Abstract
Background
Despite the importance of the Galápagos Islands for the development of central concepts in ecology and evolution, the understanding of many ecological processes in this archipelago is still very basic. One such process is pollination, which provides an important service to both plants and their pollinators. The rather modest level of knowledge on this subject has so far limited our predictive power on the consequences of the increasing threat of introduced plants and pollinators to this unique archipelago.
Scope
As a first step toward building a unified view of the state of pollination in the Galápagos, a thorough literature search was conducted on the breeding systems of the archipelago's flora and compiled all documented flower–visitor interactions. Based on 38 studies from the last 100 years, we retrieved 329 unique interactions between 123 flowering plant species (50 endemics, 39 non-endemic natives, 26 introduced and eight of unknown origin) from 41 families and 120 animal species from 13 orders. We discuss the emergent patterns and identify promising research avenues in the field.
Conclusions
Although breeding systems are known for <20 % of the flora, most species in our database were self-compatible. Moreover, the incidence of autogamy among endemics, non-endemic natives and alien species did not differ significantly, being high in all groups, which suggests that a poor pollinator fauna does not represent a constraint to the integration of new plant species into the native communities. Most interactions detected (approx. 90 %) come from a single island (most of them from Santa Cruz). Hymenopterans (mainly the endemic carpenter bee Xylocopa darwinii and ants), followed by lepidopterans, were the most important flower visitors. Dipterans were much more important flower visitors in the humid zone than in the dry zone. Bird and lizard pollination has been occasionally reported in the dry zone. Strong biases were detected in the sampling effort dedicated to different islands, time of day, focal plants and functional groups of visitors. Thus, the existing patterns need to be confronted with new and less biased data. The implementation of a community-level approach could greatly increase our understanding of pollination on the islands and our ability to predict the consequences of plant invasions for the natural ecosystems of the Galápagos.
Keywords: Galápagos, flower visitation, mutualistic interactions, oceanic islands, plant breeding systems, plant–animal interactions, pollination networks
INTRODUCTION
Around 90 % of the world's flowering plant species are pollinated by animals (Ollerton et al., 2011) and the reproduction of approx. 70 % is likely to be, to some extent, pollen limited (Ashman et al., 2004). Thus, pollination represents an important ecological process, which is provided by a wide array of animals, including insects, birds, mammals and reptiles, with positive consequences to the long-term population stability of flowering plants (Kearns et al., 1998). Recently, a growing realization that much ecosystem functioning is founded on the interactions among species (Duffy et al., 2007) has lifted the focus of many conservation programmes from a species-centred to a community-centred approach (Jordano et al., 2007; Tylianakis et al., 2010). For example, pollination failure is now seen as an important threat to the long-term survival of plants (Biesmeijer et al., 2006). Consequently, when planning conservation strategies for any indigenous flora one has to take its pollinator fauna into consideration (Bond, 1994). In spite of that, most studies addressing the conservation of rare plants still ignore the importance of their pollinators (Memmott et al., 2007).
Given the poor biodiversity, but unique evolutionary history typical of the biota of many oceanic islands, insular plants seem to be particularly vulnerable to the disruption of their reproductive mutualisms (Traveset and Richardson, 2006). A pervasive threat to such mutualisms is biological invasions, which are among the main drivers of global change and have, in particular, degraded island communities (MEA, 2005), of which the Galápagos are no exception. The rapid growth in the number of alien species, particularly plants, together with the inherent pressures of a growing human presence (Trueman et al., 2010), are the principal threats to the conservation of the biota of these islands (Bensted-Smith, 2002).
Many Galápagos plants have small, drab-coloured flowers with overall poor rewards, being associated with a poor pollinator fauna typical of oceanic islands (Linsley and Usinger, 1966; Rick, 1966; McMullen, 2009a). Moreover, a high prevalence of self-compatible species has been reported for the Galápagos (McMullen, 1990). Information on animal–flower visitation in this archipelago has been collected by many observers, from explorers to ornithologists to botanists. This information is, as a result, highly scattered throughout the literature and there is no recent treatment summarizing this body of knowledge. Therefore, despite the importance of the Galápagos for the development of central concepts in ecology and evolution, the understanding of many ecological processes in this archipelago is still basic. This poor understanding has therefore hindered our ability to predict the effects of introduced plants and pollinators in the Galápagos ecosystems. The compilation of such information is a first step towards a general overview of the ecological networks of the Galápagos archipelago, which can ultimately assist their conservation and restoration (Kaiser-Bunbury et al., 2010).
The objective of this study was to gather all available information on the breeding systems and on the flower-visitors of the Galápagos flora. We anticipated that we would use the compiled datasets to address three main issues. First, we would assess whether the proportion of autogamous species differs between endemics, non-endemic natives and alien species. If the incidence of autogamy among aliens proved to be significantly greater than that for natives, then pollen limitation might represent a smaller constraint for introduced species in a scenario of competition for pollinators (Morales and Traveset, 2009). Second, we would determine the main orders of pollinators functioning in each of the two main habitats, i.e. the arid lowlands and moist uplands. Finally, gathered knowledge would allow us to highlight particular areas where pollination research in the Galápagos archipelago can be particularly relevant in theoretical and applied terms and make such information available. We hope that this synthesis will catalyse a new set of studies that will be able to build upon the existing knowledge compiled here and take our understanding of the function of pollination systems in the Galapagos to a whole new level both in terms of scope (i.e. the community level) and detail.
METHODS
Study site
The Galápagos archipelago spans the equator (1 °40'N to 1 °36'S, 89 °16' to 92 °01'W), in the Eastern Pacific, approx. 960 km west of mainland Ecuador (Fig. 1). With an area of 7882 km2, it consists of 123 islands of volcanic origin, rising from a few metres to approx. 1700 m a.s.l. (Tye et al., 2002). Seven of the islands are larger than 100 km2 and 18 are larger than 1 km2 (Snell et al., 1996). The oldest lava flows on the eastern islands have been aged to less than four million years, whereas the youngest islands, Fernandina and Isabela, to the west, are <0·5 million years old (White et al., 1993). The isolation and location of the archipelago with respect to oceanic currents and trade winds have favoured a high degree of endemism. Fifty-nine per cent of all vertebrates are endemic (Tye et al., 2002) with endemism being especially high among terrestrial birds (84 %).
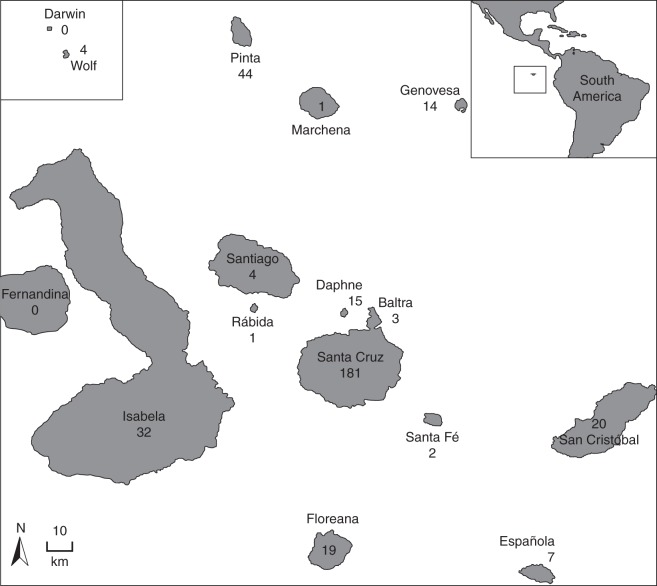
Fig. 1.
Global position of the Galápagos archipelago including the number of plant–visitor interactions compiled from the literature for each island.
The flora of the Galápagos is closely related to that of South America (Hooker, 1847; Porter, 1984). Variation in dispersal efficiency among plant families resulted in a disharmonic flora compared with its mainland origin, a typical property of oceanic islands (Baur, 1891; Porter, 1983). The Galápagos flora consists of 557 native (including doubtfully native) vascular plant species, of which 180–190 are endemic, and approx. 825 alien (Jaramillo et al., 2011, but see also Van Leeuwen et al., 2008).
The native vegetation is distributed into distinct zones along an altitude gradient varying in climatic conditions (Wiggins and Porter, 1971). These zones are (a) the littoral zone, (b) the arid zone, which dominates the archipelago and has the greatest diversity of plants, (c) a transition zone, and (d) a humid zone with the highest precipitation and primary productivity in the archipelago (Wiggins and Porter, 1971; Tye et al., 2002). Only seven islands are high enough to have developed a humid zone (San Cristóbal, Santa Cruz, Floreana, Fernandina, Santiago, Isabela and Pinta).
