Abstract
The hydrolysis of cell wall pectins by tomato (Lycopersicon esculentum) polygalacturonase (PG) in vitro is more extensive than the degradation affecting these polymers during ripening. We examined the hydrolysis of polygalacturonic acid and cell walls by PG isozyme 2 (PG2) under conditions widely adopted in the literature (pH 4.5 and containing Na+) and under conditions approximating the apoplastic environment of tomato fruit (pH 6.0 and K+ as the predominate cation). The pH optima for PG2 in the presence of K+ were 1.5 and 0.5 units higher for the hydrolysis of polygalacturonic acid and cell walls, respectively, compared with activity in the presence of Na+. Increasing K+ concentration stimulated pectin solubilization at pH 4.5 but had little influence at pH 6.0. Pectin depolymerization by PG2 was extensive at pH values from 4.0 to 5.0 and was further enhanced at high K+ levels. Oligomers were abundant products in in vitro reactions at pH 4.0 to 5.0, decreased sharply at pH 5.5, and were negligible at pH 6.0. EDTA stimulated PG-mediated pectin solubilization at pH 6.0 but did not promote oligomer production. Ca2+ suppressed PG-mediated pectin release at pH 4.5 yet had minimal influence on the proportional recovery of oligomers. Extensive pectin breakdown in processed tomato might be explained in part by cation- and low-pH-induced stimulation of PG and other wall-associated enzymes.
The role of PG (EC 3.2.1.15) in the depolymerization of pectic polymers in tomato (Lycopersicon esculentum) fruit cell walls has been demonstrated in a number of studies (Huber, 1983; Seymour et al., 1987; Giovannoni et al., 1989; DellaPenna et al., 1990; Smith et al., 1990). Nonetheless, mole-size downshifts exhibited by pectins derived from cell walls or ethanol-insoluble solids of ripening tomato fruit are considerably more limited than those occurring in in vitro reactions with purified PG (Seymour et al., 1987; Huber, 1992). Specifically, in in vitro reactions at pH 4.5 with 150 mm NaCl, PG is capable of rapidly generating oligouronides and galacturonic acid from tomato fruit cell walls (Huber and Lee, 1988). The extent of oligomer production in vivo is less certain. Huber and O'Donoghue (1993) reported negligible levels of low-DP uronides (DP < 10) in cv Sunny tomato fruit, and only in fruit at the ripe stage and beyond, whereas Melotto et al. (1994) recovered pectic oligomers (DP ≤ 14) in cv Castlemart tomato fruit as early as the breaker stage of ripening.
Assays for PG have routinely used buffers at pH values from 4.5 to 4.8, usually containing NaCl at 150 mm or higher (Pressey and Avants, 1973). Early studies of tomato-fruit PG (Patel and Phaff, 1958, 1960) reported an optimum at pH 4.5 for the hydrolysis of PGA, with a second maximum near pH 3.5 for tetragalacturonic acid and lower-DP substrates. The practice of including NaCl in in vitro reactions for tomato PG is based on the findings that activities of both isoforms (PG1 and PG2) of the enzyme are strongly enhanced by Na+ (Pressey and Avants, 1973).
There is reason to question whether the conditions used for assaying PG and other enzymes of wall origin approximate the cell wall environment in situ. Ruan et al. (1995, 1996) reported a pH of near 6.0 for apoplastic sap derived from immature-green cv Floradade tomato fruit. Frozen-thawed fruit yielded fluid at pH 4.8, representing the bulk pH of the fruit (Ruan et al., 1996). Although the apoplastic pH found for tomato fruit is in the range for apoplastic fluid from other plant tissues (Grignon and Sentenac, 1991), Themmen et al. (1982) reported that tomato PG was inactive against native cell walls at pH 6.0.
In this study we examined the catalytic potential of PG2 under a range of conditions anticipated for the apoplast of tomato fruit. The primary objective was to determine whether these reaction conditions might explain the differences in the capacity for PG-mediated pectin hydrolysis in vitro compared with that observed for ripening fruit.
MATERIALS AND METHODS
Tomato (Lycopersicon esculentum) fruit were harvested from greenhouse (cv Ailsa Craig) or field (cv Solarset) plantings at the University of Florida (Gainesville). Fruit were surface sterilized with 2 mm NaOCl, rinsed in tap water, and dried at room temperature. External pericarp that was free of locule and placental tissues was prepared from fruit at the mature-green stage of development and stored at −30°C.
Cell Wall Preparation and PG Purification
Cell walls were prepared as described previously (Chun and Huber, 1997) using Tris-buffered phenol (Huber, 1991) to denature endogenous enzymes. PG2 was purified from ripe cv Solarset tomato pericarp using the protocol of Ali and Brady (1982).
