Abstract
The Dominantly Inherited Alzheimer Network (DIAN) is a collaborative effort of international Alzheimer disease (AD) centers that are conducting a multifaceted prospective biomarker study in individuals at-risk for autosomal dominant AD (ADAD). DIAN collects comprehensive information and tissue in accordance with standard protocols from asymptomatic and symptomatic ADAD mutation carriers and their non-carrier family members to determine the pathochronology of clinical, cognitive, neuroimaging, and fluid biomarkers of AD. This article describes the structure, implementation, and underlying principles of DIAN, as well as the demographic features of the initial DIAN cohort.
Keywords: Alzheimer disease, autosomal dominant, biomarkers of Alzheimer disease, PSEN1, PSEN2, APP, amyloid-beta, preclinical Alzheimer disease
Aging populations in developed countries ensure that Alzheimer disease (AD) will reach epidemic proportions unless therapies are developed to cure or prevent it. Unfortunately, to date all “disease-modifying” experimental therapies for AD have failed to demonstrate clinical benefit in individuals with symptomatic AD. [Note: “symptomatic AD” encompasses both mild cognitive impairment due to AD [1] and AD dementia.[2]] One possibility for these failures is that the drugs were administered too late in the course of the AD pathological process. That is, by the time the earliest symptoms of AD appear, substantial (and currently irreversible) synaptic and neuronal loss already has occurred in critical brain regions.[3,4]
The concept of a “preclinical” (asymptomatic) stage of AD preceding the symptomatic stage by many years developed from clinicopathologic studies of cognitively normal older adults with neuropathologic AD.[5] Markers of AD molecular pathology in cognitively normal older adults now provide in vivo evidence of preclinical AD.[6] In cognitively normal persons, the future occurrence of symptomatic AD is predicted by the cerebrospinal fluid (CSF) levels of amyloid-beta1-42 (Aβ42) and phosphorylated tau species.[7] The CSF levels of these proteins, which are key constituents of the hallmark pathologic lesions of AD, Aβ plaques and neurofibrillary tangles, also predict progression along the continuum of symptomatic AD from subtle to more severe cognitive impairment [8,9] Similarly, positron emission tomography (PET) imaging with the 11C tracer for fibrillar Aβ, Pittsburgh Compound B (PIB),[10] in cognitively normal individuals also identifies those who progress to cognitive impairment[11] and symptomatic AD.[12] A full demonstration of the predictive power of these molecular biomarkers for AD, however, will require following large samples of cognitively healthy older adults for many years to determine whether biomarker-positive persons eventually develop symptomatic AD.
Individuals from families with autosomal dominant AD (ADAD), while representing <1% percent of all persons with AD, have been immensely valuable for the understanding of the molecular and biochemical abnormalities that underlie the disease. Highly penetrant mutations in 3 genes, presenilin 1 (PSEN1), presenilin 2 (PSEN2), and amyloid precursor protein (APP), cause ADAD and are linked mechanistically in that each mutation alters the normal processing of APP such that brain Aβ or the ratio of the Aβ42/Aβ40 isoforms is elevated. These observations have been integral for the development of the “amyloid hypothesis” of AD[13] that posits that the overproduction or underclearance (or both) of Aβ42 isa critical factor in the pathogenesis of AD. This hypothesis has recently been strengthened by the discovery of a unique APP mutation that inhibits beta-secretase cleavage of APP, decreases Aβ production, and is protective against AD.[14] The mutations causing ADAD also have led directly to cell culture and transgenic murine models of AD, which in turn fostered the development of potential mechanism-based therapies for AD.[15,16]
ADAD families offer a unique and compelling opportunity to assess biomarkers in preclinical AD. Asymptomatic mutation carriers (MCs) are virtually certain to develop symptomatic AD and usually at a predictable age (generally at or near the age when their parent became affected). Further, comorbid disorders such as cerebrovascular disease that can confound biomarker validation in older adults generally are absent in ADAD due to the early age of symptom onset. Hence, biomarker studies of ADAD families are much more efficient than studies of late-onset AD (LOAD) individuals as the timing, order, and magnitude of AD pathophysiologic changes can be established in asymptomatic MCs who are destined to develop AD dementia. The characterization of these changes is critically important for the validation of prognostic and diagnostic AD biomarkers and for the design of “prevention” trials. Although ADAD and LOAD are not identical, they share similar clinical and neuropathological phenotypes[17] and thus findings from ADAD samples likely will be relevant for LOAD.