The climate of the Galápagos is atypical compared with other tropical oceanic islands due to the influence of several weather systems and oceanic currents (Colinvaux, 1984). There are two distinct climatic seasons in Galápagos. The hot season, prevailing from January to May, is characterized by a warm sea, high air temperatures (from 24 to 29 °C) and a highly fluctuating annual rainfall (64–2769 mm at the coast). The cool season, from June to December, is characterized by a prolonged cloud cover and perpetual drizzle in the highlands, almost no rain in the lowlands, and temperatures ranging between 19 and 23 °C (Trueman and d'Ozouville, 2010). The cyclic El Niño events cause prolonged intense rains, followed by a period of drought (La Niña) (Snell and Rea, 1999).
Due to the harsh conditions faced by settlers and the early establishment of the Galápagos National Park (in 1959), the archipelago has remained relatively unspoiled (Gardener et al., 2010). Throughout the last century, the establishment of permanent human settlements, and particularly the deliberate introduction of alien plants and animals, severely impacted large areas of the archipelago (Mauchamp, 1997; Sulloway, 2009; Guézou et al., 2010). Nevertheless, the Galápagos is today one of the best preserved oceanic archipelagos, where human impacts on many ecological processes are still relatively low, particularly on uninhabited islands (PNG, 2005).
Literature search
A literature search was conducted on www.scholar.google.com, www.isiknowledge.com/WOS and Web of Science and in the library of the Charles Darwin Foundation in Puerto Ayora, Santa Cruz. We compiled information from the 17 available studies on the reproductive biology of 83 species belonging to 41 families, including information on the level of self-pollination (autogamy) and self-compatibility. We further consulted 38 publications from the last 100 years and retrieved 329 unique flower–visitor interactions between 123 flowering plant species from 41 families and 120 animal species from 13 orders and four classes (Insecta, Aves, Reptilia and Arachnida). This dataset included (a) flower–visitation interactions, (b) islands, habitats and seasons encompassed by each study, (c) study periods (diurnal, nocturnal or both), and (d) whether information included all flower-visitors or only pollinators, i.e. with active pollen transport. Interactions were recorded for a total of 15 islands, including the five inhabited ones; one-third of the studies actually were performed on Santa Cruz, the most populated island (Fig. 1). Approximately half of the studies (n = 24) took place in the dry zone, ten in the humid zone, and 13 in the transition zone. Some studies encompassed more than one zone, whereas others did not provide such information. A similar number of studies was carried out during the cold and hot periods, although this information was missing from 21 publications. Most observations were made during the day; only eight publications (all from the last 5 years) also included some nocturnal observations. Finally, most often ‘pollination’ was inferred from observations of flower visits, and only eight studies (22 %) actually evaluated pollen transport by the flower visitor.
While most studies identified plants to species level, the taxonomic affiliation of the flower visitors was often poorly resolved and many studies only reported the family or order of the visitors. In our database, we included all records of interactions where plants were identified at least to genus level and visitor at least to order level. Our review identified 120 flower-visitors (Supplementary Data Table S1), of which 62 % were identified to species, 22 % to genus, 12 % to family and the remaining 4 % to the level of order.
Statistical analysis
We used likelihood ratio tests (G-test) to look for different patterns emerging from the interactions retrieved from the literature. Namely, three groups of tests were used to explore differences (1) on the proportion of autogamous species between endemic, non-endemic natives and alien plants, (2) between the total diversity of each order known for Galapagos and the number of species from those same orders that have been recorded as flower visitors, and (3) between the dry and humid zones in the proportion of interactions established between plants of flower visitors belonging to the different orders.
RESULTS
Plant breeding systems
Reliable information was available for 70 plant species of which 34 were endemic, 21 non-endemic natives, 13 alien, and two possibly native (Table 1). Most species (n = 56; 80 %) were self-compatible, and 11 species (16 %) were dioecious. The proportion of autogamous species did not differ between alien, endemic and native species (85 %, 74 % and 78 %, respectively; G = 0·15; d.f. = 2; P = 0·93). Among the endemic species, three did not self-pollinate and might thus be self-incompatible: Jasminocereus thouarsii, Cordia revoluta and Sarcostemma angustissimum. Among the self-compatible species, 96 % show some level of autonomous selfing, whereas two species (4 %) exhibit induced selfing, requiring pollinators for setting seed: the endemic Gossypium barbadense, and the introduced Diodia radula. Pooling species that exhibit induced selfing and dioecious species together, 16 out of 70 species (23 %) depend on a vector for pollination. That leaves many species in the Galápagos flora still needing to be tested for autogamy. All reported dioecious species (n = 11) were native or endemic except for the introduced Carica papaya.
Table 1.
Compilation of known information regarding the breeding systems of the Galápagos vascular flora
| Family | Species | Origin | Autonomously self-pollinates | Self-compatible | References |
|---|---|---|---|---|---|
| Acanthaceae | Justicia galapagana | En | Yes | Yes | 2, 4, 6 |
| Tetramerium nervosum | Na | Yes | Yes | 4, 6 | |
| Amaranthaceae | Alternanthera echinocephala | Na | Yes | Yes | 4, 6 |
| Apocynaceae | Vallesia glabra | Na | Inconclusive results | 4, 6 | |
| Asclepiadaceae | Sarcostemma angustissimum | En | No* | 12 | |
| Asteraceae | AdeNostemma platyphyllum | In | Yes | Yes | 4, 6 |
| Ageratum conyzoides | In | Yes | Yes | 4, 6 | |
| Baccharis gnidiifolia | Na | Dioecious | – | 1, 9 | |
| Baccharis steetzii | En | Dioecious | – | 1, 6 | |
| Bidens pilosa | In | Yes | Yes | 4, 6 | |
| Darwiniothamnus tenuifolius | En | Yes† | Yes | 4, 6, 8, 12 | |
| Jaegeria gracilis | En | Yes | Yes | 4, 6 | |
| Lecocarpus pinnatifidus | En | Yes | Yes | 11 | |
| Pectis tenuifolia | En | Yes | Yes | 12 | |
| Scalesia helleri | En | Yes | Yes | 2 | |
| Scalesia affinis | En | Yes | Yes | 2, 12, 13 | |
| Scalesia aspera | En | Yes | Yes | 6 | |
| Scalesia baurii | En | Yes | Yes | 7 | |
| Scalesia cordata | En | Yes | Yes | 16 | |
| Scalesia pedunculata | En | Yes | Yes | 4, 6 | |
| Avicenniaceae | Avicennia germinans | Na | Yes | Yes | 4, 6 |
| Batidaceae | Batis maritima | Na | Dioecious | – | 1, 9 |
| Boraginaceae | Cordia leucophlycthis | En | Yes‡ | Yes§ | 2, 4, 6 |
| Cordia lutea | Na | Yes¶ | Yes | 2, 4, 6 | |
| Cordia revoluta | En | No* | 12 | ||
| Tournefortia psilostachya | Na | Yes | Yes | 4, 6 | |
| Tournefortia pubescens | En | Yes | Yes | 4, 6 | |
| Tournefortia rufo-sericea | En | Yes | Yes | 2, 4, 6, 14 | |
| Burseraceae | Bursera graveolens | Na | Dioecious | – | 1, 9 |
| Bursera malacophylla | En | Dioecious | – | 1, 6, 9 | |
| Cactaceae | JasmiNocereus thouarsii | En | No | 2, 12, 17 | |
| Opuntia echios | En | Inconclusive results | 2, 4, 6, 12 | ||
| Opuntia helleri | En | Inconclusive results# | 3, 5, 6 | ||
| Caesalpiniaceae | Cassia occidentalis | Na | Inconclusive results | 6 | |
| Parkinsonia aculeata | Na | Yes | Yes | 4, 6 | |
| Senna occidentalis | Na | Inconclusive results | 4 | ||
| Caricaceae | Carica papaya | In | Dioecious | – | 1, 9 |
| Convolvulaceae | Ipomoea habeliana | En | Yes | Yes | 15 |
| Stictocardia campanulata | NaQ | Inconclusive results | 2 | ||
| Cyperaceae | Cyperus elegans | En | Yes | Yes | 4, 6 |
| Euphorbiaceae | Croton scouleri | En | Dioecious | – | 4, 6, 9 |
| Lamiaceae | Hyptis rhomboidea | In | Yes | Yes | 4, 6 |
| Lobeliaceae | Lobelia xalapensis | Na | Yes | Yes | 4, 6 |
| Lythraceae | Cuphea racemosa | In | Yes | Yes | 4, 6 |
| Malvaceae | Bastardia viscosa | Na | Yes | Yes | 4, 6 |
| Gossypium barbadense | En | No | Yes | 4, 6 | |
