Abstract
Seventy eight natural products from chemical libraries containing compounds from marine organisms (sponges, algae, fungi, tunicates and cyanobacteria) and terrestrial plants, were screened for the inhibition of bacterial quorum sensing (QS) using a reporter strain Chromobacterium violaceum CV017. About half of the natural products did not show any QS inhibition. Twenty four percent of the tested compounds inhibited QS of the reporter without causing toxicity. The QS inhibitory activities of the most potent and abundant compounds were further investigated using the LuxR-based reporter E. coli pSB401 and the LasR-based reporter E. coli pSB1075. Midpacamide and tenuazonic acid were toxic to the tested reporters. QS-dependent luminescence of the LasR-based reporter, which is normally induced by N-3-oxo-dodecanoyl-L-homoserine lactone, was reduced by demethoxy encecalin and hymenialdisin at concentrations 46.6 μM and 15μM, respectively. Hymenialdisin, demethoxy encecalin, microcolins A and B and kojic acid inhibited responses of the LuxR-based reporter induced by N-3-oxo-hexanoyl-L-homoserine lactone at concentrations 40.2 μM, 2.2 μM, 1.5 μM, 15 μM and 36 μM, respectively. The ability to prevent microfouling by one of the compounds screened in this study (kojic acid; final concentrations 330 μM and 1 mM) was tested in a controlled mesocosm experiment. Kojic acid inhibited formation of microbial communities on glass slides, decreasing the densities of bacteria and diatoms in comparison with the control lacking kojic acid. The study suggests that natural products with QS inhibitory properties can be used for controlling biofouling communities.
Keywords: quorum sensing, inhibitors, antifouling, natural products, biofilms
Introduction
In the marine environment, all natural and artificial substrata are quickly colonized by marine micro- and macro-organisms in a process that is known as “biofouling”. Micro- and macro-foulers can cause severe industrial problems by increasing drag force, promoting metal corrosion and reducing heat transfer efficiency of heat exchangers (Yebra et al. 2006; Schultz et al. 2011). Biocides that are used to control biofouling are not effective against some bacterial and diatom species (Cassé and Swain 2006; Molino et al. 2009a,b), which colonize entire surfaces coated with antifouling paints and affect their performance. Therefore, development of new ways to regulate densities of microbes in and on antifouling coatings is urgently required.
Interference with bacterial quorum sensing (QS) has been proposed as one potential approach for controlling biofouling (Dobretsov et al. 2009; Choudhary and Schmidt-Dannert 2010; Qian et al. 2010; Xiong and Liu 2010). QS is a population density-dependent gene regulatory mechanism, which relies on the production and perception of threshold concentrations of low molecular weight signal molecules that activate transcriptional regulators (Antunes et al. 2010). In Gram negative bacteria, QS is affected by N-acyl homoserine lactones (AHLs). These QS molecules are typically produced by the AHL synthethases (homologues of the LuxI) and actively or passively redistributed in the environment (Waters and Bassler 2005; Dickschat 2010). When the intracellular concentration of AHLs reaches a certain threshold level, the AHL molecules bind to the LuxR-type receptor, and this leads to the formation of active dimers allowing interactions with QS-dependent promoter sequences (Boyer and Wisniewski-Dye 2009). Once active complexes within promoter sequences are established, transcription of QS genes responsible for luminescence, biofilm formation, virulence and other relevant processes is effected (Waters and Bassler 2005; Irie and Parsek 2008).
Because bacterial QS is central to the interactions of bacteria with their eukaryotic hosts, it is not surprising that many multicellular organisms evolved different mechanisms to interfere with bacterial QS (reviewed by Dobretsov et al. 2009; Goecke et al. 2010). One of the well-studied examples of organisms producing QS inhibitors is the red alga Delisea pulchra (Manefield et al. 1999), which produces a suite of halogenated furanones that reduce bacterial adhesion to algal surfaces and inhibit bacterial swarming (Maximilien et al. 1998). Several recent studies demonstrated that extracts of Great Barrier Reef marine invertebrates (Skindersoe et al. 2008b) and cyanobacteria from Florida waters (Dobretsov et al. 2010) are similarly capable of inhibiting bacterial QS.
It has been proposed that QS inhibitors can be used for antimicrobial protection in aquaculture (Defoirdt et al. 2004; Dobretsov et al. 2009). In the laboratory, it has been shown that synthetic furanones inhibited development of microbial biofilms (Dobretsov et al. 2007). In short field and laboratory experiments, furanones produced by D. pulchra strongly inhibited attachment of marine bacteria on rocks and seaweeds (Maximilien et al. 1998).
In this study, we screened 78 natural products from marine invertebrates (mostly sponges), terrestrial plants, fungi and cyanobacteria for the inhibition of bacterial QS reporters. The activities of the most potent and abundant QS inhibitors, such as demethoxy encecalin, midpacamide, tenuazonic acid, hymenialdisin, microcolins A and B and kojic acid, were further investigated using different reporter strains. The AF performance of kojic acid was tested in a mesocosm experiment. The main aims of the study were to investigate: 1) the effects of natural products on QS pigment production in Chromobacterium violaceum CV017; 2) the activity of demethoxy encecalin, midpacamide, tenuazonic acid, hymenialdisin, microcolins A and B and kojic acid using the LuxR-based and the LasR-based reporters; 3) AF performance of kojic acid in a mesocosm experiment.
Material and methods
Compounds used in this study
All natural products analyzed in this study were previously isolated by the research groups of P. Proksch and V. Paul (Table 1). These compounds had been isolated from sponges, tunicates, fungi, plants and cyanobacteria and represent major groups of natural products. All isolated compounds were fully characterized structurally by mass spectrometry as well as by one and two dimensional NMR spectroscopy (1H, 13C, COSY, HMBC). All compounds were dissolved in methanol (Fisher Scientific, USA) yielding a stock solution (0.2 mg ml−1).
Table 1.
Origin of tested natural products and their effect on quorum sensing of Chromobacteruium violaceum CV017 and its growth. Quorum sensing inhibition reported as mean of 3 replicates ± SD minimum inhibitory concentrations–MIC (μM). Toxicity effect of compounds is presented as a minimal amount of natural product necessary to inhibit growth of the reporter strain. Compounds are sorted according to their bioactivity. The first group contains compounds that only inhibited QS of C. violaceum CV017. The second group includes compounds that inhibited QS of C. violaceum CV017 but have some toxicity. The third group represents compounds that inhibited growth of CV017. The fourth group includes compounds that did not have any bioactive properties.