Slightly over 500 ADAD families worldwide have been described in the literature and many families are geographically dispersed. Research with these families has been limited to relatively few academic medical centers, each studying a small number of individuals with site-specific protocols. There thus is a clear rationale for the Dominantly Inherited Alzheimer Network (DIAN), a global ADAD network that uses standard and uniform assessment protocols to obtain longitudinal clinical, cognitive, genetic, neuroimaging, biofluid, and neuropathological data. This multi-center network has resulted in a much larger patient registry and biospecimen repository than any one center could achieve on its own to allow the full characterization of the cascade of pathophysiological processes in preclinical AD. DIAN also provides the infrastructure to support future studies in ADAD, including clinical trials of experimental therapies that may be beneficial for the secondary prevention of symptomatic ADAD.
Overall Approach and Structure
DIAN (U19 AG032438; JC Morris, Principal Investigator) was established in 2008 by the National Institute on Aging (NIA) to longitudinally collect clinical, cognitive, and biomarker information and tissue in asymptomatic and symptomatic MCs and their non-carrier (NC) family members, who serve as a naturally occurring control group. The aims of DIAN are to: 1) establish a multicenter international registry of individuals (MCs and NCs, asymptomatic and symptomatic) who are biological adult children of a parent with a known mutation causing AD; 2) compare asymptomatic MCs and NCs to determine the pathochronology of clinical, cognitive, neuroimaging, and biofluid indicators of AD; and 3) compare the clinical and neuropathological phenotypes of symptomatic MCs with those of LOAD. The DIAN is organized around 8 Cores: Administrative, Clinical, Biostatistics, Neuropathology, Biomarkers, Genetics, Imaging, and Informatics. All Core Leaders (CLs) are faculty at Washington University and for many years have worked together productively on AD-related research, including its dominantly inherited forms.
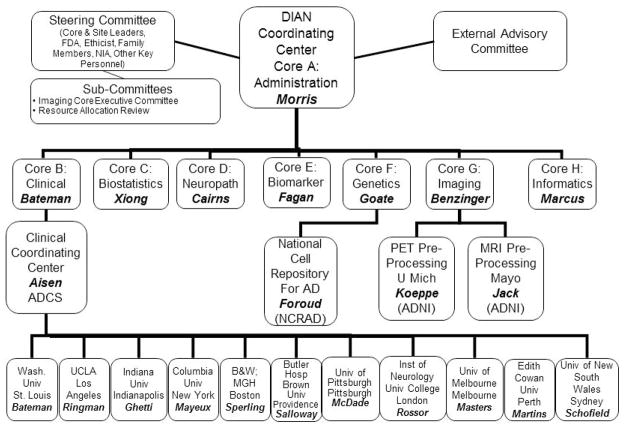
Washington University (St. Louis; USA) is the recipient of the NIA grant and serves both as the DIAN-wide scientific and administrative center and as a performance site. To inaugurate the study, interest in joining DIAN was solicited from academic institutions with experience in ADAD investigations. Based on access to adequate numbers of potential DIAN participants, the resources and capacity to conduct all elements of the DIAN protocol in a uniform manner, and commitment to DIAN’s goals, 9 other performance sites were selected to form the initial Network (all original sites were English-speaking to facilitate uniform adoption of the standard assessments without concerns about translation issues). Current performance sites and the administrative structure for DIAN are shown in Figure 1.
Figure 1. Administrative Structure of the Dominantly Inherited Alzheimer Network (DIAN).
Core Leaders are: JC Morris, RJ Bateman, C Xiong, NJ Cairns, AM Fagan, AM Goate, T Benzinger, and D Marcus, all of Washington University in St. Louis. Site Leaders are: RJ Bateman, JM Ringman, B Ghetti, R Mayeux, RA Sperling, S Salloway, E McDade, MN Rossor, CL Masters, RN Martins, and PR Schofield. The Alzheimer’s Disease Cooperative Study (ADCS; P Aisen, principal investigator) is the Clinical Coordinating Center for DIAN. C Jack (Mayo Clinic-Rochester) and R Koeppe (University of Michigan) provide quality review for positron imaging tomography (PET) and magnetic resonance imaging (MRI). T Foroud (Indiana University) directs the National Cell Repository for Alzheimer’s Disease (NCRAD), which maintains lymphoblast cell lines from DIAN participants. FDA: Federal Drug Administration; NIA: National Institute on Aging; ADNI: Alzheimer’s Disease Neuroimaging Initiative, Wash Univ: Washington University; UCLA: University of California Los Angeles; BWH/MGH: Brigham and Women’s Hospital/Massachusetts General Hospital.
