Abstract
Background and Aims
The study of local adaptation in plant reproductive traits has received substantial attention in short-lived species, but studies conducted on forest trees are scarce. This lack of research on long-lived species represents an important gap in our knowledge, because inferences about selection on the reproduction and life history of short-lived species cannot necessarily be extrapolated to trees. This study considers whether the size for first reproduction is locally adapted across a broad geographical range of the Mediterranean conifer species Pinus pinaster. In particular, the study investigates whether this monoecious species varies genetically among populations in terms of whether individuals start to reproduce through their male function, their female function or both sexual functions simultaneously. Whether differences among populations could be attributed to local adaptation across a climatic gradient is then considered.
Methods
Male and female reproduction and growth were measured during early stages of sexual maturity of a P. pinaster common garden comprising 23 populations sampled across the species range. Generalized linear mixed models were used to assess genetic variability of early reproductive life-history traits. Environmental correlations with reproductive life-history traits were tested after controlling for neutral genetic structure provided by 12 nuclear simple sequence repeat markers.
Key Results
Trees tended to reproduce first through their male function, at a size (height) that varied little among source populations. The transition to female reproduction was slower, showed higher levels of variability and was negatively correlated with vegetative growth traits. Several female reproductive traits were correlated with a gradient of growth conditions, even after accounting for neutral genetic structure, with populations from more unfavourable sites tending to commence female reproduction at a lower individual size.
Conclusions
The study represents the first report of genetic variability among populations for differences in the threshold size for first reproduction between male and female sexual functions in a tree species. The relatively uniform size at which individuals begin reproducing through their male function probably represents the fact that pollen dispersal is also relatively invariant among sites. However, the genetic variability in the timing of female reproduction probably reflects environment-dependent costs of cone production. The results also suggest that early sex allocation in this species might evolve under constraints that do not apply to other conifers.
Keywords: Pinus pinaster, conifers, sex-dependent threshold size for first reproduction, size-dependent sex allocation, clinal variation, neutral genetic structure
INTRODUCTION
It is widely appreciated that plants are enormously variable in key life-history traits such as seed number, germination rate, growth rates and time to flowering. To the extent that life-history variation has an additive genetic component, these observations are puzzling, because natural selection is expected to deplete genetic variation for traits that affect individual fitness (reviewed by Barton and Keightley, 2002). Much of the variation in life-history traits within populations is attributable to phenotypic plasticity (Sultan, 2000), but it is also well established that plant populations contain large amounts of additive genetic variation for life-history traits, including seed size and number (Mazer, 1987), plant relative growth rate (Sánchez-Gómez et al., 2010), time to flowering (Montague et al., 2008), and flower size and number (Worley and Barrett, 2000). Understanding the nature and maintenance of this variation remains an important challenge for plant evolutionary biologists. One idea is that life-history variation is rendered effectively neutral as a result of antagonistic pleiotropy, i.e. fitness trade-offs between different life-history traits (Walsh and Blows, 2009), or by fluctuating selection from among generations (Bonser and Aarssen, 2009; Childs et al., 2010). Another idea is that much of the variation we observe is maintained in a balance between purifying selection, which depletes it, and its replenishment by mutation or immigration (Barton and Keightley, 2002).
The importance of immigration as a process that introduces new genetic variation for life-history traits into a population will ultimately depend on the maintenance of this variation among populations by adaptive responses to natural selection locally, i.e. on the adaptive divergence among populations for the life-history traits concerned. Measuring population genetic divergence for life-history traits is thus an important task, yet discriminating between variation due to phenotypic plasticity and that due to genetic divergence requires the measurement of traits expressed by individuals from different populations under comparable environmental conditions (i.e. tested in common gardens or reciprocal transplant experiments). In standard common-garden experiments, genotypes sampled from a range of different populations are grown together in a common environment. Unlike reciprocal transplant experiments, common gardens are unable to test the hypothesis of local adaptation for all populations, but they allow an assessment of genetic divergence among more populations that could be accommodated in fully reciprocal transplant experiments. Common garden experiments thus offer a promising avenue towards understanding the maintenance of life-history variation in geographically widespread species.
The age and/or size of first reproduction, i.e. the onset of reproduction, and the number of offspring produced represent particularly important life-history traits. Slight changes in size at maturity and fecundity can have dramatic effects on lifetime reproductive output in perennial species, because early reproduction can be costly in terms of future reproductive potential (Kozłowski, 1992; Roff, 2000). Theory predicts that selection will cause plants with a large life expectancy and a strongly positive relationship between size and fecundity to delay their onset of flowering (Roff, 1992). It is widely accepted that plants tend to use size rather than age as a developmental cue for reproduction (de Jong and Klinkhamer, 2005; but see Metcalf et al., 2003). However, size and age are clearly linked, not least because the age schedule of survival and the disturbance regime, which affects life expectancy, can have an important impact on size at reproduction (Klinkhamer and de Jong, 1987; Burd et al., 2006; Weiner et al., 2009).
Plant size at first reproduction has received relatively little attention in long-lived polycarpic trees (Thomas, 1996; Dodd and Silvertown, 2000; Niklas and Enquist, 2003; Wright et al., 2005), although it is well studied in monocarps (Wesselingh et al., 1997; Rees et al., 1999; Callahan and Pigliucci, 2002; Kuss et al., 2008; Kagaya et al., 2009) and perennial herbs (Méndez and Karlsson, 2004; Brys et al., 2011). These studies have not only documented substantial variability in size at first reproduction among populations, but they have also shown high within-population variability. Moreover, they confirmed theoretical predictions on the role of environmental effects that act through changes in disturbance regimes and growth conditions (see Méndez and Karlsson, 2004).
Although it may sometimes be useful to refer to size at first reproduction as a single trait, individuals in sexual populations transmit their genes through both male and female functions, and the size threshold for each may differ. Thus, in the case of dioecious species, males often begin flowering earlier than females (Delph, 1999). Similarly, in monoecious or hermaphroditic species, male function often precedes female function (Freeman et al., 1981). In some species, this decoupling of male and female functions in relation to size, i.e. a sex-dependent threshold size for first reproduction (TSFR), can result in the presence of individuals functioning as pure males, as females or as hermaphrodites expressing both sexes simultaneously during their early stages of sexual maturity (de Jong and Klinkhamer, 2005; Zhang, 2006). In the extreme, populations can express sequential hermaphroditism, as in the classical example of the jack-in-the-pulpit, where plants are male when small and either female or hermaphroditic one to several reproductive seasons later (Kinoshita, 1987). Such transitions in the functional gender of individuals as they grow have been interpreted in terms of adaptive responses to natural selection when the fitness gains through male and female functions depend on size (Cadet et al., 2004; de Jong and Klinkhamer, 2005; Zhang, 2006).
Size effects on fitness expectations through male vs. female functions can be direct or indirect (Klinkhamer et al., 1997). In wind-pollinated species, pollen dispersal may be enhanced directly by growth in height, so that tall individuals preferentially express their male function (Pickup and Barrett, 2012). In animal-pollinated species, this ‘direct effect’ of height on male fitness is less likely. Here, larger plants are more likely to enhance their female function if the female fitness gain curve flattens off less quickly with investment than the male curve, as seems likely; these are the so-called ‘budget effects’ of size on sex allocation (Klinkhamer et al., 1997). Because a plant's resource base, and therefore its growth rate and ultimate size, will inevitably depend on habitat quality, we might then expect selection through these indirect budget effects to vary among populations of a species exposed to environments that differ across the species' geographical range. For example, the relative costs of male versus female functions may be different under different environments, such that female function may be borne optimally by large individuals in some, but not all, populations (see Klinkhamer et al., 1997). If so, differences in sex-specific TSFR may evolve in accordance with the differing trade-offs between reproduction through each sexual function and growth. Such geographical variation in this important life-history trait would allow a more subtle understanding of the action of natural selection on sex allocation that is possible through broader comparisons among species, for example with different pollination modes. There has, however, been very little research on among-population variation in TSFR through male versus female functions (hereafter male or female TSFR) despite the abundant recent literature dealing with sex allocation in plants.