PG2 Hydrolysis of PGA
The hydrolytic capacity of purified PG2 was performed using de-esterified PGA (Sigma) and cell walls from mature-green cv Ailsa Craig pericarp tissue. PGA was prepared at 4 mg mL−1 in 20 mm NaOAc and 150 mm NaCl or 20 mm KOAc and 100 mm KCl. The pH was adjusted as required with dilute HCl or NaOH to values in the range of 4.0 to 6.5. One-half milliliter of substrate (2 mg) and 10 μL of purified PG2 (approximately 2 μg of protein [Smith et al., 1985]) were incubated for 1 h at 34°C, and hydrolysis was determined reductometrically (Milner and Avigad, 1967).
PG2 Hydrolysis of Cell Walls from Mature-Green Pericarp
The hydrolysis of cell wall-integrated pectin by purified PG2 was measured as described previously (Chun and Huber, 1997). Cell walls (approximately 5 mg) were hydrated in 2 mL of ice-cold 25 mm KCl and maintained on ice for 30 min with continuous stirring after the addition of 2.2 μg of PG2 protein (0.44 μg mg−1 of cell wall). Enzyme-loaded cell walls were filtered, rinsed with 8 mL of cold 25 mm KCl, and transferred to catalysis buffer (conditions described in figure legends) at 34°C. After a 2-h reaction period, the suspensions were chilled in an ice bath and filtered (Whatman GF/C), and the pH was measured before adjusting to pH 7.0 to arrest further PG activity. Uronic acids in the filtrates were determined using the hydroxydiphenyl assay (Blumenkrantz and Asboe-Hansen, 1973).
Gel Chromatography of Cell Wall Pectic Polymers Released by PG2
Pectic polymers recovered from PG2 hydrolysis of cell walls were applied to Sepharose CL-6B-100 (29.5 cm long × 1.5 cm wide) or Bio-Gel P-4 (extra-fine, 48 cm long × 1.5 cm wide) columns operated in 200 mm ammonium acetate, pH 5.0 (Mort et al., 1991). Pectic polysaccharides (250–500 μg of galacturonic acid equivalents) in a total volume of 1 mL were applied and 1-mL (Bio-Gel P-4) or 2-mL (Sepharose CL-6B-100) fractions were collected. Aliquots (0.5 mL) were used for measuring uronic acid levels (Blumenkrantz and Asboe-Hansen, 1973). The Bio-Gel P-4 column was calibrated with PG2 hydrolysates of PGA and galacturonic acid (Sigma).
RESULTS
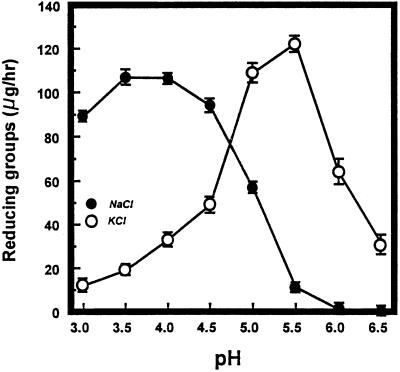
The effect of pH on PG2-mediated hydrolysis of PGA showed significantly different responses to 100 mm KCl compared with 150 mm NaCl (Fig. 1). The concentrations of K+ and Na+ initially selected were those necessary to achieve maximum stimulation of PG2 activity in in vitro reactions. The maximum rate of hydrolysis (monitored reductometrically) of PGA in 20 mm NaOAc and 150 mm NaCl or 20 mm KOAc and 100 mm KCl occurred at pH 3.5 to 4.0 and pH 5.0 to 5.5, respectively. In the presence of 100 mm KCl, PG activity persisted at approximately 50% and 25% at pH 6.0 and 6.5, respectively, of that at the optimum pH 5.5. In contrast, PG2 was not active with PGA at pH 6.0 and 6.5 in the presence of 150 mm NaCl.
Figure 1.
PG2 hydrolysis of PGA in response to pH. Reaction mixtures contained 0.5 mL of PGA (4 mg mL−1) in 20 mm NaOAc and 150 mm NaCl or 20 mm KOAc and 100 mm KCl, adjusted to the desired pH with dilute HCl or NaOH. After the addition of 50 μL of purified PG2 (approximately 2 μg of protein), the samples were incubated for 1 h at 34°C. Activity was measured reductometrically using galacturonic acid as the standard. •, 150 mm NaCl; ○, 100 mm KCl.
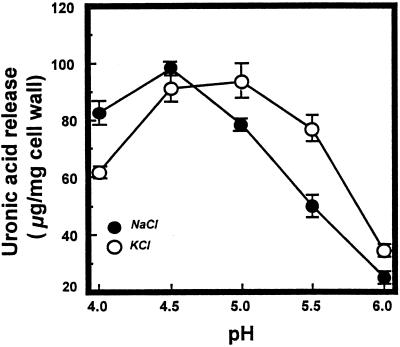
Subsequent experiments tested the influence of pH and cation on PG2 hydrolysis of cell walls from mature-green cv Ailsa Craig tomato fruit. Cell walls were treated to inactivate endogenous enzymes (Huber, 1992) and preloaded with rate-limiting quantities of PG2 (Chun and Huber, 1997) before incubation under catalytic conditions. PG1 (Tucker et al., 1981) was not tested separately, but cell walls from mature-green fruit contain the β-subunit protein (Zheng et al., 1992), as confirmed by the recovery of PG1 and PG2 in high-saline washes of walls provided with purified PG2 (Chun and Huber, 1997). The effects of the β-subunit protein on pectin solubility do not appear to be altered by the buffered phenol used to inactivate wall-associated enzymes (Chun and Huber, 1997).