| Abbreviation | Name | Origin | Groups of compounds | Reference | QS (μM) | Growth (mole × 10−6) | |
|---|---|---|---|---|---|---|---|
| Species | Group | ||||||
| First group of compounds | |||||||
| 1 | Demethoxy encecalin | Baccharis cassinaefolia | Plant | Benzopyran | Proksch and Rodriguez 1982 | 3.92 ± 1.11 | - |
| 2 | Orientin | Polygonum orientale | Plant | Flavonoid | Weber 2007 | 4.46 ± 1.3 | - |
| 3 | Kuanoniamin D | Cystodytes sp. | Tunicate | Alkaloid | Eder et al. 1998 | 5.55 ± 3.11 | - |
| 4 | Malyngamide A | Lyngbya majuscula | Cyanobacteria | Amide | Cardellina et al.1979 | 7.46 ± 2.11 | - |
| 5 | Ageliferin | Agelas conifera | Sponge | Alkaloid | Hertiani et al. 2010 | 11.29 ± 1.93 | - |
| 6 | Microcolin A | Lyngbya sp. | Cyanobacteria | Peptide | Koehn et al. 1992 | 15.23 ± 2.75 | - |
| 7 | Mauritamide B | Agelas nakamurai | Sponge | Alkaloid | Hertiani et al. 2010 | 36.76 ± 4.47 | - |
| 8 | Pinoresinol | Pinus ssp. | Plant | Lignan | Weber 2007 | 41.85 ± 6.13 | - |
| 9 | Microcolin B | Lyngbya sp. | Cyanobacteria | Peptide | Koehn et al. 1992 | 43.21 ± 3.14 | - |
| 10 | Gallic acid | Plants | Plant | Phenol | Bayer 2009 | 64.66 ± 12.27 | - |
| 11 | Glucobrassicin | Brassica napus var. napus | Plant | Alkaloid | Weber 2007 | 133.71 ± 16.39 | - |
| 12 | Meleagrin | Penicillium chrysogenum | Fungus | Alkaloid | Rusman 2006 | 138.42 ± 22.18 | - |
| 13 | Alterporriol E | Alternaria porri | Fungus | Polyketide | Aly et al. 2008 | 298.26 ± 57.30 | - |
| 14 | Kojic acid | Aspergillus spp | Fungus | Pyranone | Indriani 2008 | 239.25 ± 8.92 | - |
| 15 | 4-(4,5-dibromo-1- methyl-1H-pyrrole- 2-carboxamido) butanoic acid | Agelas sp. | Sponge | Alkaloid | 2010 | 271.73 ± 9.13 | - |
| 16 | Hymenialdisin | Hymeniacidon aldis | Sponge | Alkaloid | Supriyono et al. 1995 | 308.52 ± 8.19 | - |
| 17 | Dulcitol | Spatoglossum sp. | Plant | Sugar | Queiroz et al. 2008 | 380.02 ± 56.23 | - |
| 18 | Midpacamide | Agelas mauritiana | Sponge | Alkaloid | Hertiani et al. 2010 | 458.61 ± 34.11 | - |
| 19 | Tenuazonic acid | Alternaria tenuis | Fungus | Alkaloid | Hassan 2007 | 517.03 ± 39.71 | - |
| Second group of compounds | |||||||
| 20 | Malyngamide B | Lyngbya majuscula | Cyanobacteria | Amide | Cardellina et al. 1978 | 5.89 ± 1.77 | 17.27 ± 1.26 |
| 21 | (+)Avarol | Dysidea avara | Sponge | Terpene | Putz 2009 | 6.36 ± 2.78 | 0.40 ± 0.04 |
| 22 | Alternariol monomethyl ether | Alternaria sp. | Fungus | Polyketide | Aly et al. 2008 | 7.32 ± 1.25 | 7.35 ± 1.16 |
| 23 | Aaptamin | Aaptos aaptos | Sponge | Alkaloid | Supriyono 1997 | 8.76 ± 2.38 | 0.61 ± 0.02 |
| 24 | 8-OH-manzamien A | Acanthostrongylo phore ingens | Sponge | Alkaloid | Edrada 1998 | 12.41± 1.34 | 0.50 ± 0.03 |
| 25 | Lyngbyastatin 3 | Lyngbya majuscula | Cyanobacteria | Peptide | Williams et al. 2003 | 12.00 ± 3.41 | 16.19 ± 1.67 |
| 26 | Aeroplysinin | Aeroplysisnin sp. | Sponge | Alkaloid | Ebel 1997 | 16.55 ± 5.51 | 0.31 ± 0.02 |
| 27 | (−)Dibromophakelline | Pseudaxinyssa cantharella | Sponge | Alkaloid | Hertiani 2007 | 17.99 ± 5.10 | 0.46 ± 0.05 |
| 28 | Alterlactone | Alternaria sp. | Fungus | Polyketide | Aly et al. 2008 | 24.31 ± 5.29 | 1.74 ± 0.13 |
| 29 | Emodin | Rhamnus purshiana | Plant | Polyketide | Debbab 2007 | 25.90 ± 5.14 | 0.93 ± 0.04 |
| 30 | Encecalin | Eupatorium californica | Plant | Benzopyran | Proksch and Rodriguez 1982 | 30.12 ± 6.39 | 1.29 ± 0.09 |
| 31 | Agelanesin C | Agelas sp. | Sponge | Alkaloid | Hertiani et al.2010 | 36.30 ± 8.94 | 0.91 ± 0.01 |
| 32 | Cyclo Colorenon (1)* | Porella vernicosa | Plant | Terpene | Handayani 1998 | 91.60 ± 16.22 | 1.38 ± 0.04 |
| 33 | Aerothionin | Aplysina aerophoba | Sponge | Alkaloid | Ebel 1997 | 244.47 ± 19.71 | 0.27 ± 0.02 |
| 34 | (−) Agelasidine D | Agelas clathrodes | Sponge | Alkaloid | Hertiani et al.2010 | 454.90 ± 14.22 | 0.46 ± 0.06 |
| 35 | Altersolanol A | Alternaria solani | Fungus | Polyketide | Aly et al. 2008 | 594.71 ± 48.39 | 0.41 ± 0.04 |
| Third group of compounds | |||||||
| 36 | Curacin D | Lyngbya majuscula | Cyanobacteria | Lipid | Marquez et al. 1998 | - | 69.88 ± 3.54 |
| 37 | Alternariol sulphate | Alternaria sp. | Fungus | Polyketide | Aly et al. 2008 | - | 0.59 ± 0.06 |
| Forth group of compounds | |||||||
| 38 | Alteric acid | Alternaria sp. | Fungus | Polyketide | Aly et al. 2008 | - | - |
| 39 | Alternariol | Alternaria tenuis | Fungus | Polyketide | Aly et al. 2008 | - | - |
| 40 | Altenuene 4′-Epialtenuene | Alternaria sp. | Fungus | Polyketide | Aly et al. 2008 | - | - |
| 41 | Altenusin | Alternaria tenuis | Fungus | Polyketide | Aly et al. 2008 | - | - |
| 42 | Alterporriol D | Alternaria porri | Fungus | Polyketide | Aly et al. 2008 | - | - |
| 43 | Ampelanol | Ampelomyces sp. | Fungus | Polyketide | Hassan 2007 | - | - |
| 44 | Aposhaerin A | Aposphaeria sp. | Fungus | Polyketide | Hassan 2007 | - | - |
| 45 | Atromentine | Aglaia odorata | Fungus | Phenol | Duong 2006 | - | - |
| 46 | Chaetomin | Chaetomium cochliodes | Fungus | Alkaloid | Aly et al. 2008 | - | - |
| 47 | Cochliodinol | Chaetomium globosum | Fungus | Alkaloid | Aly et al. 2008 | - | - |
| 48 | Citrinin | Penicillium citrinum | Fungus | Phenol | Hiort et al. 2004 | - | - |
| 49 | Cyclo(prolyl-Valyl)* | Aspergillus flavipes | Fungus | Peptide | Indriani 2008 | - | - |
| 50 | Cyclo(L-tyr-L-pro)* | Alternaria alternata | Fungus | Peptide | Indriani 2008 | - | - |
| 51 | Cytochalasin E | Rosellinia necatrix | Fungus | Alkaloid | Indriani 2008 | - | - |
| 52 | Equisetin | Fusarium equiseti | Fungus | Alkaloid | Kjer 2010 | - | - |
| 53 | Ageraton | Ageratum houstonianum | Plant | Benzofuran | Kunze 1995 | - | - |
| 54 | Aglaiol | Aglaia odorata | Plant | Terpene | Duong 2006 | - | - |
| 55 | (+) Curcudiol | Didiscus flavus | Plant | Terpene | Hertiani 2007 | - | - |
| 56 | Piscidinol A | Phellodendron chinense | Plant | Terpene | Duong 2006 | - | - |
| 57 | Septicine | Tylophora asthmatica | Plant | Alkaloid | Moustafa 2009 | - | - |
| 58 | Ellagic acid | Plants | Plant | Phenol | Bayer 2009 | - | - |
| 59 | Agelasine I | Agelas sp. | Sponge | Alkaloid | Hertiani et al. 2010 | - | - |
| 60 | Agelanesin A | Agelas sp. | Sponge | Alkaloid | Hertiani et al. 2010 | - | - |
| 61 | Agelanesin B | Agelas sp. | Sponge | Alkaloid | Hertiani et al. 2010 | - | - |
| 62 | Agelanin A | Agelas sp. | Sponge | Alkaloid | Hertiani et al. 2010 | - | - |
| 63 | Agelanin B | Agelas sp. | Sponge | Alkaloid | Hertiani et al. 2010 | - | - |
| 64 | (+) Agelasidine-C | Agelas nakamurai | Sponge | Alkaloid | Hertiani et al. 2010 | - | - |
| 65 | Agelongine | Agelas longissima | Sponge | Alkaloid | Hertiani et al. 2010 | - | - |
| 66 | (−)-Ageloxime D | Agelas nakamurai | Sponge | Alkaloid | Hertiani et al. 2010 | - | - |
| 67 | Aldisine | Hymeniacidon aldis | Sponge | Alkaloid | Hertiani 2007 | - | - |
| 68 | Aplysamine-2 | Psammaplysilla purpurea | Sponge | Alkaloid | Hertiani 2007 | - | - |
| 69 | E/Z-aplysinopsin | Verongia spengelii | Sponge | Alkaloid | Hertiani 2007 | - | - |
| 70 | (+)Avarone | Dysidea avara | Sponge | Terpene | Putz 2009 | - | |
| 71 | Bastadin- 4 | Ianthella basta | Sponge | Alkaloid | Ortlepp et al.2007 | - | - |
| 72 | 2 Bromoaldisine | Hymeniacidon aldis | Sponge | Alkaloid | Hertiani 2007 | - | - |
| 73 | 4-(4-Bromo-1H- pyrrole-2- carboxamido) butanoic acid | Agelas nakamurai | Sponge | Alkaloid | Hertiani 2007 | - | - |
| 74 | 4-Bromopyrrole-2- carboxamide | Agelas nakamurai | Sponge | Alkaloid | Hertiani 2007 | - | - |
| 75 | Dienone dimethoxyketal | Pseudoceratina purpura | Sponge | Alkaloid | Fendert 2000 | - | - |
| 76 | Hymenidin | Agelas clathrodes | Sponge | Alkaloid | Supriyono 1997 | - | - |
| 77 | Mauritamide C | Agelas nakamurai | Sponge | Alkaloid | Hertiani et al. 2010 | - | - |
| 78 | Dragonamide C | Lyngbya cf. polychroa | Cyanobacteria | Peptide | Gunasekera et al. 2008 | - | - |
QS inhibition bioassays
A reporter strain Chromobacterium violaceum CV017 was used for screening for QS inhibitors. This biosensor strain produces N-hexanoyl homoserine lactone, which induces production of the purple pigment violacein via the AHL receptor CviR (Chernin et al. 1998). Methanol solutions of the compounds were added into wells of microtiter plates (Nunc, Denmark), solvents were evaporated and extracts were re-dissolved in 3μl of dimethyl sulfoxide (DMSO). DMSO in empty cells was used as a control. Experiments were conducted according to Dobretsov et al. (2010). Briefly, bacterial cells from overnight culture of CV017 were centrifuged and washed with sterile distilled water. Five ml of soft LB agar (Difco) were mixed with 500μl of washed culture of CV017, and 100μl of this mixture were applied to each well. The plates were incubated overnight at 30 °C. A reduction in violacein production was compared to the control treatments visually. The bioassays were repeated three times and the mean minimum inhibitory concentration (MIC) in μM was calculated.