The leader of each performance site joins all CLs, DIAN’s Associate and Executive Directors, representatives from the NIA and the Food and Drug Administration, a bioethicist, and the principal investigators of two successful, well-established multicenter AD research programs, the Alzheimer’s Disease Cooperative Study (ADCS) and the Alzheimer’s Disease Neuroimaging Initiative (ADNI), on DIAN’s Steering Committee. Individuals from ADAD families also are full Steering Committee members. The Steering Committee meets via teleconference every other month and has an annual in-person meeting. The clinical operations for DIAN are coordinated and monitored by the ADCS. To ensure that DIAN data can be compared with data obtained from individuals with LOAD, DIAN uses clinical, cognitive, biomarker, and imaging assessments that are standard for the field. For example, the imaging qualification for each DIAN performance site, the imaging protocols, and continuous quality control of DIAN imaging data are performed by ADNI components. Biofluids are collected, processed and analyzed consistent with ADNI protocols. Blood samples from DIAN participants are sent to the National Cell Repository for Alzheimer’s Disease (NCRAD) to develop lymphoblastoid cell lines. A DIAN Resource Allocation Review Committee oversees the DIAN biorepository (blood; CSF; DNA; brain tissue). The operations, progress toward goals, and new initiatives for DIAN are reviewed annually by a 6-member External Advisory Committee (EAC), comprised of ADAD experts in North America who are not otherwise participating in DIAN. The relationships of the DIAN Committees to the Cores and performance sites also are shown in Figure 1.
Administration Core
Responsibilities and communication
The DIAN Administration Core (JC Morris, CL) is responsible for the management and coordination of all DIAN components and activities. The Administration Core establishes procedures and policies to manage resources, personnel, subcontractors, committees and subcommittees. Communication between DIAN leadership, CLs, site leaders and coordinators, subcontractors, NIA partners, and Steering Committee members is achieved with meetings, electronic messages, and conference calls.
An electronic Manual of Operations is maintained as a guide for administrative operations, including invoicing workflows, quality control procedures for data obtained through DIAN’s Cores, site liaison documents, and accounting pro forma documents. In addition to the internal management of DIAN, the Administration Core also arranges annual review by the EAC and interfaces with external complementary programs and initiatives.
The DIAN Website (www.DIAN-info.org) serves as the electronic repository of public information about DIAN. It provides information (currently in English and German; Spanish is in development) to the public about: the purpose of DIAN and a description of the procedures and information about how to volunteer; helpful resources; frequently asked questions; the original grant application; DIAN publications, and contact information for the leadership and participating sites. The website also allows investigators to request DIAN data and tissue.
Recruitment, training and standardization
With the Clinical Core and the ADCS, the Administration Core monitors the recruitment of DIAN participants and the quality of the data and tissue collection. It is also responsible for training and standardization of research procedures. The Core planned and executed the DIAN Training and Standardization Initiation Meeting in Chicago in July, 2008, where all site leaders, coordinators, imagers, neuropsychologists and clinicians reviewed the administration and scoring of the DIAN protocol and procedures. When site personnel change or a new site joins DIAN, the site leader and coordinator travel to Washington University in St. Louis for 4 days of training, including observation of a DIAN participant evaluation. DIAN also makes use of webinars when specific issues or protocol changes need to be communicated to all sites or for refresher training.
Standardization is achieved by rigorous scrutiny of collected data and specimens using methods appropriate to the testing modality. Every 10th clinical and neuropsychological participant evaluation at a site is audio-recorded and uploaded to DIAN’s secure website for review by the Clinical CL and the senior psychometrician for protocol adherence. Corrective feedback is communicated to the appropriate site personnel via email. The ADCS is the portal through which all clinical data are entered before being transferred to the Informatics Core. The ADCS employs standard error checking mechanisms (e.g. range checks) to detect errors. ADCS also conducts formal monitoring of DIAN clinical and neuropsychologic data by both remote electronic monitoring and site visit review of case report forms. Monitors work with sites to correct current errors and prevent future errors through an electronic query system and personal communication. Biofluid collection is reviewed for adequacy (amount collected) and quality (no hemolysis, etc.). Imaging data are uploaded directly to the DIAN Informatics Core where quality review assures that the scans were collected on certified scanners using the specified sequences and were reconstructed correctly. The Imaging and Informatics Cores work directly with sites to correct procedural deviations or problems.
Data sharing and publications
The Administration Core established the Datasharing/Publications and Biospecimens Review Committees and assisted in developing their policies and procedures. Data and tissue requests are submitted to DIAN via a web-based system. The DIAN Data and Tissue Sharing, Notifications, Publications, and Authorship Policies (available on the DIAN website) govern the sharing of DIAN resources and guidelines for publications. These policies are designed to meet the NIA data sharing requirements and protect participant confidentiality while rewarding DIAN investigators for their efforts. Publications using DIAN data and tissue must cite the DIAN grant and the DIAN study group. In conjunction with the Clinical, Biostatistics, and Informatics Cores, the Administration Core conducts biannual DIAN data freezes. These data freezes provide an opportunity to perform quality control and also to generate verified datasets for investigators, both internal and external to DIAN, who request DIAN data.