Forest trees offer a valuable opportunity to test hypotheses concerning variation in size-dependent sex expression and sex allocation (SDS). Not only do they often have large distribution ranges (Petit and Hampe, 2006), but the several-fold differences in size between small and large plants within populations has probably favoured the selection of SDS patterns (Burd and Allen, 1988). For instance, variability in the gender of monoecious individuals in conifers does seem to be associated with variability in size related to environmental stress (Cobb et al., 2002; Kang, 2007). Interestingly, this is in some ways similar to the well-established observation of an increased tendency towards dioecy with environmental stress in species that vary in the degree to which their sexes are separate (Ashman, 2006; and see Discussion).
In this study, we use a large common garden to assess patterns of genetic variation in early reproductive life-history traits among populations of the widespread Mediterranean species Pinus pinaster (maritime pine). P. pinaster is a monoecious, wind-pollinated, wind-dispersed conifer with a remarkably wide ecological niche (Tapias et al., 2004). Previous work, based on data from common-garden experiments, has described among-population variation in size at reproduction for this species (Santos-del-Blanco et al., 2010). Here, we investigate whether P. pinaster shows differences in the threshold size at first reproduction through male versus female function among populations from a broad geographical range across a strong climatic gradient, from Atlantic (i.e. mild winters, high annual precipitation and low altitude) to dry continental environments. We sought evidence for a genetic trade-off between allocation to growth and early reproduction, and we tested the prediction that the median threshold size at first reproduction through each of the two sexual functions should correlate with the environment of the source populations. Finally, by comparing the among-population genetic structure in terms of life-history traits with that displayed by neutral genetic markers, we tested the hypothesis that observed clinal variation in the former has resulted from responses to natural selection as opposed to drift or historical effects (Alleaume-Benharira et al., 2006; Grivet et al., 2011).
MATERIALS AND METHODS
Common garden
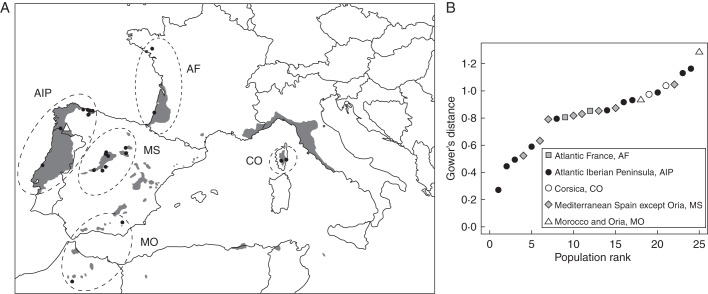
We established our common garden at Parderrubias, Ourense Province, north-west Spain (Fig. 1A). This common garden belongs to a broader series of experiments aimed at describing variability in adaptive traits in Pinus pinaster, and was chosen for the present study as it was placed in a transitional area from Atlantic to Mediterranean climate, as indicated by Gower's distance (Fig. 1B). The common garden comprised 194 open-pollinated families from a total of 23 natural populations distributed over the greater part of the species' natural range, including Atlantic Iberian Peninsula and France, Mediterranean Spain, Corsica and Morocco (Fig. 1, Supplementary Data Table S1). Two additional non-natural populations present in the common garden were excluded from the present study.
Fig. 1.
Distribution map of Pinus pinaster source populations (circles) and common garden (triangle). Shaded areas indicate the species' natural distribution range; see key in (B) for population abbreviations. (B) Gower's (absolute) environmental distance between each population to the common garden location. Values closer to 0 indicate greater similarity between the population of origin and the common garden site with respect to the environmental variables used for the calculation of the distance. Symbols represent genetic groups, as indicated in the key.
Half-sib families in natural populations were sampled from trees that were spaced at least 100 m apart. In total, 2767 2-year-old seedlings were established from seed following standard nursery procedures, and were planted in the common garden in 2005 in a resolvable alpha design, with four replicates of 71 incomplete blocks and four contiguous plants from the same family per block. This design was originally chosen because it allows efficient control of spatial heterogeneity, which is a common problem in large common gardens of forest trees. Additional seedlings from a local provenance were used as a border as well as to fill in the gaps of missing plants. Individuals were planted at a spacing of 2 m × 3 m. The soil in the common garden was a humic cambisol. Despite generally uniform climatic conditions across the common garden, there was inevitably small-scale environmental heterogeneity due to a soil depth gradient, and this was probably responsible for some of the (random) variation in plant size. Of the 2767 trees initially planted, 2240 were still alive when we last measured them in 2010.
Measurement of traits and environmental variables
We measured reproductive and growth-related traits in 2007, 2009 and 2010, a period that covered the transition from a point where most individuals were still vegetative to one when most had become reproductive. We also calculated a number of derived size-dependent fitness components (Méndez and Karlsson, 2004), as set out in Table 1. Correlation among size-dependent fitness components was tested using Pearson's correlation coefficient. Population means of size-dependent fitness components were also included in association tests with environmental data (see below). Following previous research (Climent et al., 2008; Grivet et al., 2011), three spatial and six climatic variables were considered (Table 1). Climatic data for Iberian populations were obtained from a functional phytoclimatic model based on raw data from meteorological stations (Gonzalo-Jiménez, 2010). Climatic data for non-Iberian populations were obtained from WorldClim-Global Climate Data at 5′ resolution (Hijmans et al., 2005).
Table 1.
Common garden measured traits, derived size-dependent fitness components and native location environmental data of Pinus pinaster trees
| Variable |
Description | |
|---|---|---|
| Measured traits | ||
| H | Height (cm) | Total height of each tree in 2007, 2009 and 2010 |
| F. Rep | Female reproduction | Presence or absence of seed cones in each tree, yearly from 2007 |
| M.Rep | Male reproduction | Presence or absence of pollen cones in each tree in 2009 and 2010 |
| Derived size-dependent fitness components | ||
| μ | Intercept of reproduction-size logistic regression at the population level | |
| α | Slope of reproduction-size logistic regression at the population level | |
| TSFR | Median threshold size for first reproduction (cm) | Size at which the probability for a tree within a population to have reached sexual maturity was 50 %, equal to μ/α |
| SRI | Smallest reproductive individual (cm) | Size of the smallest reproductive individual in the population |
| LVI | Largest vegetative individual (cm) | Size of the largest vegetative individual in the population |
| RAN | Range between LVI and SRI | |
| %VEG | Percentage of vegetative trees (cm) | Percentage of non-reproductive trees larger than the population TSFR |
| %REP | Percentage of reproductive trees (cm) | Percentage of reproductive trees in the population |
| Environmental data | ||
| Alt | Altitude (m) | |
| Long | Longitude (m) | |
| Lat | Latitude (m) | |
| AMT | Annual mean temperature (°C) | |
| MTWM | Mean temperature of the warmest month (°C) | |
| MTCM | Mean temperature of the coldest month (°C) | |
| CI | Continentality index (°C) | Difference between mean temperature of the warmest month and mean temperature of the coldest month |
| AP | Annual precipitation (mm) | |
| PDM | Precipitation during the warmest quarter (mm) | Precipitation in June, July and August |
Molecular analysis
Needles from between six and 30 individuals per population (mean = 16) were collected and dried in silica gel for subsequent DNA extraction. Twelve nuclear microsatellites (simple sequence repeats, SSRs) were genotyped: ITPH4516 (Mariette et al., 2001); RPtest1, ctg275, ctg4363, NZPR1078 and NZPR544 (Chagné et al., 2004); A6F03 (Guevara et al., 2005); pEST2669 (Steinitz et al., 2011); and gPp14, epi3, epi5 and epi6 (F. Sebastiani and G. G. Vendramin, Istituto di Genetica Vegetale, CNR, Florence, pers. comm., June 2011; Supplementary Data Table S2). Forward primers were 5′ end-labelled with fluorochromes (HEX, FAM, VIC or PET) and amplified using the Qiagen Multiplex PCR Kit (Qiagen, Venlo, the Netherlands) following the manufacturer's instructions. Amplified allele fragments were separated using an ABI 3730 genetic analyser (Applied Biosystems, Carlsbad, CA, USA) and fragment sizes were determined with reference to the GeneScan™ –500 LIZ® Size Standard (Applied Biosystems) using GeneMapper software version 4·0 (Applied Biosystems).
