers of restriction sites were obtained with DdeI and AvaII (8 restriction targets each) and HaeIII (12 targets), as shown in Table 2.
FIG. 2.
Restriction profiles obtained with most enzymes used for RFLP analysis. The arrowheads indicate the positions of faint bands.
TABLE 2.
Presence of the restriction endonuclease cleavage site obtained by RFLP assays
| Sero- type | Strain | Cleavage sites with the following restriction endonucleases:
|
|||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EcoRI | BamIII | XhoI |
AatII
|
PvuII
|
BstEII
|
MboI
|
DdeI
|
AvaII
|
HaeIII
|
||||||||||||||||||||||||||||||||||||||
| 1 | 1 | 1 | 1 | 2 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| A2 | Spajarup | − | − | − | + | + | + | + | + | + | − | − | + | + | + | − | + | + | + | + | + | − | − | − | − | + | − | + | − | + | − | + | − | + | − | − | + | + | − | − | + | + | − | − | − | − | − |
| 152 | − | − | − | + | + | + | + | + | + | − | − | + | + | + | − | + | + | + | + | + | − | − | − | − | + | − | + | + | − | − | + | − | + | − | − | + | + | − | − | + | + | − | − | + | − | − | |
| 533 | − | − | − | + | + | + | + | + | + | − | − | + | + | + | − | + | + | + | + | + | − | − | − | − | + | − | + | − | + | − | + | − | + | − | − | + | + | − | − | + | + | − | − | + | − | − | |
| 534 | − | − | − | + | + | + | + | + | + | − | − | + | + | + | − | + | + | + | + | + | − | − | − | − | + | − | + | − | + | − | + | − | + | − | − | + | + | − | − | + | + | − | − | + | − | − | |
| 1146 | − | − | − | + | + | + | + | + | + | − | − | + | + | + | − | + | + | + | + | + | − | − | − | − | + | − | + | + | − | − | + | − | + | − | − | + | + | − | − | + | + | − | − | + | − | − | |
| 55R | − | − | − | + | + | + | + | + | + | − | − | + | + | + | − | + | + | + | + | + | − | − | − | − | + | − | + | − | + | − | + | − | + | − | − | + | + | − | − | + | + | − | − | + | − | − | |
| 88R | − | − | − | + | + | + | + | + | + | − | − | + | + | + | − | + | + | + | + | + | − | − | − | − | + | − | + | − | + | − | + | − | + | − | − | + | + | − | − | + | + | − | − | + | − | − | |
| A3 | Abild | − | − | − | + | + | − | + | + | − | + | − | + | − | + | + | + | − | + | + | + | + | + | − | − | + | − | + | + | + | − | − | − | + | − | − | − | + | − | − | − | − | − | − | + | − | − |
| CV-HB-I | − | − | − | + | + | − | + | + | − | + | − | + | − | + | + | + | − | + | + | − | − | + | − | − | − | − | + | + | + | − | − | − | + | − | − | − | + | − | − | − | − | − | − | + | − | − | |
| 578 | − | − | − | + | + | − | + | + | − | + | − | + | − | + | + | + | − | + | + | + | + | + | − | − | + | − | + | + | + | − | − | − | + | − | − | − | + | − | − | − | − | − | − | + | − | − | |
| 2284 | − | − | − | + | + | − | + | + | − | + | − | + | − | + | + | + | − | + | + | + | + | + | − | − | + | − | + | + | + | − | − | − | + | − | − | − | + | − | − | − | − | − | − | + | − | − | |
| 2290 | − | − | − | + | + | − | + | + | − | + | − | + | − | + | + | + | − | + | + | + | + | + | − | − | + | − | + | + | + | − | − | − | + | − | − | − | + | − | − | − | − | − | − | + | − | − | |
| 2464 | − | − | − | + | + | − | + | + | − | + | − | + | − | + | + | + | − | + | + | + | + | + | − | − | + | − | + | + | + | − | − | − | + | − | − | − | + | − | − | − | − | − | − | + | − | − | |
| 24R | − | − | − | + | + | − | + | + | − | + | − | + | − | + | + | + | − | + | + | + | + | + | − | − | + | − | + | + | + | − | − | − | + | − | − | − | + | − | − | − | − | − | − | + | − | − | |
| A9 | Jasper | + | + | + | + | + | − | + | − | − | + | + | + | + | − | − | − | − | + | + | + | + | − | + | + | + | + | − | − | − | − | − | − | + | + | + | − | − | + | + | − | − | + | + | − | − | + |