The influence of pH and cation on the hydrolysis of cell walls by purified PG2 is illustrated in Figure 2. The pH optima for reactions in 100 mm KCl (pH 5.0) and 150 mm NaCl (pH 4.5) were not as divergent as observed for the substrate PGA; however, activity in 100 mm K+ was 20%, 56%, and 50% higher than that in Na+ at pH 5.0, 5.5, and 6.0, respectively. Cell walls incubated with inactive PG2 released less than 5 μg of uronic acid equivalents mg−1 under all reaction conditions examined, indicating that pectin solubilization from PG2-treated cell walls was caused nearly exclusively by PG2. The maximum recovery of soluble uronic acids in response to PG2 occurred at pH 4.5 to 5.0 and represented about 40% of total cell wall uronic acid and about 58% of the maximum quantities of uronic acids solubilized in response to saturating levels of the enzyme (data not shown).
Figure 2.
PG2 hydrolysis of cell walls from mature-green cv Ailsa Craig tomato fruit. Cell walls (5 mg) preloaded with purified PG2 (0.44 μg protein mg−1 of cell wall) were incubated in 4 mL of 20 mm NaOAc and 150 mm NaCl or 20 mm KOAc and 100 mm KCl for 2 h at 34°C. Reaction mixtures were filtered and soluble uronic acids measured as described in Methods. Activity is expressed as micrograms of galacturonic acid equivalents released per milligram of cell wall. •, 150 mm NaCl; ○, 100 mm KCl.
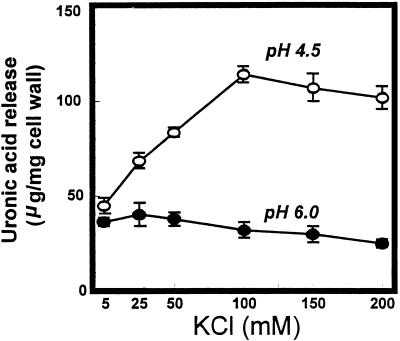
Additional experiments were designed to determine the influence of [K+] on PG2-mediated hydrolysis of cell walls at pH 6.0, the estimate for the apoplast pH in developing tomato fruit (Ruan et al., 1996), and at pH 4.5, the pH routinely used in PG assays and representative of the bulk pH of tomato fruit (Al-Shaibani and Greig, 1979; Ruan et al., 1996). Cell wall degradation at pH 6.0 was unaffected or slightly inhibited with increasing K+ over the range from 5 to 200 mm (Fig. 3). At pH 4.5, pectin release increased linearly with increasing K+ to 100 mm. Activity in the presence of 5 mm KCl did not exceed that occurring in buffer (20 mm KOAc) alone.
Figure 3.
PG2 hydrolysis of cell walls from mature-green cv Ailsa Craig tomato fruit at pH 4.5 and 6.0 in response to increasing [K+]. Cell walls (5 mg) in 4 mL of 20 mm KOAc at the indicated pH and KCl concentrations were incubated for 2 h at 34°C. Reaction mixtures were filtered and soluble uronic acids determined. Activity is expressed as micrograms of galacturonic acid equivalents released per milligram of cell wall. ○, pH 4.5; •, pH 6.0.
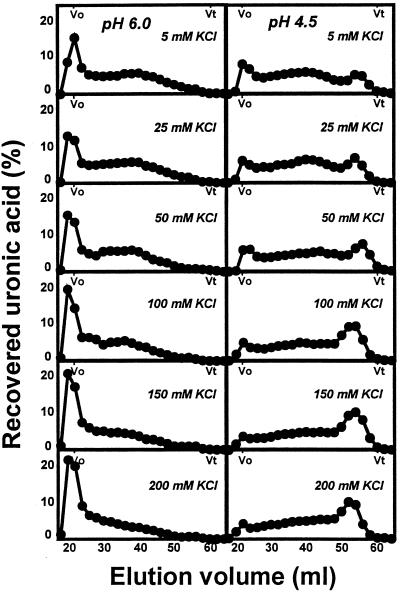
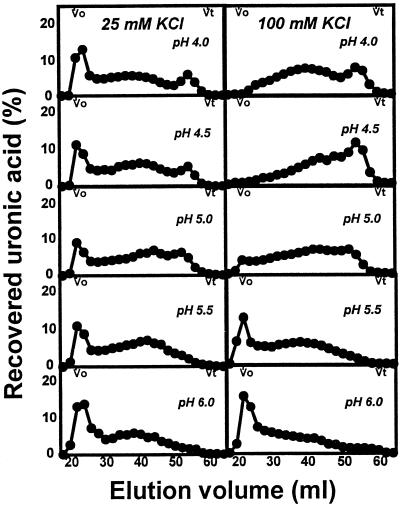
The molecular mass distributions of pectins enzymically solubilized from mature-green cv Ailsa Craig cell walls treated with PG2 at pH 6.0 or 4.5 in response to increasing [K+] are shown in Figure 4. Pectins solubilized by PG2 at pH 6.0 included an excluded, high-molecular-mass fraction and a polydisperse population distributed over the fractionation range of the gel. Increasing K+ resulted in a proportional increase in high-molecular-mass polymers. Profiles of pectins released at pH 4.5 included a low-molecular-mass peak eluting from 50 to 58 mL, becoming proportionally more prominent with increasing K+ to 100 mm. The increase in this fraction at pH 4.5 in response to increasing K+ closely paralleled the trend for total uronic acid solubilization under identical reaction conditions (Fig. 3).