Data analysis
Genetic variation among populations for flowering probability
Based on the common-garden data, individual male and female reproductive status was coded as a binary variable at each sampling time for each individual (4, 6 and 7 years old). Given the 2-year developmental cycle of female strobili, female reproduction was recorded as present for any tree bearing 1st- or 2nd-year female cones.
The probability of reproduction was modelled by a generalized linear mixed model (GLMM) with a binomial family distribution and logit link function fitted by Laplace approximation, as implemented by the lme4 package (Bates et al., 2011) on the R platform (R Development Core Team, 2012). Total height (i.e. size), provenance and their interaction were used as fixed terms, and family was included as a random factor. Block structure was not included in these models, as preliminary analyses showed no influence of spatial heterogeneity on the allometry of reproduction (i.e. the block effect or spatial auto-correlation disappeared when tree size variation was included in the models). This first model was used to test the existence of genetic variation among populations for the relationship between size (estimated by height) and reproduction, both by using a likelihood ratio test (LRT) for models with and without the interaction term, and in terms of overall Akaike information criterion.
Ontogenetic and climatic effects on reproduction were necessarily confounded in our experiment, as all trees were the same age. Because we did not include age/year information in the model, our results should thus be interpreted as reflecting the overall behaviour of trees during their transition to maturity, but also to some extent influenced by among-year climatic variation.
We fitted a GLMM for both male and female reproduction using Markov chain Monte Carlo (MCMC) methods, with uninformative priors and fixing residual variance at 1 (MCMCglmm R package; Hadfield, 2011). This approach was preferred over restricted maximum-likelihood methods (Hadfield, 2010), which did not achieve satisfactory convergence for some populations when random effects were included, and also because MCMC methods allowed a more accurate estimation of errors in estimates of the median threshold size for reproduction. The Markov chain was run for 5500 000 iterations, sampling each 5000 after a burn-in period of 500 000 iterations. These settings proved suitable, as successive values of the chain were uncorrelated. Convergence of the chain was also checked graphically. From these models, posterior mode values for the parameters μ (intercept) and α (slope associated with height) were extracted as size-dependent fitness components commonly used in these studies (Méndez and Karlsson, 2004; Brys et al., 2011).
Age-dependent size distribution of reproductive stages
We classified each individual tree as female, male, cosexual or juvenile depending on the sex expressed for its first reproductive event; juveniles were those individuals that had still not started reproducing in 2010. We also recorded the height of individuals at which the first reproductive event took place. We tested whether plant size for each class of reproductive status differed significantly overall and among populations by using the mixed model Hijk = μ + Ri * Pj + Fk + eijk, where H was plant height, μ was the general mean, R was the reproductive class (female, male, cosexual or juvenile), P was the population (fixed factor), F was the family (random factor) and e was the random error term. The GLMM was implemented in R. Finally, we recorded the changes in sex choice for all individuals across the last two years (Fig. 2).
Fig. 2.
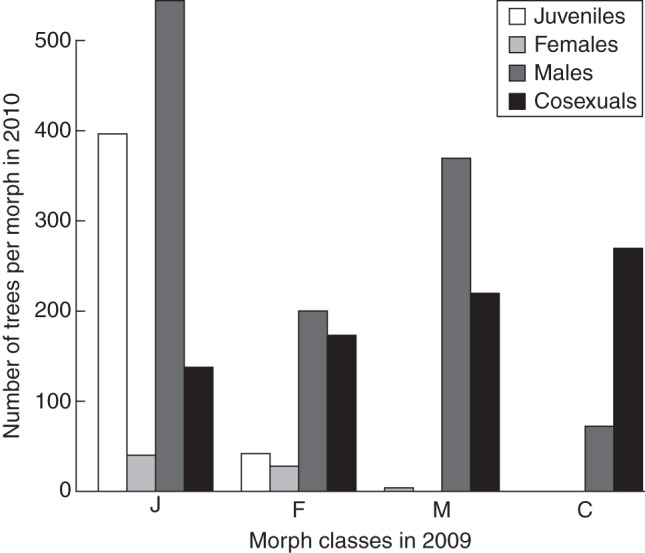
Transitions between reproductive developmental stages across Pinus pinaster populations grown in a common garden in north-west Spain. The x-axis represents morph classes at age 6, and within each class bars represent the number of individuals behaving as juveniles (J), females (F), males (M) or cosexuals (C) at age 7.
Genetic population structure and environmental correlations
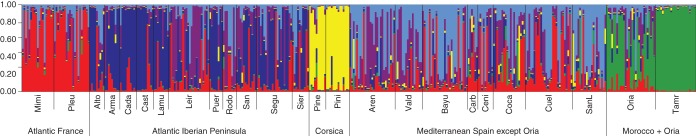
Maritime pine is characterized by strong population genetic structure at the range-wide scale (e.g. Bucci et al., 2007). When testing for environmental correlations at wide spatial scales, geographical genetic structure in maritime pine results in large numbers of false positives (estimated at approx. 84 % by Grivet et al., 2011). Thus, here we used linear models to test for genetic associations between early reproductive life-history traits and environmental data that controlled for neutral population structure (i.e. associations between plant traits and environmental data that could be due to demographical history) using molecular markers (nuclear SSRs) as covariates. Neutral population structure was assessed using the Bayesian clustering method implemented in STRUCTURE 2·2 (Pritchard et al., 2000). We ran an admixture model with correlated allele frequencies between clusters. Ten runs were performed for each number of clusters from K = 1 to K = 10, with a burn-in length of 50 000 and a run length of 500 000 iterations. The optimal number of population clusters, K, was calculated following Evanno et al. (2005) and the guidelines provided by STRUCTURE developers in the manual. Population means for individual assignment probability (Fig. 3) to each of the K clusters (i.e. the Q matrix, Yu et al., 2006) were used to control for neutral population structure in environmental associations (see similar appoaches in Eckert et al., 2010; Grivet et al., 2011). Additionally, to allow a more synthetic interpretation, we carried out a principal component analysis (PCA) with varimax rotation to reduce the number of environmental variables. To decide the number of principal components to retain, we ran a parallel analysis with 1000 iterations (Hayton et al., 2004) and selected only those principal components with eigenvalues for observed data larger than those obtained in simulations. The analysis was implemented by the psych package (Revelle, 2011) on the R platform (R Development Core Team, 2012). PCA loadings of retained components were used in correlation analysis with plant traits. Finally, we used a likelihood ratio test to compare a model including only neutral population genetic structure with one including both neutral population genetic structure and PCA loadings or single environmental variables to explain phenotypic differences among populations.
Fig. 3.
Barplot of individual assignment probability to each of the optimal K = 6 clusters representing five geographical genetic groups in Pinus pinaster as produced by STRUCTURE 2·2 software. Each individual is represented as a line segment which is vertically partitioned into K-coloured components, representing the individual's estimated proportions of ancestry in the K clusters. Population abbreviations are given in Supplementary Data Table S1.
RESULTS
Phenotypic variation in early sex expression
Trees began to reproduce sexually from 5 years of age, i.e. in 2008, and most were reproductive by year 7 (in 2010). The majority of these early individuals expressed only their male function, with similar lower numbers expressing either only female or both sexual functions (Table 2). In 2009, about half of the plants were juvenile, some remained juvenile in 2010, many began to express their male function and a minority expressed their female function, either purely or together with their male function. Individuals bearing only female or male cones in 2009 tended to produce only male cones or both male and female cones in 2010, so that individuals producing only female cones over two consecutive years were rare. Finally, all trees with female and male cones in 2009 continued producing male cones, but some abandoned their female function (Fig. 2).