| Sida rhombifolia | In | Yes | Yes | 4, 6 | |
| Melastomaceae | Miconia robinsoniana | En | Inconclusive results | 4, 6 | |
| Mimosaceae | Acacia macracantha | Na | Inconclusive results | 4, 6 | |
| Prosopis juliflora | Na | Yes | Yes | 4, 6 | |
| Vigna luteola | Na | Inconclusive results | 4, 6 | ||
| Nolanaceae | Nolana galapagensis | En | Yes | Yes | 2 |
| Nyctaginaceae | Commicarpus tuberosus | Na | Yes | Yes | 2, 4, 6 |
| Pisonia floribunda | En | Dioecious | – | 9 | |
| Orchidaceae | Epidendrum spicatum | En | Inconclusive results | 4, 6 | |
| Habenaria moNorrhize | Na | Yes | Yes | 4, 6 | |
| IoNopsis utricularioides | Na | Inconclusive results | 4, 6 | ||
| Passifloraceae | Passiflora colinvauxii | En | Inconclusive results | 4, 6 | |
| Passiflora foetida | En | Yes | Yes | 4, 6 | |
| Piperaceae | Peperomia galapagensis | En | Yes | Yes | 4, 6 |
| Plumbaginaceae | Plumbago scandens | Na | Yes | Yes | 4, 6, 8, 12 |
| Poaceae | Paspalum conjugatum | In | Yes | Yes | 4, 6 |
| Setaria geniculata | Na | Yes | Yes | 4, 6 | |
| Polygonaceae | Polygonum opelousanum | Na | Yes | Yes | 4, 6 |
| Portulacaceae | Portulaca oleracea | In | Yes | Yes | 4, 6 |
| Rhamnaceae | Scutia spicata | En | Yes | Yes | 4, 6 |
| Rosaceae | Rubus nivaeus | In | Yes | Yes | 10 |
| Rubiaceae | Borreria sp. | En | Yes | Yes | 2 |
| Chiococca alba | Na | Yes† | Yes | 2, 12 | |
| Diodia radula | In | No | Yes | 4, 6 | |
| Rutaceae | Zanthoxylum fagara | Na | Dioecious | – | 1, 9 |
| Sapindaceae | Cardiospermum galapageium | En | Inconclusive results | 2 | |
| Simaroubaceae | Castela galapageia | En | Dioecious** | – | 2, 6, 9 |
| Solanaceae | Acnistus ellipticus | En | Yes‡ | Yes§ | 2, 4, 6 |
| Capsicum frutescens | In | Yes | Yes | 4, 6 | |
| Lycium minimum | En | Yes | Yes | 2 | |
| Solanum cheesmanii †† | En | Yes | Yes | 2, 4, 6 | |
| Sterculiaceae | Waltheria ovata | Na | Yes | Yes | 12 |
| Urticaceae | Urera caracasana | NaQ | Dioecious | – | 9 |
| Verbenaceae | Clerodendrum molle | Na | Yes | Yes | 4, 6, 18 |
| Lantana peduncularis | En | Yes | Yes | 4, 6 | |
| Verbena litoralis | In | Yes | Yes | 4, 6 | |
| Zygophyllaceae | Tribulus cistoides | NaQ | Yes | Yes | 4, 6 |
Plant origin: Na, native, En, endemic; NaQ, questionably native; In, introduced.
References: 1, Wiggins and Porter (1971); 2, Rick (1996); 3, Grant and Grant (1981); 4, McMullen (1987); 5, McMullen (1989); 6, McMullen (1990); 7, McMullen and Naranjo (1994); 8, McMullen and Videman (1994); 9, McMullen (1999); 10, Landázuri (2002); 11, Philipp et al. (2004) 12, Philipp et al. (2006); 13, Nielsen et al. (2007); 14, McMullen (2007); 15, McMullen (2009b), 16, Philipp and Nielsen (2010); 17, Jaramillo et al. (2010); 18, McMullen (2011).
* Results based on the seed set of only four bagged flowers; † self-incompatible according to Philipp et al., (2006); ‡ inconclusive according to McMullen (1987); § inconclusive according to McMullen (1990); ¶ C. K. McMullen (unpubl. res.); # little self-pollination according to Grant and Grant (1981); ** inconclusive according to Rick (1966); †† Lycopersicum cheesmanii in the original text.
Flower visitation
Data on flower visits by animals can be found in Supplementary Data Table S1. Table 2 shows the list of studies considered in the review along with information on islands, habitats and seasons encompassed in them. Overall, 488 interactions were quantified, reflecting 329 different animal–plant species interactions. Although more interactions have been reported for endemic plant species (41 %), non-endemic natives (32 %) and alien species (20 %) are also well represented in the database; a few species (2 %) were classified as questionably native, and 5 % were identified only to genus level, so their origin is unknown. This information is not available for most animal species as they were not identified to the species level.
Table 2.
Brief characterization of the systems studied and the methodology adopted by each study considered in the review
| Visit type: pollination (P) or visitation (V) | Islands |
Habitat: dry (D), transition T) or humid (H) | Season: hot (H) or cold (C) | Time of day: nocturnal (N) or diurnal (D) | Reference | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| South Plaza | Isabela | Santa Cruz | San Cristobal | GeNovesa | Floreana | Pinta | Santiago | Marchena | Wolf | Española | Santa Fé | Baltra | Daphne | Rábida | |||||
| V | · | x | x | x | x | x | x | x | x | · | x | x | · | · | x | DTH | HC | DN | Williams, 1911 |
| V | · | · | · | x | · | · | · | · | · | · | · | · | x | · | · | · | · | · | Beebe, 1923 |
| V | · | · | · | · | · | · | · | · | · | · | · | · | x | · | · | · | · | · | Beebe, 1924 |
| V | · | x | x | x | Wheeler, 1924 | ||||||||||||||
| V | · | x | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | Rick, 1963 | |
| V | · | x | x | x | · | x | · | · | · | · | x | x | · | · | · | DTH | HC | · | Linsley and Usinger, 1966 |
| V | · | · | x | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | Rick, 1966 |
| V | · | · | x | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | Eliasson, 1974 |
| V | · | · | x | · | · | · | · | · | · | · | · | x | · | · | · | · | · | · | Hayes, 1975 |
| V | · | · | · | · | · | · | · | · | · | · | x | · | · | · | · | D | HC | D | Werner, 1978 |
| V | · | · | · | · | x | · | · | · | · | · | · | · | · | · | · | D | HC | D | Grant and Grant, 1979b |
| V | · | · | · | · | x | · | · | · | · | · | · | · | · | · | · | D | H | D | Grant and Grant, 1979a |
| P | · | · | x | · | x | · | x | · | · | x | x | · | · | x | · | D | C | D | Grant and Grant, 1981 |
| V | · | · | · | · | · | · | x | · | x | · | · | · | · | · | · | DTH | HC | D | Schluter and Grant, 1982 |
| V | · | · | x | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | Aide, 1983 |
| V | · | · | · | · | · | · | · | · | · | · | · | · | · | x | · | D | H | D | Millington and Grant, 1983 |
| V | · | · | · | · | · | · | x | · | · | · | · | · | · | · | · | DT | HC | D | Schluter, 1984 |
| V | · | · | · | · | · | · | · | · | · | · | · | · | · | x | · | D | HC | D | Boag and Grant, 1984 |
| V | · | · | x | · | · | · | · | · | · | · | · | · | · | · | · | DTH | C | D | McMullen, 1985 |
| V | · | · | · | · | · | · | · | · | · | · | · | · | · | x | · | D | H | D | Price, 1985 |
| V | · | · | x | · | · | · | · | · | · | · | · | · | · | · | · | DTH | C | · | McMullen, 1986 |
| V | · | x | · | · | · | · | · | · | · | · | · | · | · | · | x | · | · | · | Elisens, 1989 |
| V | · | · | x | · | · | · | · | · | · | · | · | · | · | · | · | DT | C | D | McMullen, 1989 |
| V | · | · | x | · | · | · | x | · | · | · | · | · | · | · | · | DTH | · | · | McMullen, 1990 |
| P | x | · | · | · | · | · | · | · | · | · | · | · | · | · | · | D | H | D | Putz and Naughton, 1992 |
| V | · | · | x | · | · | · | x | · | · | · | · | · | · | · | · | DT | C | · | McMullen and Naranjo, 1994 |
| V | · | · | · | · | · | · | · | · | · | · | · | · | · | x | · | D | H | D | East, 1995 |
| V | · | · | · | · | · | · | · | · | · | · | · | · | · | x | · | D | H | D | Grant, 1996 |
| V | · | · | x | · | · | · | · | · | · | · | · | · | · | · | · | H | H | D | Landázuri, 2002 |
| P | · | · | · | · | · | x | · | · | · | · | · | · | · | · | · | TH | H | D | Philipp et al·, 2004 |
| V | · | x | x | x | · | · | · | x | · | · | x | · | · | · | · | DTH | HC | D | Boada, 2005 |
| P | · | x | · | · | · | · | · | · | · | · | · | · | · | · | · | D | H | DN | Philipp et al·, 2006 |
| V | · | · | · | · | · | · | x | · | · | · | · | · | · | · | · | T | · | DN | McMullen, 2007 |
| P | · | · | x | · | · | · | · | · | · | · | · | · | · | · | · | T | C | DN | Smith et al., 2008 |
| P | · | · | · | · | · | · | x | · | · | · | · | · | · | · | · | D | C | N | McMullen, 2009b |
| V | · | · | x | · | · | · | · | · | · | · | · | · | · | · | · | D | H | DN | Jaramillo et al., 2010 |
| P | · | x | · | · | · | · | · | · | · | · | · | · | · | · | · | H | C | D | Philipp and Nielsen, 2010 |
| P | · | · | x | · | · | · | · | · | · | · | · | · | · | · | · | D | C | DN | McMullen, 2011 |
For each study, the islands, main habitats and seasons from where plant–visitor interactions were described are presented. It is also shown if studies include records from diurnal and/or nocturnal observations, and whether studies reported all visits from animals to flower parts or if an examination of pollen transported on the animals bodies or deposited by the animals on the stigma was conducted.