A toxicity assay was performed according to (Dobretsov et al. 2010) in order to test the effect of compounds on the growth of the reporter strain C. violaceum CV017. Briefly, solutions of compounds in DMSO were applied onto glass fiber disks (diameter=1 cm) and these disks were placed onto LB agar (Difco, USA) inoculated with C. violaceum CV017. Growth inhibition around the disk corresponds to antibacterial activity of the compound against the reporter strains. This experiment was repeated 3 times. These results were expressed as a mean minimal amount of compound in moles that inhibit growth of the reporter. DMSO was used as a control.
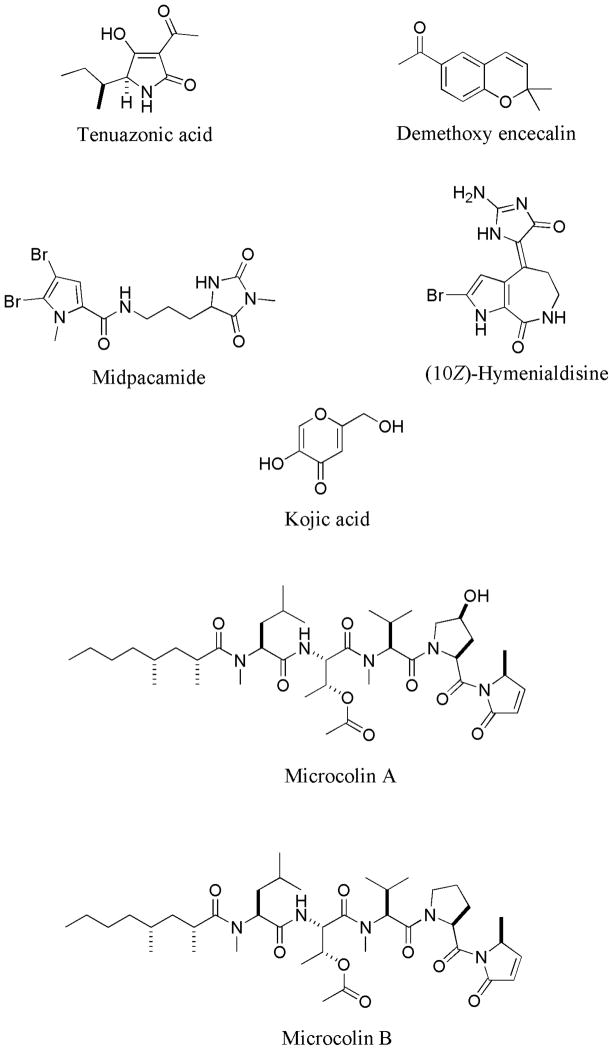
In order to further investigate QS inhibitory properties, the most active QS inhibitors were selected. Since some of these inhibitors were isolated in low quantity, only demethoxy encecalin, tenuazonic acid, midpacamide, hymenialdisin and microcolins A and B (Fig. 1, Table 1) were used in this study. Additionally, we tested QS inhibitory properties of kojic acid – a compound that was used for the mesocosm experiment (see below). Several bioassays were performed using E. coli-based reporters. Before the bioassays all compounds were re-dissolved in DMSO. Midpacamide was tested at 0.4 – 46 μM, tenuazonic acid was assayed at 0.1– 102 μM, demethoxy encecalin - at 0.04– 6.6μM, hymenialdisin - at 0.007– 15μM, microcolins A and B - at 0.015–150 μM and kojic acid - at 0.4–330 μM. Possible toxic effects of compounds on metabolic activity or luminescence of the reporters were tested using a control construct containing a pTIM2442 plasmid in E. coli DH5α (Alagely et al. 2011), in which the lux cassette is controlled by a constitutive phage lambda promoter. Compounds were tested at the maximal inhibitory concentrations. Midpacamide was tested at 46 μM, tenuazonic acid was tested at 102 μM, demethoxy encecalin was tested at 6.6 μM, hymenialdisin was tested at 15 μM, microcolins A and B - at 150 μM and kojic acid - at 330 μM. This experiment was performed with 8 replicates. For the LasR-based bioassay (Pseudomonas aeruginosa LasR/ LasI QS system), we used the LasR-based reporter (pSB1075) (Winson et al. 1998). It emits light in response to AHLs with long (>C10) acyl side chains. We performed direct and indirect bioassays with non-toxic compounds according to Alagely et al. (2011). In the direct bioassays, the reporter E. coli pSB1075 was exposed to the compounds dissolved in DMSO; in indirect bioassays, N-3-oxo-dodecanoyl-L-homeserine lactone (3-oxo-C12-HSL) (final concentration of 2μM) was also added in order to stimulate QS for this reporter. This experiment was performed with 8 replicates. Additionally, we tested the effect of non-toxic compounds on QS in a LuxR-based reporter E. coli pSB401 (Winson et al. 1998) that contained the LuxR PluxI-luxCDABE transcriptional fusion which emits light in response to AHLs with medium (C6–10) acyl side chains. In the direct bioassays, the reporter E. coli pSB401 was exposed to the compounds of interest dissolved in DMSO. In indirect bioassay, N-3-oxo-hexanoyl-L-homeserine lactone (3-oxo-C6-HSL) (final concentration of 10 μM) was also added in order to stimulate QS of pSB401. Direct and indirect experiments were performed with 8 replicates each. Since the reporters pSB1075 and pSB401 could give variable counts per second (CPS) for a number of technical and biological reasons (see Alagely et al. 2011), two DMSO controls - positive (reporters with AHLs) and negative (reporters without AHLs) - were included. All concentrations of tested compounds were tested together with the same positive and negative controls and the experiments were replicated 8 times. For all bioassays, compounds were added to the wells of a black microtiter plate and serially diluted. Reporter suspensions (in LB soft agar) were thoroughly mixed with 3% DMSO solutions of compounds. Luminescence and optical density of the reporter suspensions (OD595) were measured every hour using a multimode microtiter plate reader Victor-3 (Perkin Elmer). The data obtained in indirect bioassays are presented as “relative bioluminescence” in order to take into account the population density of the reporters. To calculate relative bioluminescence (RB), we use the following formula:
Figure 1.
Structures of tenuazonic acid, demethoxy encecalin, midpacamide, hymenialdisin, microcolins A and B and kojic acid.
Where Bs is bioluminescence of each sample measured in CPS, and OD595 is optical density of the reporter culture measured at 595 nm. The differences between the treatments and the positive control were compared by ANOVA followed by a Dunnet test (Zar 1996).
Mesocosm experiment
Since several grams of QS inhibitor were required for the mesocosm experiment, we selected kojic acid - a QS inhibitor discovered within this study – which is commercially available. Kojic acid was dissolved in unfiltered seawater from the Marina Bandar Rawdha (Muscat, Oman) to give 1 mM and 330 μM final concentrations. Three 3L sterile plastic containers were filled with 1L of the Kojic acid solutions. One sterile microscope slide (size 25 × 75mm) was immersed horizontally in each container. Sterile glass slides placed into 1L of unfiltered seawater from the Marina Bandar Rawdha were used as a control. Each treatment was replicated 3 times and the experiment was conducted 2 times. Each experiment was analyzed separately. Containers with slides were kept under continuous illumination (light intensity 2500 lux) in controlled conditions (temperature =25°C) for 7 days. At the end of experiment, slides were taken out and fouling was fixed with 3% formaldehyde solution in seawater. The slides were stained with the DNA-binding fluorochrome 4,6-diamidino-2-phenylindole (DAPI, Fluka Chemie AG, Switzerland) solution (0.5 μg ml−1). The number of bacteria in 10 randomly selected fields of view was counted under an epifluorescence microscope (Axiophot, Zeiss, Germany; magnification 1000x; λEx = 359 nm, λ Em = 441 nm). The number of diatoms in 10 randomly selected fields of view was counted under a microscope (Nikon Eclipse, USA; magnification 400x). For counting bacteria and diatoms tables of random x and y coordinates were generated using MS Excel program and these were used to select random field of views. Treatments were coded; codes were masked prior to the scoring of the treatments. Densities of bacteria and diatoms were log-transformed to normalize the data. The normality assumption was verified with the Shapiro-Wilk test (Zar 1996). Differences in densities of microorganisms between the treatments and the control were compared by ANOVA followed by a Dunnet test (Zar 1996).