Preservation of confidentiality
Individual research results, including genotyping, are not disclosed to the participant. In the situation where a participant wishes to learn his/her mutation status, the Core covers the cost of genetic counseling and commercial genetic testing (independent of DIAN) and works closely with the site to support the participant and address the consequences of the test results. All DIAN investigators are committed to the protection of participant confidentiality and all sites in the United States (US) have obtained Certificates of Confidentiality from the NIA to protect against involuntary disclosure requested by subpoena. Data transmission and storage meets or exceeds the standards for secure data management. This vigilance, which follows a philosophy of providing only the minimum information necessary, includes attention to the display of data, the format for sharing data, and controlling access to data.
Expansion – New sites and languages
The Administration Core manages the expansion of DIAN and translation of DIAN materials into languages other than English. In addition to English, DIAN materials now are available in Spanish and German. Another US site (University of Pittsburgh) has joined DIAN, and Mayo Clinic-Jacksonville plus two sites in Germany that are supported by the Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE) are poised to join. Site initiation includes the following: completion of a survey of site resources (availability of required services, scanners, and eligible participants); training at Washington University; completion of regulatory approvals, certification of scanners, preliminary testing (MRI of a healthy volunteer, test sample shipment, etc.), and a site activation visit by the principal investigator for DIAN. Sites must commit to the uniform collection of data and tissue according to DIAN protocols and procedures.
All DIAN materials read to or by participants, including brochures, test materials, consent forms, and the computerized psychometric battery, have been translated into Spanish and German.
Clinical Core
Recruitment
Individuals are eligible to be enrolled if they are adult children (18 years or older) of a clinically affected parent in an ADAD family in which the parent (or consanguineous relative) has a known mutation causing symptomatic AD. The primary sources of recruitment into DIAN are the ADAD families that are identified by DIAN performance sites, including kindreds that were previously established and kindreds that have emerged in response to publicity about DIAN. The Clinical Core (RJ Bateman, CL) also has established the DIAN Expanded Registry website (www.DIANExpandedRegistry.org) for individuals from ADAD families who may be interested in proposed therapeutic trials; this website serves as another recruitment tool for DIAN. Other recruitment sources include NCRAD, physicians with patients with positive genetic testing results, and individuals from ADAD families who seek information from relevant nonprofit organizations (the Alzheimer’s Association; the Alzheimer Research Forum) and the NIA’s Alzheimer’s Disease Education and Referral Center. Potential participants are screened for eligibility by a DIAN coordinator, who refers eligible candidates to the most appropriate DIAN site based on factors such as geography, prior involvement with site investigators, bilingual staff for Spanish speaking participants, etc. The major reason potential participants are not enrolled is the absence of a confirmed mutation in the family (primarily because testing has not been done). If the pedigree is consistent with the presence of ADAD, DIAN offers commercial genetic testing and counseling if the family has indicated interest in participation in the study.
Operations and Assessments
A template for the DIAN visit is shown in Table 1. Most participants live distant to performance sites and thus travel with their study partner to the nearest site, with travel expenses covered by DIAN, to complete all procedures during a single visit. If they do live in the same city where a DIAN site is located, they can complete the procedures at their convenience (within 3 months).
Table 1.
Sample Visit Schedule for DIAN Participants
| Day 1 | Day 2 | Day 3 | |
|---|---|---|---|
| 0700 | Lumbar puncture & fasting blood collection (after MRI) 2.5 hrs | ||
| 0800 | Informed consent discussion: 1 hr | PET PIB and PET FDG (Fasting): 2.5 hrs | |
| 0900 | Intake, Genetics and NCRAD non-fasting blood collection, Clinical Assessment: 2 hrs | ||
| 1000 | |||
| 1100 | Physical/Neurological Exams: 1 hr | Lunch | Break |
| 1200 | Lunch | MRI (before LP): 1.5 hrs | Lunch |
| 1300 | DIAN Psychometrics: 2.5 hrs | Review of Participant Experience |
The identical assessment is administered at the initial and each follow-up visit, with the exception of the nonfasting blood draw for genetic studies (initial visit only). All assessments are completed in asymptomatic and symptomatic participants. The interval between assessments is every 3 years for asymptomatic individuals, except for participants within 3 years of the parent’s age at onset (AAO) of symptomatic AD when assessments are completed annually. Asemi-structured interview has been developed to establish the parent’s last known normal age, age at symptom onset, and age at death. Symptomatic individuals are assessed annually until cognitive decline prevents their participation in a majority of study procedures. In the years that the participant is not evaluated on site, telephone follow-up is conducted annually with the participant and study partner. If cognitive decline is suspected as a result of this telephone follow-up, an in-person assessment at the performance site is triggered. All DIAN investigators involved in the participant’s clinical, cognitive, imaging, and biofluid assessments are without explicit knowledge of the participant’s mutation status.