| 2310 | + | + | + | + | + | − | + | − | − | + | + | + | + | − | − | − | − | + | + | + | + | − | + | + | + | + | − | − | − | − | − | − | + | + | + | − | − | + | + | − | − | + | + | − | − | + | |
| 405 | + | + | + | + | + | − | + | − | − | + | + | + | + | − | − | − | − | + | + | + | + | − | + | + | + | + | − | − | − | − | − | − | + | + | + | − | − | + | + | − | − | + | + | − | − | + | |
| A1 | West Buxton | + | + | − | − | − | − | − | − | − | − | + | + | + | − | − | − | − | + | + | + | + | − | + | + | + | − | + | + | + | + | − | + | + | + | − | + | + | − | − | − | + | − | + | − | + | + |
| 24FO | + | + | − | − | − | − | − | − | − | − | + | + | + | − | − | − | − | + | + | + | + | − | + | + | + | − | + | + | + | + | − | + | + | + | − | + | + | − | − | − | + | − | + | − | + | + | |
| A5 | Tellina | − | − | − | + | − | − | − | + | − | − | − | − | − | + | + | − | − | + | + | − | − | + | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | − | − |
| B1 | TV-1 | − | − | − | + | + | + | + | + | + | − | − | + | − | + | + | + | − | + | + | + | − | − | − | − | + | − | + | − | + | − | + | − | + | − | − | + | + | − | − | + | + | − | − | + | − | − |
| A6 | Canada 1 | − | − | − | + | − | − | − | + | − | + | − | − | + | + | − | − | − | + | + | + | − | + | − | − | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | − | + |
| A7 | Canada 2 | + | − | − | + | − | − | + | + | − | − | − | − | − | + | − | − | − | + | + | − | − | − | − | − | + | − | + | − | − | − | − | − | − | − | + | + | − | − | − | − | − | − | + | + | − | − |
| A8 | Canada 3 | + | − | − | + | − | − | + | + | + | − | − | − | − | + | − | − | − | + | + | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | + | + | − | − |
| A4 | Hecht | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | + | + | − | − | − | + | + | − | − | − | − | − | + | − | + | − | + | − | − | − | − | − | − | − | − | − | − | − |
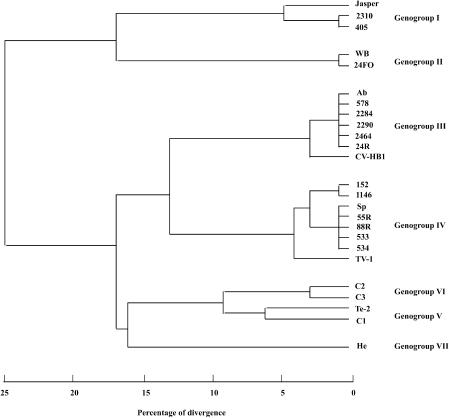
Table 2 also shows the presence or absence of the recognition sites for all the enzymes in the cDNA fragments corresponding to the type strains and the Galician isolates of the aquabirnaviruses assayed. These data were then analyzed by the SPSS 6.1 statistical package to obtain percentages of similarity between strains calculated from matching coefficients, and a phylogenetic tree was constructed (Fig. 3). Seven homology groups were established by using 7% divergence as the cutoff point. Two groups, genogroups III and IV, comprised most of the Galician strains. Genogroup III included type strain Abild (serotype A3), as well as local isolates 578, 2284, 2290, 2464, and 24R and reference strain CV-HB1. Genogroup IV comprised reference strains TV-1 (serotype B1) and Spajarup (serotype A2) and Galician isolates 152, 533, 534, 1146, 55R, and 88R. Genogroup I included isolates 2310 and 405, which were related to the Jasper type strain. Genogroup II contained Galician isolate 24FO and strain West Buxton (serotype A1). Genogroups V and VI exhibited a low percentage of divergence (close to 10%). Genogroup V contained strains Tellina (serotype A5) and Canada 1 (serotype A6), and genogroup VI included strains Canada 2 (serotype A7) and Canada 3 (serotype A8). No local isolates were included in these groups or in genogroup VII, which contained the Hecht type strain (serotype A4). As shown at Fig. 3, the percentages of divergence between genogroups I and II and the remaining genogroups were around 25%. Genogroups III and IV were separated by less than 15% divergence, and genogroups V, VI, and VII were separated by more than 15% divergence. Strain Hecht (genogroup VII) exhibited a higher level of relatedness to strains Abild, Sparajup, and Tellina and the Canadian serotypes (genogroups III, IV, V, and VI) than to the American strains Jasper and West Buxton (genogroups I and II).
FIG. 3.
Phylogenetic tree and genogroups for the aquabirnavirus strains assayed, based on divergence and similarity data obtained from RFLP analysis. WB, West Buxton; Ab, Abild; Sp, Sparajup; C1, Canada 1; C2, Canada 2; C3, Canada 3; Te-2, Tellina; He, Hecht.
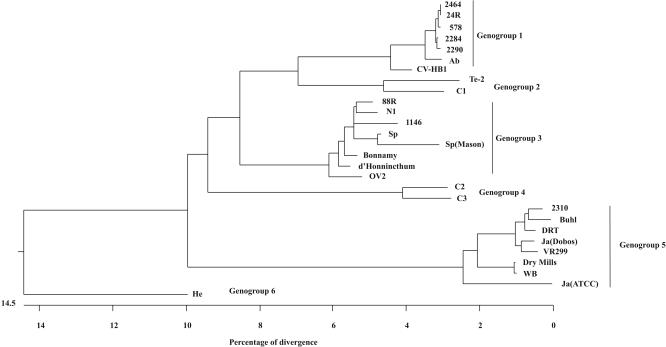
As expected, sequence analysis of the eight selected strains of Galician aquabirnaviruses revealed the presence of two ORFs in genome segment A and one ORF in segment B. In segment A, a small ORF, ranging from 444 to 447 nucleotides long, overlapped the 5′ end of the large ORF encoding the polyprotein (2,919 nucleotides). On genome segment B the size of the VP1 ORF was variable, ranging from 2,535 to 2,538 nucleotides depending on the strain sequenced. Alignments of the amino acid sequences deduced from nucleotide sequences corresponding to the polyprotein gene revealed that the type strains and local isolates could be distributed into six genogroups, as illustrated in Fig. 4 by using the same cutoff value that was used previously. Genogroup 1 comprised the majority of the local strains sequenced (strains 578, 2284, 2290, 2464, and 24R), which were similar to type strain Abild, corresponding to serotype A3. It also included reference strain CV-HB1. Tellina and the Canadian type strain Canada 1 constituted genogroup 2, and the remaining two Canadian serotype strains (Canada 2 and Canada 3) belonged to genogroup 4. Local isolates were not represented in either of these genogroups. Isolates 1146 and 88R showed sequence similarity to strain Sparajup, and these strains were in genogroup 3, which also included reference strains Sp-Mason, Bonnamy, d'Honnincthun, N1, and OV-2. Genogroup 5 was made up of serotype A1 strains, including West Buxton, Dry Mills, Buhl, VR299, and DRT, as well as Galician isolate 2310, and serotype A9 strain Jasper. It is interesting that whereas both sequences available for strain Jasper, Ja-Dobos from Duncan and Dobos (9) (accession number M18049) and Ja-ATCC (accession number AF342735), were included in the same genogroup, genogroup 5, Ja-Dobos exhibited a higher level of relatedness with the serotype A1 type strains than Ja-ATCC exhibited. The atypical Hecht strain, the only known representative of serotype A4, constituted genogroup 6. Pairwise distances based on deduced amino acid sequences (data not shown) showed that the percentages of similarity within each group were always higher than 95% and that the divergence between groups was never higher than 19%. The highest levels of similarity were obtained within genogroup 1 (type strain Abild). A comparison of the amino acid sequences deduced from the VP5 (small ORF) and VP1 (segment B ORF) nucleotide sequences resulted in a genogrouping (data not shown) for the reference and local isolates sequenced equivalent to that obtained with the large ORF.
FIG. 4.
Phylogenetic relationships among type strains and Galician aquabirnaviruses as determined by amino acid sequence homology of the polyprotein ORF. WB, West Buxton; Ab, Abild; Sp, Sparajup; C1, Canada 1; C2, Canada 2; C3, Canada 3; Te-2, Tellina; He, Hecht.