Figure 4.
Sepharose CL-6B-100 profiles of uronic acids released from mature-green cv Ailsa Craig cell walls by PG2 at pH 6.0 or 4.5 in response to increasing [K+]. Cell walls (5 mg) in 4 mL of 20 mm KOAc, pH 4.5 or 6.0, containing from 5 to 200 mm KCl were incubated for 2 h at 34°C. Two-milliliter fractions were collected and 0.5-mL aliquots measured for soluble uronic acids. Uronic acid levels in each fraction are expressed as a percentage of total uronic acids recovered from the column. Vo, Void volume; Vt, total volume.
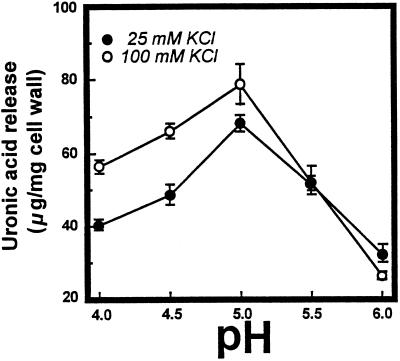
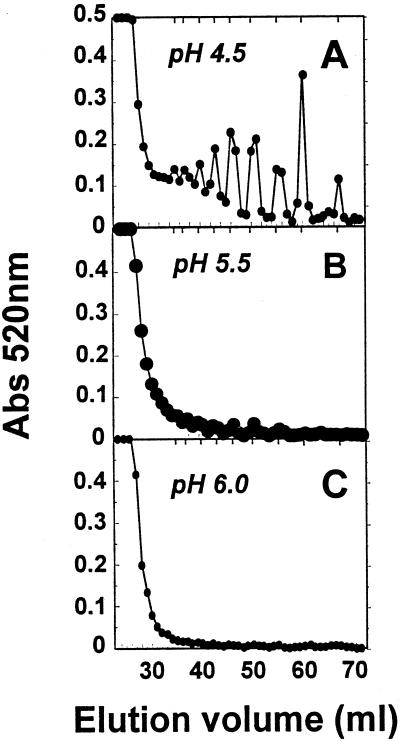
The yields and molecular mass distribution of pectins enzymically solubilized from cell walls over the range of pH from 4.0 to 6.0 in the presence of 25 or 100 mm KCl are shown in Figures 5 and 6. The K+ levels selected are representative of those for the apoplast (approximately 20 mm) and bulk tissue (approximately 100 mm) of tomato fruit (Ruan et al., 1996). Pectin release in 100 mm K+ was enhanced 45%, 40%, and 16% at pH 4.0, 4.5, and 5.0, respectively, compared with 25 mm K+, whereas activities at pH values > 5.0 were similar at the two K+ levels. At pH 4.0 and 4.5, depolymerization was extensive at the higher K+ level, as indicated by the near absence of excluded, high-molecular-mass polymers and the accumulation of products eluting near the column total volume (Fig. 6). Low-molecular-mass products were also evident in reactions performed at 25 mm K+; however, high-molecular-mass polymers persisted at all pH values. The presence of low-DP reaction products was determined using Bio-Gel P-4 chromatography. In reactions performed in the presence of 100 mm K+, oligomeric (DP ≤ 10) and monomeric uronic acids constituted 30% of the products at pH 4.5, <5% at pH 5.5 and <1% at pH 6.0 (Fig. 7). Oligomer production was clearly favored at lower pH values; however, oligomers were also noted at pH 6.0 in response to excess levels (20×) of PG2 protein (Bio-Gel P-4 profiles not shown).
Figure 5.
Effect of pH on uronic acid release from mature-green cv Ailsa Craig cell walls in response to PG2 at 25 or 100 mm KCl. Activity is expressed as micrograms of galacturonic acid equivalents released per milligram of cell wall.
Figure 6.
Sepharose CL-6B-100 profiles of uronic acids released from mature-green cv Ailsa Craig cell walls by PG2 at 25 or 100 mm KCl over the pH range from 4.0 to 6.0. Cell walls (5 mg) in 2 mL of 20 mm KOAc at the indicated pH and KCl concentrations were incubated with the enzyme for 2 h at 34°C. Vo, Void volume; Vt, total volume.