Informed consent documents are sent to the participant for their perusal before the initial visit. These documents are reviewed and all procedures are explained at the beginning of the visit. The study partner is available (in person or by telephone) to provide observations about the participant’s health history and clinical, cognitive, and functional status. Both initial and follow-up clinical assessments use the Uniform Data Set (UDS),[18,19] the standard instrument of the US federally-funded Alzheimer Disease Centers. The UDS includes a study partner interview to assess possible decline in the participant’s cognitive, behavioral, and functional abilities and an interview and examination of the participant. It incorporates commonly used measures and scales, including the Functional Assessment Questionnaire (FAQ),[20] the Neuropsychiatric Inventory (NPI),[21,22] and the Geriatric Depression Scale.[22] It provides sufficient information for the clinician to synthesize and determine the participant’s Clinical Dementia Rating (CDR),[23] where CDR 0 indicates cognitive normality and CDR 0.5, 1, 2, and 3 indicate very mild, mild, moderate, and severe dementia. For operational purposes, CDR 0.5 also captures participants designated as mild cognitive impairment. The UDS cognitive measures also are administered in DIAN at baseline and follow-up but are supplemented with a novel computerized battery, the Executive, Language, Spatial, Memory (ELSMEM), to more stringently assess the typically young, cognitively intact DIAN participants. All participants are requested to complete all procedures of the protocol (Table 2) but are assured that they may refuse any procedure. Table 3 illustrates that refusal of a procedure is relatively rare with procedure completion rates ranging between 90% and 100% (lumbar puncture at 82%). DIAN has an attrition rate of 5%; of 255 participants enrolled as of 5/15/2012, 9 have been discontinued (largely due to progression of symptomatic AD) and 5 have withdrawn. This low rate is a testament to the expertise of DIAN personnel and to the dedication of DIAN participants. Current sample characteristics are presented in Table 4; enrollment is ongoing.
Table 2.
DIAN Assessments
| Procedure | Baseline | Follow-up | |
|---|---|---|---|
| Study explanation | X | ||
| Consent | X | ||
| Blood for Genetic Analysis | X | ||
| Demographics, Family Hx, Inclusion/Exclusion Criteria | X | ||
| Medical History, Physical Exam, Neurological Exam, Vital signs, Medications (From the UDS) | X | X | |
| Clinical Evaluation – (CDR, GDS, NPI FAQ) | X | X | |
| Psychometric Battery | UDS Measures | X | X |
| Computer psychometrics | X | X | |
| 3 Tesla MRI, FDG PET, PET PIB | X | X | |
| LP | X | X | |
Table 3.
Initial Visit Study Completion Rates as of 5/15/2012 (N = 255)
| Procedure | N |
|---|---|
| CDR | 255 (100%) |
| International Personality Item Pool (IPIP) | 236 (93%) |
| Psychometrics – Pencil-Paper | 253 (99%) |
| Psychometrics – Computer Battery | 239 (94%) |
| MRI | 241 (95%) |
| PET-PIB | 232 (91%) |
| FDG-PET | 233 (91%) |
| Lumbar Puncture | 208 (82%) |
Table 4.
Participant Entry Characteristics from Data Freeze 3 (DF3) 29FEB2012. Data from 242 DIAN participants are included in DF3. 27 are still undergoing genetic quality control to confirm mutation status and are not included in the demographic data; however cognitive status is available for all 242 and 176 of the 242 (72.7%) are asymptomatic.
| Total N = 215 | Asymptomatic | Symptomatic | ||
|---|---|---|---|---|
| N | NC N=74 | MC N=75 | N = 66 | |
| Age (mean ± SD) | 41.1 ± 9.0 | 34.4 ± 9.0 | 45.2 ± 10.4 | |
| Female Gender (%) | 44 (59.5%) | 44 (58.7%) | 38 (57.6%) | |
| Parental Age of Onset | 46.4 ± 6.7 | 47.4 ± 6.6 | 45.9 ± 8.3 | |
| Education | 14.8 ± 2.5 | 14.4 ± 2.6 | 13.7 ± 2.5 | |
| MMSE [25] | 29.3 ± 1.1 | 29.0 ± 1.4 | 22.8 ± 7.0 | |
| ApoE4+ | 1 E4 | 21 | 15 | 11 |
| 2 E4 | 0 | 1 | 4 | |
MC = Mutation Carrier; NC = Noncarrier
MMSE = Mini-Mental State Examination
The Clinical Core established the ADAD Forum, a confidential web-based forum accessible only to ADAD family members. The ADAD Forum is hosted in affiliation with the Alzheimer’s Association and managed independently of DIAN so that family members have a private medium in which to connect with other ADAD families and discuss common concerns.