Results
QS reporter inhibition bioassays
All tested compounds (Table 1) can be separated into four groups according to their bioactivity. The first group contains compounds that only inhibited QS of C. violaceum CV017. The second group includes compounds that inhibited QS of C. violaceum CV017 and had some antibiotic properties. The third group represents compounds that only inhibited growth of CV017. The fourth group includes compounds that did not have any bioactive properties in our bioassays. A high proportion (51%) of the natural products did not show any activity. The proportion of compounds that only inhibited QS of C. violaceum CV017 without toxicity was the second highest (24%). Twenty percent of compounds inhibited QS of CV017 but had some antibiotic properties. Only a few compounds had only antibacterial activity. Compounds from all tested groups of organisms exhibited some QS inhibitory activity. All major groups of investigated natural products demonstrated some QS inhibitory properties (Table 1).
In the C. violaceum CV017 bioassay, QS inhibitory concentrations of tested compounds varied from 3.92 μM to 517 μM (Table 1). Many of the tested natural products had minimum inhibitory concentrations below 100 μM. Demethoxy encecalin from the plant Baccharis cassinaefolia was the most effective QS inhibitor in this investigation.
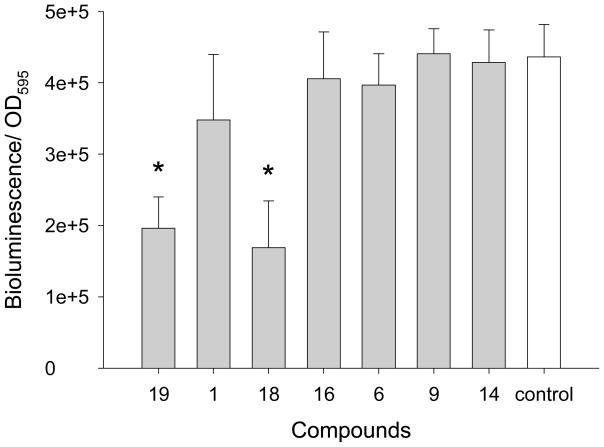
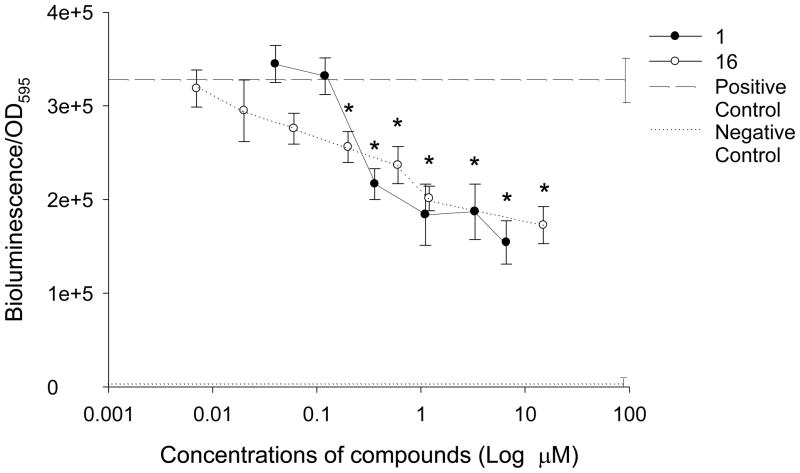
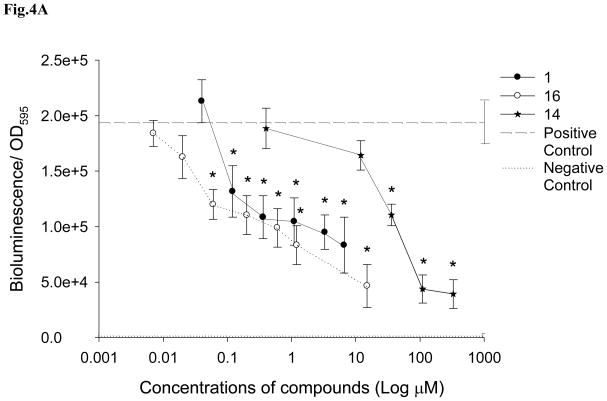
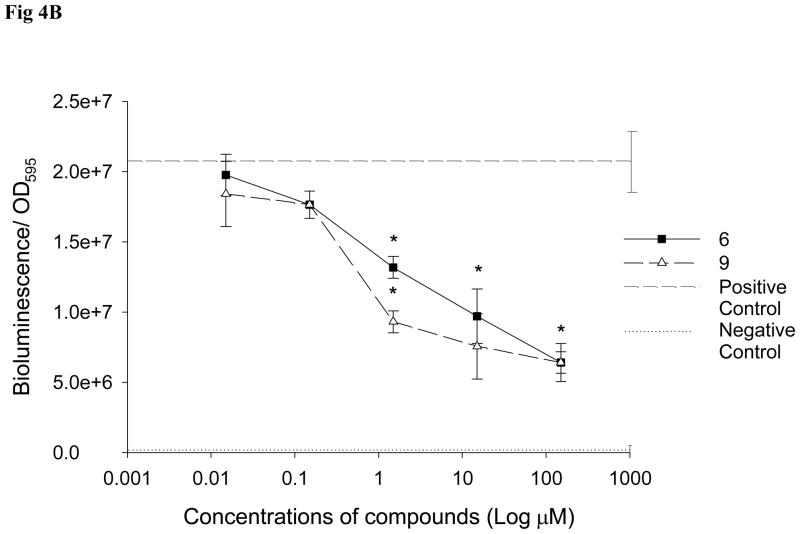
QS inhibitory properties of selected compounds (demethoxy encecalin, tenuazonic acid, midpacamide, hymenialdisin, microcolins A and B and kojic acid) (Fig. 1) were further investigated in LasR based and LuxR-based bioassays. Prior to the tests, possible toxic effects of compounds on metabolic activity or luminescence of the reporters were evaluated with the E. coli pTIM2442 reporter. This constitutively luminescent reporter demonstrated that both midpacamide and tenuazonic acid significantly (ANOVA, Dunnet test, p<0.05) inhibited luminescence of the reporter in the absence of AHLs at the maximal inhibitory concentrations (Fig. 2), suggesting that they were either toxic or inhibited luminescence either directly (i.e. by affecting the luciferase enzyme) or indirectly (by affecting metabolism). This constitutive reporter, when used in conjunction with the toxicity assays, allowed us to eliminate compounds that were both generally toxic and those that inhibited the bioassay. Therefore, compounds that were either toxic or otherwise negatively affected the pTIM2442 reporter were not used for further studies. Demethoxy encecalin, hymenialdisin, microcolins A and B and kojic acid were not toxic and did not interfere with luminescence of E. coli pTIM2442. Bioactivity of these compounds was further studied. Demethoxy encecalin and microcolins A and B, hymenialdisin and kojic acid did not affect luminescence of pSB1075 in the direct experiments (data not shown). In the indirect bioassay, both demethoxy encecalin and hymenialdisin at concentrations above 0.36μM and above 0.2μM, correspondingly, significantly reduced QS dependent luminescence of the reporter E. coli pSB1075 induced by 3oxo-C12-HSL (Fig. 3). Microcolin A, microcolin B and kojic acid did not significantly (ANOVA, Dunnet test, p<0.05) inhibit QS dependent luminescence of the reporter E. coli pSB1075 at the tested concentrations. Background relative bioluminescence of pSB1075 without 3oxo-C12-HSL (negative control) was consistently under 2800 CPS, and bioluminescence of this reporter was always below 500 CPS. None of the tested compounds induced luminescence of the reporter E. coli pSB401in the direct experiments (data not shown), suggesting that none was capable of stimulating QS responses in this reporter based on the LuxR system of Vibrio fischeri. Both hymenialdisin and demethoxy encecalin at concentrations above 0.06μM and 0.12μM, correspondingly, significantly (ANOVA, Dunnet test, p<0.05) reduced QS dependent luminescence of the reporter E. coli pSB401 induced by 3-oxo-C6-HSL (Fig. 4A). Kojic acid inhibited QS dependent luminescence of the reporter induced by 3-oxo-C6-HSL only at concentrations above 36μM (Fig. 4A). Microcolins A and B significantly (ANOVA, Dunnet test, p<0.05) reduced QS dependent luminescence of the reporter induced by 3-oxo-C6-HSL at concentrations above 1.5μM (Fig. 4B). Background relative bioluminescence of pSB401 without 3-oxo-C6-HSL (negative control) was consistently under 1500 CPS, and bioluminescence of this reporter was always below 114 CPS.
Figure 2.
The effect of tenuazonic acid (#19), demethoxy encecalin (#1), midpacamide (#18), hymenialdisin (#16) and microcolins A (#6) and B (#9) as well as kojic acid (#14) on bioluminescence of E. coli DH5α containing a pTIM2442 plasmid. The data are shown as mean + SD relative bioluminescence (bioluminescence/OD595) of the reporter with added compounds in dimethyl sulfoxide (DMSO) (n=8) or without (only DMSO, control, n=8). Toxic compounds highlighted by asterisks have significantly (Dunnet, p<0.05) lower relative bioluminescence compared to the control. Midpacamide was tested at 46 μM, tenuazonic acid was tested at 102 μM, demethoxy encecalin was tested at 6.6 μM, hymenialdisin was tested at 15 μM, microcolins A and B - at 150 μM and kojic acid - at 330 μM. Measurements were taken every 1 h but results obtained at 4h are shown.
