Abstract
Mantle cell lymphoma (MCL) is challenging to manage, with a median survival of 3–5 years. While intensive strategies are often appropriate for younger patients, these approaches are often not appropriate for older patients. In 2006, we reported our initial results using modified R-hyperCVAD (rituximab with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) with maintenance rituximab. The complete response rate was 64%, and median progression-free survival (PFS) 37 months. Herein, we update our results, now with a median follow-up of 62 months. The median PFS is unchanged and the median overall survival (OS) is 70 months. The proportion of patients surviving at 5 years is 62%, comparable to studies using intensive strategies in similar patient populations. No late toxicities were noted in our cohort. These long-term results suggest that the modified R-hyperCVAD regimen with maintenance rituximab is an excellent option for older patients with newly diagnosed mantle cell lymphoma.
Keywords: Mantle cell lymphoma, non-Hodgkin’s lymphoma, rituximab maintenance, modified R-hyperCVAD
Introduction
Mantle cell lymphoma (MCL) constitutes approximately 6–8% of non-Hodgkin lymphomas and is challenging to manage, with many reports indicating a median survival of 3–4 years [1–4] and a recent study showing up to 5 years’ median survival [5]. It is considered incurable with standard therapies for non-Hodgkin lymphoma [6]. While high response rates are seen with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), the progression-free survival is short [7,8]. When eligible, selected patients may benefit from autologous stem cell transplant (ASCT) consolidation in first remission [9–13]. However, this strategy did not prolong overall survival in a randomized clinical trial, and furthermore, many patients are not eligible for transplant [14]. Very favorable results have been reported for the R-hyperCVAD regimen (rituximab with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with rituximab plus methotrexate and cytarabine) [15]. However, this regimen can be prohibitively toxic for patients over the age of 65 and some younger patients with comorbidities [15,16]. The median age for patients with newly diagnosed MCL is 64 years, and thus regimens that do not include stem cell transplant or highly aggressive chemotherapy are needed. Furthermore, data suggest that intensive strategies may not always be necessary to prolong survival [17].
With this in mind, in 2000, we devised a modified version of R-hyperCVAD (in which patients received only part A of R-hyperCVAD) followed by maintenance rituximab (MR) and tested it in a phase II pilot study. Data on the results of 22 patients treated under this protocol were previously published after 3 years median follow-up [18]. The overall response rate was 77% and the complete response rate was 64%. Median progression-free survival (PFS) was 37 months and median overall survival (OS) was not reached. The 5-year follow-up of this trial is presented here, as well as outcome analysis by the Mantle Cell Lymphoma International Prognostic Index (MIPI) [19].
Patients and methods
Patient eligibility
This was a prospective study performed in the Wisconsin Oncology Network, an affiliation between the University of Wisconsin Comprehensive Cancer Center and several community-based practices. Eligible patients must have had untreated MCL confirmed by a central hematopathologist. Eligibility criteria included the following: at least 18 years of age, an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, an absolute neutrophil count of ≥1500/μL, platelet count ≥100 000/μL (unless the cytopenias were caused by marrow infiltration of lymphoma or splenomegaly), a serum creatinine level of <2 mg/dL, a serum bilirubin of <1.5 mg/dL, and an aspartate transaminase (AST) <2.5 times the upper limit of normal. Patients were ineligible if they were pregnant or lactating, had an active second malignancy, an uncontrolled infection, central nervous system (CNS) lymphoma, human immunodeficiency virus (HIV), or New York Heart Classification III or IV disease, or were incapable of giving informed consent. The University of Wisconsin Human Subjects Committee and the local institutional review boards of participating sites approved this trial.