The large majority (89 %) of interactions were documented on a single island (Fig. 2), most of them from Santa Cruz, and the most commonly reported interaction was the widespread native cloudless sulphur butterfly (Phoebis sennae) on the flowers of the native Cordia lutea. The proportion of flower visitors in different arthropod orders is shown in Fig. 3. Such distribution differs from that of the total species diversity in each order in Galápagos (G = 63·95; d.f. = 7; P < 0·001); e.g. only 6 % of the known species of Diptera (17 out of 304) are reported as flower visitors, whereas the figure for Hymenoptera is 32 % (21 out of 65 species).
Fig. 2.
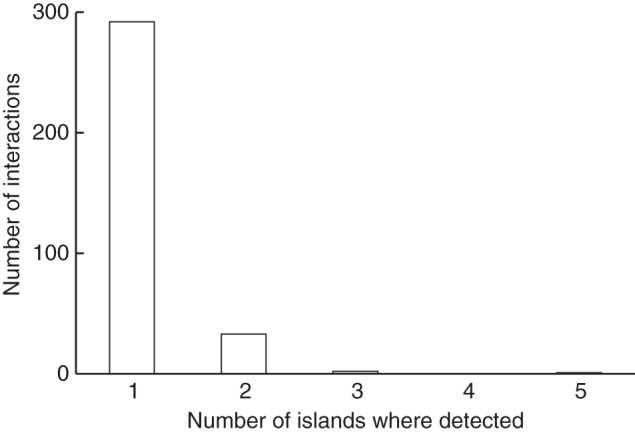
Frequency distribution of specific flower–visitor interactions identified in multiple islands. The island with the highest number of interactions recorded is Santa Cruz.
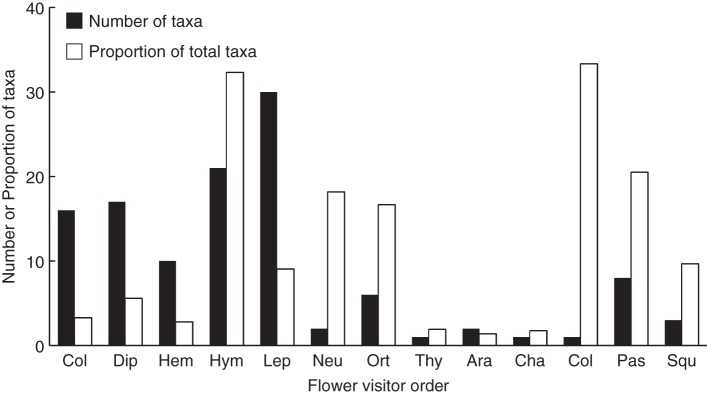
Fig. 3.
Number of taxa from each order reported as flower visitor and the proportion it represents of the total number of species in each of those orders known to exist in Galápagos (as indicated in the key). Order codes: Insecta: Col, Coleoptera; Dip, Diptera; Hem, Hemiptera; Hym, Hymenoptera; Lep, Lepidoptera; Neu, Neuroptera; Ort, Orthoptera; Thy, Thysanoptera; Arachnida: Ara, Aranae; Aves: Cha, Charadriiformes; Col, Columbiformes; Pas, Passeriformes; Reptilia: Squ, Squamata.
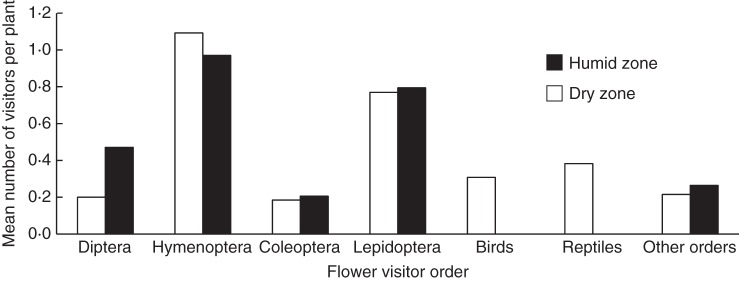
Most animal orders visited the plants of the dry and humid zones equally (Fig. 4). The only exceptions to this pattern were dipterans, of which more frequently visited plants of the humid zone (G = 5·25, d.f. = 1, P < 0·022), and birds and reptiles, which were only reported visiting flowers in the dry zone (G = 16·83, d.f. = 1, P < 0·001 and G = 10·94, d.f. = 1, P = 0·001, respectively).
Fig. 4.
Mean number of species from each order recorded as flower-visitors of plants from the two main habitat types in Galápagos. Diptera, birds and reptiles are the only groups showing significant differences between habitats (G-test; P < 0·05).
Animal-pollinated plants
Among plant families, Asteraceae was the best represented in terms of visited species (n = 21), followed by Malvaceae (12 species) and Cactaceae, Solanaceae and Fabaceae (seven species each). For nearly half of the families, the information compiled included visits to a single species.
Of the 123 plant species, some are highly generalized, i.e. visited by many potential pollinators; for instance, Tournefortia rufo-sericea, Darwiniothamnus tenuifolius and Opuntia helleri are visited by species from six orders (including insects, birds and reptiles). However, most plant species are visited by animals belonging to a single order, frequently Hymenoptera or Lepidoptera. It is important to note that six out of the seven most generalized species have been extensively studied by McMullen and colleagues and, thus, the high diversity of interactions recorded unquestionably reflects the larger sampling effort received by these species and highlights the incompleteness of the existing data.
Floral visitors groups
Over one-third of the compiled interactions involved Hymenoptera, followed by Lepidoptera, Diptera, Coleoptera, birds and reptiles (Fig. 5). Below, we present results of these main groups:
Fig. 5.
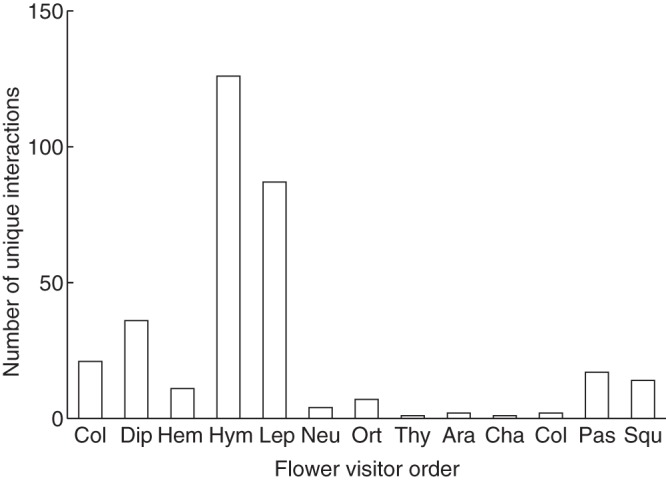
Frequency of unique flower–visitor interactions performed by animals of each order. Order codes: Insecta: Col, Coleoptera; Dip, Diptera; Hem, Hemiptera; Hym, Hymenoptera; Lep, Lepidoptera; Neu, Neuroptera; Ort, Orthoptera; Thy, Thysanoptera; Arachnida: Ara, Aranae; Aves: Cha, Charadriiformes; Col, Columbiformes; Pas, Passeriformes; Reptilia: Squ, Squamata.
Hymenoptera
This order includes the visits from 22 species to 91 plant species. The endemic carpenter bee (Xylocopa darwini) and several ant species (Fam. Formicidae) are particularly important flower visitors. Xylocopa darwini appears as the most generalized pollinator, visiting 84 species encompassing a wide range of flower morphologies (from the open inflorescences of the Asteraceae to the tubular flowers of Cordia leucophlyctis and Clerodendrum molle). Twelve of the 22 ant species in the Galápagos are documented to visit flowers, including both endemic (e.g. Camponotus spp.) and alien (e.g. Monomorium floricola, Tapinoma melanocephalum) species.
Lepidoptera
Thirty-one species of lepidopterans were observed visiting the flowers of 44 plant species. Most of them are moths and hawkmoths and only four are butterflies (out of ten butterfly species known from the Galápagos). The most generalized are the diurnal butterflies Phoebis sennae (14 plant species visited), Leptotes parrhasioides (ten) and Urbanus dorantes (eight), and the diurnal moth Atteva hysginiella (eight).