Figure 7.
Bio-Gel P-4 profiles of uronic acids released from mature-green cv Ailsa Craig cell walls in response to PG2. Cell walls (5 mg) in 2 mL of 20 mm KOAc and 100 mm KCl were incubated for 2 h at 34°C. Shown are uronic acids released at pH 4.5 (A), pH 5.5 (B), and pH 6.0 (C). Total uronic acids in each fraction were determined at A520 (Abs 520nm). Vertical ticks at the top of each profile show the elution positions of uronic acid oligomers generated from PG2 hydrolysis of PGA and galacturonic acid.
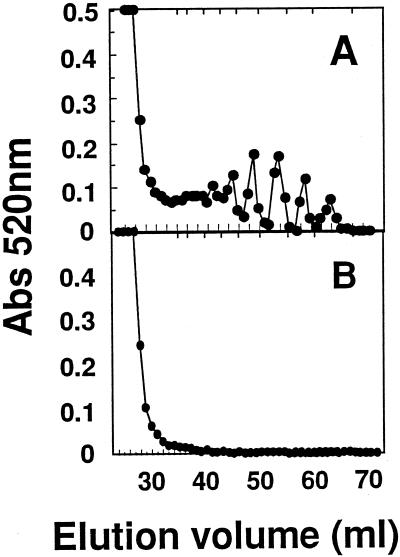
PG2-mediated pectin release from mature-green cv Ailsa Craig cell walls at pH 4.5 and 100 mm KCl was reduced from 75% to 80% in the presence of 2 mm CaCl2, whereas the proportion of low-DP products was less affected (Fig. 8A). At pH values suppressing the generation of oligomers (pH 6.0), cell walls provided with EDTA or subjected to alkaline deesterification before PG2 treatment released 4- to 5-fold higher levels of pectin compared with control samples; however, the greater yields were not accompanied by the generation of products that fractionated on Bio-Gel P-4 (Fig. 8B).
Figure 8.
Bio-Gel P-4 profiles of uronic acids released from mature-green cv Ailsa Craig cell walls by PG2 in the presence of EDTA or Ca2+. Cell walls (5 mg) in 2 mL of 20 mm KOAc and 100 mm KCl were incubated for 2 h at 34°C. Shown are uronic acids released at pH 4.5 and 1.5 mm CaCl2 (A) and at pH 6.0 and 2 mm EDTA (B). Total uronic acids in each fraction were determined at A520 (Abs 520nm).
DISCUSSION
This study demonstrates that the hydrolysis pattern of cell wall pectin by PG2 is strongly influenced by pH and ionic conditions over a range anticipated in vivo. Early reports for tomato fruit PG (McCready et al., 1955; Patel and Phaff, 1958, 1960) established that hydrolysis of pectic substrates was optimal at pH 4.5 (100 mm Ac), with a second optimum at pH 3.5 for hydrolysis of tri- and tetragalacturonic acids. These early studies, together with the observation that PG activity is stimulated by NaCl (Pressey and Avants, 1973), have resulted in the adoption of pH 4.0 to 4.5 and 100 to 150 mm NaCl for assaying PG from many types of fruits. Recent analyses, however, indicate that these conditions are not representative of the apoplastic environment of developing tomato fruit (Ruan et al., 1996).
The hydrolysis of PGA by PG2 in the presence of K+ levels reported for tomato fruit apoplast was different from that noted using traditional assay conditions. Compared with Na+, K+ resulted in an upward shift in pH optimum and persistence of catalysis at higher pH values (5.5 and 6.0). [Na+] in apoplastic exudate from tomato fruit averaged only 0.5 mm (Ruan et al., 1996), nearly 40-fold lower than apoplastic [K+] (Ruan et al., 1996) and 200-fold lower than Na+ levels commonly used in assays for PG (Pressey and Avants, 1973).
Effects of cations and pH were also noted for the hydrolysis of native, cell wall-associated pectin. The cell walls used for substrate were treated with buffered phenol (Huber, 1991) and have not been found to contain residual active enzymes (Huber, 1992; Hedge and Maness, 1996; MacDougall et al., 1996). The release of pectic polymers from mature-green cell walls by PG2 was greater at lower pH (4.0–5.0) than at higher pH and was accompanied by the production of oligomeric and monomeric uronic acids. PG-mediated hydrolysis of pectins is enhanced by pectin methylesterase (EC 3.2.1.11) (Seymour et al., 1987; Burns and Pressey, 1988) and β-galactosidases (de Veau et al., 1993); however, our PG2 preparations exhibited no activity of these enzymes in assays up to 6 h, and yielded two bands corresponding in molecular mass to PG2A and PG2B (Ali and Brady, 1982) on SDS-PAGE (not shown). With the possible exception of PG1, which can be formed after binding of PG2 to cell walls (Chun and Huber, 1997), the present study demonstrates that PG2 can achieve significant solubilization and depolymerization of native pectic polymers without the concurrent participation of other enzymes. The cation levels used in our studies did not promote pectin solubilization in the absence of PG, indicating that cation-facilitated disaggregation of pectin (Fishman et al., 1989) may require concurrent or previous enzyme involvement.