Treatment schedule
Details of the treatment schedule are published elsewhere. Briefly, rituximab 375 mg/m2 was administered on day 1 of all treatment cycles except cycle 1. Following completion of the rituximab infusion, chemotherapy was initiated: cyclophosphamide days 1–3 (six doses, total dose 1800 mg/m2), doxorubicin 25 mg/m2/day as a continuous infusion days 1–2 (total dose 50 mg/m2), vincristine 2 mg given intravenously (i.v.) on day 3, and dexamethasone 40 mg given orally on days 1–4. The first five patients also received vincristine 2 mg i.v. push on day 11 and dexamethasone 40 mg orally on days 11–14. All patients received filgrastim 5 μg/kg/day subcutaneously beginning on day 5 and continuing until the absolute neutrophil count was greater than 4000/mm3. Cycles were repeated every 28 days. Patients were treated until they achieved a complete response plus two additional cycles, up to a maximum of six cycles. Patients achieving a partial response (PR) or complete response/complete response unconfirmed (CR/CRu) after modified R-hyperCVAD received rituximab 375 mg/m2 given weekly for 4 consecutive weeks, repeated every 6 months for a total of four courses. MR began 6 months after completion of chemotherapy. Patients with progressive disease at any point came off study treatment.
Patient evaluation and response criteria
Disease restaging occurred after each even-numbered chemotherapy cycle, every 3 months while on maintenance therapy, and every 6 months following the completion of maintenance therapy until year 5, and then yearly thereafter. Restaging included a physical examination, all routine blood tests, computed tomography (CT) scans of the chest/abdomen/pelvis, and a bone marrow evaluation if assessing for a CR. Response criteria were according to the International Workshop to Standardize Response Criteria for non-Hodgkin Lymphoma [20].
Statistical methods
The primary end point of this phase II pilot study was CR rate. The sample size determination was based upon precision, defined by the lower limit of a one-sided 90% confidence interval. Using a sample size of 20 eligible patients, an observed CR rate of 60% or more would provide preliminary information that this treatment regimen was worthy of further study. Secondary end points included estimates of overall response rate (ORR), PFS, and OS. All response rate estimates are reported with 90% confidence intervals, and PFS and OS were estimated by the Kaplan–Meier product limit method.