Coleoptera
Beetles were represented by 16 species visiting 11 plant species. This low number of records contrasts with their large diversity (486 spp.) in the archipelago. The most generalized species (Oxacis sp., Oedemeridae) was only reported to visit the flowers of three species. Two plants visited by a large diversity of beetles were Cordia lutea (by six species) and Miconia robinsoniana (by four species).
Diptera
The flowers of 24 plant species (mostly Asteraceae) were visited by 17 species of dipterans. This is also a low figure, considering the rich Diptera fauna (304 species) of Galápagos. The syrphid Toxomerus crockeri is by far the most generalized species, having been observed on ten species from seven families.
Birds
Up to ten species of birds have been recorded as flower visitors of seven plant species, including four Opuntia species, Portulaca howelli, Tribulus cistoides and Waltheria ovata. Two species of cactus finches (Geospiza scandens and G. cornirostris) are particularly renowned for exploring nectar and pollen in the flowers of several Opuntia spp. (Rick, 1966; Grant and Grant, 1981). However, several other species, including ground finches, mockingbirds, the Galápagos dove and even one water bird (Arenaria interpres) visit flowers and possibly act as pollinators (Putz and Naughton, 1992).
Reptiles
Among reptiles, only lava lizards (Microlophus spp.) have been reported to visit flowers. This group consists of seven endemic species in the Galapagos, three of which were recorded to visit flowers of 13 species in three islands (Daphne Mayor, Pinta and Española). They are usually reported to consume the entire flower (e.g. Schluter, 1984), and only one study refers to the consumption of pollen and nectar (East, 1995).
DISCUSSION
Over the last two centuries, researchers have dedicated much work to describe the Galápagos biodiversity. These efforts resulted in a detailed knowledge of the flora and fauna of the islands and a solid baseline of more ‘elusive’ groups, such as the entomofauna (Bungartz et al., 2009). In contrast, much less is known about the interactions among species, which ultimately sustain the functionality of the ecosystems (Bond, 1994). Our review highlights a very limited and biased knowledge on one important kind of interaction, viz. plant–pollinator interactions. The limitations of the existing information is revealed primarily by a greater number of interactions being retrieved from islands (e.g. Santa Cruz, Pinta, Isabela; Table 2) and species (e.g. Cordia lutea, McMullen, 2012; Clerodendrum molle, McMullen, 2011; Justicia galapagana, McMullen, 1994) which received greater sampling effort or were the target of focal studies. Secondly, the incompleteness of the dataset is evident by the existence of many conspicuous and common interactions which have not yet been described (authors' observations; see also Fig. 6). For this reason, and while the volume of existing data is no longer anecdotal, it should be complemented by more detailed and unbiased studies. With this in mind, we summarize below the main patterns found with the existing information and highlight specific areas where pollination studies in the Galápagos can be particularly important to the conservation of plants and animals.
Fig. 6.
Examples of flower–visitor interactions not yet described in the Galápagos. All pictures were taken between January and March 2011 and represent possibly common, yet so far overlooked, visits of animals to Galápagos flowers. Insects: (A) Xylocopa darwini (Hymenoptera) pollinating Centratherum punctatum (Asteraceae) in San Cristóbal; (B) Agraulis vanillae (Lepidoptera) pollinating Bidens pilosa (Asteraceae) in Santa Cruz; (C) Eumorpha labruscae yupanquii (Sphingidae) pollinating Hibiscus rosa-sinensis (Malvaceae) in Santa Cruz; (D) Blaesoxipha sp. (Sarcophagidae) pollinating Croton scouleri (Euphorbiaceae) in San Cristóbal. Birds: (E) Mimus parvulus pollinating Opuntia galapageia (Cactaceae) in Pinta; (F) Geospiza fuliginosa pollinating Opuntia galapageia (Cactaceae) in Pinta. Reptiles: (G) Microlophus pacificus pollinating Opuntia galapageia (Cactaceae) in Pinta.
Patterns on breeding systems
Despite plant breeding systems being known for <20 % of the Galápagos flora, it seems conclusive that most species are self-compatible and thus do not depend entirely on pollinators to produce seeds, as already reported by McMullen (1987, 1990). This reproductive strategy is also common in other oceanic islands, and is attributable to the poor insular pollinator faunas compared with those on continents (Barrett, 1996 and references therein). Baker (1955) was the first to suggest that self-compatible individuals would be favoured for island colonization given that a single propagule is sufficient to start a sexually reproducing colony, while Ehrendorfer (1979) argued that many of the plant genera that have colonized islands have autogamous representatives in the islands but normally outbreeding populations in the mainland. Lloyd (1980) attributed the high level of selfing in Galápagos partly to their relatively young age and partly to their poor insect fauna and open habitat. Whether such a high level of selfing has evolved in situ in Galápagos or instead is a result of a filter effect acting upon colonizers to the islands is unknown. Future studies on the breeding systems of closely related species in the nearby continent should shed light on this (McMullen, 1990).
The proportion of dioecious species in Galápagos is very low (<2 %), when compared with other archipelagos such as Hawaii (approx. 28 %) or New Zealand (approx. 15 %). Carlquist (1974) attributed this low incidence to the Galápagos' recent origin; younger islands have fewer potential pollinators, which are required for effective pollination of dioecious species. A few Galápagos species show leaky dioecy, such as the natives Laguncularia racemosa, Maytenus octogona and Atriplex peruviana (McMullen, 1999). Half of the dioecious species (Baccharis steetzii, Bursera malacophylla, Croton scouleri, Castela galapageia and Pisonia floribunda) are endemic to Galápagos, but belong to genera that are dioecious elsewhere, suggesting that dioecy is unlikely to have evolved in these islands (McMullen, 1987). Future studies on the same or related species in the mainland will be necessary to assess if dioecy has evolved in or ex situ on Galápagos for other genera.
The prevalence of anemophily (wind pollination) in the archipelago is unknown (but see McMullen and Close, 1993). Anemophilous species tend to produce large amounts of pollen, but most of the species tested by McMullen (1990) showed a low pollen production. Likewise, floral morphologies indicative of wind pollination (e.g. protruding stamens, feathery stigmas) are relatively scarce in the Galápagos flora (McMullen, 1987, 1999), and actually anemophily appears to have been selected against in a number of species (Colinvaux and Schofield, 1976; McMullen and Close, 1993). The reasons for such a low level of anemophily are probably related to the abiotic conditions: in the lowlands, most flowering takes place in the warm rainy season, while in the highlands, many flowers are produced during the cool garúa season and both (rain and humidity) reduce the efficiency of wind pollination (McMullen and Close, 1993).
Patterns on plant–pollinator interactions
Most records compiled during the first half of the last century were unsystematic observations taken during scientific expeditions with more general purposes (e.g. Williams, 1911; Beebe, 1923, 1924; Wheeler, 1924). For example, Beebe (1923) commented on the diurnal foraging of hawkmoths which ‘all day in the brightest sunshine could be found hovering before small blossoms’. These were followed by more specific studies aiming at exploring the pollination biology of selected plant species by McMullen (e.g. 1985, 1993, 2007, 2009b, 2011) and others (e.g. Nielsen et al., 2000; Philipp et al., 2004). The work of Boada (2005) draws the attention to the necessity of considering the community of pollinators in the conservation of rare plants, whereas the study by Philipp et al. (2006) was the first to frame pollination in a more realistic scenario of multiple interacting plants and pollinators.
Hymenopterans, mainly the endemic carpenter bee Xylocopa darwinii and ants, followed by lepidopterans, were quantitatively the most important flower visitors. The carpenter bee has been confirmed as the most generalized pollinator of the Galápagos flora. Regarding ants, a high proportion (55 %) of those found in the archipelago have been found on flowers and, although they have been suggested as possible pollinators, in some cases, they might simply be bug-tending while visiting flowers (Boada, 2005). Due to their abundance and potential impact on ecosystems, the role of ants as pollinators do deserve more attention, particularly during the night, as suggested by McMullen (2011).
We found no association between the relative importance of each order as flower-visitors and their absolute diversity in the Galápagos fauna. This finding might reflect real differences in the importance of pollen and nectar as resources for the species of different orders (e.g. hemipterans are not commonly found on flowers). The pattern, however, might also indicate a bias in preferential sampling of more conspicuous groups of flower visitors, such as Lepidoptera.
Studies reporting animal visitors on flowers seem to have covered the main Galápagos habitats (dry lowlands, intermediate zone and humid highlands) approximately in the proportions that they are found in the islands. With the information available so far, it seems that dipterans are more important pollinators in the humid zone, while birds and reptiles are only known to visit plants in the dry zone. Likewise, comparable efforts have been made in the cold and the warm seasons. However, the vast majority of the studies have exclusively collected diurnal interactions, thus forming an important bias in the information gathered. The few studies that have monitored what was happening during the ‘night shift’ (Devoto et al., 2011) discovered a different but similarly active pollinating community (Philipp et al., 2006; McMullen, 2007, 2009b, 2011).