Figure 3.
The effect of demethoxy encecalin (#1) and hymenialdisin (#16) on QS dependent bioluminescence of the LasR-based reporter E. coli pSB1075 induced by 3oxo-C12-HSL (final concentration of 2 μM). Data show mean + SD relative bioluminescence (bioluminescence/OD595) of the reporter with added compounds (n= 8). Compound concentrations that significantly (Dunnet, p<0.05) inhibited QS of the reporter are marked with asterisks. Dash line - positive control (reporters with AHLs) (n= 8) and dotted line - negative control (reporters without AHLs) (n= 8). All treatments and controls contained dimethyl sulfoxide (DMSO). Measurements were taken every 1 h but results obtained at 4h are shown.
Figure 4.
The effect of A: demethoxy encecalin (#1), hymenialdisin (#16) and kojic acid (#14), and B: microcolin A (#6) and microcolin B (#9) on QS dependent bioluminescence of the LuxR-based reporter E. coli pSB401 induced by 3-oxo-C6-HSL (final concentration of 10 μM). Data show relative mean + SD bioluminescence (bioluminescence/OD595) of the reporter with added compounds (n= 8). Compound concentrations that significantly (Dunnet, p<0.05) inhibited QS of the reporter are marked with asterisks. Dashed line -positive control (reporters with AHLs) (n= 8) and dotted line - negative control (reporters without AHLs) (n= 8). All treatments and controls contained dimethyl sulfoxide (DMSO). Measurements were taken every 1 h but results obtained at 4h are shown.
Mesocosm experiment
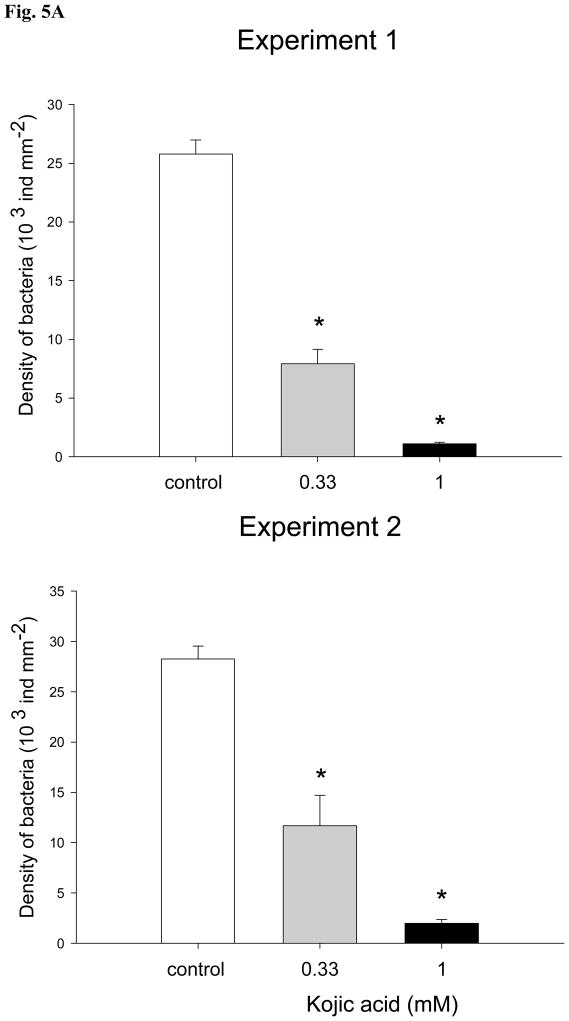
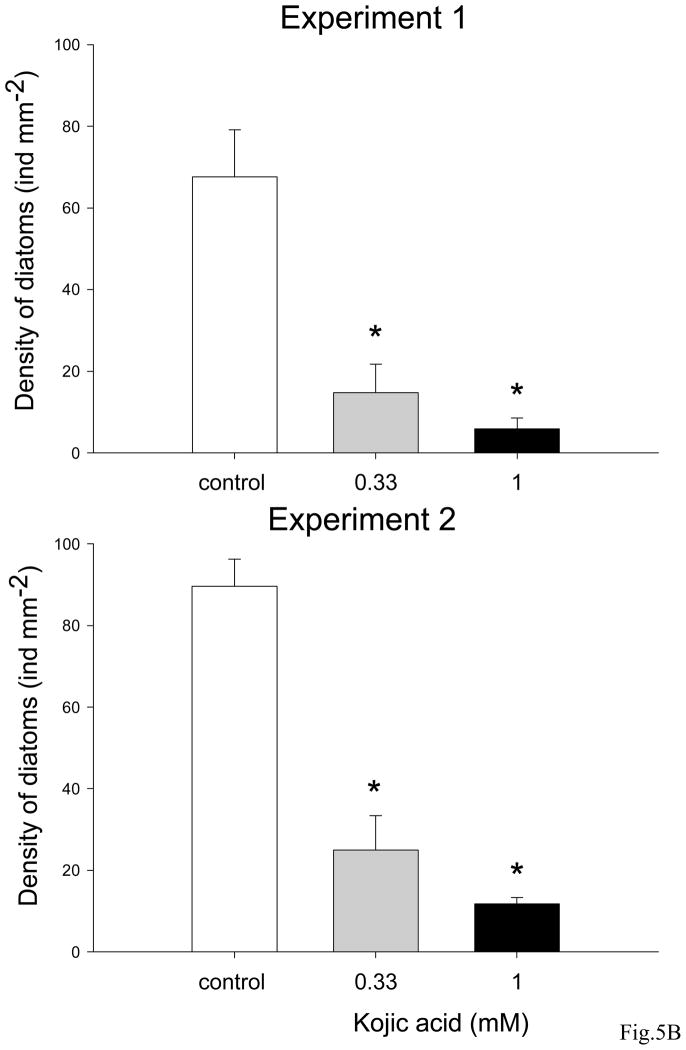
Kojic acid reduced formation of microbial communities on glass slides (Fig. 5A and B). In both experiments bacterial densities in the presence of 330 μM and 1 mM of kojic acid were significantly reduced (ANOVA, Dunnet test, p<0.05) in comparison with ones on the control slides (Fig. 5A). Similarly, significantly lower (ANOVA, Dunnet test, p<0.05) densities of diatoms were observed in biofilms developed with kojic acid solutions in two repeated experiments (Fig. 5B).
Figure 5.
Mean densities of A: bacteria (ind mm2) and B: diatoms (ind mm2) on glass slides exposed to 330 μM and 1 mM kojic acid solutions prepared with unfiltered seawater from the Marina Bandar Rawdha (Muscat, Oman). Three glass slides were incubated in containers filled with unfiltered seawater for 7 days to allow development of biofouling. Sterile glass slides placed into unfiltered seawater without addition of kojic acid were used as a control. Bars are the means of 3 replicates + SD. Data that are significantly different from the control (ANOVA, Dunnet test: p<0.05) are indicated by an asterisk above the bars. Data are from 2 independent experiments.
Discussion
In this study, 78 different natural products from marine organisms (sponges, algae, fungi, tunicates and cyanobacteria) and terrestrial plants were screened for their ability to inhibit bacterial QS. Marine natural products have rarely been screened for QS inhibitory compounds (reviewed by Dobretsov et al. 2009; Ni et al. 2009) in comparison with synthetic compounds (Muh et al. 2006; Soulere et al. 2010). Our results demonstrate that a large proportion of tested compounds (51%) did not interfere with bacterial QS and only 24% of compounds inhibited QS of C. violaceum CV017. Inhibition of the C. violaceum reporter could be due to the compound’s ability to inhibit QS in the reporter (either directly – by blocking AHL perception, or indirectly by affecting, for example, the transcription of the AHL receptor gene or the stability of the QS transcript). Inhibition of the C. violaceum reporter could also be due to the direct or indirect inhibition of the synthesis of the tryptophan derivative violacein, a purple pigment which serves as a read-out for this bioassay. Some of the tested natural products inhibited the C. violaceum reporter at concentrations above 100 μM. These concentrations are extremely high, and are unlikely to occur in the marine environment (Hmelo and Van Mooy 2009). Therefore, it is likely that these compounds are not truly functioning as QS inhibitors in nature. This fact may explain the relatively high rate of finding QS inhibitors in this study and in other investigations (Skindersoe et al. 2008b).
Some of the C. violaceum CV017 QS inhibitors found in this study have antibiotic properties. Due to the different application method, QS inhibitory concentrations of compounds cannot be directly compared to the amount of compounds used for the toxicity bioassay. Further, toxicity of some QS inhibitors was determined by the pTIM2442 reporter. This suggested that indeed some QS inhibitors demonstrate some antibiotic activity. This fact is not novel (Skindersoe et al. 2008a). Previously, 12 antibiotics at sub-lethal concentrations were screened for their QS inhibitory activity (Skindersoe et al. 2008a). The antibiotics azithromycin, ceftazidime, and ciprofloxacin at concentrations of 0.1–11 μM inhibited QS of a LuxR based reporter based on the QS circuit of Vibrio fischeri. In a previous study, the cyanobacterial antibiotic malyngolide produced by the cyanobacterium Lyngbya majuscula inhibited QS at concentrations ranging from 3.57 μM to 57 μM (Dobretsov et al. 2010). The mechanism of this QS inhibition remains unknown but it was suggested that antibiotics can change bacterial membrane permeability, thus affecting flux of QS signals (Skindersoe et al. 2008a).
QS activity of some compounds similar to those tested in this study have been investigated earlier. It has been shown that malyngamide C and 8-epi-malyngamide C inhibited luminescence of Las-R based E. coli reporter induced with 3-oxo-C12-HSL at concentrations of 10μM -1mM (Kwan et al. 2010), likely by inhibiting transcription of the lasR gene. In our study, malyngamides A and B, which are structurally different from malyngamide C, inhibited QS-dependent violacein production by C. violaceum CV017 at similar concentrations. Extracts of the plants Moringa oleifera and Acacia nilotica that contained gallic and ellagic acids had anti-QS potential (Singh et al. 2009a; Singh et al. 2009b). Epigallocatechin gallate (salt of gallic acid) and ellagic acid inhibited Las-R based and Lux-R based QS at concentrations of 15–30μM (Huber et al. 2003). In our study, only gallic but not ellagic acid inhibited QS of C. violaceum CV017 at 64.7μM. Different reporters used in both studies likely explain such differences in the results.
Because the inhibition of the Chromobacterium violaceum reporter is likely to identify a number of bioactive substances, not all of which are inhibitory to the QS regulatory cascades, the activity of the compounds was tested using semi-synthetic LuxR and LasR reporters based on E. coli. These reporters contain AHL receptor genes on a multi-copy plasmid (Winson et al. 1998; Alagely et al. 2011). Unfortunately, we were unable to investigate the effect of all promising QS inhibitors in this experiment because most of the natural products tested in this study were available at low quantities. Midpacamide and tenuazonic acid were toxic to the reporters at the tested concentrations. Demethoxy encecalin and hymenialdisin interfered with induced luminescence of LasR and LuxR reporters, while kojic acid and microcolin A and B only interfered with LuxR reporters. Inhibitory concentrations of hymenialdisin, demethoxy encecalin, kojic acid and microcolins A and B were comparable with ones of natural furanones (Maximilien et al. 1998; Martinelli et al. 2004), ellagic acid (Huber et al. 2003), malyngolide (Dobretsov et al. 2010) and manoalide (Skindersoe et al. 2008b).
Usually, QS inhibitors have been tested in the laboratory against monocultures of pathogens (see review by Dobretsov et al. 2009) or environmental isolates (Maximilien et al. 1998). Only a few studies investigated the effect of quorum sensing inhibitors, such as furanones, on multispecies of bacteria in the laboratory (Dobretsov et al. 2007) and over 2h in the field (Maximilien et al. 1998). In the latter study, crude extracts from the red alga Delisea pulchra and pure furanone-1 and -2 at the concentration 1 μg cm−2 applied to Perspex disks or glass Petridishes inhibited attachment of bacteria to less than 20% of control numbers. In this study, we tested antifouling performance of the QS inhibitor - kojic acid - against environmental microbes. This compound was selected because of its QS inhibitory activity in the experiments and its commercial availability that ensured sufficient quantities of the compound. In a preliminary field experiment (data not shown) kojic acid incorporated into a non-toxic paint matrix at a concentration of 0.5% significantly reduced densities of bacteria and diatoms growing on the paint and decreased macro-fouling over 1 month. Interpretation of these results poses unique technical and scientific challenges that make it difficult to attribute inhibition of micro- and macro-fouling solely to QS inhibitory activity of kojic acid. Therefore, a controlled mesocosm experiment with kojic acid at non-toxic (330 μM) and 3 fold higher concentrations was conducted. In this experiment, which was repeated two times, kojic acid at non-toxic concentrations inhibited 2.5 – 3.2 fold bacterial density and 4.7 – 3.6 fold diatom density in biofilms on glass slides.
How did kojic acid affect micro-fouling in our experiment? It is possible that kojic acid inhibited QS of bacteria and this led to low bacterial attachment/recruitment and biofilm formation. This was supported by the data showing that kojic acid at tested concentrations inhibited QS of the reporters and was not toxic to the reporters or the diatom Amphora coffeaeformis (data not presented). Kojic acid is widely used as a food additive for preventing enzymatic browning, and in cosmetic preparations as a skin-lightening or bleaching agent because of its tyrosinase inhibitory action (Cabanes et al. 1994; Burdock et al. 2001). Unfortunately, direct measurements of AHLs in the biofilms treated and not treated with kojic acid are technically challenging and would not help proven this hypothesis. Previous studies suggested that QS inhibitors, such as furanones, affect microbial composition and densities of certain groups of bacteria (Dobretsov et al. 2007), shifting the composition of microbial communities from being dominated by Gram-negative bacteria to those dominated by Gram-positive species (Maximilien et al. 1998; Kjelleberg et al. 2001). In this case, a decrease in AHL concentrations might reflect changes in microbial composition. Changes in bacterial species composition and chemical compound production could possibly result in changes in diatom communities, as presence of particular bacteria affect recruitment of diatoms (Gawne et al. 1998; Wigglesworth-Cooksey and Cooksey 2005). Alternatively, there is a possibility that kojic acid reduced formation of microbial communities by means other than QS inhibition. For example, kojic acid could have a toxic effect on some marine bacteria and diatoms more sensitive to this acid than the tested reporters. It is possible that kojic acid could inhibit other regulatory cascades that affect biofilm formation. Brominated furanones are known to inhibit multiple regulatory pathways leading to biofilm formation even without interference with QS (Janssens et al. 2008). Overall, results of this experiment demonstrate a high antifouling potential of kojic acid.
In conclusion, results of this study suggest that screening of natural products is a promising way to find novel QS inhibitors. Natural products with QS inhibitory properties can control formation of microbial communities and potentially can be used in the future for antifouling applications.
Acknowledgments
The authors would like to thank four anonymous reviewers who provided helpful suggestions that significantly improved this manuscript. The authors would also like to thank Ms Badruya Al-Ghafri for her technical assistance. The work of SD was supported by the George E. Burch Fellowship in Theoretical Medicine and Affiliated Sciences at the Smithsonian Institution (USA), the SQU internal grant IG/AGR/FISH/09/03 and the HM Fund for Strategic Research SR/AGR/FISH/10/01. MT’s contribution was supported by funding from Protect Our Reefs Foundation/Mote Marine Laboratory under CRIS project FLA-SWS-04591. P.P. wishes to thank BMBF for support. VJP and SG thank the National Institutes of Health, NIGMS grant P41GM086210, for support. This is contribution No. 861 of the Smithsonian Marine Station at Fort Pierce.
References
- Alagely A, Rajamani S, Teplitski M. Luminescent reporters and their applications for the characterization of signals and signal-mimics that alter LasR-mediated quorum sensing. Methods Mol Biol. 2011;692:113–130. doi: 10.1007/978-1-60761-971-0_9. [DOI] [PubMed] [Google Scholar]
- Aly AH, Edrada-Ebel RA, Indriani ID, Wray V, Müller WEG, Totzke F, Zirrgiebel U, Schächtele C, Kubbutat MHG, Lin WH, Proksch P, Ebel R. Cytotoxic metabolites from the fungal endophytic Alternaria sp. and their subsequent detection in its host plant Polygonum senegalense. J Nat Prod. 2008;71:972–980. doi: 10.1021/np070447m. [DOI] [PubMed] [Google Scholar]
- Antunes LC, Ferreira RB, Buckner MM, Finlay BB. Quorum sensing in bacterial virulence. Microbiology. 2010;156:2271–2282. doi: 10.1099/mic.0.038794-0. [DOI] [PubMed] [Google Scholar]
- Bayer M. PhD dissertation. Düsseldorf University; 2009. Naturstoffe aus chinesischen Mangrovenpflanzen - Untersuchung der insektiziden, tumorhemmenden und vor UV-Licht schützenden Aktivitäten. [Google Scholar]
- Boyer M, Wisniewski-Dye F. Cell-cell signalling in bacteria: not simply a matter of quorum. FEMS Microbiol Ecol. 2009;70:1–19. doi: 10.1111/j.1574-6941.2009.00745.x. [DOI] [PubMed] [Google Scholar]
- Burdock GA, Soni MG, Carabin IG. Evaluation of health aspects of kojic acid in food. Regul Toxicol Pharmacol. 2001;33:80–101. doi: 10.1006/rtph.2000.1442. [DOI] [PubMed] [Google Scholar]
- Cabanes J, Chazarra S, Garcia-Carmona F. Kojic acid, a cosmetic skin whitening agent, is a slow-binding inhibitor of catecholase activity of tyrosinase. J Pharm Pharmacol. 1994;46:982–985. doi: 10.1111/j.2042-7158.1994.tb03253.x. [DOI] [PubMed] [Google Scholar]
- Cardellina JH, Dalietos D, Marner F-J, Mynderse JS, Moore RE. (−)-trans-7(S)-Methoxytetradec-4-enoic acid and related amides from the marine cyanophyte Lyngbya majuscula. Phytochemistry. 1978;17:2091–2095. [Google Scholar]
- Cardellina JH, Marner F-J, Moore RE. Malyngamide A, a novel chlorinated metabolite of the marine cyanophyte Lyngbya majuscula. J Am Chem Soc. 1979;101:240–242. [Google Scholar]
- Cassé F, Swain GW. The development of microfouling on four commercial antifouling coatings under static and dynamic immersion. International Biodeterioration & Biodegradation. 2006;57:179–185. [Google Scholar]
- Chernin LS, Winson MK, Thompson JM, Haran S, Bycroft BW, Chet I, Williams P, Stewart GSAB. Chitinolytic activity in Chromobacterium violaceum: substrate analysis and regulation by quorum sensing. J Bacteriol. 1998;180:4435–4441. doi: 10.1128/jb.180.17.4435-4441.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary S, Schmidt-Dannert C. Applications of quorum sensing in biotechnology. Appl Microbiol Biotechnol. 2010;86:1267–1279. doi: 10.1007/s00253-010-2521-7. [DOI] [PubMed] [Google Scholar]
- Debbab A. PhD thesis. Université Mohammed V; 2007. Nouveaux Composés Naturels Extraits des Champignons Endophytiques isolés de Plantes Medicinales Marocaines Caractérisation Chimique et Anticancereuse. http://toubkal.imist.ma/handle/123456789/1817. [Google Scholar]
- Defoirdt T, Boon N, Bossier P, Verstraete W. Disruption of bacterial quorum sensing: An unexplored strategy to fight infections in aquaculture. Aquaculture. 2004;240:69–88. [Google Scholar]
- Dickschat JS. Quorum sensing and bacterial biofilms. Nat Prod Rep. 2010;27:343–369. doi: 10.1039/b804469b. [DOI] [PubMed] [Google Scholar]
- Dobretsov S, Dahms HU, Yili H, Wahl M, Qian PY. The effect of quorum-sensing blockers on the formation of marine microbial communities and larval attachment. FEMS Microbiol Ecol. 2007;60:177–188. doi: 10.1111/j.1574-6941.2007.00285.x. [DOI] [PubMed] [Google Scholar]
- Dobretsov S, Teplitski M, Paul V. Mini-review: quorum sensing in the marine environment and its relationship to biofouling. Biofouling. 2009;25:413–427. doi: 10.1080/08927010902853516. [DOI] [PubMed] [Google Scholar]
- Dobretsov S, Teplitski M, Alagely A, Gunasekera SP, Paul VJ. Malyngolide from the cyanobacterium Lyngbya majuscula interferes with quorum sensing circuitry. Environmental Microbiology Reports. 2010;2:739–744. doi: 10.1111/j.1758-2229.2010.00169.x. [DOI] [PubMed] [Google Scholar]
- Duong NT. PhD dissertation. Düsseldorf University; 2006. Isolation and structure elucidation of insecticidal secondary metabolites from Aglaia species collected in Vietnam. http://docserv.uni-duesseldorf.de/servlets/DerivateServlet/Derivate-3467. [Google Scholar]
- Ebel R, Brenzinger M, Kunze A, Gross HJ, Proksch P. Wound activation of protoxins in the marine sponge Aplysina aerophoba. J Chem Ecol. 1997;23:1451–1462. [Google Scholar]
- Eder C, Schupp P, Proksch P, Wray V, Steube K, Müller C, van Soest RWM. Bioactive pyridoacridine alkaloids from the Micronesian sponge Oceanapia sp. J Nat Prod. 1998;61:301–305. doi: 10.1021/np9702704. [DOI] [PubMed] [Google Scholar]
- Edrada RA. PhD dissertation. Düsseldorf University; 1998. Isolation and structure elucidation of bioactive secondary metabolites from Philippine marine sponges and soft corals. http://www.pharmazie.uni-duesseldorf.de/Institute/pharm_bio/arbeitskreise/diss. [Google Scholar]
- Fendert T. PhD dissertation. Würzburg University; 2000. Charakterisierung der enzymatischen Abwehrreaktion in Schwämmen der Gattung Aplysina und Isolierung von Bromotyrosinalkaloiden aus Aplysina insularis. http://www.opus-bayern.de/uni-wuerzburg/volltexte/2002/116/ [Google Scholar]
- Gawne B, Wang Y, Hoagland KD, Gretz MR. Role of bacteria and bacterial exopolymer in the attachment of Achnanthes longipes (Bacillariophyceae) Biofouling. 1998;13:137–156. [Google Scholar]
- Goecke F, Labes A, Wiese J, Imhoff JF. Chemical interactions between marine macroalgae and bacteria. Mar Ecol Progr Ser. 2010;409:267–300. [Google Scholar]
- Gunasekera SP, Ross C, Paul V, Mathew S, Luesch H. Dragonamides C and D, two linear lipopeptides from the marine cyanobacterium brown Lyngbya polychoroa. J Nat Prod. 2008;71:887–890. doi: 10.1021/np0706769. [DOI] [PubMed] [Google Scholar]
- Handayani D. PhD dissertation. Düsseldorf University; 1998. Isolierung und Strukturaufklärung biologisch aktiver Naturstoffe aus marinen Invertebraten Südostasiens. http://docserv.uni-duesseldorf.de/servlets/DocumentServlet?id=3153. [Google Scholar]
- Hassan A. PhD dissertation. Düsseldorf University; 2007. Novel natural products from endophytic fungi of Egyptian medicinal plants: Chemical and biological characterization. http://docserv.uni-duesseldorf.de/servlets/DerivateServlet/Derivate-5325/AmalHassan_2007.pdf. [Google Scholar]
- Hertiani T. PhD dissertation. Dusseldorf University; 2007. Isolation and structure elucidation of bioactive secondary metabolites from Indonesian marine sponges. http://docserv.uni-duesseldorf.de/servlets/DerivateServlet/Derivate-5651/Triana%20Hertiani-Dissertation.pdf. [Google Scholar]
- Hertiani T, Edrada-Ebel RA, Ortlepp S, van Soest RWM, de Voogd NJ, Wray V, Hentschel U, Kozytska S, Müller WEG, Proksch P. From anti-fouling to biofilm inhibition: new cytotoxic secondary metabolites from two Indonesian Agelas sponges. Bioorganic Medicinal Chemistry. 2010;18:1297–1311. doi: 10.1016/j.bmc.2009.12.028. [DOI] [PubMed] [Google Scholar]
- Hiort J, Maksimenka K, Reichert M, Lin WH, Wray V, Steube K, Schaumann K, Proksch P, Ebel R, Bringmann C. New natural products from the sponge-derived fungus Asperigillus niger. J Nat Prod. 2004;67:1532–1543. doi: 10.1021/np030551d. [DOI] [PubMed] [Google Scholar]
- Hmelo L, Van Mooy BAS. Kinetic constraints on acylated homoserine lactone-based quorum sensing in marine environments. Aquatic Microb Ecol. 2009;54:127–133. [Google Scholar]
- Huber B, Eberl L, Feucht W, Polster J. Influence of polyphenols on bacterial biofilm formation and quorum-sensing. Z Naturforsch C. 2003;58:879–884. doi: 10.1515/znc-2003-11-1224. [DOI] [PubMed] [Google Scholar]
- Indriani ID. PhD dissertation. Düsseldorf University; 2008. Biodiversity of marine-derived fungi and identification of their metabolites. http://docserv.uni-duesseldorf.de/servlets/DocumentServlet?id=7333. [Google Scholar]
- Irie Y, Parsek MR. Quorum sensing and microbial biofilms. Curr Top Microbiol Immunol. 2008;322:67–84. doi: 10.1007/978-3-540-75418-3_4. [DOI] [PubMed] [Google Scholar]
- Janssens JCA, Steenackers H, Robijns S, Gellens E, Levin J, Zhao H, Hermans K, De Coster D, Verhoeven TL, Marchal K, Vanderleyden J, De Vos DE, De Keersmaecker SCJ. Brominated furanones inhibit biofilm formation by Salmonella enterica serovar typhimurium. Appl Envir Microb. 2008;74:6639–6648. doi: 10.1128/AEM.01262-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehn FE, Longley RE, Reed JK. Microcolin A and B, new imminosuppressive peptides from the blue-green algae Lyngbya majuscula. J Nat Prod. 1992;55:613–619. doi: 10.1021/np50083a009. [DOI] [PubMed] [Google Scholar]
- Kjer J. PhD dissertation. Düsseldorf University; 2010. New natural products from endophytic fungi from mangrove plants - structure elucidation and biological screening. http://docserv.uni-duesseldorf.de/servlets/DerivateServlet/Derivate-17938/Dissertation_JuliaKjer.pdf. [Google Scholar]
- Kunze A. PhD dissertation. Düsseldorf University; 1995. Chromene aus Asteraceen als chemischer Fraßschutz gegen herbivore Insekten. http://www.pharmazie.uni-duesseldorf.de/Institute/pharm_bio/arbeitskreise/diss. [Google Scholar]
- Kjelleberg S, Steinberg P, Givskov M, Gram L, Manefield M, De Nys R. Do marine natural products interfere with prokaryotic AHL regulatory systems? Aquat Microb Ecol. 2001;13:85–93. [Google Scholar]
- Kwan JC, Teplitski M, Gunasekera SP, Paul VJ, Luesch H. Isolation and biological evaluation of 8-epi-malyngamide C from the Floridian marine cyanobacterium Lyngbya majuscula. J Nat Prod. 2010;73:463–466. doi: 10.1021/np900614n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manefield M, de Nys R, Kumar N, Read R, Givskov M, Steinberg P, Kjelleberg S. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology. 1999;145:283–291. doi: 10.1099/13500872-145-2-283. [DOI] [PubMed] [Google Scholar]
- Marquez B, Verdier-Pinard P, Hamel E, Gerwick WH. Curacin D, an antimitotic agent from the marine cyanobacterium Lyngbya majuscula. Phytochemistry. 1998;49:2387–2389. doi: 10.1016/s0031-9422(98)00365-3. [DOI] [PubMed] [Google Scholar]
- Martinelli D, Grossmann G, Sequin U, Brandl H, Bachofen R. Effects of natural and chemically synthesized furanones on quorum sensing in Chromobacterium violaceum. BMC Microbiology. 2004;4:25. doi: 10.1186/1471-2180-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximilien R, de Nys R, Holmstrm C, Gram L, Givskov M, Crass K, Kjelleberg S, Steinberg PD. Chemical mediation of bacterial surface colonisation by secondary metabolites from the red alga Delisea pulchra. Aquat Microb Ecol. 1998;15:233–246. [Google Scholar]
- Molino PJ, Campbell E, Wetherbee R. Development of the initial diatom microfouling layer on antifouling and fouling-release surfaces in temperate and tropical Australia. Biofouling. 2009a;25:685–693. doi: 10.1080/08927010903089912. [DOI] [PubMed] [Google Scholar]
- Molino PJ, Childs S, Hubbard EMR, Carey JM, Burgman MA, Wetherbee R. Development of the primary bacterial microfouling layer on antifouling and fouling release coatings in temperate and tropical environments in Eastern Australia. Biofouling. 2009b;25:149–162. doi: 10.1080/08927010802592917. [DOI] [PubMed] [Google Scholar]
- Moustafa E. PhD dissertation. Düsseldorf University; 2009. Insecticidal plant constituents - Isolation, structure elucidation, and biological testing. http://docserv.uni-duesseldorf.de/servlets/DerivateServlet/Derivate-3467/1467.pdf. [Google Scholar]
- Muh U, Schuster M, Heim R, Singh A, Olson ER, Greenberg EP. Novel Pseudomonas aeruginosa quorum-sensing inhibitors identified in an ultra-high-throughput screening. Antimicrobial Agents and Chemotherapy. 2006;50:3674–3679. doi: 10.1128/AAC.00665-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni N, Li M, Wang J, Wang B. Inhibitors and antagonists of bacterial quorum sensing. Medicinal Research Reviews. 2009;29:65–124. doi: 10.1002/med.20145. [DOI] [PubMed] [Google Scholar]
- Ortlepp S, Sjögren M, Dahlström M, Weber H, Ebel R, Edrada R, Thoms C, Shupp P, Bohlin L, Proksch P. Antifouling activity of bromotyrosine-derived sponge metabolites and synthetic analogues. Mar Biotech. 2007;9:776–785. doi: 10.1007/s10126-007-9029-x. [DOI] [PubMed] [Google Scholar]
- Proksch P, Rodriguez E. High performance liquid chromatography of chromenes and benzofurans from the genus Encelia. J Chromatogr. 1982;240:543–546. [Google Scholar]
- Putz A. PhD dissertation. Düsseldorf University; 2009. Secondary metabolites from marine sponges, with focus on the chemical ecology and biochemical characterisation of the stress induced biotransformation of Aplysina alkaloids. http://docserv.uni-duesseldorf.de/servlets/DerivateServlet/Derivate-11835/Annika%20Putz.pdf. [Google Scholar]
- Qian PY, Xu Y, Fusetani N. Natural products as antifouling compounds: recent progress and future perspectives. Biofouling. 2010;26:223–234. doi: 10.1080/08927010903470815. [DOI] [PubMed] [Google Scholar]
- Queiroz KCS, Medeiros VP, Queiroz LS, Abreu LRD, Rocha HAO, Ferreira CV, Juca MB, Aoyama H, Leite EL. Inhibition of reverse transcriptase activity of HIV by polysaccharides of brown algae. Biomed Pharmacother. 2008;62:303–307. doi: 10.1016/j.biopha.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Rusman Y. PhD dissertation. Düsseldorf University; 2006. Isolation of new secondary metabolites from sponge-associated and plant-derived endophytic fungi. http://deposit.ddb.de/cgi-bin/dokserv?idn=983305986&dok_var=d1&dok_ext=pdf&filename=983305986.pdf. [Google Scholar]
- Singh BN, Singh BR, Singh RL, Prakash D, Dhakarey R, Upadhyay G, Singh HB. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem Toxicol. 2009a;47:1109–1116. doi: 10.1016/j.fct.2009.01.034. [DOI] [PubMed] [Google Scholar]
- Singh BN, Singh BR, Singh RL, Prakash D, Sarma BK, Singh HB. Antioxidant and anti-quorum sensing activities of green pod of Acacia nilotica L. Food Chem Toxicol. 2009b;47:778–786. doi: 10.1016/j.fct.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Skindersoe ME, Alhede M, Phipps R, Yang L, Jensen PO, Rasmussen TB, Bjarnsholt T, Tolker-Nielsen T, Hoiby N, Givskov M. Effects of antibiotics on quorum sensing in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2008a;52:3648–3663. doi: 10.1128/AAC.01230-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skindersoe ME, Ettinger-Epstein P, Rasmussen TB, Bjarnsholt T, De Nys R, Givskov M. Quorum sensing antagonism from marine organisms. Mar Biotech. 2008b;10:56–63. doi: 10.1007/s10126-007-9036-y. [DOI] [PubMed] [Google Scholar]
- Soulere L, Sabbah M, Fontaine F, Queneau Y, Doutheau A. LuxR-dependent quorum sensing: computer aided discovery of new inhibitors structurally unrelated to N-acylhomoserine lactones. Bioorg Med Chem Lett. 2010;20:4355–4358. doi: 10.1016/j.bmcl.2010.06.081. [DOI] [PubMed] [Google Scholar]
- Supriyono A, Schwarz B, Wray V, Witte L, Müller WEG, van Soest RWM, Sumaryono W, Proksch P. Bioactive alkaloids from the tropical marine sponge Axinella carteri. Z Naturforsch. 1995;50c:669–674. doi: 10.1515/znc-1995-9-1012. [DOI] [PubMed] [Google Scholar]
- Schultz MP, Bendick JA, Holm ER, Hertel WM. Economic impact of biofouling on a naval surface ship. Biofouling. 2010;27:87–98. doi: 10.1080/08927014.2010.542809. [DOI] [PubMed] [Google Scholar]
- Waters CM, Bassler BL. Quorum sensing: Cell-to-cell communication in bacteria. Annual Review of Cell and Developmental Biology. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- Weber N. PhD dissertation. Düsseldorf University; 2007. Antioxidative Polyphenole chinesischer Leguminosen und weiterer chinesischer Gemüse. http://docserv.uni-duesseldorf.de/servlets/DerivateServlet/Derivate-5676/NadineWeber.pdf. [Google Scholar]
- Wigglesworth-Cooksey B, Cooksey KE. Use of Fluorophore-Conjugated Lectins To Study Cell-Cell Interactions in Model Marine Biofilms. Appl Environ Microbiol. 2005;71:428–435. doi: 10.1128/AEM.71.1.428-435.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PG, Moore RE, Paul VJ. Isolation and structure determination of Lyngbyastatin 3, a Lyngbyastatin 1 homologue from the marine cyanobacterium Lyngbya majuscula. determination of the configuration of the 4-amino-2,2-dimethyl-3-oxopentanoic acid unit in Majusculamide C, Dolastatin 12, Lyngbyastatin 1, and Lyngbyastatin 3 from cyanobacteria. J Nat Prod. 2003;66:1356–1363. doi: 10.1021/np0302145. [DOI] [PubMed] [Google Scholar]
- Winson MK, Swift S, Fish L, Throup JP, Jorgensen F, Chhabra SR, Bycroft BW, Williams P, Stewart GS. Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol Lett. 1998;163:185–192. doi: 10.1111/j.1574-6968.1998.tb13044.x. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Liu Y. Biological control of microbial attachment: a promising alternative for mitigating membrane biofouling. Appl Microbiol Biotechnol. 2010;86:825–837. doi: 10.1007/s00253-010-2463-0. [DOI] [PubMed] [Google Scholar]
- Yebra DM, Kiil S, Weinell CE, Dam-Johansen K. Effects of marine microbial biofilms on the biocide release rate from antifouling paints - A model-based analysis. Progress in Organic Coatings. 2006;57:56–66. [Google Scholar]
- Zar JH. Biostatistical analysis. Prentice Hall International, Inc; Upper Saddle River, NJ, USA: 1996. [Google Scholar]