Table 2.
Percentages of Pinus pinaster trees remaining non-reproductive (J), or having reached sexual maturity as females (F), males (M) or cosexuals (C) until year 7
| Population | GG | % J | % F | % M | % C | n |
|---|---|---|---|---|---|---|
| Mimi | AF | 15 | 13 | 43 | 30 | 96 |
| Pleu | AF | 12 | 36 | 20 | 31 | 108 |
| Alto | AIP | 9 | 12 | 68 | 11 | 65 |
| Arma | AIP | 14 | 8 | 58 | 20 | 71 |
| Cada | AIP | 14 | 3 | 76 | 7 | 87 |
| Cast | AIP | 15 | 15 | 50 | 20 | 74 |
| Lamu | AIP | 15 | 12 | 55 | 18 | 67 |
| Leir | AIP | 15 | 12 | 61 | 12 | 132 |
| Puer | AIP | 16 | 3 | 66 | 15 | 61 |
| Sanc | AIP | 10 | 31 | 33 | 26 | 39 |
| Segu | AIP | 7 | 13 | 55 | 24 | 165 |
| Sier | AIP | 11 | 7 | 72 | 11 | 46 |
| Pine | CO | 59 | 11 | 30 | 0 | 27 |
| Pini | CO | 11 | 6 | 70 | 13 | 54 |
| Oria | MO | 20 | 27 | 40 | 13 | 143 |
| Tamr | MO | 43 | 32 | 20 | 5 | 125 |
| Aren | MS | 12 | 16 | 48 | 24 | 126 |
| Bayu | MS | 20 | 35 | 31 | 13 | 143 |
| Carb | MS | 15 | 35 | 27 | 23 | 48 |
| Ceni | MS | 5 | 16 | 47 | 31 | 55 |
| Coca | MS | 32 | 25 | 41 | 1 | 68 |
| Cuel | MS | 14 | 44 | 30 | 12 | 153 |
| Rodo | MS | 17 | 16 | 46 | 21 | 76 |
| SanL | MS | 18 | 22 | 40 | 20 | 137 |
| Vald | MS | 14 | 18 | 54 | 15 | 74 |
| Average or total | 16 | 19 | 48 | 17 | 2240 | |
N, number of trees per population. GG, genetic group; see Fig. 1 for GG codes and Supplementary Data Table S1 for population codes.
Overall, juveniles were the smallest class of individual (206·0 ± 23·9 cm), followed by individuals expressing their female function (233·2 ± 23·6 cm), then those expressing their male function (321·1 ± 23·3 cm) and finally those expressing both functions (372·9 ± 25·2 cm). However, this association between gender expression and plant size also depended significantly on the population of origin (population × gender interaction, P < 0·0001).
Associations between reproduction and size
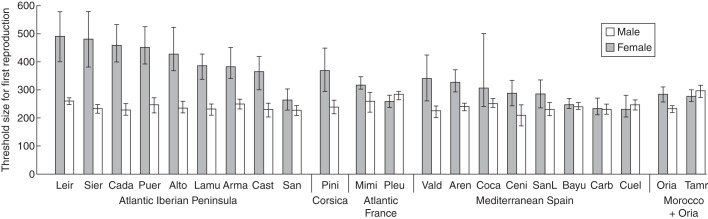
Median TSFR varied significantly among populations for both male (P < 0·0001) and female functions (P < 0·0001). At the population level, the size effect was highly significant for all populations except the Pineta population (Corsica), which was dropped in subsequent analyses. Across all other populations, intercepts were similar for male and female logistic regressions, while slopes where approximately double for male compared with female reproduction, reflecting the faster transition from juvenile to male reproductive stage. Median male TSFR was relatively uniform among populations and lower than female TSFR, which varied substantially among populations (Fig. 4).
Fig. 4.
Median threshold size for first reproduction through male and female functions (as indicated) in Pinus pinaster populations grown in the common garden in north-west Spain. Bars represent the posterior mode of Bayesian estimates, with lower and upper 95 % credible intervals. Population abbreviations are given in Supplementary Data Table S1.
Average height of the largest vegetative individual (LVI) was sensibly larger for plants considering female function, whereas heights were only slightly smaller for female function compared with male function regarding the smallest reproductive individual (SRI). Accordingly, size ranges were larger for female function (Table 3). Size-dependent fitness components at the population level are given in Supplementary Data Tables S3 and S4.
Table 3.
Mean values for size-dependent fitness components for male and female reproduction, averaged across 22 natural Pinus pinaster populations; abbreviations are as defined in Table 1
| Male | Female | |
|---|---|---|
| μ | –8·07 ± 1·79 | –5·75 ± 0·90 |
| α | 0·033 ± 0·007 | 0·018 ± 0·005 |
| TSFR | 241·7 ± 19·7 | 339·1 ± 81·9 |
| LVI | 322 ± 50 | 393 ± 39 |
| SRI | 113 ± 17 | 105 ± 22 |
| RAN | 209 ± 52 | 254 ± 100 |
| %VEG | 4·0 ± 2·4 | 1·7 ± 2·3 |
Correlations and trade-offs between size-dependent fitness components
There was low variability for male function, and correlations between early male reproductive traits with other traits were non-significant (data not shown). In contrast, significant correlations were found between female fitness components, height and the percentage of individuals reaching sexual maturity through female function (Table 4). Height was positively correlated with female TSFR (i.e. delayed female reproduction) and negatively correlated with the number of individuals that became reproductive through female function, thus indicating an overall negative correlation between growth in height and female function at the population level.
Table 4.
Among-population correlations for early female reproductive traits in Pinus pinaster; abbreviations are as defined in Table 1.
| fμ | fα | fTSFR | fLVI | fSRI | fRAN | %fVEG | %J | %F | |
|---|---|---|---|---|---|---|---|---|---|
| H | –0·305 | –0·360 | 0·691*** | 0·654*** | 0·744*** | 0·269 | –0·363 | –0·634** | –0·747*** |
| fμ | –0·523* | –0·148 | –0·505* | –0·553** | –0·222 | 0·117 | –0·036 | 0·315 | |
| fα | –0·730*** | –0·161 | –0·094 | –0·128 | 0·521* | 0·152 | 0·517* | ||
| fTSFR | 0·624** | 0·544** | 0·372 | –0·694*** | –0·231 | –0·865*** | |||
| fLVI | 0·557** | 0·815 | –0·337 | –0·149 | –0·579** | ||||
| fSRI | –0·026 | –0·324 | –0·420 | –0·689*** | |||||
| fRAN | –0·180 | 0·113 | –0·217 | ||||||
| %fVEG | 0·284 | 0·768*** | |||||||
| %J | 0·322 |
Significant at: ***P < 0·001, **P < 0·01, *P < 0·05.
Environmental correlations
Parallel analysis associated with PCA for environmental traits revealed that only one principal component (PC1), explaining 64 % of the variance, should be retained. Overall, this principal component reflected favourable growth conditions. This factor was positively related to longitude, mean temperature of the coldest month (MTCM), precipitation during the warmest quarter (PDM) and annual precipitation (AP), and negatively to altitude, mean temperature of the warmest month (MTWM) and continentality index (CI). Latitude and annual mean temperature (AMT) had loadings below 0·7.
Male TSFR was not correlated with PC1 or any single spatial or climatic variable (Table 5 and Supplementary Data Table S5), but we found significant positive associations between PC1 and female TSFR, height and the percentage of individuals expressing their male function, and negative associations between PC1 and the percentage of individuals expressing their female function. Among single climatic parameters, models for MTCM and CI showed the highest significance levels (see Supplementary Data Table S5). Trees with larger female TFSR tended to come from sites with more favourable growing conditions, as those with milder winters and a reduced degree of continentality (Fig. 5).