Results
Clinical characteristics
Between October 2000 and March 2004, 22 patients with untreated MCL were enrolled into the study. Details of patient characteristics have been published previously. Briefly, the median age was 63 (range 40–81). Most patients had an ECOG performance status of 0 or 1. Twenty of 22 (91%) patients were male, and 19/22 (86%) had stage IV disease. An elevated lactate dehydrogenase (LDH) was noted in 41%. Most patients had a nodal presentation while two patients had a leukemic presentation with massive splenomegaly. When stratified according to the MIPI, seven patients were classified as low-risk, eight as intermediate-risk, and seven as high-risk [19].
Response
Two patients expired before any response assessment and were considered non-responders. Three patients experienced progressive disease (PD) during induction therapy, including both patients with leukemic presentations. The remaining 17 patients responded to the R-hyperCVAD induction (14 CR and three PR) for an ORR of 77% (90% confidence interval [CI] 58–91%) and complete response rate (CRR) of 64% (90% CI 44–80%). Four patients achieved CR after two cycles of therapy and received four total cycles, while the remaining 13 patients received six total cycles.
PFS and OS
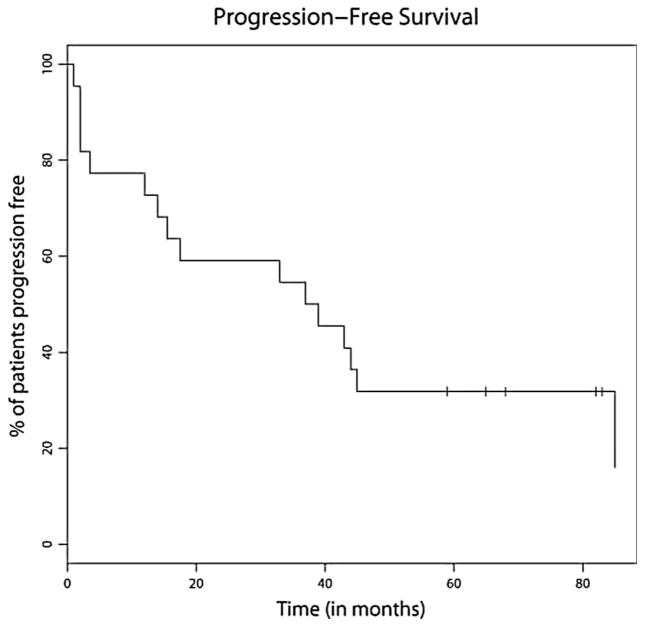
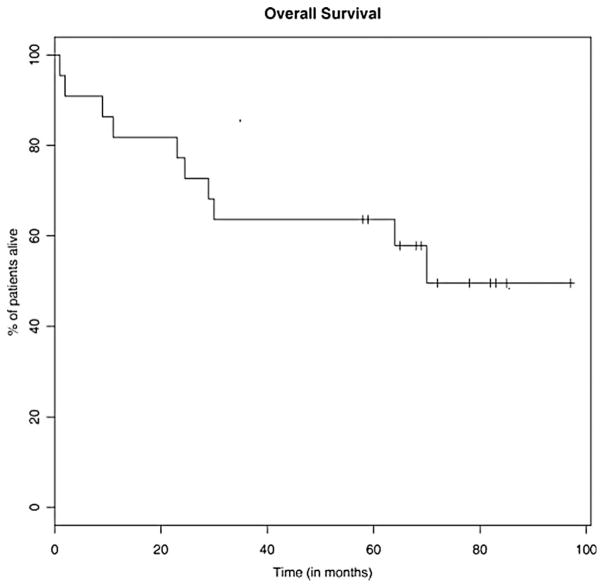
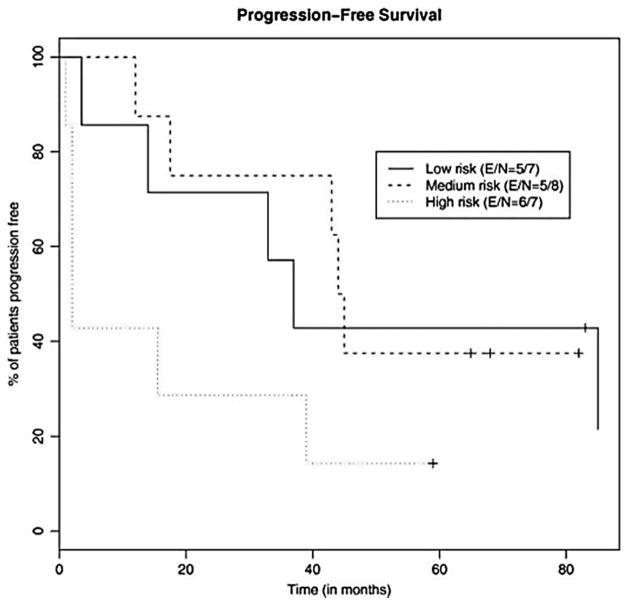
After a median follow-up of 62 months, the median PFS was 38 months (range 1–85) (Figure 1) and the median OS was 70 months (range 1–97+) (Figure 2). Median PFS for the low-, intermediate-, and high-risk patients was 37 months, 45 months, and 2 months, respectively (Figure 3). Median OS for the low-, intermediate-, and high-risk patients was not reached, not reached, and 23 months, respectively (Figure 4). For the entire cohort, the proportion of patients surviving at 5 years was 62%.
Figure 1.
Kaplan–Meier estimate for PFS. Median PFS 38 months.
Figure 2.
Kaplan–Meier estimate for OS. Median OS 70 months.
Figure 3.
Kaplan–Meier analysis of the probability of PFS for each risk group as designated by the MIPI.
Figure 4.