Although PG is capable of producing oligomeric products from native cell wall pectins under favorable conditions in vitro (Huber and Lee, 1988), the extent to which low-DP products are produced during ripening is uncertain. Pectic oligomers were not detected in ripe cv Sunny tomato fruit (Huber and O'Donoghue, 1993), whereas fragments of DP 4 to 12 were recovered from cv Castlemart fruit as early as the breaker stage of ripening (Melotto et al., 1994). The data in the present study indicate that pH and ionic conditions approximating the apoplastic environment of immature-green (Ruan et al., 1996) and mature-green (D.P.F. Almeida and D.J. Huber, unpublished data) tomato fruit do not favor the production of low-DP pectic fragments. Limitations in pectin hydrolysis might also be imposed by interactions of PG2 with the β-subunit protein (Knegt et al., 1991; Zheng et al., 1992; Chun and Huber, 1997), although the subunit appears to exert greater influence on the quantity of pectins hydrolyzed than on the extent of hydrolysis (Chun and Huber, 1997).
The diffusional freedom or mobility of wall-associated proteins has also been implicated as a means of regulating the activity of PG. Little wall-associated PG2 is released during in vitro catalysis (Chun and Huber, 1997), and sustained pectin solubilization requires continued provision of fresh enzyme (Rushing and Huber, 1990), consistent with a relatively immobile status of the enzyme. The parallel interruption of PG deposition and pectin solubilization in silver-thiosulfate-treated tomato fruit (Smith et al., 1989) and the finding that PG2 deposition occurs initially in discrete cell wall domains (Steele et al., 1997) provide evidence for the limited mobility of PG in vivo.
Ca2+ provides a potent means for negative control of pectin hydrolysis. Although the cation strongly suppressed the quantity of pectin solubilized by PG, the proportional generation of oligomers was much less affected. In agreement with this finding, the 4- to 5-fold stimulation in PG-mediated pectin solubilization at pH 6.0 by Ca2+ removal (i.e. EDTA) did not enhance oligomer recovery. That the extent of depolymerization is more closely controlled by reaction environment than by the quantity of active enzyme is further supported by the observation that cell walls from tomato fruit at the early stages of ripening exhibit a low capacity for autolytic pectin release (< 5% ripe fruit cell walls) yet generate a high proportional recovery of oligomers under suitable reaction conditions (Huber and Lee, 1988).
Our data indicate that reductions in pH and increases in [K+] enhance the activity (greater pectin solubilization) and might influence the hydrolysis pattern of PG. These observations predict that disruption of fruit tissue integrity, with the resulting decrease in bulk pH and elevation in the levels of ions, including K+, should promote greater activity of and more exhaustive hydrolysis by PG. Changes in apoplastic pH and [K+] in response to fruit-handling injury or as a consequence of senescence-related membrane dysfunction might explain the differences in uronic acid oligomer levels reported for tomato fruit (Huber and O'Donoghue, 1993; Melotto et al., 1994). As our data show, a decrease of as little as 1.0 pH unit significantly increases the capacity of PG2 to produce low-DP products. The influence of pH in controlling oligomer production may be caused by direct effects on PG2 protein or by altering ionic interactions between PG2 and the anionic pectin. The decreased binding tenacity of cell walls for PG2 over the pH range from 4.5 to 6.0 (Pressey, 1986) might be accompanied by changes in the effective enzyme population in the vicinity of the substrate.
The conditions adopted herein for characterizing PG2 hydrolysis of cell walls were based on reports of the apoplastic composition of immature cv Floradade tomato fruit. Although the apoplastic environment of young, developing fruit may reflect processes dependent on or causing expansion growth, we have found that the pH of apoplastic fluid from fully expanded, mature-green cv Ailsa Craig tomato fruit was similar (5.8–6.0; D.P.F. Almeida and D.J. Huber, unpublished data) to the value reported for immature-green fruit (Ruan et al., 1996).
The idea that PG activity is restricted by apoplastic conditions might explain the minimal effects of the PG-antisense gene on pectin depolymerization despite major reductions in the levels of PG enzyme (Smith et al., 1990). PG-antisense fruit do show more persistent viscosity of extracted juice products compared with wild-type lines (Schuch et al., 1991; Kramer et al., 1992). Homogenates of tomato fruit exhibit pH values of 4.3 to 4.5 (Al-Shaibani and Greig, 1979) and K+ levels near 50 mm (Ruan et al., 1996). These conditions are strongly promotive of PG and would explain why the juice products from wild-type fruit compared with the PG-antisense fruit are more divergent in behavior than the intact, healthy fruit. Conditions conducive to higher PG activity and other cell wall hydrolases could also arise as a consequence of stresses that compromise the integrity of membranes. The long-term consequence of these stress factors might depend in part on their effects on cell wall hydrolases, and tissues expressing reduced levels of specific enzymes may show greater resilience to altered apoplastic conditions. The reduced susceptibility to bruising injury and cracking of PG-antisense fruit compared with normal fruit (Schuch et al., 1991; Kramer et al., 1992) is consistent with this explanation.