Table 5.
Environmental associations of Pinus pinaster reproductive life-history traits (first column) with environmental variables represented by population scorings on first component of PCA analysis
| Corrected |
Uncorrected |
|||
|---|---|---|---|---|
| Trait | Slope | P | Slope | P |
| fTSFR | 109·10 ± 26·97 | 0·001 | 56·34 ± 13·30 | 0·000 |
| mTSFR | 8·36 ± 8·90 | n.s. | –1·89 ± 4·39 | n.s. |
| H | 32·21 ± 11·80 | 0·016 | 24·30 ± 4·16 | 0·000 |
| %M | 15·28 ± 6·15 | 0·025 | 10·43 ± 2·97 | 0·002 |
| %F | –12·34 ± 4·85 | 0·023 | –7·74 ± 2·04 | 0·001 |
Corrected values indicate slopes, standard errors and P-values for the association after including neutral genetic structure corrections (i.e. likelihood ratio test between a full model and a reduced model with just neutral genetic structure); uncorrected values indicate association parameters without correcting for neutral genetic structure (i.e. just environmental data and traits data in the model). n.s., not significant.
Fig. 5.
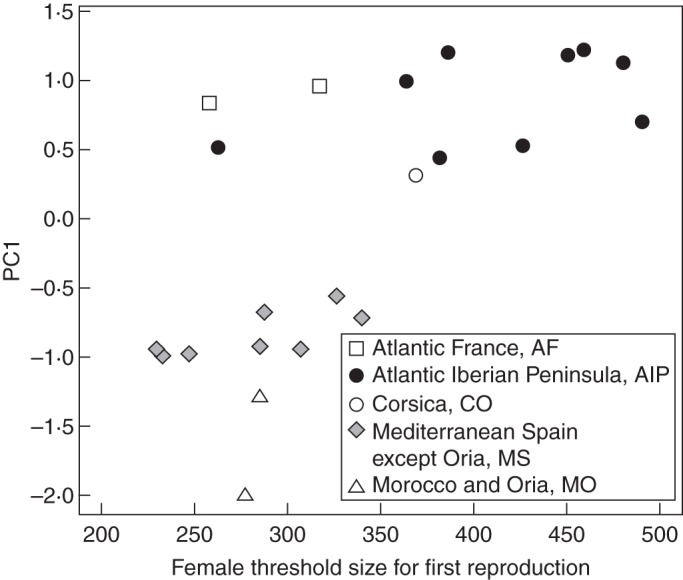
Variation of Pinus pinaster median female threshold size for first reproduction along an environmental harshness gradient, as estimated by population scorings on PC1. Symbols represent genetic groups, as indicated in the key.
Nuclear SSR summary information is provided in Supplementary Data Table S2. A STRUCTURE analysis using Bayesian clustering based on SSRs showed the existence of five clearly differentiated genetic groups in P. pinaster corresponding roughly to Atlantic France, Atlantic Iberian Peninsula, Corsica, Mediterranean Spain (except the Oria population in southern Spain) and Morocco plus Oria (Fig. 3 and Supplementary Data Table S6). Atlantic and Mediterranean Iberian populations displayed varying degrees of admixture, which has been suggested to be the result of historical gene flow among Mediterranean and Atlantic maritime pine glacial refugia (de Lucas et al., 2009). Once neutral population genetic structure was integrated in the models, the overall correlations of early reproductive life-history traits with favourable growth conditions (as resumed in PC1) remained significant. However, several correlations for single environmental parameters became non-significant, including one for CI (see Supplementary Data Table S5). This indicated that either some true environmental associations were confounded with neutral genetic structure imposed by demographic history (and thus were not reliable), or that they were false-positives.
DISCUSSION
Our results provide evidence for clear genetic differences in key life-history traits among populations of P. pinaster sampled across a wide environmental range. Because much of this genetic variation was associated with a strong environmental gradient among the sites we sampled, some of which remained significant after neutral genetic structure was accounted for, the observed life-history trait clines are likely to be the result of local adaptation rather than simply the outcome of divergence through drift or historical accident. This is among the first reports of reproductive life-history trait variation in a widespread forest species (Climent et al., 2008; Santos-del-Blanco et al., 2010) but also, as far as we know, the first report of adaptive genetic differentiation for both male and female threshold sizes for reproduction in a (monoecious) plant species.
The P. pinaster individuals in our study tended to start reproducing preferentially as males, and they tended to maintain their male function fairly constantly through time. Accordingly, male median TSFR was lower than female TSFR. This finding is somewhat surprising, given that most conifer species tend to start reproducing as females (Williams, 2009). In perennial plants and, most notably, in trees, the existence of threshold sizes for reproduction arises from the need to invest all available resources in a large vegetative body during early developmental stages to maximize further reproduction (reviewed by Thomas, 2011). The existence of greater female costs of reproduction has been related to a generally lower threshold size for male reproduction in dioecious and monoecious plants and to failures in setting fruit in species with perfect flowers (Iwasa, 1991; de Jong and Klinkhamer, 2005). This pattern, largely observed in woody angiosperms, differs from that typically observed in conifers, which display earlier expression of female function. P. pinaster would thus seem to have evolved a pattern of early reproduction and sex allocation more similar to woody angiosperms than other conifers.
Although the trend for early male reproduction in P. pinaster is interesting and unusual, the more striking pattern we observed is that male TSFR is much more canalized, both within and among populations, than female TSFR: individuals in our common garden started producing male cones at around 2·5 m in height, whereas female function commenced in individuals over a very wide range of heights (Fig. 4). Why should male TSFR be so uniform, and why should the female TSFR vary so widely?
An increase in maleness with size in wind-pollinated plants is a well-established prediction from SDS theory (de Jong and Klinkhamer, 2005), and forest trees have been identified as suitable organisms to test it (Cruden and Lyon, 1985). The prediction rests on the presumed advantage to plants that release their pollen from greater heights, regardless of the seed dispersal mode (Friedman and Barrett, 2009). However, in conical-shaped conifers, like many pine species, male cones are not located in the treetop but rather on lower secondary branches, and it is the female cones that are borne by upper vigorous branches. This segregation is more evident in adult trees (Shmida et al., 2000), but we have observed it also in our young maritime pine specimens. This architectural pattern has been proposed either as a strategy to avoid selfing, as male and female flowering are synchronous in this species (Miguel-Pérez et al., 2002), and/or as a consequence of the larger size of female cones which could only be borne on stronger upper vertical branches (Ne'eman et al., 2011). Either way, the hypothesis that increased height favours male function would not offer a convincing explanation for size-dependent sex allocation in conifers (Fox, 1993). Moreover, the size advantage hypothesis only applies in the presence of clear differences in size among individuals within a population (Friedman and Barrett, 2009), which is not common in Mediterranean conifer forests. The established theory for size-dependent sex allocation thus would not seem to apply to conifers in a straightforward manner.
Given that the position of male and female cones is conserved across conical-shaped conifers and thus appears to be phylogenetically constrained, for whatever reason, a more plausible explanation for canalization of male TSFR is simply that pollen needs to be released above a threshold height to be lifted by horizontal winds and updrafts; if so, it would not be surprising if the same physical constraint applied to all individuals, irrespective of their provenance. The high value for the slope of the male flowering probability curve with size (α = 0·033) indicates low genetic variation for this trait and a common sharp threshold for male TSFR within and across populations. We therefore hypothesize that the low among-population variation in the threshold sizes for male reproduction found in our study points to the existence of similar male fitness gain curves and might be the result of rather uniform conditions for pollen release and transport across populations. To our knowledge, this possibility has not yet been considered in the SDS literature.
Female TSFR not only showed high variability within populations, but also clear evidence for high among-population differentiation; this variation, in turn, was significantly correlated with most early reproductive life-history traits (Table 4). For example, we found a positive correlation among populations between the average height of individuals and female TSFR, and a negative correlation between the average height in the population and the number of individuals first reproducing as females (as opposed to early males). These patterns emerge as negative correlations of population means for reproductive and growth traits measured in a common garden environment, so our results should be considered at the genetic, not phenotypic or physiological, level (Reznick, 1985; de Jong and Klinkhamer, 2005). Thus, the observed patterns probably reflect genetic trade-offs between growth and female reproduction that have been selected under different environments.
A particularly interesting finding is that much of the observed among-population variation was associated with climatic differences among sites. At least some of this variation might underlie possible differences in the intensity of within-stand competition (e.g. competition might be more important at more mesic sites; Grime, 1977; Grivet et al., 2011). Thus, in favourable (yet strongly competitive) environments, early investment in the more costly female function would increase the risk to individuals of being suppressed by their neighbours, so a delay of reproduction might be advantageous (Thomas, 2011). By contrast, in unfavourable (but less competitive) environments, selection would tend to favour stress tolerance at the expense of growth or competitive ability, so that a low female TSFR would be expected (Roff, 1992).
In accordance with this reasoning, we found lower female TSFR and a higher proportion of individuals first expressing their female function in populations from sites offering less favourable conditions for growth, a pattern that remained significant after accounting for neutral genetic structure. It would thus seem plausible that, on the one hand, populations have become differentiated for female TSFR in response to selection under possible differences in the competitive environment and disturbance regime among sites, and, on the other hand, a canalized male TSFR has evolved under the possible site-independent physical constraints facing pollen dispersal in conifers.
The idea that selection will have caused populations of P. pinaster to diverge for reproductive traits across its range is largely in keeping with observed clinal variation in morphological traits for a number of widespread species (Davis et al., 2005); in some cases, this clinal variation has evolved over short time periods, for example flowering-time variation in Verbascum thapsus (Ansari and Daehler, 2010) and Lythrum salicaria (Montague et al., 2008; Colautti et al., 2010). Similarly, artificial selection experiments on size at reproduction in Cynoglossum officinale also elicited a fast and direct response (Wesselingh and de Jong, 1995), and the cultivation of Eucalyptus trees in India resulted in the rapid evolution of fecundity traits (Varghese et al., 2009). These studies argue for the existence of large genetic variation for size at reproduction, allowing fast among-population differentiation due to strong selection.
If the above explanation is able to account for among-population patterns in TSFR, how might we explain the differences in within-population variability in this important trait between male and female functions? A differential plastic response in female TSFR of populations grown out of their native habitat could account for variability registered in the common garden. This variability might not be present in native habitats, where a more uniform female TSFR could exist. However, we found no evidence of correlation between environmental distance and range of median threshold sizes in each population (t21 = 0·761, P > 0·45). Another possibility is that the greater variance in TSFR in female function within populations is attributable to the evolution of a bet-hedging strategy, either through stochastic or plastic expression of the same underlying genotypes, or through the frequency-dependent maintenance of genetic variation for TSFR under environmental stochasticity (Rees et al., 2004; Metcalf et al., 2008; Weiner et al., 2009; Childs et al., 2010). The role of environmental stochasticity on reproductive strategies has been described mainly for monocarps, which can suffer high rates of mortality before reaching maturity. Woody species usually have more stable demographic patterns than monocarps, probably buffered by ongoing trade-offs between investment in reproduction and growth or maintenance. Nevertheless, Mediterranean pine species suffer unpredictable fire return intervals as well as episodic droughts, both of which are likely to play a major role in reproductive strategies (Pausas et al., 2008). A bet-hedging strategy in a long-lived woody species prone to environmental stochasticity might be expected to evolve for the more expensive reproduction function, i.e. for cone production, as observed. Interestingly, previous studies on Pinus spp. report lower heritabilities for male compared with female cone production, for example in P. pinaster (Merlo and Fernández López, 2004) and P. sylvestris (Savolainen et al., 1993), suggesting that a similar explanation might apply to several species evolving under a rather broad range of environmental challenges.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
R. Alía and J. Majada established and maintained the common garden (TREESNIPS QLK3-CT2002-01973). The data used in this research are part of the Spanish Network of Genetic Trials (GENFORED). We thank E. Ballesteros, E. Alamillo, F. del Caño, D. Barba and N. Godoy (†) for fieldwork. Thanks are extended to A. I. de-Lucas, J. P. Jaramillo-Correa and G. G. Vendramin for assistance in nuclear microsatellite genotyping, to D. Grivet for advice on environmental correlations and to J. J. Robledo-Arnuncio for valuable discussions. The manuscript also benefited from comments by R. Wesselingh and S. P. Bonser. This study was funded by the Spanish Ministry of Economy and Competitiveness through projects Mitigenfor (RTA 2011-00016-00-00), Restaura (PSS-310000-2008-4), AdapCon (CGL2011-30182-C02-01) and LinkTree (ERAnet-BiodivERsA; EUI2008-03713). Additional funding was provided by the European Community's Seventh Framework Program (FP7/ 2007-2013) under grant agreement no. 211868 (Project Noveltree) KBBE-2007-1-2-05. Finally, this work was also supported by the Spanish Ministry of Education through a PhD grant to L.S.B. (FPU-AP-03302).
LITERATURE CITED
- Alleaume-Benharira M, Pen I, Ronce O. Geographical patterns of adaptation within a species' range: interactions between drift and gene flow. Journal of Evolutionary Biology. 2006;19:203–215. doi: 10.1111/j.1420-9101.2005.00976.x. [DOI] [PubMed] [Google Scholar]
- Ansari S, Daehler CC. Life history variation in a temperate plant invader. Verbascum thapsus along a tropical elevational gradient in Hawaii. Biological Invasions. 2010;12:4033–4047. [Google Scholar]
- Ashman TL. The evolution of separate sexes: a focus on the ecological context. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. New York: Oxford University Press; 2006. pp. 204–222. [Google Scholar]
- Barton N, Keightley P. Understanding quantitative genetic variation. Nature Reviews Genetics. 2002;3:11–21. doi: 10.1038/nrg700. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B. Package lme4: linear mixed-effects models using S4 classes. 2011 R package version 0·999375-42. http://CRAN.R-project.org/package=lme4 . [Google Scholar]
- Bonser SP, Aarssen LW. Interpreting reproductive allometry: individual strategies of allocation explain size-dependent reproduction in plant populations. Perspectives in Plant Ecology, Evolution and Systematics. 2009;11:31–40. [Google Scholar]