Another important bias detected is that the vast majority of interactions come from a single island, mostly in the central islands, while only 10 % have been reported from at least two islands and no data are reported from peripheral islands such as Fernandina and Wolf. This differential sampling is mainly determined by the intrinsic logistical limitations of working on uninhabited islands. It is thus likely that patterns identified on such islands will vary when more and better data become available. So far, only one study has investigated an entire pollination network in the Galápagos, even if suffering from sampling limitations (Philipp et al., 2006). However, there is still a marked lack of knowledge on the structure and functioning of such communities in the archipelago, especially for interaction networks including different habitats and different islands. Such community level studies will also allow us to estimate the level of generalization in both plants and pollinators (e.g. Olesen et al., 2002; Padrón et al., 2009). With the information available so far, plant species such as Tournefortia rufo-sericea, Darwiniothamnus tenuifolius, Opuntia helleri and Cordia lutea, all visited by a wide assemblage of potential pollinators, including insects, birds and reptiles, are likely to act as network ‘hubs’. Regarding animals, the emerging pattern is that hymenopterans are the group visiting more flowers, followed by lepidopterans, dipterans and coleopterans. Besides the carpenter bee, several species of butterflies and the diurnal moth Atteva hysginiella are highly generalized visitors. Nevertheless, surprises might arise when more robust datasets, particularly those including nocturnal observations, become available.
How important is flower visitation by vertebrates in Galápagos?
Some ornithological–evolutionary studies (e.g. Grant and Grant, 1979a, 1981) stress the importance of birds as potential pollinators of several species from the dry zone. For example, Grant and Grant (1981) showed that the flowers of at least four species of Opuntia are important resources for finches (Geospiza spp.), doves (Zenaida galapagoensis) and mockingbirds (Mimus spp.) on six islands. Nectar and pollen seem to be valuable alternative food items for birds during the end of the dry season when the shortage of insect prey coincides with the flowering season of some plants and the beginning of the bird breeding season (Grant, 1996). During this period, cactus finches can spend nearly 90 % of their time foraging in Opuntia flowers (Grant, 1996). It is possible that observations on other plants and islands will reveal more plant–bird pollination interactions, as shown in other archipelagos, e.g. in the Macaronesia (Valido and Olesen, 2010).
Similarly, flowers and nectar can be important energy resources for lava lizards, which have been reported consuming flowers (Werner, 1978; Schluter, 1984; East, 1995). However, no study has evaluated the potential role of the Galápagos lava lizards as pollinators (as found in other archipelagos; Olesen and Valido, 2003). The role of other reptiles and small mammals as pollinators has likewise not yet been evaluated.
Future avenues of research and implications for conservation and management
Studying interaction networks of complex and mega-diverse systems is a challenge for future ecological investigations. Fully understanding the co-evolution of very diverse wildlife systems will be possible only once we have understood the processes underlying their interaction networks (Guimarães et al., 2011). On the one hand, these studies provide important aspects of different forms of interaction, whereas on the other, they reveal key aspects of importance for diversity persistency and robustness against species loss (Wardle et al., 2011).
In terms of biodiversity conservation, knowing the structure of mutualistic networks will allow us to explore (a) the resilience of mutualistic networks to global change (i.e. biological invasions, fragmentation, etc.), (b) the impact of the loss of a specific mutualist for certain species and for the whole community, and (c) the role of a rare species in a community. In the face of the severe threats to the Galápagos ecosystems, a community-level working approach is required to be able to assess causes and consequences of biological invasions (Simberloff, 2004) and to plan effective ecological restoration (Palmer et al., 1997). The knowledge of plant–animal interactions, including structural and functional aspects of the ecosystem, can significantly contribute to strengthen the capacity to deal with the problem of invasive species in the archipelago (Heleno et al., 2010). Knowing how they integrate and how they impact on the native biota and its interactions will allow setting better and more cost-effective management plans (Gosper et al., 2005).
Impact of alien species
According to some authors, Xylocopa darwinii and probably other insects prefer to visit alien plants to natives, because they often have larger flowers (Linsley et al., 1966; McMullen, 1987, 1989). Other authors, however, did not identify any effect of alien species on pollinators' preferences (Philipp et al., 2006). From the compiled dataset we could not find any clear preference by this bee or by any of the generalist lepidopteran species. More studies are required to assess whether alien species are more successful because of larger flowers with more attractive colours and possibly with higher quantities of nectar and/or pollen. At present, the apparent bias towards endemic and native plants visited is most likely a result of preferential interest of ecologists in documenting the visits to endemic and native flowers. However, given the alarming increase in the proportion of the flora that is alien (approx. 65 %), introduced species will also need to be studied alongside the natives.
Conclusions
With regard to plant breeding systems, our literature review confirms that most plants are selfers and that dioecy is rather rare, although we need to bear in mind that information is available for <20 % of the native flora. Specific tests to detect self-incompatibility are needed, as well as studies comparing breeding systems from closely related species, both in the islands and the mainland. A high number of interactions between plants and flower visitors has been recorded, but this information is much biased because most studies have been performed in the most populated island Santa Cruz. An important knowledge gap on plant–pollinator interactions is in the highlands of the tallest island, Isabela, as well as in peripheral islands (Fernandina, Wolf and Darwin). Studies on nocturnal flower visitors are also urgently needed, mainly to assess the importance of moths as pollinators, which are one of the most diverse group of potential flower-visitors in Galápagos, but also because many insects might be active at night due to the hot and dry conditions during the day and the low incidence of night-active predators. Finally, a community approach is much needed to improve our understanding of the patterns of pollination interactions and to be able to predict how the increasing number of alien species is going to infiltrate and impact on the native communities of this unique archipelago.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We are grateful to the staff of the Charles Darwin Foundation, particularly to Sally Taylor for help in consulting the library and to the Galápagos Natural Park for facilitating the work in the islands. We are also grateful to John Pannell for the invitation to participate in the Population Biology Meeting in Oxford and to collaborate in this special issue of Annals of Botany. Rosa Leimu gave us good suggestions to improve the clarity of the manuscript. Finally, we thank the rest of our colleagues in the BBVA project for their kind support and a welcoming environment. This work was supported by a biodiversity project funded by the BBVA Foundation (Spain), co-ordinated by A.T. The first author also received a fellowship from the Spanish International and Development Cooperation Agency (AECID).
LITERATURE CITED
- Aide M. CDRS Annual Report. Galapagos Islands, Ecuador: Charles Darwin Research Station; 1983. The influence of Xylocopa darwini on floral evolution in the Galapagos. [Google Scholar]
- Ashman TL, Knight TM, Steets JA, et al. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology. 2004;85:2408–2421. [Google Scholar]
- Baker HG. Self-compatibility and establishment after ‘long-distance’ dispersal. Evolution. 1955;9:347–349. [Google Scholar]
- Barrett SCH. The reproductive biology and genetics of island plants. Philosophical Transactions of the Royal Society. B: Biological Sciences. 1996;351:725–733. [Google Scholar]
- Baur G. The origin of the Galapagos Islands. American Naturalist. 1891;25:217–229. [Google Scholar]