It is not known whether the restrictions in pectin hydrolysis in ripening tomato fruit are characteristic of pectin metabolism in other fruits. Depolymerization of pectic polymers in ripening cvs Hass and Fuerte avocado fruit was more extensive than that observed in cv Sunny tomato fruit, even though extractable PG activity was similar (Huber and O'Donoghue, 1993). If the pH of avocado fruit apoplast is near the values reported for tomato fruit, then the higher pH optimum (5.5–6.0) for avocado PG (Huber and O'Donoghue, 1993) compared with the tomato enzyme may explain the more extensive depolymerization occurring during ripening of this fruit.
The present study focused on pH and cation levels as they influence PG. Effects on other enzymes are anticipated, and the numerous cell wall enzymes described in fruit tissues exhibit widely different responses to pH and cation levels (Huber, 1983). Apoplastic conditions could provide an elegant means for regulating the sequence of disassembly of specific wall polymers during ripening.
Abbreviations:
- Ac
acetate
- DP
degree of polymerization
- PGA
polygalacturonic acid
- PG1 and PG2
polygalacturonase isozymes 1 and 2, respectively
Footnotes
This work was supported in part by U.S. Department of Agriculture Competitive Grant no. 93-37304-9575. This is journal series no. R06232 of the Florida Agricultural Experiment Station.
LITERATURE CITED
- Ali ZM, Brady CJ. Purification and characterization of the polygalacturonases of tomato fruits. Aust J Plant Physiol. 1982;9:155–169. [Google Scholar]
- Al-Shaibani AMH, Greig JK. Effects of stage of maturity, storage, and cultivar on some quality attributes of tomatoes. J Am Soc Hortic Sci. 1979;104:880–882. [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Burns JK, Pressey R. Enhancement of the release of protoplasts and pectin from tomato locular gel by pectin methylesterase. J Am Soc Hortic Sci. 1988;113:624–626. [Google Scholar]
- Chun J-P, Huber DJ. Polygalacturonase isozyme 2 binding and catalysis in cell walls from tomato fruit: pH and β-subunit effects. Physiol Plant. 1997;101:283–290. [Google Scholar]
- DellaPenna D, Lashbrook C, Toenjes K, Giovannoni JJ, Fischer RL, Bennett AB. Polygalacturonase isozymes and pectin depolymerization in transgenic rin tomato fruit. Plant Physiol. 1990;94:1882–1886. doi: 10.1104/pp.94.4.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Veau EJ, Gross KC, Huber DJ, Watada A. Degradation and solubilization of pectin by beta-galactosidases purified from avocado mesocarp. Physiol Plant. 1993;87:279–285. [Google Scholar]
- Fishman ML, Gross KC, Gillespie DT, Sondey SM. Macromolecular components of tomato fruit pectin. Arch Biochem Biophys. 1989;274:179–191. doi: 10.1016/0003-9861(89)90429-3. [DOI] [PubMed] [Google Scholar]
- Giovannoni JJ, DellaPenna D, Bennett AB, Fischer RL. Expression of a chimeric polygalacturonase gene in transgenic rin (ripening inhibitor) tomato fruit results in polyuronide degradation but not fruit softening. Plant Cell. 1989;1:53–63. doi: 10.1105/tpc.1.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignon C, Sentenac H. pH and ionic conditions in the apoplast. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:103–128. [Google Scholar]
- Hegde S, Maness NO. Sugar composition of pectin and hemicellulose extracts of peach fruit during softening over two harvest seasons. J Am Soc Hortic Sci. 1996;121:1162–1167. [Google Scholar]
- Huber DJ. The role of cell wall hydrolases in fruit softening. Hortic Rev. 1983;5:169–219. [Google Scholar]
- Huber DJ. Acidified phenol alters tomato cell wall pectin solubility and calcium content. Phytochemistry. 1991;30:2523–2527. [Google Scholar]
- Huber DJ. The inactivation of pectin depolymerase associated with isolated tomato fruit cell wall: implications for the analysis of pectin solubility and molecular weight. Physiol Plant. 1992;86:25–32. [Google Scholar]
- Huber DJ, Lee JH. Uronic acid products release from enzymically active cell wall from tomato fruit and its dependency on enzyme quantity and distribution. Plant Physiol. 1988;87:592–597. doi: 10.1104/pp.87.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber DJ, O'Donoghue EM. Polyuronides in avocado (Perseaamericana) and tomato (Lycopersiconesculentum) fruits exhibit markedly different patterns of molecular weight downshifts during ripening. Plant Physiol. 1993;102:473–480. doi: 10.1104/pp.102.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knegt E, Vermeer E, Pak C, Bruinsma J. Function of the polygalacturonase convertor in ripening tomato fruit. Physiol Plant. 1991;82:237–242. [Google Scholar]