- Brys R, Shefferson RP, Jacquemyn H. Impact of herbivory on flowering behaviour and life history trade-offs in a polycarpic herb: a 10-year experiment. Oecologia. 2011;166:293–309. doi: 10.1007/s00442-010-1842-7. [DOI] [PubMed] [Google Scholar]
- Bucci G, González-Martínez SC, Le-Provost G, et al. Range-wide phylogeography and gene zones in Pinus pinaster Ait. Molecular Ecolology. 2007;16:2137–2153. doi: 10.1111/j.1365-294X.2007.03275.x. revealed by chloroplast microsatellite markers. [DOI] [PubMed] [Google Scholar]
- Burd M, Allen TFH. Sexual allocation strategy in wind-pollinated plants. Evolution. 1988;42:403–407. doi: 10.1111/j.1558-5646.1988.tb04145.x. [DOI] [PubMed] [Google Scholar]
- Burd M, Read J, Sanson GD, Jaffré T. Age-size plasticity for reproduction in monocarpic plants. Ecology. 2006;87:2755–2764. doi: 10.1890/0012-9658(2006)87[2755:apfrim]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Cadet C, Metz JAJ, Klinkhamer PGL. Size and the not so single sex: disentangling the effects of size and budget on sex allocation in hermaphrodites. The American Naturalist. 2004;164:779–792. doi: 10.1086/425624. [DOI] [PubMed] [Google Scholar]
- Callahan HS, Pigliucci M. Shade-induced plasticity and its ecological significance in wild populations of Arabidopsis thaliana. Ecology. 2002;83:1965–1980. [Google Scholar]
- Chagné D, Chaumeil P, Ramboer A, et al. Cross-species transferability and mapping of genomic and cDNA SSRs in pines. Theoretical and Applied Genetics. 2004;109:1204–1214. doi: 10.1007/s00122-004-1683-z. [DOI] [PubMed] [Google Scholar]
- Childs DZ, Metcalf CJE, Rees M. Evolutionary bet-hedging in the real world: empirical evidence and challenges revealed by plants. Proceedings of the Royal Society B: Biological Sciences. 2010;277:3055–3064. doi: 10.1098/rspb.2010.0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Climent J, Prada MA, Calama R, Chambel MR, Sánchez de Ron D, Alia R. To grow or to seed: ecotypic variation in reproductive allocation and cone production by young female Aleppo pine (Pinus halepensis, Pinaceae) American Journal of Botany. 2008;95:833–842. doi: 10.3732/ajb.2007354. [DOI] [PubMed] [Google Scholar]
- Cobb NS, Trotter RT, III, Whitham TG. Long-term sexual allocation in herbivore resistant and susceptible Pinyon pine (Pinus edulis) Oecologia. 2002;130:78–87. doi: 10.1007/s004420100785. [DOI] [PubMed] [Google Scholar]
- Colautti RI, Eckert CG, Barrett SCH. Evolutionary constraints on adaptive evolution during range expansion in an invasive plant. Proceedings of the Royal Society B: Biological Sciences. 2010;277:1799–806. doi: 10.1098/rspb.2009.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruden RW, Lyon DL. Patterns of biomass allocation to male and female functions in plants with different mating systems. Oecologia. 1985;66:299–306. doi: 10.1007/BF00379868. [DOI] [PubMed] [Google Scholar]
- Davis MB, Shaw RG, Etterson JR. Evolutionary responses to changing climate. Ecology. 2005;86:1704–1714. [Google Scholar]
- Delph L. Sexual dimorphism in life history. In: Geber MA, Dawson TE, Delph LF, editors. Gender and sexual dimorphism in flowering plants. Berlin: Springer; 1999. pp. 149–173. [Google Scholar]
- Dodd ME, Silvertown J. Size-specific fecundity and the influence of lifetime size variation upon effective population size in Abies balsamea. Heredity. 2000;85:604–609. doi: 10.1046/j.1365-2540.2000.00822.x. [DOI] [PubMed] [Google Scholar]
- Eckert AJ, van Heerwaarden J, Wegrzyn JL, et al. Patterns of population structure and environmental associations to aridity across the range of loblolly pine (Pinus taeda L., Pinaceae) Genetics. 2010;185:969–982. doi: 10.1534/genetics.110.115543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Fox JF. Size and sex allocation in monoecious woody plants. Oecologia. 1993;94:110–113. doi: 10.1007/BF00317310. [DOI] [PubMed] [Google Scholar]
- Freeman DC, McArthur ED, Harper KT, Blauer AC. Influence of environment on the floral sex ratio of monoecious plants. Evolution. 1981;35:194–197. doi: 10.1111/j.1558-5646.1981.tb04875.x. [DOI] [PubMed] [Google Scholar]
- Friedman J, Barrett SCH. Wind of change: new insights on the ecology and evolution of pollination and mating in wind-pollinated plants. Annals of Botany. 2009;103:1515. doi: 10.1093/aob/mcp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo-Jiménez J. Diagnosis fitoclimática de la españa peninsular: hacia un modelo de clasificación funcional de la vegetación y de los ecosistemas peninsulares españoles. 2010 Organismo Autónomo de Parques Nacionales. [Google Scholar]
- Grime J. Evidence for the existence of three primary strategies in plants and Its relevance to ecological and evolutionary theory. American Naturalist. 1977;111:1169–1194. [Google Scholar]
- Grivet D, Sebastiani F, Alía R, et al. Molecular footprints of local adaptation in two Mediterranean conifers. Molecular Biology and Evolution. 2011;28:101–16. doi: 10.1093/molbev/msq190. [DOI] [PubMed] [Google Scholar]
- Guevara M, Chagné D, Almeida M, et al. Isolation and characterization of nuclear microsatellite loci in Pinus pinaster Ait. Molecular Ecology Notes. 2005;5:57–59. [Google Scholar]
- Hadfield J. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. Journal of Statistical Software. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- Hadfield J. 2011 Package “MCMCglmm” Reference manual: http://cran.r-project.org/web/packages/MCMCglmm/MC . [Google Scholar]
- Hayton JC, Allen DG, Scarpello V. Factor retention decisions in Exploratory Factor Analysis: a tutorial on Parallel Analysis. Organizational Research Methods. 2004;7:191–205. [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL J, et al. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. [Google Scholar]
- Iwasa Y. Sex change evolution and cost of reproduction. Behavioral Ecology. 1991;2:56–68. [Google Scholar]
- de Jong TJ, Klinkhamer PGL. Evolutionary ecology of plant reproductive strategies. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Kagaya M, Tani T, Kachi N. Variation in flowering size and age of a facultative biennial, Aster kantoensis (Compositae), in response to nutrient availability. American Journal of Botany. 2009;96:1808–1813. doi: 10.3732/ajb.0800327. [DOI] [PubMed] [Google Scholar]
- Kang H. Changes in gender expression in korean populations of Pinus densiflora over a five-year period. Journal of Plant Biology. 2007;50:181–189. [Google Scholar]
- Kinoshita E. Sex change and population dynamics in Arisaema (Araceae) I. Arisaema serratum (Thunb.) Schott. Plant Species Biology. 1987;2:15–28. [Google Scholar]
- Klinkhamer PGL, de Jong TJ. Plant size and seed production in the monocarpic perennial Cynoglossum Officinale L. New Phytologist. 1987;106:773–783. doi: 10.1111/j.1469-8137.1987.tb00178.x. [DOI] [PubMed] [Google Scholar]
- Klinkhamer PGL, de Jong TJ, Metz H. Sex and size in cosexual plants. Trends in Ecology & Evolution. 1997;12:260–265. doi: 10.1016/s0169-5347(97)01078-1. [DOI] [PubMed] [Google Scholar]
- Kozłowski J. Optimal allocation of resources to growth and reproduction: Implications for age and size at maturity. Trends in Ecology & Evolution. 1992;7:15–9. doi: 10.1016/0169-5347(92)90192-E. [DOI] [PubMed] [Google Scholar]
- Kuss P, Rees M, Ægisdóttir HH, Ellner SP, Stöcklin J. Evolutionary demography of long lived monocarpic perennials: a time lagged integral projection model. Journal of Ecology. 2008;96:821–832. [Google Scholar]
- de Lucas AI, González-Martínez S, Hidalgo E, Bravo F, Heuertz M. Admixture, one-source colonization or long-term persistence of maritime pine in the Castilian Plateau?: insights from nuclear microsatellite markers. Investigación agraria. Sistemas y recursos forestales. 2009;18:3–12. [Google Scholar]
- Mariette S, Chagne D, Decroocq S, et al. Microsatellite markers for Pinus pinaster Ait. Annals of Forest Science. 2001;58:203–206. [Google Scholar]
- Mazer S. Quantitative genetics of life history and fitness components in Raphanus raphanistrum L. (Brassicaceae): ecological and evolutionary consequences of seed-weight. American Naturalist. 1987;130:891–914. [Google Scholar]
- Merlo E, Fernández López J. Análisis del balance parental reproductivo en un huerto semillero de Pinus pinaster Ait. Investigación Agraria. Sistemas y Recursos Forestales. 2004;13:387–398. [Google Scholar]
- Metcalf CJE, Rose KE, Rees M. Evolutionary demography of monocarpic perennials. Trends in Ecology & Evolution. 2003;18:471–480. [Google Scholar]
- Metcalf CJE, Rose KE, Childs DZ, Sheppard AW, Grubb PJ, Rees M. Evolution of flowering decisions in a stochastic, density-dependent environment. Proceedings of the National Academy of Sciences. 2008;105:10466–10470. doi: 10.1073/pnas.0800777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Pérez I, González-Martínez SC, Alía R, Gil L. Growth phenology and mating system of Maritime pine (Pinus pinaster Aiton) in central Spain. Investigación Agraria. Sistemas y Recursos Forestales. 2002;11:193–204. [Google Scholar]
- Montague JL, Barrett SCH, Eckert CG. Re-establishment of clinal variation in flowering time among introduced populations of purple loosestrife (Lythrum salicaria, Lythraceae) Journal of Evolutionary Biology. 2008;21:234–245. doi: 10.1111/j.1420-9101.2007.01456.x. [DOI] [PubMed] [Google Scholar]
- Méndez M, Karlsson PS. Between-population variation in size-dependent reproduction and reproductive allocation in Pinguicula vulgaris (Lentibulariaceae) and its environmental correlates. Oikos. 2004;104:59–70. [Google Scholar]
- Ne'eman G, Goubitz S, Werger MJ, Shmida A. Relationships between tree size, crown shape, gender segregation and sex allocation in Pinus halepensis, a Mediterranean pine tree. Annals of Botany. 2011;108:197–206. doi: 10.1093/aob/mcr104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklas KJ, Enquist BJ. An allometric model for seed plant reproduction. Evolutionary Ecology Research. 2003;5:79–88. [Google Scholar]
- Pausas JG, Llovet J, Rodrigo A, Vallejo R. Are wildfires a disaster in the Mediterranean basin?–A review. International Journal of Wildland Fire. 2008;17:713–723. [Google Scholar]
- Petit RJ, Hampe A. Some evolutionary consequences of being a tree. Annual Review of Ecology, Evolution and Systematics. 2006;37:187–214. [Google Scholar]
- Pickup M, Barrett SCH. Reversal of height dimorphism promotes pollen and seed dispersal in a wind-pollinated dioecious plant. Biology Letters. 2012;8:245–8. doi: 10.1098/rsbl.2011.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees M, Childs DZ, Rose KE, Grubb PJ. Evolution of size-dependent flowering in a variable environment: partitioning the effects of fluctuating selection. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2004;271:471–475. doi: 10.1098/rspb.2003.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees M, Sheppard AW, Briese D, Mangel M. Evolution of size-dependent flowering in Onopordum illyricum: a quantitative assessment of the role of stochastic selection pressures. American Naturalist. 1999;154:628–651. doi: 10.1086/303268. [DOI] [PubMed] [Google Scholar]
- Reznick D. Costs of reproduction: an evaluation of the empirical evidence. Oikos. 1985;44:257–267. [Google Scholar]
- Roff DA. Age and size at maturity. In: Roff DA, editor. The evolution of life histories: theory and analysis. New York: Chapman and Hall; 1992. pp. 179–241. [Google Scholar]
- Roff DA. Trade-offs between growth and reproduction: an analysis of the quantitative genetic evidence. Journal of Evolutionary Biology. 2000;13:434–445. [Google Scholar]
- Sánchez-Gómez D, Majada J, Alía R, Feito I, Aranda I. Intraspecific variation in growth and allocation patterns in seedlings of Pinus pinaster Ait. Annals of Forest Science. 2010;67 submitted to contrasting watering regimes: can water availability explain regional variation? 505–504. [Google Scholar]
- Santos-del-Blanco L, Notivol E, Zas R, Chambel MR, Majada J, Climent J. Variation of early reproductive allocation in multi-site genetic trials of Maritime pine and Aleppo pine. Forest Systems. 2010;19:381–392. [Google Scholar]
- Savolainen O, Kärkkäinen K, Harju A, Nikkanen T, Rusanen M. Fertility variation in Pinus sylvestris: a test of sexual allocation theory. American Journal of Botany. 1993;80:1016–1020. [Google Scholar]
- Shmida A, Lev-Yadun S, Goubitz S, Ne'Eman G. Sexual allocation and gender segregation in Pinus halepensis, P. brutia and P. pinea. In: Ne'Eman G, Trabaud L, editors. Leiden: Backhuys Publisher; 2000. pp. 91–104. Ecology, biogeography and management of Pinus halepensis and P. brutia Forest Ecosystems in the Mediterranean Basin. [Google Scholar]
- Steinitz O, Troupin D, Vendramin G G, Nathan R. Genetic evidence for a Janzen-Connell recruitment pattern in reproductive offspring of Pinus halepensis trees. Molecular Ecology. 2011;20 doi: 10.1111/j.1365-294X.2011.05203.x. 5152–4164. [DOI] [PubMed] [Google Scholar]
- Sultan SE. Phenotypic plasticity for plant development, function and life history. Trends in Plant Science. 2000;5:537–42. doi: 10.1016/s1360-1385(00)01797-0. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2012. www.r-project.org . [Google Scholar]
- Revelle W. psych: Procedures for personality and psychological research. 2011 Northwestern University, Evanston (R package, version 1·1.12) [Google Scholar]
- Tapias R, Climent J, Pardos JA, Gil L. Life histories of Mediterranean pines. Plant Ecology. 2004;171:53–68. [Google Scholar]
- Thomas SC. Relative size at onset of maturity in rain forest trees: a comparative analysis of 37 Malaysian species. Oikos. 1996;76:145–154. [Google Scholar]
- Thomas SC. Age-related changes in tree growth and functional biology: the role of reproduction. In: Meinzer FC, Lachenbruch B, Dawson TE, editors. Size- and age-related changes in tree structure and function. Dordrecht: Springer; 2011. pp. 33–64. [Google Scholar]
- Varghese M, Kamalakannan R, Harwood CE, Lindgren D, McDonald MW. Changes in growth performance and fecundity of Eucalyptus camaldulensis and E. tereticornis during domestication in southern India. Tree Genetics and Genomes. 2009;5:629–640. [Google Scholar]
- Walsh B, Blows MW. Abundant genetic variation+ strong selection= multivariate genetic constraints: a geometric view of adaptation. Annual Review of Ecology, Evolution, and Systematics. 2009;40:41–59. [Google Scholar]
- Weiner J, Campbell LG, Pino J, Echarte L. The allometry of reproduction within plant populations. Journal of Ecology. 2009;97:1220–1233. [Google Scholar]
- Wesselingh RA, de Jong TJ. Bidirectional selection on threshold size for flowering in Cynoglossum officinale (hound's-tongue) Heredity. 1995;74:415–424. [Google Scholar]
- Wesselingh RA, Klinkhamer PGL, de Jong TJ, Boorman LA. Threshold size for flowering in different habitats: effects of size-dependent growth and survival. Ecology. 1997;78:2118–2132. [Google Scholar]
- Williams CG. Conifer reproductive biology. Berlin: Springer; 2009. [Google Scholar]
- Worley AC, Barrett SCH. Evolution of floral display in Eichhornia paniculata (Pontederiaceae): direct and correlated responses to selection on flower size and number. Evolution. 2000;54:1533–1545. doi: 10.1111/j.0014-3820.2000.tb00699.x. [DOI] [PubMed] [Google Scholar]
- Wright SJ, Jaramillo MA, Pavon J, Condit R, Hubbell SP, Foster RB. Reproductive size thresholds in tropical trees: variation among individuals, species and forests. Journal of Tropical Ecology. 2005;21:307–315. [Google Scholar]
- Yu J, Pressoir G, Briggs WH, et al. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nature Genetics. 2006;38:203–208. doi: 10.1038/ng1702. [DOI] [PubMed] [Google Scholar]
- Zhang DY. Evolutionary stable reproductive investment and sex allocation in plants. In: Harder LD, Barrett SCH, editors. The ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 41–59. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.