Other cores
The responsibilities of the other DIAN Cores center on tissue and data. The Biomarker (AM Fagan, CL), Genetics (AM Goate, CL), and Neuropathology (NJ Cairns, CL) Cores serve as repositories of CSF, plasma and serum, DNA, and brain tissue. DIAN tissue is processed by these Cores to yield neuropathological data, plasma and CSF measures of Aβ and tau, ADAD family pedigrees, APP/PSEN1/PSEN2 mutation data, and apolipoprotein E (APOE) genotype. The Imaging Core (T Benzinger, CL) oversees the scanner certification, scan collection, quality control and processing of MRI, (3T, structural and functional); amyloid PET imaging (regional PIB uptake), and fluordeoxyglucose (18F) (FDG-PET) measures of brain metabolism. Data are managed, securely stored, and made available to investigators internal and external to DIAN by the Biostatistics (C Xiong, CL) and Informatics (D Marcus, CL) Cores following the DIAN data sharing policies. The Biostatistics Core also provides analytic consultation and analysis-quality datasets.
Future Perspective
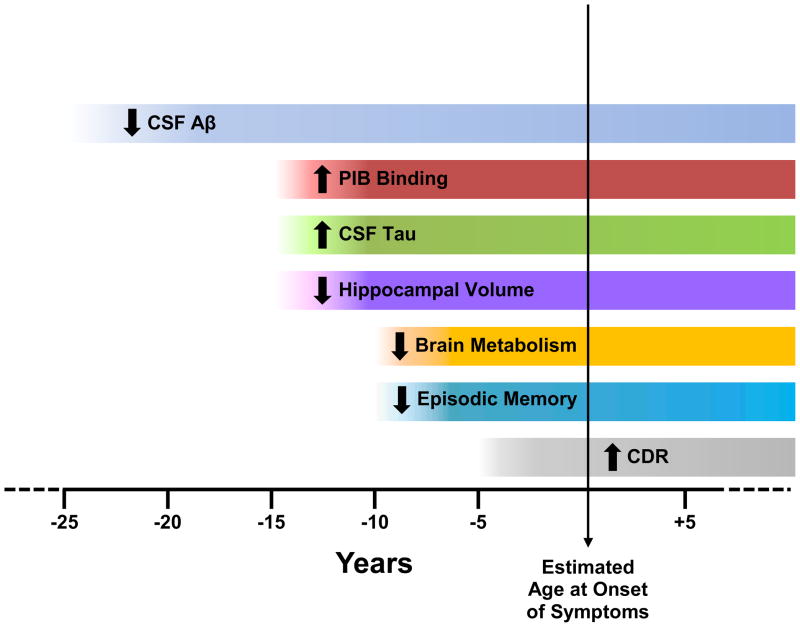
The initial publication of cross-sectional results from DIAN validate the use of AD biomarkers to identify preclinical AD, as only MCs (both asymptomatic and symptomatic) demonstrated biomarker abnormalities.[24] Although preliminary, the findings propose an instructive timeline for the pathophysiology of preclinical AD, beginning with elevated CSF Aβ42 (predicted by the mutation-induced altered processing of APP) that presumably is present at birth but at perhaps 20–25 years prior to estimated AAO of symptomatic AD begins to decline, perhaps as Aβ42 is sequestered in cerebral cortical deposits as amyloid plaques or as clearance mechanisms fail. Subsequent to the reduction in CSF level of Aβ42, a sequence of other biomarker changes become apparent relative to estimated years to symptom onset (Figure 2): elevation of CSF tau, elevation of plasma Aβ, increased retention of PIB, and brain atrophy occur ~15 years before, cerebral hypometabolism and decline in episodic memory performance occur ~10 years before, and declines in global cognition begin ~5 year before. Caveats to this proposed timeline include the cross-sectional nature of the data and relatively small numbers of participants, particularly at the extremes of the age range, and thus justify continuation of the longitudinal DIAN study. The characterization of the timing and degree of biomarker changes in asymptomatic MCs, however, is critical for the design of secondary prevention trials to delay or prevent symptomatic AD.