- Kramer M, Sanders R, Bolkan H, Waters C, Sheey RE, Hiatt WR. Postharvest evaluation of transgenic tomatoes with reduced levels of polygalacturonase: processing, firmness and disease resistance. Postharvest Biology and Technology. 1992;1:241–255. [Google Scholar]
- MacDougall AJ, Needs PW, Rigby NM, Ring SG. Calcium gelation of pectic polysaccharides isolated from unripe tomato fruit. Carbohydr Res. 1996;923:235–249. [Google Scholar]
- McCready RM, McComb EA, Jansen EF. The action of tomato and avocado polygalacturonase. Food Res. 1955;20:186–191. [Google Scholar]
- Melotto E, Greve LC, Labavitch JM. Cell wall metabolism in ripening fruit. VII. Biologically active pectin oligomers in ripening tomato (Lycopersicon esculentum Mill.) fruits. Plant Physiol. 1994;106:575–581. doi: 10.1104/pp.106.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner Y, Avigad G. A copper reagent for the determination of hexuronic acids and certain ketohexoses. Carbohydr Res. 1967;4:359–361. [Google Scholar]
- Mort AJ, Moerschbacher BM, Pierce ML, Maness NO. Problems encountered during the extraction, purification, and chromatography of pectic fragments, and some solutions to them. Carbohydr Res. 1991;215:219–227. [Google Scholar]
- Patel DS, Phaff HJ. On the action of purified “tomato polygalacturonase.”. Food Res. 1958;23:693–694. [Google Scholar]
- Patel DS, Phaff HJ. Properties of purified tomato polygalacturonase. Food Res. 1960;25:47–57. [Google Scholar]
- Pressey R. Extraction and assay of tomato polygalacturonases. HortScience. 1986;21:490–492. [Google Scholar]
- Pressey R, Avants JK. Two forms of polygalacturonase in tomatoes. Biochim Biophys Acta. 1973;309:363–369. doi: 10.1016/0005-2744(73)90035-1. [DOI] [PubMed] [Google Scholar]
- Ruan Y-L, Mate C, Patrick JW, Brady CJ. Non-destructive collection of apoplast fluid from developing tomato fruit using a pressure dehydration procedure. Aust J Plant Physiol. 1995;22:761–769. [Google Scholar]
- Ruan Y-L, Patrick JW, Brady CJ. The composition of apoplast fluid recovered from intact developing tomato fruit. Aust J Plant Physiol. 1996;23:9–13. [Google Scholar]
- Rushing JW, Huber DJ. Mobility limitations of bound polygalacturonase in isolated cell wall from tomato pericarp tissue. J Am Soc Hortic Sci. 1990;115:97–101. [Google Scholar]
- Schuch W, Kanczler J, Robertson D, Hobson G, Tucker G, Grierson D, Bright S, Bird C. Fruit quality characteristics of transgenic tomato fruit with altered polygalacturonase activity. HortScience. 1991;26:1517–1520. [Google Scholar]
- Seymour GB, Lasslett Y, Tucker GA. Differential effects of pectolytic enzymes on tomato polyuronides in vivo and in vitro. Phytochemistry. 1987;26:3137–3139. [Google Scholar]
- Smith CJS, Watson CF, Morris PC, Bird CR, Seymour GB, Gray JE, Arnold C, Tucker GA, Schuch W, Harding S and others. Inheritance and effect on ripening of antisense polygalacturonase genes in transgenic tomatoes. Plant Mol Biol. 1990;14:369–379. doi: 10.1007/BF00028773. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Smith R, Seymour G, Tucker GA. Inhibition of cell wall degradation by silver (I) ions during ripening of tomato fruit. J Plant Physiol. 1989;134:514–516. [Google Scholar]
- Steele NM, McMann MC, Roberts K. Pectin modification in cell walls of ripening tomatoes occurs in distinct domains. Plant Physiol. 1997;114:373–381. doi: 10.1104/pp.114.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Themmen APN, Tucker GA, Grierson D. Degradation of isolated tomato cell walls by purified polygalacturonase in vitro. Plant Physiol. 1982;69:122–124. doi: 10.1104/pp.69.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker GA, Robertson NG, Grierson D. The conversion of tomato-fruit polygalacturonase isoenzyme 2 into isoenzyme 1 in vitro. Eur J Biochem. 1981;115:87–90. doi: 10.1111/j.1432-1033.1981.tb06201.x. [DOI] [PubMed] [Google Scholar]
- Zheng L, Heupel RC, DellaPenna D. The beta subunit of tomato fruit polygalacturonase isoenzyme 1: isolation, characterization, and identification of unique structural features. Plant Cell. 1992;4:1147–1156. doi: 10.1105/tpc.4.9.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]