DISCUSSION
A summary of the genogrouping results established by the different assays for the aquabirnaviruses is shown in Table 3. The first interesting point comes from a comparison of the sequences obtained for the three ORFs. The same six genogroups could be established by nucleotide sequencing independent of the gene sequenced. In addition, with some exceptions, the genogroups corresponded significantly to previously established serotypes. Thus, the American serotypes A1 and A9 (with reference strains West Buxton and Jasper, respectively) constituted genogroup 5 and could be separated only if two subgroups were subjectively differentiated by a level of divergence as low as 2.5%. Similarly, genogroups 2 and 4 included two serotypes each, with levels of divergence between 4 and 5%. Early reports on typing by genomic sequencing analysis yielded only three genogroups (13, 15); genogroup I corresponded to the American strains (serotypes A1 and A9), genogroup II included reference strains of serotypes A2, A3, A5, A6, A7, and A8, and serotype A4 constituted the third genogroup. However, in these reports only short fragments (310 and 359 bp, respectively) were sequenced. More recently, some of us (3) described a quite different genogrouping based on the complete sequence of segment A and the VP2 coding region. Although the numbering of the groups does not exactly coincide with the numbering reported here, the groupings are basically equivalent. Small differences between the results of the studies are probably due to the differences in the local isolates and reference strains employed. Significantly, genogroups 2 and 4 each also included two indistinguishable serotypes; genogroup 2 included serotypes A5 (reference strain Tellina) and A6 (Canada 1), and genogroup 4 included serotypes A7 (Canada 2) and A8 (Canada 3).
TABLE 3.
Summary of the genogrouping of aquabimaviruses generated by RFLP and sequencing analyses
| RFLP genogroup | Sequencing genogroupa | Genogroups proposedb
|
Corresponding serotypec | Strains studied
|
Electropherogroupsd
|
|||
|---|---|---|---|---|---|---|---|---|
| Genogroup | Genotypee | Reference strains | Local isolates | Reference strain | Local isolate(s) | |||
| I | 5 | I | I.1 | A9 | Jasper (ATCC)f | 2310,g 405 | EG6 | EG2 |
| II | 5 | I | I.2 | A1 | West Buxton, DM, Buhl, DRT, VR-299 | 24FO | EG2 | EG5 |
| III | 1 | II | A3 | Abild, CV-HB1 | 578, 2284, 2290, 2464, 24R | EG3 | EG3 | |
| IV | 3 | III | III.1 | A2 | Spajarup, Bonnamy, d'Honnincthom, OV-2, N1 | 152, 533, 534, 1146, 55R, 88R | EG4 | EG1, EG4, EG5 |
| IV | III | III.2 | B1 | TV-1 | EG4 | |||
| V | 2 | IV | IV.1 | A5 | Tellina | EG2 | ||
| V | 2 | IV | IV.2 | A6 | Canada 1 | EG2 | ||
| VI | 4 | V | V.1 | A7 | Canada 2 | EG2 | ||
| VI | 4 | V | V.2 | A8 | Canada 3 | EG2 | ||
| VII | 6 | VI | A4 | Hecht | EG5 | |||
Results from the comparison of the deduced amino acid sequences.
Final genotypes proposed on the basis of the results of both RFLP and sequencing analyses.
Serogroups and serotypes as reported by Hill and Way (14).
Correspondence with genotyping (by genome electropherotypes, as proposed by Cutrin et al. [6]).
Genotypes differentiated by the two-step restriction analysis assay.
ATCC, American Type Culture Collection.
Strain 2310 is related to Ja-Dobos, as determined by polyprotein sequencing.
RFLP analysis yielded a genogrouping quite similar to that obtained from genomic sequencing. Seven genogroups (designated by roman numerals) were established with a maximum level of divergence of around 25%. Genogroups I and II, including reference strains Jasper (serotype A9) and West Buxton (serotype A1), respectively, could be separated by a level of divergence of around 17%, whereas these strains were included in the same genogroup, genogroup 5 (2.5% divergence), by sequencing. Genogroups III, V, VI, and VII corresponded to genogroups 1, 2, 4, and 6, respectively, obtained by sequencing (Table 3). Although the divergence between genogroups V and VI was similar to the divergence obtained by sequencing (between genogroups 2 and 4), these genogroups might be considered members of a unique cluster by RFLP analysis, whereas genogroups 2 and 4 represent quite separate clusters. It is interesting that although a clear correspondence between genogroups IV and 3 was observed (by RFLP and sequencing), the divergence (approximately 4%) observed between the TV-1 (serotype B1) and Spajarup type strains could not be demonstrated by sequencing due to the lack of available sequences for TV-1.