- Beebe W. Notes on Galapagos Lepidoptera. Zoologica. 1923;5:51–59. [Google Scholar]
- Beebe W. Galapagos: world's end. London: Putnams's Sons; 1924. [Google Scholar]
- Bensted-Smith R. Visión para la biodiversidad de las islas Galápagos. 2002 Puerto Ayora, Galápagos, Fundación Charles Darwin para las islas Galápagos y Fondo Mundial para la Naturaleza. [Google Scholar]
- Biesmeijer JC, Roberts SPM, Reemer M, et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science. 2006;313:351–354. doi: 10.1126/science.1127863. [DOI] [PubMed] [Google Scholar]
- Boada R. Insects associated with endangered plants in the Galapagos Islands, Ecuador. Entomotropica. 2005;20:77–88. [Google Scholar]
- Boag GT, Grant PR. Darwin's finches (Geospiza) on isla Daphne Major, Galápagos: breeding and feeding ecology in a climatically variable environment. Ecological Monographs. 1984;54:463–489. [Google Scholar]
- Bond WJ. Do mutualisms matter – assessing the impact of pollinator and disperser disruption on plant extinction. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 1994;344:83–90. [Google Scholar]
- Bungartz F, Herrera HW, Jaramillo P, et al. List of all known species from the Galapagos Islands – lista de todas las especies conocidas de las Islas Galápagos. Puerto Ayora, Galapagos: Charles Darwin Foundation/Fundación Charles Darwin; 2009. [Google Scholar]
- Carlquist S. Island biology. Columbia, NY: Columbia University Press; 1974. [Google Scholar]
- Colinvaux PA. The Galápagos climate: present and past. In: Perry R., editor. Key environments: Galápagos. Oxford: Pergamon Press; 1984. [Google Scholar]
- Colinvaux PA, Schofield EK. Historical ecology of the Galapagos Islands. I. A Holocene pollen record from El Junco, Isla San Cristobal. Journal of Ecology. 1976;64:989–1012. [Google Scholar]
- Devoto M, Bailey S, Memmott J. The ‘night shift’: nocturnal pollen-transport networks in a boreal pine forest. Ecological Entomology. 2011;36:25–35. [Google Scholar]
- Duffy JE, Carinale BJ, France KE, McIntyre PB, Thébault E, Loreau M. The functional role of biodiversity in ecosystems: incorporating trophic complexity. Ecology Letters. 2007;10:522–538. doi: 10.1111/j.1461-0248.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- East KT. Pollen digestion in Galápagos lava lizards. Noticias de Galápagos. 1995;55:8–14. [Google Scholar]
- Ehrendorfer F. Reproductive biology in island plants. In: Bramwell D., editor. Plants and islands. London: Academic Press; 1979. [Google Scholar]
- Eliasson UH. Studies in Galápagos plants. XIV. The genus Scalesia Arn. Opera Botanica. 1974;36:1–117. [Google Scholar]
- Elisens WJ. Genetic variation and evolution of the Galápagos shrub snapdragon. National Geographic Research (USA) 1989;5:98–110. [Google Scholar]
- Gardener MR, Atkinson R, Renteria JL. Eradications and people: lessons from the plant eradication program in Galapagos. Restoration Ecology. 2010;18:20–29. [Google Scholar]
- Gosper CR, Stansbury CD, Vivian-Smith G. Seed dispersal of fleshy-fruited invasive plants by birds: contributing factors and management options. Diversity and Distributions. 2005;11:549–558. [Google Scholar]
- Grant BR. Pollen digestion by Darwin's finches and its importance for early breeding. Ecology. 1996;77:489–499. [Google Scholar]
- Grant BR, Grant PR. Exploitation of Opuntia cactus by birds on the Galapagos. Oecologia. 1981;49:179–187. doi: 10.1007/BF00349186. [DOI] [PubMed] [Google Scholar]
- Grant PR, Grant KT. Breeding and feeding ecology of the Galápagos Dove. The Condor. 1979a;81:397–403. [Google Scholar]
- Grant PR, Grant N. Breeding and feeding of Galápagos Mockingbirds, Nesomimus parvulus. Auk. 1979b;96:723–736. [Google Scholar]
- Guézou A, Trueman M, Buddenhagen CE, et al. An extensive alien plant inventory from the inhabited areas of Galapagos. PLoS ONE. 2010;5:e10276. doi: 10.1371/journal.pone.0010276. http://dx.doi.org/10.1371/journal.pone.0010276 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães PR, Jordano P, Thompson JN. Evolution and coevolution in mutualistic networks. Ecology Letters. 2011;14:877–885. doi: 10.1111/j.1461-0248.2011.01649.x. [DOI] [PubMed] [Google Scholar]
- Hayes AH. The larger moths of the Galapagos Islands (Geometroidea: Sphingoidea & Noctuoidea) Proceedings of the California Academy of Sciences. Series 4. 1975;40:145–208. [Google Scholar]
- Heleno RH, Lacerda I, Ramos JA, Memmott J. Evaluation of restoration effectiveness: community response to the removal of alien plants. Ecological Applications. 2010;20:1191–1203. doi: 10.1890/09-1384.1. [DOI] [PubMed] [Google Scholar]
- Hooker J. An enumeration of the plants of the Galapagos archipelago with descriptions of those which are new. Transactions of the Linnean Society of London. 1847;20:163–233. [Google Scholar]
- Jaramillo P, Trigo MM, Ramírez E, Mauchamp A. Insect pollinators of Jasminocereus thouarsii, an endemic cactus of the Galapagos islands. Galapagos Research. 2010;67:21–25. [Google Scholar]
- Jaramillo P, Guézou A, Mauchamp A, Tye A. CDF checklist of Galapagos flowering plants – FCD Lista de especies de plantas con flores de Galápagos. In: Bungartz F, Herrera H, Jaramillo P, et al., editors. Charles Darwin Foundation Galapagos Species Checklist – Lista de Especies de Galápagos de la Fundación Charles Darwin. Puerto Ayora, Galapagos: Charles Darwin Foundation/Fundación Charles Darwin; 2011. http://www.darwinfoundation.org/datazone/checklists/vascular-plants/magnoliophyta/ [Google Scholar]
- Jordano P, García C, Godoy JA, García-Castaño JL. Differential contribution of frugivores to complex seed dispersal patterns. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3278–3282. doi: 10.1073/pnas.0606793104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser-Bunbury CN, Traveset A, Hansen DM. Conservation and restoration of plant-animal mutualisms on oceanic islands. Perspectives in Plant Ecology, Evolution and Systematics. 2010;12:131–143. [Google Scholar]
- Kearns CA, Inouye DW, Waser NM. Endangered mutualisms: The conservation of plant-pollinator interactions. Annual Review of Ecology and Systematics. 1998;29:83–112. [Google Scholar]
- Landázuri O. Distribución, fenologia reproductiva y dinámica del banco de semillas de mora (Rubus niveus Thunb) en la parte alta de la isla Santa Cruz, Galápagos. 2002 PhD Thesis, Universidad Central del Ecuador, Quito. [Google Scholar]
- Linsley EG, Usinger RL. Insects of the Galápagos Islands. Proceedings of the California Academy of Sciences. 1966;33:113–196. [Google Scholar]
- Linsley EG, Rick CM, Stephens SG. Observations on the floral relationships of the Galápagos carpenter bee. The Pan-Pacific Entomologist. 1966;42:1–18. [Google Scholar]
- Lloyd DG. Demographic factors and mating patterns in angiosperms. In: Solbrig OT., editor. Demography and evolution in plant populations. Oxford: Blackwell Science; 1980. [Google Scholar]
- McMullen CK. Observations on insect visitors to flowering plants of Isla Santa Cruz – Part I. The endemic carpenter bee. Noticias de Galápagos. 1985;42:24–25. [Google Scholar]
- McMullen CK. CDRS Annual Report. Galapagos Islands, Ecuador.: Charles Darwin Research Station; 1986. A study of self-compatibility and pollination agents of representative Galápagos Islands angiosperms. [Google Scholar]
- McMullen CK. Breeding systems of selected Galápagos Islands angiosperms. American Journal of Botany. 1987;74:1694–1705. [Google Scholar]
- McMullen CK. The Galápagos carpenter bee, just how important is it? Noticias de Galápagos. 1989;48:16–18. [Google Scholar]
- McMullen CK. Reproductive biology of Galápagos Islands angiosperms. Monographs in Systematic Botany of the Missouri Botanical Garden. 1990;32:35–45. [Google Scholar]
- McMullen CK. Flower-visiting insects of the Galápagos Islands. Pan-Pacific Entomologist. 1993;69:95–106. [Google Scholar]
- McMullen CK. Pollinator availability: a possible explanation of inter-island floral variation in Justicia galapagana (Acanthaceae) Noticias de Galápagos. 1994;54:22–27. [Google Scholar]
- McMullen CK. Flowering plants of the Galápagos. Ithaca, NY: Comstock Publishing Associates; 1999. [Google Scholar]
- McMullen CK. Pollination biology of the Galápagos endemic, Tournefortia rufo-sericea (Boraginaceae) Botanical Journal of the Linnean Society. 2007;153:21–31. [Google Scholar]
- McMullen CK. Insular flora: more than ‘wretched-looking little weeds. In: Roy TD., editor. Galápagos – preserving Darwin's legacy. Albany, New Zealand: David Baterman; 2009a. [Google Scholar]
- McMullen CK. Pollination biology of a night-flowering Galápagos endemic, Ipomoea habeliana (Convolvulaceae) Botanical Journal of the Linnean Society. 2009b;160:11–20. [Google Scholar]
- McMullen CK. Nocturnal and diurnal pollination of Clerodendrum molle (Verbenaceae) in the Galápagos Islands. Plant Systematics and Evolution. 2011;292:15–23. [Google Scholar]