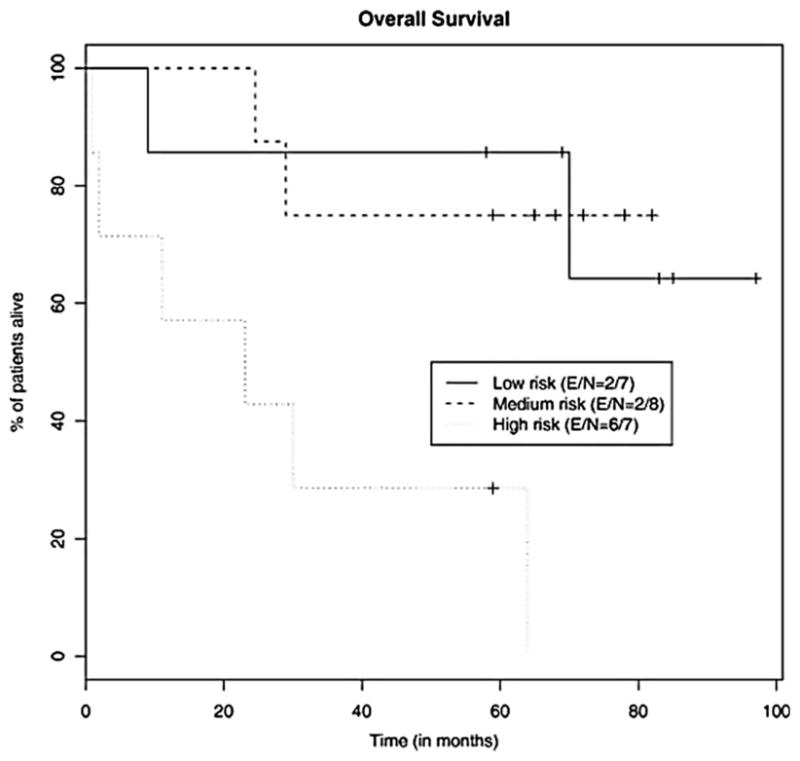
Kaplan–Meier analysis of the probability of OS for each risk group as designated by the MIPI.
Toxicity
Details of toxicity have been published previously. The major toxicity during induction therapy was expected myelosuppression. During the 2 years of MR, no grade 4 toxicities and only five incidences of grade 3 toxicity were noted. One patient developed delayed neutropenia, which responded promptly to filgrastim. No significant infections were observed during or after completion of MR. There were no observed cases of therapy related leukemia or myleodysplastic syndrome.
Discussion
Modified R-hyperCVAD with MR showed good response rates in 22 patients with previously untreated MCL, with an ORR of 77% and CRR of 64%. Now with median follow-up of 62 months, we report a median PFS of 38 months and median OS of 70 months. These results compare favorably to other trials which have explored non-intensive immunochemotherapy approaches, where the median PFS after R-CHOP chemotherapy was typically around 18 months [7,8]. Our version of modified R-hyperCVAD was actually more CHOP-like than hyperCVAD-like, leading us to speculate that the majority of the PFS prolongation we observed was due to MR rather than something unique in our induction strategy.
The role of MR in MCL has been controversial. Unlike diffuse large B-cell lymphoma, where MR for 2 years does not appear to be beneficial after R-CHOP [21], MR has been shown to prolong PFS in incurable lymphomas [22–24]. As MCL is generally an incurable B-cell lymphoma, we hypothesized that MR would have a beneficial effect in MCL. Two small randomized clinical trials in MCL have generated conflicting results. The German Low Grade Lymphoma Study Group enrolled patients with relapsed follicular lymphoma (FL) and MCL, and after induction chemotherapy with either chemotherapy or R-chemotherapy, randomized patients to MR or observation for 9 months. A statistically significant improvement in response duration was seen in the 47 patients with MCL who received MR, with a higher proportion of those patients experiencing an ongoing remission beyond 2 years (45% vs. 9%, p = 0.049) [25]. The Swiss Group for Clinical Cancer Research (SAKK) enrolled 104 patients with a mixture of untreated and relapsed MCL and randomized them to a single 4-week rituximab treatment or a prolonged rituximab schedule of a 4-week treatment followed by a single dose every 8 weeks × 4 doses [26]. The extended schedule did not improve response rates, response duration, or event-free survival. The reasons for these discrepant results are unclear, but we speculate that the quality of the response to induction therapy may affect the likelihood of MR benefit. The question may soon be put to rest in MCL, as an independent Data and Safety Monitoring Board (DSMB) recently recommended early closure of a large randomized trial which demonstrated a significant benefit for patients receiving MR after R-CHOP chemotherapy (M. Dreyling, personal communication).