Figure 2. Alzheimer Biomarker Pathochronology in Autosomal Dominant AD.
Model derived from preliminary data presented in Bateman et al., 2012.[24] Note: The levels of CSF Aβ initially are elevated (~25y before estimated age at onset) but then are marked by progressive decline.
The DIAN study will provide the infrastructure for randomized, placebo-controlled secondary prevention trials of mechanism-based therapies in ADAD.[24] The trials will be designed to allow several therapies to be evaluated simultaneously, with an initial primary outcome of target engagement (e.g., reduction from baseline of PIB retention) and multiple secondary outcomes of downstream AD biomarkers. The compound(s) with the optimal target engagement, improvement of neurodegenerative biomarkers, and sufficient safety profile then will be selected for a second trial in which cognitive benefit will be the primary outcome. Because the large majority of DIAN participants do not know, nor wish to know, their mutation status, confidentiality will be preserved in the DIAN clinical trials by enrolling both MCs and NCs but assuring that NCs are assigned to placebo therapy to avoid their exposure to potential drug side effects. One-quarter of MCs also will be assigned to placebo therapy.
Because the NIA award that established DIAN did not including funding for clinical trials, the DIAN Trials Unit (TU) (RJ Bateman, Director) will sponsor the studies. The DIAN TU is supported by the DIAN Pharma Consortium (currently comprised of 10 pharmaceutical companies; see Financial Disclosure section) and the Alzheimer’s Association; NIA support also is being sought. All trial advice is given in a pre-competitive environment; the DIAN Pharma Consortium solely advises on the design and implementation of the trials, not on compound selection. The launch of the DIAN trials, which will preferentially enroll DIAN participants to capitalize on available “run-in” data, is expected in late 2012 or early 2013.
Executive Summary.
The Dominantly Inherited Alzheimer Network (DIAN) was established in 2008 as an international study of autosomal dominant Alzheimer disease (ADAD), with a focus on characterizing the pathochronology of AD biomarker changes in asymptomatic mutation carriers who are destined to develop symptomatic AD.
Washington University (St. Louis, Missouri, USA) is the coordinating center for DIAN, which has 11 current performance sites in the US, United Kingdom, and Australia. Additional sites are anticipated; the DIAN protocols are available in English, Spanish, and German languages.
DIAN participants complete uniform and comprehensive baseline and follow-up assessments that yield a rich repository of clinical, cognitive, imaging, genetic, biofluid, and neuropathologic data and tissue. 255 DIAN participants were enrolled as of May 15, 2012, the largest international cohort of ADAD individuals in the world. Total enrollment is ongoing and anticipated to reach 400.
DIAN has estimated the rate and sequence of biomarker changes in asymptomatic MCs, providing validation of these biomarkers in detecting preclinical AD, antecedent to any cognitive or other symptoms.
Asymptomatic MCs now are the target of DIAN clinical trials, designed as secondary prevention studies (the brain lesions of AD are present, thus the treatment goal is not to prevent the disease but its cognitive consequences). Based on the order and magnitude of biomarker changes in asymptomatic MCs and the stage of drug development, experimental therapies that target Aβ will be selected initially for the DIAN trials, which are expected to launch before or during 2013.
Acknowledgments
Financial disclosure/Acknowledgements
Supported by NIA U19 AG032438 (JC Morris, PI) and a private nonprofit foundation. One of more of the authors have affiliations or financial involvement with the following entities: Abbott (PA, MW), Amgen (PA), Anavex (PA), Araclon (MW), Astellas (PA), AstraZeneca (PA, RB, AG, DH, MW), Baxter (PA), Bayer (PA, BG, SS, MW), BioClinica, Inc. (MW) Biogen Idec (RB, MW), Biomarin (PA), Blackwell Medical Publishing (JM), BlueArc, Inc. (DM), Brain Resource Ltd (PS), Bristol-Myers Squibb (PA, DH, RS), C2N Diagnostics (RB, DH), Cardeus (PA), Daiichi (PA), Dainippon (PA), Dickstein Shapiro (AG), Eisai Inc. (PA, RB, JM, RS, MW), Elan Corporation (PA, RB, MW), Eli Lilly (PA, RB, TB, DM, KQ, SS, RS, MW), EnVivo (RB), Esteve (JM), Exonhit Therapeutics (MW), Finnegan HC (AG), GE Healthcare (WK, SS), Gene Network Sciences (MW), Genentech (RB, AG, MW), GlaxoSmithKline (JM, MW), Hoffmann La Roche (RB), Howrey & Associates (AG), Innogenetics (MW), Ipsen (MW), Janssen (PA, RB, JM, MR, RS, MW), KLJ Associates (MW), Kujawski & Associates (TB), Medivation, Inc. (PA, MW), Medpace (MW), Medtronic (RB), Merck & Co. (PA, RB, MW), Mithridion inc. (RB), NeuroPhage (PA), NeuroVigil (MW), Novartis (PA, RB, JM, MW), Otsuka (PA, JM), Pfizer Inc. (PA, RB, AG, DH, JM, MR, RS, MW), Radiologics, Inc. (DM), Roche (PA, RS, MW), Sanofi-Aventi (RB), Schering Plough (MW), Servier (MW), Siemens (MW), Solvay (PA), Synarc (MW), Taconic (AG), Taylor and Francis (JM), TauRx Therapeutics (MW), Theravance (PA), Wyeth (PA, MW). Financial involvement refers to consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
The DIAN Pharma Consortium (which supports the DIAN TU, but not the parent DIAN registry) consists of Biogen Idec, Elan, Genentech, Janssen, Eli Lilly, Mithridion, Novartis, Pfizer, Roche, and Sanofi.
We thank the DIAN participants and their families for their dedication and altruism and the research and support staff at each of the DIAN sites for their contributions to the study.
References
- 1.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKhann GM, Knopman DS, Chertkow H, Hynes M, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimer’s & Dementia. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez-Isla T, Price JL, McKeel DW, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4*.Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Jr, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. Substantial neuronal loss already has occurred in critical brain regions by the time the earliest symptoms of AD appear. [DOI] [PubMed] [Google Scholar]
- 5.Price JL, McKeel DW, Buckles VD, Roe CM, Xiong C, Grundman M, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6*.Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, et al. APOE predicts Aβ but not tau Alzheimer’s pathology in cognitively normal aging. Ann Neurol. 2010;67:122–131. doi: 10.1002/ana.21843. Increasing cerebral Aβ deposition with age is the pathobiological phenotype of APOE4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7**.Fagan AM, Roe CM, Xiong C, Morris JC, Holtzman DM. Cerebrospinal fluid tau/s-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. In cognitively normal persons, the future occurrence of symptomatic AD is predicted by the CSF levels of Aβ42 and phosphorylated tau species. [DOI] [PubMed] [Google Scholar]
- 8.Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD, et al. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69:631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 9.Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O. Cerebrospinal fluid levels of B-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry. 2012;69:98–106. doi: 10.1001/archgenpsychiatry.2011.155. [DOI] [PubMed] [Google Scholar]
- 10*.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. This report describes the first human study of the amyloid imaging tracer, Pittsburgh Compound B (PIB), providing quantitative information on amyloid deposits in living persons. [DOI] [PubMed] [Google Scholar]
- 11.Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, et al. Longitudinal assessment of AB and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69:181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, et al. Pittsburgh Compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer’s disease. Arch Neurol. 2009;66:1469–1475. doi: 10.1001/archneurol.2009.269. PET imaging with Pittsburgh Compound B (PIB) in cognitively normal individuals identifies those who will progress to symptomatic AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 14.Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012 doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 15.Schenk D, Barbour R, Dunn K, Gordon G, Grakeda J, Guldo T, et al. Immunization with amyloid-s attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–174. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 16.Qu B-X, Xiang Q, Li L, Johnston SA, Hynan LS, Rosenberg RN. AB42 gene vaccine prevents AB42 deposition in brain of double transgenic mice. J Neurol Sci. 2007;260:204–213. doi: 10.1016/j.jns.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snider BJ, Norton J, Coats MA, Chakraverty S, Hou CE, Jervis R, et al. Novel Presenilin 1 mutation (S170F) causing Alzheimer disease with Lewy bodies in the third decade of life. Arch Neurol. 2005;62:1821–1830. doi: 10.1001/archneur.62.12.1821. [DOI] [PubMed] [Google Scholar]
- 18.Morris JC, Weintraub S, Chui HC, Cummings J, DeCarli C, Ferris S, et al. The Uniform Data Set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alz Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 19.Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): The Neuropsychological Test Battery. Alz Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities of older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 21.Cummings JL, Mega M, Gray K. The neuropsychiatric inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 22.Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24:709–710. [PubMed] [Google Scholar]
- 23.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 24**.Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012 doi: 10.1056/NEJMoa1202753. Epub ahead of print. Autosomal dominant AD is associated with a series of pathophysiological alterations, including CSF AD biomarker changes, brain amyloid deposition, brain metabolism decreases, and progressive cognitive impairment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. Mini-mental State: A practical method for grading the cognitive state of patients for the clinicians. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]