Abstract
Tubers are the most common brain lesions in tuberous sclerosis complex (TSC), and typically remain stable in size and appearance. We present the case of a young male with global developmental impairment and autistic features as well as multiple and mixed daily seizures. The patient had a prominent right frontal cortical tuber characterized by a calcified component, which changed on consecutive magnetic resonance imaging between the age of 4 and 11 years, at which time the patient underwent a lesionectomy. A poor long-term outcome is reported since the patient presents an intractable mixed seizure disorder status post-epilepsy surgery and no significant neuropsychological improvements. Histopathology findings show typical characteristics of tubers in TSC as well as numerous calcifications within the resected nodular lesion. This case supports the notion that tubers with calcified components are not necessarily static lesions and can change with time. Investigation of the relationship between the presence of calcification in tubers and epileptogenecity in a large group of patients would provide insights into the pathogenesis of the seizures and cognitive impairment and hopefully, eventually provide better treatment options for patients with TSC.
Tuberous sclerosis complex (TSC) is a genetic disorder, which can affect multiple organs including the central nervous system. Several cerebral abnormalities are characteristic of TSC, including subependymal nodules (SEN) and white matter abnormalities, but tubers are the most common lesions of TSC.1 Tubers have been reported in 82 to 100% of patients and are often epileptogenic.2-4 They vary widely in size, location, and appearance,5,6 but in contrast to SENs, tubers are thought to remain stable in size over time.
We report the case of a young patient whose magnetic resonance imaging (MRI) detected a prominent right frontal cortical tuber with a calcified component that changed over time in both size and appearance. This cerebral lesion was resected and we describe both radiological and histological findings of this unusual lesion. The Massachusetts General Hospital institutional review board approved this study and informed consent was obtained from the patient’s parents.
CASE REPORT
Clinical course
The patient is a 15-year-old left-handed male with a known spontaneous TSC2 mutation. He was diagnosed with TSC at birth, but the diagnosis was suspected antenatally because of cardiac abnormalities detected on ultrasound that were subsequently confirmed as cardiac rhabdomyoma. Other clinical manifestations of TSC in this patient include renal angiomyolipomas, retinal hamartomas, as well as several dermatological features including angiofibromas and ash leaf macules. Neurologically, the patient has a history of global developmental impairment with autistic features, and mild spastic quadriparesis. He cannot walk or speak, but is responsive to his name and produces vocalizations. He developed infantile spasms on his first day of life, and subsequently developed intractable epilepsy characterized by daily seizures. Multiple antiepileptic drugs, a vagus nerve stimulator, and ketogenic diet were tried, without sustained or significant seizure reduction.
Considering the intractable seizures and their daily occurence, a presurgical evaluation was conducted. Long-term electroencephalogram monitoring showed two main epileptic foci in the right frontal and the left occipito-parietal regions, with more frequent discharges over the right as compared to the left hemisphere. A pronounced and discrete cortical tuber located in the right frontal area with calcification and associated perilesional edema was seen on MRI. Magnetic resonance spectroscopy showed a reduced N-acetylaspartate (NAA) signal in the vicinity of this lesion, suggesting a reduction of neuronal density. Positron-emission tomography revealed multifocal hypometabolic regions, although the most prominent area was over the right frontal region. The patient underwent a right frontal lesionectomy at the age of 11 years. After surgery, his seizure frequency and intensity initially decreased markedly, but unfortunately, both seizure frequency and intensity have progressively increased and he now has an intractable mixed seizure disorder post-epilepsy surgery.
Neuroradiology
Considering the unusual characteristics and progression of the calcified component of this tuber, we retrospectively reviewed all the patient’s MRIs acquired prior to the neurosurgery to characterize its progression over time. Available MRIs included annual MRIs performed as part of his routine care (starting at age 4y). Seven MRIs were performed prior to his surgical procedure. MR imaging was performed on a 1.5 Tesla GE Signa system (GE Signa, Madison, WI) or on a 1.5 Tesla Picker International scanner (Picker International Inc, High-lands Heights, OH, USA). Sequences obtained with the GE Signa system included three-dimensional spoiled gradient-recalled with steady-state acquisition (3D SPGR; TR 32, TE 8, NEX 1, flip angle 25, matrix 256×192, 1.2mm slice thickness, 0mm gap) acquired in axial plane, fast spin-echo (FSE) axial T2 (TR 6000, TE 110, NEX 2, ETL 12, matrix 320×256, 3.5mm slice thickness, 0mm gap), and axial fluid attenuation inversion recovery (FLAIR; TR 10000, TE 137, TI 2200, NEX 0.5, matrix 256×192, 3.5mm slice thickness, 0mm gap). Sequences obtained with the 1.5 Tesla Picker International system included axial and sagittal T1 SE (TR 433, TE 13.5, NEX 2, flip angle 90, matrix 256×256, 5.5mm slice thickness, 1.5mm gap), axial T2 FSE (TR 3500, TE 96, NEX 2, ETL 8, Matrix 256×384, 5.5mm slice thickness, 1.5mm gap), and axial FLAIR (TR 9000, TE 96, TI 2100, NEX 1, matrix 192×256, 5.5mm slice thickness, 1.5mm gap).
Typical radiological brain features of TSC, such as cortical and subcortical tubers, SEN, and white matter abnormalities are observed on MRIs. While most of the tubers are static in appearance, one tuber in the right frontal lobe was noted to evolve with time. Based on visual inspection and manual measurements performed on 3D SPGR or T1 SE sequences, this lesion progressively increased in size (5.5×1.9×3.5mm at 4y 4mo; 6.8×3.3×3.5mm at 5y 11mo; 10.3×6.8×5.9mm at 7y; 11.3×7.8×6.8mm at 8y; 12.7×8.4×10.0mm at 8y 7mo; 16.8×9.1×12.4mm at 9y 9mo; 18.4×11.9×13.2mm at 10y 6mo) and changed in appearance over time.
Figure 1 shows the progression of this lesion on T1 SE or 3D SPGR, and on T2 FSE MRI sequences. On the initial study at 4 years 4 months, the lesion is T1 hypointense and T2 hyperintense without findings that suggest calcification (Fig. 1a,e). At 8 years of age, the tuber begins to demonstrate areas of T1 and T2 hypointensity suggestive of areas of calcification (Fig. 1b,f). As this tuber continues to increase in size, it becomes more heterogeneous in appearance with increasing areas of calcification demonstrated by susceptibility artifact with T1 and T2 hypointensity. Additionally, areas of T1 signal isointense to gray matter and T2 hyperintensity increase in size as well.
Figure 1.
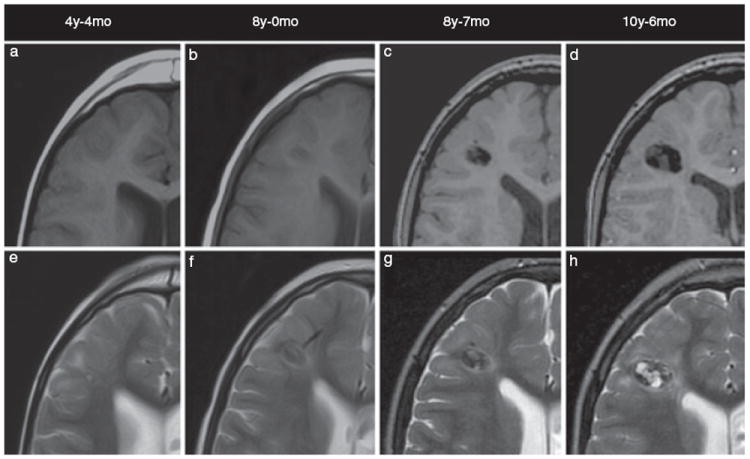
Axial T1 SE (a, b), 3D SPGR (c, d), and T2 FSE (e–h) magnetic resonance imaging shows progression in the size and appearance of the right frontal cortical tuber over time. Images are presented chronologically.
Pathology
The surgical specimen consisted of multiple fragments of soft tissue measuring each 1.0×0.3×0.3cm in aggregate. Histological examination shows a nodular lesion with numerous calcifications of various sizes embedded in brain parenchyma (Fig. 2a). The intervening brain parenchyma is composed of reactive astrocytes as well as a population of cells with mixed glial and neuronal features, characteristic of tubers in TSC. They are relatively small, and packed together with eosinophilic glial-like cytoplasm but more neuronal nuclear appearances. The cortex adjacent to the nodule shows preservation of layered architecture, with scattered atypical glioneuronal cells and rare dystrophic calcifications (Fig. 2b). There are scattered neurons in the underlying white matter, without the formation of a clear heterotopic group.
Figure 2.
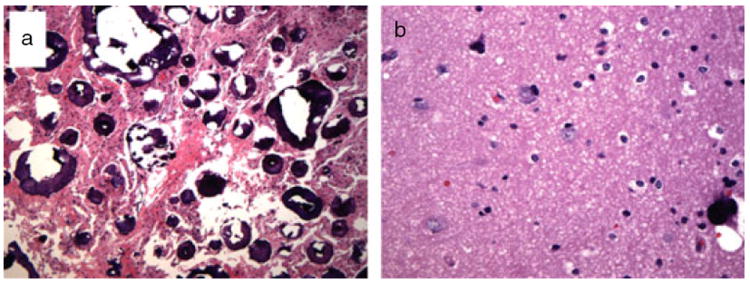
Histological findings show (a) a nodular lesion with tuber-type cells and numerous calcifications as well as (b) dystrophic calcifications located in the cortex adjacent to the nodule.
DISCUSSION
Calcification of SENs is frequent (93%) in patients with TSC, especially after 18 months of age,7 and increase in size with time in 35% of patients.8 Calcification in tubers has been reported in 43 to 62% of patients.8-10 and prevalence increases with age.7,11,12 However, unlike SENs, cortical tubers do not usually increase in size over time in adults, and remain proportional to the rest of the brain in growing children. We report the case of a young patient with a prominent right frontal cortical tuber characterized by a calcified component confirmed on pathological results, which changes over time in both size and appearance on consecutive MRIs. To our knowledge, this is the first report of a progressive calcified tuber with a radiological-pathological correlation associated with this uncommon lesion in TSC.
In a recent multiple case study, Chu-Shore et al.13 reported the cases of two children with TSC and progressive cyst-like changes in tubers on subsequent MRIs. Based on their results, they suggested that tubers in TSC are not static lesions and can exhibit evolving cyst-like characteristics over time. Based on our results, calcification of tubers can also progress, resulting in a change in appearance with time.
The calcified component of tubers has been reported to contribute to epileptogenicity,11,14 but this relationship has not been consistently observed.8 Large tubers with a calcified component are reportedly more likely to be an epileptogenic focus compared to smaller tuber without calcification.10 In the present case, given the improvement in seizure frequency after surgery, the lesion was clearly involved in the epileptogenicity of this region. This may in part be related to the tuber itself as well as the mass effect on the surrounding tissue. The cause of the mass effect remains unclear, but could be either secondary to seizure activity, possibly consisting as an inflammatory response; or a reactive response to the enlarging calcified tuber itself, inducing a real mass effect. Nevertheless, in this patient, epileptogenicity is probably a result of multiple foci, which could explain the continued mixed seizure types that have evolved since surgery. The post-surgical perilesional oedema could have also contributed to the progressive increase of seizure frequency and intensity after the lesionectomy. Given that calcified tubers may act as an epileptogenic focus, further understanding of the contributory role these lesions play in patients with TSC and intractable epilepsy is important for targeting treatment (including surgical resection) and hopefully, eventually improving outcome.
What this paper adds.
Calcified tubers can exhibit progression rather than remaining static.
Lesionectomy had a poor long-term outcome for epilepsy control.
Acknowledgments
We are grateful to the Radiology Educational Media Services of the Massachusetts General Hospital for their help with graphic illustrations. This work was supported by scholarships from the Canadian Institutes of Health Research, awarded to Dr Anne Gallagher, as well as the Carol and James Herscot Center for Tuberous Sclerosis Complex.
ABBREVIATIONS
- TSC
Tuberous sclerosis complex
- SEN
Subependymal nodules
- MRI
Magnetic resonance imaging
References
- 1.Inoue Y, Nemoto Y, Murata R, et al. CT and MR imaging of cerebral tuberous sclerosis. Brain Dev. 1998;20:209–21. doi: 10.1016/s0387-7604(98)00022-9. [DOI] [PubMed] [Google Scholar]
- 2.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–56. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 3.Doherty C, Goh S, Young Poussaint T, Erdag N, Thiele EA. Prognostic significance of tuber count and location in tuberous sclerosis complex. J Child Neurol. 2005;20:837–41. doi: 10.1177/08830738050200101301. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz RA, Fernandez G, Kotulska K, Jozwiak S. Tuberous sclerosis complex: advances in diagnosis, genetics, and management. J Am Acad Dermatol. 2007;57:189–202. doi: 10.1016/j.jaad.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher A, Grant EP, Madan N, Jarett DY, Lyczkowski DA, Thiele EA. MRI findings reveal three different types of tuber in patients with tuberous sclerosis complex. J Neurol. 2010;257:1373–81. doi: 10.1007/s00415-010-5535-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridler K, Suckling J, Higgins N, Bolton P, Bullmore E. Standardized whole brain mapping of tubers and subependymal nodules in tuberous sclerosis complex. J Child Neurol. 2004;19:658–65. doi: 10.1177/08830738040190090501. [DOI] [PubMed] [Google Scholar]
- 7.Martin N, de Broucker T, Cambier J, Marsault C, Nahum H. MRI evalution of tuberous sclerosis. Neuroradiology. 1987;29:437–43. doi: 10.1007/BF00341739. [DOI] [PubMed] [Google Scholar]
- 8.Menor F, Marti-Bonmati L, Mulas F, Poyatos C, Cortina H. Neuroimaging in tuberous sclerosis: a clinicoradiological evaluation in pediatric patients. Pediatr Radiol. 1992;22:485–9. doi: 10.1007/BF02012989. [DOI] [PubMed] [Google Scholar]
- 9.Altman NR, Purser RK, Donovan Post MJ. Tuberous sclerosis: characteristics at CT and MR imaging. Radiology. 1988;167:527–32. doi: 10.1148/radiology.167.2.3357966. [DOI] [PubMed] [Google Scholar]
- 10.Koh S, Jayakar P, Dunoyer C, et al. Epilepsy surgery in children with tuberous sclerosis complex: presurgical evaluation and outcome. Epilepsia. 2000;41:1206–13. doi: 10.1111/j.1528-1157.2000.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi Y, Yoshimura K, Nakae Y, Nara T, Hamada R, Maekawa K. Neonatal tuberous sclerosis with heart and brain tumors. Acta Paediatr Jpn. 1990;32:571–4. doi: 10.1111/j.1442-200x.1990.tb00884.x. [DOI] [PubMed] [Google Scholar]
- 12.Sugita K, Itoh K, Takeuchi Y, et al. Tuberous sclerosis: report of two cases studied by computer-assisted cranial tomography within 1 week after birth. Brain Dev. 1985;7:438–43. doi: 10.1016/s0387-7604(85)80144-3. [DOI] [PubMed] [Google Scholar]
- 13.Chu-Shore CJ, Frosch MP, Grant PE, Thiele EA. Progressive multifocal cystlike cortical tubers in tuberous sclerosis complex: clinical and neuropathologic findings. Epilepsia. 2009;50:2648–51. doi: 10.1111/j.1528-1167.2009.02193.x. [DOI] [PubMed] [Google Scholar]
- 14.Holmes GL, Stafstrom CE. The tuberous sclerosis study group. Tuberous sclerosis complex and epilepsy: recent developments and future challenges. Epilepsia. 2007;48:617–30. doi: 10.1111/j.1528-1167.2007.01035.x. [DOI] [PubMed] [Google Scholar]