The 5-year OS of 62% in our cohort is comparable to the 5-year OS of 65% reported for the conventional R-hyperCVAD regimen and 64% reported in a Cancer and Leukemia Group B (CALGB) study utilizing alternative intensive treatment strategy [27,28]. We note a comparable 5-year OS despite a shorter median PFS in our trial (38 months vs. ~60 months). This observation suggests that there is a reasonable ability to administer effective salvage therapy in relapsed MCL. Unlike the experience reported by M. D. Anderson, we see no difference in outcomes when we analyze our cohort by age (using 65 years of age as the cut-off; data not shown) [27]. So while our younger patients did somewhat worse for PFS than the M. D. Anderson cohort, our older patients did somewhat better, validating the inclusion of our regimen as a reasonable standard for older patients in the National Comprehensive Cancer Network (NCCN) guidelines [29].
Finally, we were able to validate the MIPI with our patient cohort, with a particularly significant difference seen in OS between the low and high MIPI risk groups (p = 0.009).
We report the long-term follow-up from this study for three reasons: (1) to draw attention to the potential benefit for MR in MCL, (2) to emphasize the excellent OS, which is comparable to that seen in some studies using intensive strategies, and (3) to validate the inclusion of this regimen in the NCCN guidelines as an option for older patients with MCL. The US intergroup is currently designing prospective trials focusing on older patients with MCL, so that an appropriate balance between efficacy and toxicity can be evaluated in this important group of patients.
Acknowledgments
This work was supported by Genentech bio-oncology UWCCC core grant P30 CA014520-34 and the UW Forward Lymphoma Fund.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Vandenberghe E, De Wolf-Peeters C, Vaughan Hudson G, et al. The clinical outcome of 65 cases of mantle cell lymphoma initially treated with non-intensive therapy by the British National Lymphoma Investigation Group. Br J Haematol. 1997;99:842–847. doi: 10.1046/j.1365-2141.1997.4693273.x. [DOI] [PubMed] [Google Scholar]
- 2.Zucca E, Roggero E, Pinotti G, et al. Patterns of survival in mantle cell lymphoma. Ann Oncol. 1995;6:257–262. doi: 10.1093/oxfordjournals.annonc.a059155. [DOI] [PubMed] [Google Scholar]
- 3.Weisenburger DD, Armitage JO. Mantle cell lymphoma–an entity comes of age. Blood. 1996;87:4483–4494. [PubMed] [Google Scholar]
- 4.Weisenburger DD, Vose JM, Greiner TC, et al. Mantle cell lymphoma. A clinicopathologic study of 68 cases from the Nebraska Lymphoma Study Group. Am J Hematol. 2000;64:190–196. doi: 10.1002/1096-8652(200007)64:3<190::aid-ajh9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 5.Herrmann A, Hoster E, Zwingers T, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27:511–518. doi: 10.1200/JCO.2008.16.8435. [DOI] [PubMed] [Google Scholar]
- 6.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 7.Howard OM, Gribben JG, Neuberg DS, et al. Rituximab and CHOP induction therapy for newly diagnosed mantle-cell lymphoma: molecular complete responses are not predictive of progression-free survival. J Clin Oncol. 2002;20:1288–1294. doi: 10.1200/JCO.2002.20.5.1288. [DOI] [PubMed] [Google Scholar]
- 8.Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) J Clin Oncol. 2005;23:1984–1992. doi: 10.1200/JCO.2005.08.133. [DOI] [PubMed] [Google Scholar]
- 9.Khouri IF, Saliba RM, Okoroji GJ, Acholonu SA, Champlin RE. Long-term follow-up of autologous stem cell transplantation in patients with diffuse mantle cell lymphoma in first disease remission: the prognostic value of beta2-microglobulin and the tumor score. Cancer. 2003;98:2630–2635. doi: 10.1002/cncr.11838. [DOI] [PubMed] [Google Scholar]
- 10.Vandenberghe E, Ruiz de Elvira C, Loberiza FR, et al. Outcome of autologous transplantation for mantle cell lymphoma: a study by the European Blood and Bone Marrow Transplant and Autologous Blood and Marrow Transplant Registries. Br J Haematol. 2003;120:793–800. doi: 10.1046/j.1365-2141.2003.04140.x. [DOI] [PubMed] [Google Scholar]