There have been few reports of the typing of aquabirnaviruses by RFLP analysis, and the previous studies yielded results different from those described here. Heppell et al. (12) included strains belonging to the nine serotypes of serogroup A but no strains belonging to serogroup B. These authors described three genogroups and several subgroups. However, their results were quite different, probably due to the small size of the fragment subjected to restriction; i.e., they could not differentiate serotypes A3, A5, A6, and A8. Other authors employed larger fragments, corresponding to the VP2 coding region, like the fragment employed in the present study (2, 16). However, these authors did not include reference strains corresponding to the 10 serotypes of IPNV previously established by Hill and Way (14).
In the present study, similar results were obtained with the two methods employed for molecular typing. In order to unify criteria, groups were renamed with roman numerals (genogroups I to VI) (Table 3). A certain correspondence with the serotypes previously proposed by Hill and Way (14) was observed. Although most of the genogroups included two serotypes, in all cases both types could be easily differentiated if the two-step restriction analysis assay was applied, as explained below. Therefore, for the genogrouping of aquatic birnaviruses we propose establishment of subdivisions in genotypes in order to obtain complete correspondence with serotyping. As shown here, serotype A1 corresponds to genotype I.2; serotype A2 corresponds to genotype III.1; serotype A3 corresponds to genogroup II; serotype A4 corresponds to genogroup VI; serotypes A5 and A6 correspond to genotypes IV.1 and IV.2, respectively; serotypes A7 and A8 correspond to genotypes V.1 and V.2; serotype A9 corresponds to genotype I.1; and serotype B1 corresponds to genotype III.2.
Most of the local strains included in the present study were identified as members of genotype III.1 (serotype A2) and genogroup II (serotype A3), and a few of them corresponded to American serotypes A1 and A9 (genogroup I). This was expected considering that these isolates were detected in Galicia in northwestern Spain (which explains European types II and III). In addition, the presence of the American type of local isolates could be because two decades ago Galicia was an important client for importation of rainbow trout eggs from North American farms.
On the other hand, it must be noted that the local isolates used in the present study were selected based on the results of previous electropherotyping of their genomes, as reported by Cutrin et al. (6). Our results clearly indicate that electropherotyping is an incorrect approach for typing aquabirnaviruses since a substantial lack of correlation with geno- and serotyping was observed (Table 3).
Finally, although nucleic acid sequencing technology has become routine in almost every laboratory of virology due to the simplicity of restriction analysis, we propose that this technique can be used as an optimal substitute for traditional serotyping. In fact, as shown by the results of this study, typing of aquabirnaviruses is as simple as a two-step restriction analysis assay applied to the 1,179-bp cDNA fragment (corresponding to the VP2 coding region) reported here and elsewhere. In the two-step restriction analysis assay, the first step corresponds to restriction of the fragment with the PvuII enzyme, which generates five possible results (Table 4). In the second step the enzyme employed to subject the original fragment to a new restriction reaction is selected depending on the results obtained in the first step, and the final result provides complete differentiation of the 10 serotypes. This method of typing is being routinely employed in our laboratory and is a rapid and reliable method for serotyping new isolates of aquabirnaviruses.
TABLE 4.
Typing of aquabirnaviruses by the two-step restriction analysis assaya
| First step
|
Second step
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Restriction endonuclease employed | Restriction fragment profile no. | No. of sitesb | Fragment size(s) (bp) | Reference strain (serotype)c | Restriction endonuclease employed | Fragment profile no. | No. of sitesb | Fragment size(s) (bp) | Reference strain (serotype)c |
| PvuII | 1 | 0 | 1,179 | WB (A1) | EcoRI | 1 | 0 | 1,179 | He (A4) |
| He (A4) | 2 | 1 | 979, 200 | WB (A1) | |||||
| 2 | 1 | 745 | Te-2 (A5) | BstEII | 1 | 0 | 1,179 | Te-2 (A5) | |
| 434 | C1 (A6) | 2 | 1 | 787, 392 | C1 (A6) | ||||
| 3 | 1 | 622 557 | Ja (A9) | NNd | Ja (A9) | ||||
| 4 | 2 | 557 | Ab (A3) | BstII | 1 | 0 | 1,179 | C2 (A7) | |
| 434 | C2 (A7) | 2 | 1 | 787, 392 | Ab (A3) | ||||
| 188 | C3 (A8) | 3 | 1 | 743, 436 | C3 (A8) | ||||
| 5 | 3 | 434 | Sp (A2) | MboI | 6 | 5 | 434, Snfe | TV-1 (B1) | |
| 433 | TV-1 (B1) | 7 | 6 | 321,297, Smff | Sp (A2) | ||||
| 188 | |||||||||
| 124 | |||||||||
In step 1 the first endonuclease reaction was applied to the initial 1,179-bp cDNA fragment. In step 2 the second endonuclease reaction was applied to the initial 1,179-bp cDNA fragment.
Number of specific restriction sites in the initial cDNA fragment.
Reference strain (corresponding serotype) showing the corresponding specific RFLP.
NN, not needed.
Snf, five fragments smaller than 240 bp, with 745 bp added.
Snf, five small fragments around 100 to 150 bp long, representing a total of 561 bp.
Acknowledgments
Juan M. Cutrín thanks the University of Maine DNA Sequencing Facility and Patty Singer for their contributions.
This work was supported by grant MAR99-0637-C02-01 from Comisión Interministerial de Ciencia y Tecnología (DGSIC), by grant ACU01-010/2001/PC159 from Ministerio de Ciencia y Tecnología, and by grant PGIDIT02BTF23501PR from the Secretaría Xeral de I+D, Xunta de Galicia.
Footnotes
Scientific contribution no. 001/2003 of the Instituto de Acuicultura.
REFERENCES
- 1.Ahne, W., R. K. Kelly, and H. J. Schlotfeldt. 1989. Factors affecting the transmission and outbreak of infectious pancreatic necrosis (IPN), p. 19-71. In K. Lillelund and H. Rosenthal (ed.), Fish health protection strategies. Federal Ministry for Research and Technology, Bonn, Germany.
- 2.Biering, E., H. P. Melby, and S. H. Mortensen. 1997. Sero- and genotyping of some marine aquatic birnavirus isolates from Norway. Dis. Aquat. Org. 28:169-174. [Google Scholar]
- 3.Blake, S. L., J.-Y. Ma, D. A. Caporale, S. Jairath, and B. L. Nicholson. 2001. Phylogenetic relationships of aquatic birnaviruses based on deduced amino acid sequences of genome segment A cDNA. Dis. Aquat. Org. 45:89-102. [DOI] [PubMed] [Google Scholar]
- 4.Caswell-Reno, P., V. Lipipun, P. W. Reno, and B. L. Nicholson. 1989. Utilization of a group reactive and other monoclonal antibodies in an enzyme immunodot assay for identification and presumptive serotyping of aquatic birnaviruses. J. Clin. Microbiol. 27:1924-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christie, K. E., L. S. Håvarstein, H. O. Djupvik, S. Ness, and C. Endresen. 1988. Characterization of a new serotype of infectious pancreatic necrosis virus isolated from Atlantic salmon. Arch. Virol. 103:167-177. [DOI] [PubMed] [Google Scholar]
- 6.Cutrin, J. M., J. G. Olveira, J. L. Barja, and C. P. Dopazo. 2000. Diversity of infectious pancreatic necrosis virus strains isolated from fish, shellfish, and other reservoirs in northwestern Spain. Appl. Environ. Microbiol. 66:839-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobos, P. 1995. The molecular biology of infectious pancreatic necrosis virus (IPNV). Annu. Rev. Fish Dis. 5:25-54. [Google Scholar]
- 8.Dopazo, C. P., and J. L. Barja. 2002. Diagnosis and identification of IPNV in salmonids by molecular methods, p. 23-48. In C. O. Cunningham (ed.), Molecular diagnosis of salmonid diseases. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 9.Duncan, R., and P. Dobos. 1986. The nucleotide sequence of infectious pancreatic necrosis virus (IPNV) dsRNA segment A reveals one large ORF encoding a precursor polyprotein. Nucleic Acids Res. 14:5934-5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan, R., C. L. Mason, E. Nagy, J.-A. Leong, and P. Dobos. 1991. Sequence analysis of infectious pancreatic necrosis virus genome segment B and its encoded VP1 protein: a putative RNA-dependent RNA polymerase lacking the Gly-Asp-Asp motif. Virology 181:541-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Håvarstein, L. S., K. H. Kalland, K. E. Christie, and C. Endresen. 1990. Sequence of the large double-stranded ARN segment of the N1 strain of infectious pancreatic necrosis virus: a comparison with other Birnaviridae. J. Gen. Virol. 71:299-308. [DOI] [PubMed] [Google Scholar]
- 12.Heppell, J., L. Berthiaume, E. Tarrab, J. Lecomte, and M. Arella. 1992. Evidence of genomic variations between infectious pancreatic necrosis virus strains determined by restriction fragment profiles. J. Gen. Virol. 73:2863-2870. [DOI] [PubMed] [Google Scholar]
- 13.Heppell, J., L. Berthiaume, F. Corbin, E. Tarrab, J. Lecomte, and M. Arella. 1993. Comparison of amino acid sequences deduced from a cDNA fragment obtained from infectious pancreatic necrosis virus (IPNV) strains of different serotypes. Virology 195:840-844. [DOI] [PubMed] [Google Scholar]
- 14.Hill, B. J., and K. Way. 1995. Serological classification of infectious pancreatic necrosis (IPN) virus and other aquatic birnaviruses. Annu. Rev. Fish Dis. 5:55-77. [Google Scholar]
- 15.Hosono, N., S. Suzuki, and R. Kusuda. 1996. Genogrouping of birnaviruses isolated from marine fish: a comparison of VP2/NS junction regions on genome segment A. J. Fish Dis. 19:295-302. [Google Scholar]
- 16.Lee, M.-K., S. Blake, J. T. Singer, and B. L. Nicholson. 1996. Genomic variation of aquatic birnaviruses analyzed with restriction fragment length polymorphisms. Appl. Environ. Microbiol. 62:2513-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao, L., and P. Dobos. 1995. Mapping of a serotype specific epitope in the major capsid protein VP2 of infections pancreatic necrosis virus. Virology 209:684-687. [DOI] [PubMed] [Google Scholar]
- 18.Lupiani, B., C. P. Dopazo, A. Ledo, B. Fouz, J. L. Barja, F. M. Hetrick, and A. E. Toranzo. 1988. A new syndrome of mixed bacterial and viral etiology in cultured turbot (Scophthalmus maximus, L.). J. Aquat. Anim. Health 1:197-204. [Google Scholar]
- 19.McAllister, P. E., and J. Bebak. 1997. Infectious pancreatic necrosis virus in the environment: relationship to effluent from aquaculture facilities. J. Fish Dis. 20:201-207. [Google Scholar]
- 20.Mortensen, S. H., B. Hjeltnes, O. Rodsth, J. Krogsrud, and K. E. Christie. 1990. Infectious pancreatic necrosis virus serotype N1, isolated from Norwegian halibut (Hippoglossus hippoglossus), turbot (Scophthalmus maximus) and scallops (Pecten maximus). Bull. Eur. Assoc. Fish Pathol. 10:42-43. [Google Scholar]
- 21.Nicholson, B. L. 1993. Use of monoclonal antibodies in identification and characterization of fish viruses. Annu. Rev. Fish Dis. 3:241-257. [Google Scholar]
- 22.Novoa, B., A. Figueras, A. Ledo, J. L. Barja, and A. E. Toranzo. 1991. Incidence of birnavirus in cultured turbot (Scophthalmus maximus L.) in northwestern Spain. FHS/AFS News Lett. 19:2-3. [Google Scholar]
- 23.Rivas, C., C. Cepeda, C. P. Dopazo, B. Novoa, M. Noya, and J. L. Barja. 1993. Marine environment as reservoir of birnaviruses from poikilothermic animals. Aquaculture 115:183-194. [Google Scholar]
- 24.Sadasiv, E. C. 1995. Immunological and pathological responses of salmonids to infectious pancreatic necrosis virus (IPNV). Annu. Rev. Fish Dis. 5:209-223. [Google Scholar]
- 25.Tarrab, E., L. Berthiaume, S. Grothé, M. O'Connor-McCourt, J. Heppell, and J. Lecomte. 1995. Evidence of a major neutralizable conformational epitope region on VP2 of infectious pancreatic necrosis virus. J. Gen. Virol. 76:551-558. [DOI] [PubMed] [Google Scholar]