- McMullen CK. Pollination of the heterostylous Galápagos native, Cordia lutea (Boraginaceae) Plant Systematics and Evolution. 2012;298:569–579. [Google Scholar]
- McMullen CK, Close DD. Wind pollination in the Galápagos Islands. Noticias de Galápagos. 1993;52:12–17. [Google Scholar]
- McMullen CK, Naranjo SJ. Pollination of Scalesia baurii ssp. hopkinsii (Asteraceae) on Pinta Island. Noticias de Galápagos. 1994;53:25–28. [Google Scholar]
- McMullen CK, Viderman DM. Comparative studies on the pollination biology of Darwiniothamnus tenuifolius (Asteraceae) and Plumbago scandens (Plumbaginaceae) on Pinta Island and Santa Cruz Island – Galápagos. Phytologia. 1994;76:1–33. [Google Scholar]
- Mauchamp A. Threats from alien plant species in the Galápagos Islands. Conservation Biology. 1997;11:260–263. [Google Scholar]
- MEA. Ecosystems and human well-being: a synthesis. Washington, DC: Island Press; 2005. [Google Scholar]
- Memmott J, Gibson R, Carvalheiro L, et al. The conservation of ecological interactions. In: Stewart AA, New TR, Lewis OT., editors. Insect conservation biology. London: The Royal Entomological Society; 2007. [Google Scholar]
- Millington SJ, Grant PR. Feeding ecology and territoriality of the cactus finch Geospiza scandens on Isla Daphne Major, Galapagos. Oecologia. 1983;58:76–83. doi: 10.1007/BF00384545. [DOI] [PubMed] [Google Scholar]
- Morales CL, Traveset A. A meta-analysis of impacts of alien vs. native plants on pollinator visitation and reproductive success of co-flowering native plants. Ecology Letters. 2009;12:716–728. doi: 10.1111/j.1461-0248.2009.01319.x. [DOI] [PubMed] [Google Scholar]
- Nielsen LR, Philipp M, Adsersen H, Siegismund HR. Breeding system of Scalesia divisa Andersson, an endemic Asteraceae from the Galápagos Islands. In: Totland Ø, editor. The Scandinavian association for pollination ecology honours Knut Fægri. Oslo: The Norwegian Academy of Science and Letters; 2000. [Google Scholar]
- Nielsen LR, Siegismund HR, Hansen T. Inbreeding depression in the partially self-incompatible endemic plant species Scalesia affinis (Asteraceae) from Galapagos islands. Evolutionary Ecology. 2007;21:1–12. [Google Scholar]
- Olesen J, Valido A. Lizards as pollinators and seed dispersers: an island phenomenon. Trends in Ecology & Evolution. 2003;18:177–181. [Google Scholar]
- Olesen JM, Eskildsen LI, Venkatasamy S. Invasion of pollination networks on oceanic islands: importance of invader complexes and endemic super generalists. Diversity and Distributions. 2002;8:181–192. [Google Scholar]
- Ollerton J, Winfree R, Tarrant S. How many flowering plants are pollinated by animals? OIKOS. 2011;120:321–326. [Google Scholar]
- Padrón B, Traveset A, Biedenweg T, Díaz D, Nogales M, Olesen JM. Impact of alien plant invaders on pollination networks in two archipelagos. PLoS ONE. 2009;4:e6275. doi: 10.1371/journal.pone.0006275. http://dx.doi:10.1371/journal.pone.0006275 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MA, Ambrose RF, Poff NL. Ecological theory and community restoration ecology. Restoration Ecology. 1997;5:291–300. [Google Scholar]
- Philipp M, Nielsen LR. Reproductive ecology of Scalesia cordata (Asteraceae), an endangered species from the Galápagos Islands. Botanical Journal of the Linnean Society. 2010;162:496–503. [Google Scholar]
- Philipp M, Hansen LB, Adsersen H, Siegismund HR. Reproductive ecology of the endemic Lecocarpus pinnatifidus (Asteraceae) in an isolated population in the Galápagos Islands. Botanical Journal of the Linnean Society. 2004;146:171–180. [Google Scholar]
- Philipp M, Bocher J, Siegismund HR, Nielsen LR. Structure of a plant–pollinator network on a pahoehoe lava desert of the Galapagos Islands. Ecography. 2006;29:531–540. [Google Scholar]
- PNG. Plan de Manejo del Parque Nacional Galápagos: un pacto por la conservación y desarrollo sustentable del archipiélago. Puerto Ayora, Galápagos: 2005. [Google Scholar]
- Porter DM. Vascular plants of the Galapagos: origins and dispersal. In: Bowman RI, Berson M, Levitan AE., editors. Patterns of evolution in Galápagos organisms. Washington, DC: American Association for the Advancement of Science; 1983. pp. 33–96. [Google Scholar]
- Porter DM. Relationships of the Galapagos flora. Biological Journal of the Linnean Society. 1984;21:243–252. [Google Scholar]
- Price T. Reproductive responses to varying food-supply in a population of Darwin Finches – clutch size, growth-rates and hatching synchrony. Oecologia. 1985;66:411–416. doi: 10.1007/BF00378307. [DOI] [PubMed] [Google Scholar]
- Putz FE, Naughton LC. Apparent pollination of Portulaca howelli by ruddy turnstones (Arenaria interpres L.) on Isla Plaza Sur. Noticias de Galápagos. 1992;52:5. [Google Scholar]
- Rick CM. Biosystematic studies on Galapagos tomatoes. Ocasional Papers of the California Academy of Sciences. 1963;44:59–77. [Google Scholar]
- Rick CM. Some plant–animal relations on the Galapagos Islands. In: Bowman R., editor. Galápagos International Scientific Project. Berkeley, CA: University of California Press; 1966. [Google Scholar]
- Schluter D. Body size, prey size and herbivory in the Galapagos lava lizard, Tropidurus. Oikos. 1984;43:291–300. [Google Scholar]
- Schluter D, Grant PR. The distribution of Geospiza difficilis in relation to G. fuliginosa in the Galápagos Islands: tests of three hypotheses. Evolution. 1982;36:1213–1226. doi: 10.1111/j.1558-5646.1982.tb05490.x. [DOI] [PubMed] [Google Scholar]
- Simberloff D. Community ecology: is it time to move on? American Naturalist. 2004;163:787–799. doi: 10.1086/420777. [DOI] [PubMed] [Google Scholar]
- Smith SD, Izquierdo PR, Baum DA. Comparative pollination biology of sympatric and allopatric Andean iochroma (Solanaceae) Annals of the Missouri Botanical Garden. 2008;95:600–617. [Google Scholar]
- Snell H, Rea S. The 1997–1998 El Niño in Galápagos: can 34 years of data estimate 120 years of pattern? Noticias de Galápagos. 1999;60:11–20. [Google Scholar]
- Snell HM, Stone PA, Snell HL. A summary of geographical characteristics of the Galapagos Islands. Journal of Biogeography. 1996;23:619–624. [Google Scholar]
- Sulloway FJ. Tantalizing tortoises and the Darwin–Galapagos Legend. Journal of the History of Biology. 2009;42:3–31. doi: 10.1007/s10739-008-9173-9. [DOI] [PubMed] [Google Scholar]
- Traveset A, Richardson DM. Biological invasions as disruptors of plant reproductive mutualisms. Trends in Ecology & Evolution. 2006;21:208–216. doi: 10.1016/j.tree.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Trueman M, d'Ozouville N. Characterizing the Galapagos terrestrial climate in the face of global climate change. Galapagos Research. 2010;67:26–37. [Google Scholar]
- Trueman M, Atkinson R, Guezou A, Wurm P. Residence time and human-mediated propagule pressure at work in the alien flora of Galapagos. Biological Invasions. 2010;12:3949–3960. [Google Scholar]
- Tye A, Snell HL, Peck SB, Andersen H. Outstanding terrestrial features of the Galapagos archipelago. In: Bensted-Smith R., editor. A biodiversity vision for the Galapagos Islands. Puerto Ayora: Charles Darwin Foundation and World Wildlife Fund; 2002. [Google Scholar]
- Tylianakis JM, Laliberté E, Nielsen A, Bascompte J. Conservation of species interaction networks. Biological Conservation. 2010;143:2270–2279. [Google Scholar]
- Valido A, Olesen JM. Pollination on islands: examples from the Macaronesian archipelagos. In: Serrano ARM, Borges PAV, Boieiro M, Oromí P., editors. Terrestrial arthropods of Macaronesia. Lisboa, Portugal: Sociedade Portuguesa de Entomologia; 2010. [Google Scholar]
- Van Leeuwen JFN, Froyd CA, Van der Knaap WO, Coffey EE, Tye A, Willis KJ. Fossil pollen as a guide to conservation in the Galapagos. Science. 2008;322:1206. doi: 10.1126/science.1163454. [DOI] [PubMed] [Google Scholar]
- Wardle DA, Bardgett RD, Callaway RM, Van der Putten WH. Terrestrial ecosystem responses to species gains and losses. Science. 2011;332:1273–1277. doi: 10.1126/science.1197479. [DOI] [PubMed] [Google Scholar]
- Werner DI. On the Biology of Tropidurus delanonis Baur (Iguanidae) Zeitschrift für Tierpsychologie. 1978;47:337–395. doi: 10.1111/j.1439-0310.1978.tb01843.x. [DOI] [PubMed] [Google Scholar]
- Wheeler WM. The formicidae of the Harrison Williams Galapagos expedition. Zoologica. 1924;5:101–122. [Google Scholar]
- White WM, McBirney AR, Duncan RA. Petrology and geochemistry of the Galapagos islands – portrait of a pathological mantle plume. Journal of Geophysical Research – Solid Earth. 1993;98:19533–19563. [Google Scholar]
- Wiggins IL, Porter DM. Flora of the Galapagos islands. Stanford, CA: Stanford University Press; 1971. [Google Scholar]
- Williams FX. Expedition of the California Academy of Sciences to the Galapagos Islands 1905–1906. III. The butterflies and hawk-moths of the Galapagos Islands. Proceedings of the California Academy of Sciences. 1911;4:289–322. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.