- 11.Gianni AM, Magni M, Martelli M, et al. Long-term remission in mantle cell lymphoma following high-dose sequential chemotherapy and in vivo rituximab-purged stem cell autografting (R-HDS regimen) Blood. 2003;102:749–755. doi: 10.1182/blood-2002-08-2476. [DOI] [PubMed] [Google Scholar]
- 12.Mangel J, Leitch HA, Connors JM, et al. Intensive chemotherapy and autologous stem-cell transplantation plus rituximab is superior to conventional chemotherapy for newly diagnosed advanced stage mantle-cell lymphoma: a matched pair analysis. Ann Oncol. 2004;15:283–290. doi: 10.1093/annonc/mdh069. [DOI] [PubMed] [Google Scholar]
- 13.Lefrere F, Delmer A, Levy V, Delarue R, Varet B, Hermine O. Sequential chemotherapy regimens followed by high-dose therapy with stem cell transplantation in mantle cell lymphoma: an update of a prospective study. Haematologica. 2004;89:1275–1276. [PubMed] [Google Scholar]
- 14.Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105:2677–2684. doi: 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- 15.Romaguera JE, Fayad L, Rodriguez MA, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23:7013–7023. doi: 10.1200/JCO.2005.01.1825. [DOI] [PubMed] [Google Scholar]
- 16.Epner EM, Unger J, Miller T, et al. A multi center trial of hyperCVAD + rituxan in patients with newly diagnosed mantle cell lymphoma. Blood. 2007;110(Suppl 1):Abstract 387. [Google Scholar]
- 17.Martin P, Chadburn A, Christos P, et al. Intensive treatment strategies may not provide superior outcomes in mantle cell lymphoma: overall survival exceeding 7 years with standard therapies. Ann Oncol. 2008;19:1327–1330. doi: 10.1093/annonc/mdn045. [DOI] [PubMed] [Google Scholar]
- 18.Kahl BS, Longo WL, Eickhoff JC, et al. Maintenance rituximab following induction chemoimmunotherapy may prolong progression-free survival in mantle cell lymphoma: a pilot study from the Wisconsin Oncology Network. Ann Oncol. 2006;17:1418–1423. doi: 10.1093/annonc/mdl127. [DOI] [PubMed] [Google Scholar]
- 19.Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–565. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 21.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 22.Hochster H, Weller E, Gascoyne RD, et al. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: results of the randomized phase III ECOG1496 Study. J Clin Oncol. 2009;27:1607–1614. doi: 10.1200/JCO.2008.17.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Oers MH, Klasa R, Marcus RE, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood. 2006;108:3295–3301. doi: 10.1182/blood-2006-05-021113. [DOI] [PubMed] [Google Scholar]
- 24.Salles GA, Seymour JF, Feugier P, et al. Rituximab maintenance for 2 years in patients with untreated high tumor burden follicular lymphoma after response to immunochemotherapy. J Clin Oncol. 2010;28(15 Suppl):Abstract 8004. [Google Scholar]
- 25.Forstpointner R, Unterhalt M, Dreyling M, et al. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG) Blood. 2006;108:4003–4008. doi: 10.1182/blood-2006-04-016725. [DOI] [PubMed] [Google Scholar]
- 26.Ghielmini M, Schmitz SF, Cogliatti S, et al. Effect of single-agent rituximab given at the standard schedule or as prolonged treatment in patients with mantle cell lymphoma: a study of the Swiss Group for Clinical Cancer Research (SAKK) J Clin Oncol. 2005;23:705–711. doi: 10.1200/JCO.2005.04.164. [DOI] [PubMed] [Google Scholar]
- 27.Romaguera JE, Fayad LE, Feng L, et al. Ten-year follow-up after intense chemoimmunotherapy with rituximab-hyperCVAD alternating with rituximab-high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. Br J Haematol. 2010;150:200–208. doi: 10.1111/j.1365-2141.2010.08228.x. [DOI] [PubMed] [Google Scholar]
- 28.Damon LE, Johnson JL, Niedzwiecki D, et al. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J Clin Oncol. 2009;27:6101–6108. doi: 10.1200/JCO.2009.22.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.NCCN Guidelines. Available from: http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf.